Abstract
Background:
Male breast cancer (MBC) is a rare disease with limited understanding of treatment patterns and prognostic factors.
Methods:
Men with stage I-III breast cancer between 2004 and 2014 in the National Cancer Database were included. Trends in treatment modalities were described using average annual percentage change (AAPC) and estimated through Joinpoint trend analysis software. Kaplan-Meier curves and multivariate Cox proportional hazard regression model were used to compare survival between sub-groups and identify prognostic factors.
Results:
A total of 10,873 MBC cases were included, with a median age at diagnosis of 64 years. Breast conserving surgery was performed in 24%, and 70% of patients undergoing breast conservation received radiation. 44% received chemotherapy, and 62% of ER+ patients received endocrine therapy. OncotypeDx was ordered in 35% of node-negative patients with ER+/HER2- tumors. During the study period, there was a significant increase in the rates of total mastectomy, contralateral prophylactic mastectomy, post-breast-conservation radiation, OncotypeDx ordering and use of endocrine therapy (p<0.05).
In multivariate analysis, factors associated with worse overall survival were: older age, black race, higher Charlson comorbidity index, high tumor grade and stage, and undergoing total mastectomy. Residing in higher income area, PR+ tumors and administration of chemotherapy, radiation, and endocrine therapy were associated with better overall survival.
Conclusions:
Despite the lack of prospective randomized trials in MBC, our study demonstrates that treatment of this disease has evolved over the years. These findings further our understanding of the modern treatment and prognosis of MBC and identify several areas for further research.
Keywords: Male, Male Breast Cancer, Treatment, Survival, Trend, Pattern
Introduction
Male breast cancer (MBC) is a rare disease, comprising 1% of all breast cancer cases 1. Due to its rarity, prospective clinical trials specifically focused on MBC have not been conducted 2. However, the incidence of MBC has been noted to be rising over the past few decades 3, 4, and there is an increasing appreciation of differences in the tumor biology of female versus MBC, highlighting the need for studies focused on this unique population.
In many ways, male breast cancer resembles female breast cancer 5, but there are important differences. MBC tends to present at an older age, with more frequent lymph node metastases and a higher proportion of estrogen-receptor positive (ER+) tumors, compared to female breast cancer 5, 6. Risk factors for MBC are also slightly different. In contrast to female breast cancer, MBC is more likely to occur in the setting of a BRCA2 mutation rather than BRCA1 mutation 7. Also, low androgen state is a known risk factor for MBC 8.
In the last two decades, there has been significant progress in the local and systemic management of female breast cancer 9–13, but it is unclear if these advances have been applied to the management of MBC. Therefore our aim was to describe treatment patterns of MBC in the United States and identify associated prognostic factors.
Methods:
This study was performed using the National Cancer Database (NCDB), which is a clinical oncology database jointly sponsored by the American College of Surgeons and the American Cancer Society. The NCDB is sourced from hospital registry data that are collected in more than 1,500 Commission on Cancer-accredited facilities, and includes more than 70% of newly diagnosed cancer cases in the United States 14.
We identified male patients diagnosed with stage I-III invasive ductal carcinoma or invasive lobular carcinoma between 2004 and 2014 in the NCDB using a combination of histologic (ICD-O-3: 8500, 8520 and 8522), topographic, stage and sex-specific codes. Patients with unknown stage, a prior cancer diagnosis, or missing follow up data were excluded.
We estimated the median overall survival in MBC using the Kaplan-Meier method and performed comparisons among several strata using the log-rank test. We used the multivariate Cox proportional hazard regression model to identify predictors of survival in MBC. We used the Joinpoint trend analysis software to estimate the average annual percentage change (AAPC) in treatment modalities over a time period assuming a constant variance 15. We evaluated for significance in the time-trend in Joinpoint using the Monte Carlo permutation method 16. Kaplan-Meier curves were plotted in R (version 3.5.1). All other analyses were performed in SPSS (version 25).
Results:
A total of 10,873 patients with MBC were included in the analysis. The median duration of follow up was 55 months. The median age at breast cancer diagnosis was 64 years. Fifty-one percent of patients were diagnosed between ages 50–69, 15% were diagnosed before the age of 50, and 34% were diagnosed after the age of 69. Approximately 90% of patients had ER+ tumors. Triple-negative breast cancer accounted for 283 (5.5%) cases among 5148 patients with available data on ER, PR and HER2 receptor status. Most patients (43.4%) were stage II at diagnosis, followed by Stage I (37.9%). The trends in age and stage at diagnosis between 2004 and 2014 are shown in Supplemental Figure 1 and 2. Demographic and clinical characteristics are presented in Table 1.
Table 1:
Baseline characteristics
| N= 10873 | |
|---|---|
| Age at diagnosis: | |
| Mean age ± SD | 63.7 ± 12.9 |
| Median age | 64.0 |
| Range (minimum age – maximum age) | 23 – 90 |
| Race: | |
| White | 9144 (84.1%) |
| Black | 1287 (11.8%) |
| Other | 296 (2.7%) |
| Unknown | 146 (1.3%) |
| Insurance status: | |
| Not insured | 269 (2.5%) |
| Private insurance | 5010 (46.1%) |
| Government insurance | 5359 (49.3%) |
| Unknown | 235 (2.2%) |
| Median household income in area of residence:# | |
| < $ 38,000 | 1681 (15.5%) |
| $ 38,000 – $ 62.999 | 5159 (47.4%) |
| $ 63,000+ | 3928 (36.1%) |
| Unknown | 105 (1.0%) |
| Adults without high school degree in area of residence:* | |
| > 21% | 1520 (14.0%) |
| 7 – 20.99% | 6239 (57.4%) |
| < 7% | 3013 (27.7%) |
| Unknown | 101 (0.9%) |
| County of residence: | |
| Metro counties | 9107 (83.8%) |
| Urban counties | 1265 (11.6%) |
| Rural counties | 155 (1.4%) |
| Unknown | 346 (3.2%) |
| Co-morbidity Index (Charlson/Deyo score): | |
| 0 – 1 | 10384 (95.5%) |
| 2 – 3 | 489 (4.5%) |
| Histology: | |
| Ductal | 10196 (93.8%) |
| Lobular | 367 (3.4%) |
| Mixed ductal and lobular | 310 (2.9%) |
| Grade: | |
| Well differentiated | 1450 (13.3%) |
| Moderately differentiated | 5186 (47.7%) |
| Poorly differentiated/undifferentiated | 3643 (33.5%) |
| Unknown | 594 (5.5%) |
| Overall TNM Stage: | |
| Stage I | 4120 (37.9%) |
| Stage II | 4716 (43.4%) |
| Stage III | 2037 (18.7%) |
| T-stage: | |
| T1 | 4513 (41.5%) |
| T2 | 3559 (32.7%) |
| T3 | 275 (2.5%) |
| T4 | 526 (4.8%) |
| Unknown | 2000 (18.4%) |
| N-stage: | |
| N0 | 4754 (43.7%) |
| N1 | 2564 (23.6%) |
| N2 | 913 (8.4%) |
| N3 | 408 (3.8%) |
| Unknown | 2234 (20.5%) |
| Estrogen receptor status: | |
| Negative | 779 (7.2%) |
| Positive | 9648 (88.7%) |
| Unknown | 446 (4.1%) |
| Progesterone receptor status: | |
| Negative | 1692 (15.6%) |
| Positive | 8695 (80.0%) |
| Unknown | 486 (4.5%) |
| HER2 receptor status$: | |
| Negative | 4350 (40.0%) |
| Positive | 627 (5.8%) |
| Equivocal or indeterminate | 192 (1.8%) |
| Unknown | 5704 (52.5%) |
Median household income in each patient’s area of residence as derived from the 2012 American Community Survey data
Number of adults in the patient’s zip code who did not graduate from high school as derived from the 2012 American Community Survey data
HER2 status was only available for patients diagnosed in 2010 or afterwards.
Treatment Patterns: Surgical
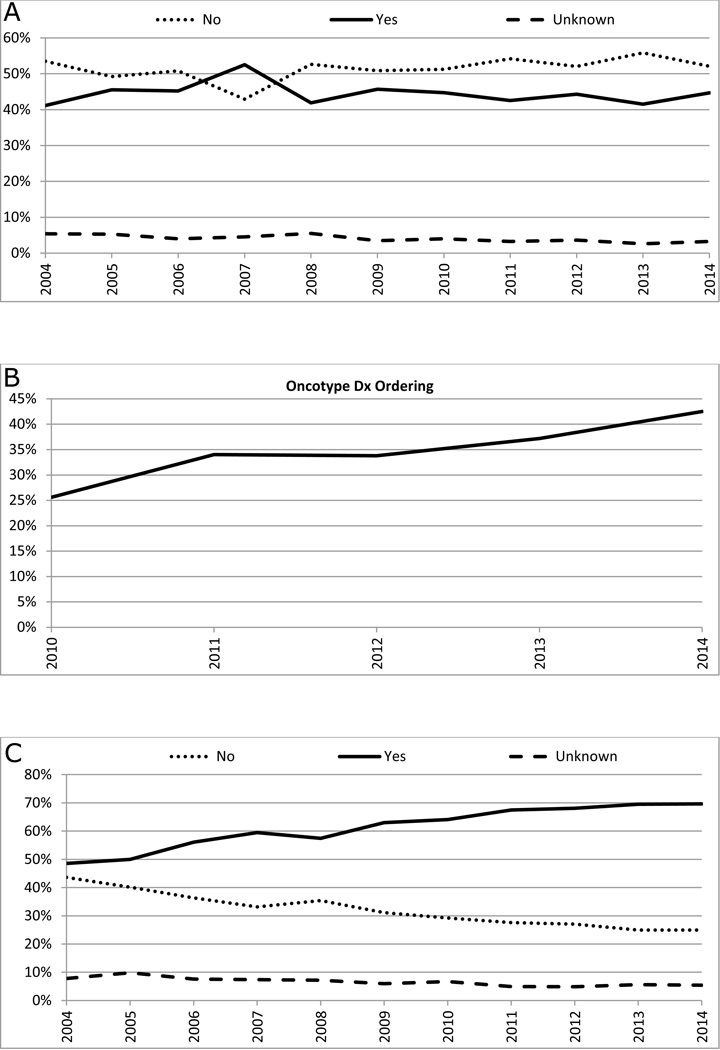
Total mastectomy was performed in 71.3%, while breast conserving surgery (BCS) was performed in 23.7% (Table 2). Between 2004 and 2014, there was a significant increase in the rate of total mastectomy (AAPC: +0.8, 95% CI: +0.2 to +1.3, p<0.05) and a decrease in the rate of BCS (AAPC: −2.2, 95% CI: −3.8 to −0.5, p<0.05, Figure 1A). 6.1% of patients underwent contralateral prophylactic mastectomy (CPM) (Table 2). There was a significant increase in CPM between 2004 and 2014 (AAPC: +10.4, 95% CI: +7.8 to +13.0, p<0.05) (Figure 1B). Sentinel lymph node surgery and/or axillary lymph node dissection was performed in 95.4% of the patients who underwent surgery (Table 2), 90.2% of patients who underwent BCS and 97.2% of patients who underwent mastectomy, and there was no significant change in this rate over time (p=0.3, Figure 1C).
Table 2:
Treatment patterns
| N= 10873 | |
|---|---|
| Type of surgery: | |
| Breast conserving surgery | 2572 (23.7%) |
| Total Mastectomy | 7755 (71.3%) |
| Unknown | 137 (1.3%) |
| No surgery | 409 (3.7%) |
| Contralateral prophylactic mastectomy (CPM):* | |
| Did not undergo CPM | 8226 (79.7%) |
| Underwent CPM | 632 (6.1%) |
| Unknown if CPM performed | 1469 (14.2%) |
| Surgical evaluation of axilla:* | |
| No | 450 (4.4%) |
| Yes | 9855 (95.4%) |
| Unknown | 22 (0.2%) |
| Adjuvant Radiation:* | |
| No | 6108 (59.1%) |
| Yes | 4070 (39.4%) |
| Unknown | 149 (1.4%) |
| Adjuvant radiation after breast conservation:# | |
| No | 728 (28.3%) |
| Yes | 1806 (70.2%) |
| Unknown | 38 (1.5%) |
| Adjuvant chest-wall radiation after mastectomy:$ | |
| No radiation | 5380 (69.4%) |
| Received radiation | 2264 (29.2%) |
| Unknown | 111 (1.4%) |
| Chemotherapy: | |
| Chemotherapy not administered | 5598 (51.5%) |
| Chemotherapy administered | 4841 (44.5%) |
| Unknown | 434 (4.0%) |
| Hormone therapy:¥ | |
| Hormone therapy not given | 3010 (31.2%) |
| Hormone therapy given | 6015 (62.3%) |
| Unknown | 623 (6.5%) |
: Analysis restricted to patients who underwent local surgery for breast cancer (N=10327)
Analysis restricted to patients who underwent breast conservation surgery (N= 2572)
: Analysis restricted to patients who underwent mastectomy (N= 7755)
: Analysis restricted to patients with estrogen receptor positive tumors (N=9648)
Figure 1: Trends in surgical treatment patterns of male breast cancer*.
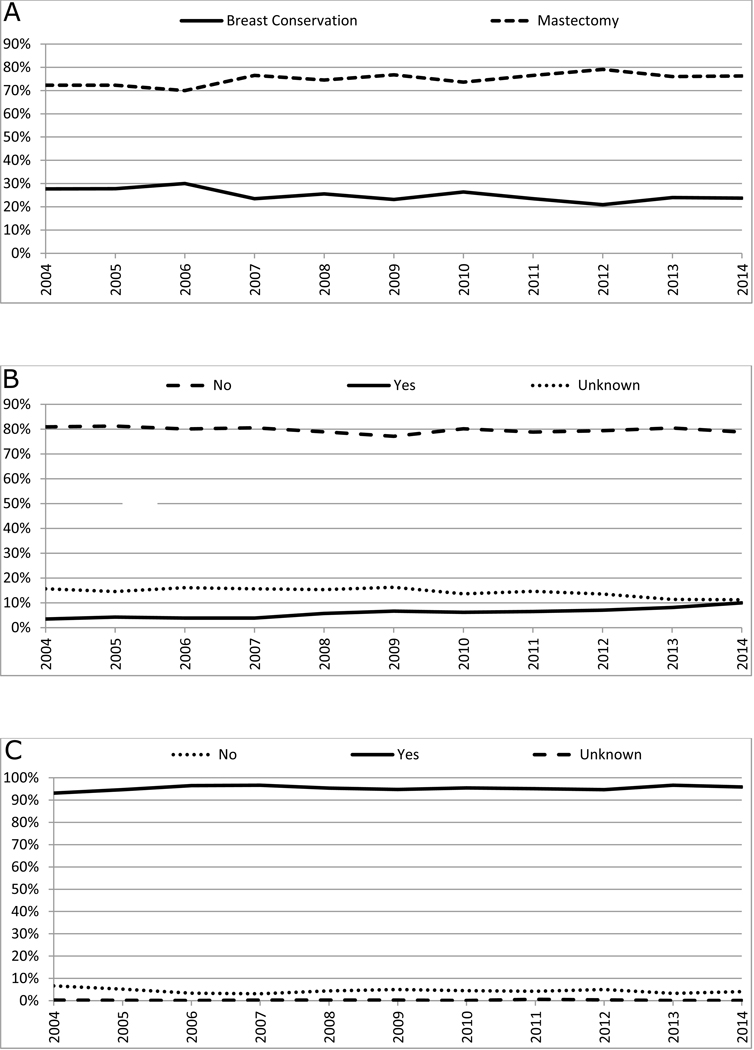
Panel A: Trends in local surgery#
Panel B: Trends in contralateral prophylactic mastectomy$
Panel C: Trends in surgical evaluation of axilla€
*: Among patients who underwent local surgery (N=10,327)
#: AAPC: Breast conservation: −2.2, 95% CI: −3.8 to −0.5, P<0.05; Mastectomy: +0.8, 95% CI: +0.2 to +1.3, P<0.05;
$: AAPC: No: −0.2, 95% CI: −0.5 to 0.1, P=0.2; Yes: +10.4, 95% CI: +7.8 to +13.0, P<0.05; Unknown: −3.1, 95% CI: −5.5 to −0.5, P<0.05;
€: AAPC: No: −2.2, 95% CI: −6.9 to 2.6, P=0.3; Yes: +0.1, 95% CI: −0.1 to +0.4, P=0.3; Unknown: −2.3, 95% CI: −14.6 to +11.9, P=0.7
Treatment Patterns: Radiation
A total of 4,070 (39.4%) patients received some form of adjuvant radiation (Table 2). Among patients who underwent BCS, adjuvant radiation was administered in 70.2% of the patients overall, in 74.5% of those aged <70, and in 59.4% of those ≥70 years. Among patients who underwent mastectomy, post-mastectomy radiation was administered in 29.2% overall, and in 49% with tumors > 5 cm or one or more positive lymph nodes. By stage, post-mastectomy radiation was administered in 6.7%, 27.0% and 64.3% of patients with stage I, II and III disease, respectively. The proportion of patients who underwent radiation after BCS increased from 66.0% in 2004 to 74.6% in 2014 (AAPC: +1.6, 95% CI: 0.9 to 2.2, p<0.05, Figure 2A), while the post-BCS radiation proportion did not change significantly for patients ≥ 70 years with ER+ tumors (Figure 2B). The proportion of patients who received post-mastectomy radiotherapy for tumors >5 cm or one or more positive lymph nodes also significantly increased during the same time-period (AAPC: +1.6, 95% CI: 0.0 to 3.1) (Figure 2C). Thirteen (0.1%) patients received proton beam therapy and 143 (5.5% of patients undergoing BCS) received brachytherapy after surgery between 2004 and 2014.
Figure 2: Trends in radiation therapy.
Panel A: Trends in radiation after breast conservation therapy*
Panel B: Trends in radiation after breast conservation therapy among older patients$
Panel C: Trends in post-mastectomy radiation€
*: Among patients who underwent breast conservation therapy (N=2,752); AAPC: No: −3.0, 95% CI: −4.5 to −1.5, P<0.05; Yes: +1.6, 95% CI: 0.9 to 2.2, P<0.05; Unknown: unable to estimate;
$: Among patients older than 70 years with ER+ tumors who underwent breast conservation (N=635); AAPC: No: −1.2, 95% CI: −3.1 to −0.8, P=0.2; Yes: +1.1, 95% CI: −0.6 to 2.7, P=0.2; Unknown: unable to estimate;
€: Among patients with tumor greater than 5 cm or node positive disease who underwent mastectomy (N=3443); AAPC: No −1.1, 95% CI: −2.2 to −0.1, P=0.05; Yes: +1.6, 95% CI: 0.0 to 3.1, P<0.05; Unknown: −9.2, 95% CI: −20.1 to +3.2, P=0.1;
Treatment Patterns: Systemic
In total, 4,841 (45.5%) patients received chemotherapy (Table 2), and there was no significant change in the overall chemotherapy rate between 2004 and 2014 (AAPC: −0.3, 95% CI: −1.8 to +1.2, p=0.6, Figure 3A). Information on sequence of chemotherapy with surgery was available from 2006 onwards, and 527(12.6%) received neoadjuvant chemotherapy among 4,165 patients who received chemotherapy between 2006 and 2014, and there was no significant change in the use of neoadjuvant chemotherapy during this time period (AAPC: +3.1, 95%CI: −3.2 to +9.8, p=0.3). We analyzed the use of multigene expression signatures among the 2,094 patients with, ER+, HER-2-negative, node-negative MBC between 2010 and 2014, and found that 765 (36.5%) had a gene expression test ordered. The most commonly ordered test was the Oncotype Dx 21-gene recurrence score (731 patients, 34.9%). A statistically significant increase in the use of Oncotype Dx between 2010 and 2014 was noted (Figure 3B). In the 731 patients who underwent Oncotype Dx testing, 403 (55.1%) were found to have a recurrence score of <18, 234 (32.0%) had scores between 18 and 31, and 66 (9.0%) had a score > 31. In patients with well-differentiated tumors, less than 1% had an OncotypeDx recurrence score > 31, while approximately 25% of patients with poorly differentiated tumors had an OncotypeDx score > 31 (Supplemental Table 1). There was a significantly higher use of chemotherapy in patients with Oncotype Dx recurrence score >31 compared to those with score <18 (72.7% vs. 4.7%, p<0.001).
Figure 3: Trends in systemic therapy.
Panel A: Trends in chemotherapy use*
Panel B: Trends in ordering of Oncotype Dx testing$
Panel C: Trends in the use of hormonal therapy among patients with estrogen receptor positive tumors€
*: AAPC: No: +0.8, 95% CI: −0.6 to 2.3, P=0.2; Yes: −0.3, 95% CI: −1.8 to 1.2, P=0.6; Unknown: −5.8, 95% CI: −8.6 to −2.8, P<0.05;
$: Among patients with node-negative, estrogen receptor-positive, and HER-2-negative tumors between 2010 and 2014 (N=2094); AAPC: +11.7, 95% CI: 3.6 to 20.3, P<0.05;
€: AAPC: No: −5.4, 95% CI: −6.2 to −4.6, P<0.05; Yes: +4.0, 95% CI: +2.5 to +5.6, P<0.05; Unknown: −5.6, 95% CI: −7.9 to −3.2, P<0.05
Among patients with ER+ tumors, 62.3% received adjuvant endocrine therapy (Table 2). The use of endocrine therapy in ER+ MBC increased significantly from 48.6% in 2004 to 69.5% in 2014 (AAPC: +4.0, 95% CI: +2.5 to +5.6, p<0.05, Figure 3C).
Survival analysis
The 5- year overall survival for the entire cohort was 79.1%. Median overall survival was 12.1 years (Supplemental Figure 3). The median overall survival for stage I MBC was not reached in this cohort, for stage II MBC was 11.5 years, and for stage III MBC was 7.2 years (Supplemental Figure 4a). Overall survival did not change significantly between 2004 and 2014 (Supplemental Figure 4b). Factors associated with overall survival in univariate analysis are shown in Table 3. In multivariate analysis, factors associated with worse overall survival were: older age, black race, higher Charlson comorbidity index, high tumor grade, high tumor and nodal stage, and undergoing total mastectomy. Residing in a high income area, progesterone-receptor positive (PR+) tumors and administration of chemotherapy, radiation or endocrine therapy were associated with better overall survival (Table 3).
Table 3:
Univariate and multivariate cox proportional hazard regression analysis for overall survival€
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratios (95% CI) |
P-value | Hazard ratios (95% CI) | P-value | |
| Age at diagnosis | 1.05 (1.04 – 1.05) | <0.001 | 1.04 (1.03 – 1.04) | <0.001 |
| Race: | ||||
| White | 1 | 1 | ||
| Black | 1.19 (1.06 – 1.35) | 0.004 | 1.22 (1.07 – 1.38) | 0.003 |
| Other | 0.64 (0.47 – 0.88) | 0.006 | 0.73 (0.53 – 1.00) | 0.051 |
| Unknown | 0.82 (0.55 – 1.22) | 0.331 | 0.84 (0.57 – 1.25) | 0.391 |
| Insurance status: | ||||
| Not insured | 1 | 1 | ||
| Private insurance | 0.62 (0.46 – 0.82) | 0.001 | 0.75 (0.57 – 1.00) | 0.052 |
| Government insurance | 1.57 (1.19 – 2.08) | 0.002 | 0.95 (0.71 – 1.26) | 0.714 |
| Unknown | 1.15 (0.76 – 1.74) | 0.504 | 1.11 (0.74 – 1.69) | 0.609 |
| Median household income in area of residence: | ||||
| < $ 38,000 | 1 | 1 | ||
| $ 38,000 – $ 62.999 | 0.80 (0.72 – 0.90) | <0.001 | 0.89 (0.79 – 1.01) | 0.063 |
| $ 63,000+ | 0.63 (0.56 – 0.71) | <0.001 | 0.78 (0.67 – 0.91) | 0.002 |
| Unknown | 2.32 (1.69 – 3.18) | <0.001 | 1.36 (0.36 – 5.15) | 0.650 |
| Adults without high school degree in area of residence: | ||||
| > 21% | 1 | 1 | ||
| 7 – 20.99% | 0.93 (0.83 – 1.04) | 0.212 | 1.01 (0.89 – 1.16) | 0.810 |
| < 7% | 0.67 (0.58 – 0.76) | <0.001 | 0.86 (0.72 – 1.01) | 0.070 |
| Unknown | 2.61 (1.88 – 3.62) | <0.001 | 1.28 (0.32 – 5.02) | 0.726 |
| County of residence: | ||||
| Metro counties | 1 | 1 | ||
| Urban counties | 1.17 (1.03 – 1.32) | 0.013 | 1.08 (0.95 – 1.22) | 0.253 |
| Rural counties | 1.27 (0.94 – 1.72) | 0.125 | 1.23 (0.90 – 1.67) | 0.191 |
| Unknown | 1.57 (1.28 – 1.92) | <0.001 | 1.23 (0.96 – 1.56) | 0.094 |
| Co-morbidity Index (Charlson/Deyo score): | ||||
| 0 – 1 | 1 | 1 | ||
| 2 – 3 | 3.26 (2.84 – 3.74) | <0.001 | 2.22 (1.93 – 2.55) | <0.001 |
| Histology: | ||||
| Ductal | 1 | 1 | ||
| Lobular | 0.84 (0.67 – 1.06) | 0.150 | 0.93 (0.73 – 1.18) | 0.528 |
| Mixed ductal and lobular | 0.72 (0.55 – 0.95) | 0.018 | 0.94 (0.72 – 1.23) | 0.659 |
| Grade: | ||||
| Well differentiated | 1 | 1 | ||
| Moderately differentiated | 1.52 (1.31 – 1.75) | <0.001 | 1.28 (1.10 – 1.49) | 0.001 |
| Poorly differentiated/Undifferentiated | 2.11 (1.82 – 2.44) | <0.001 | 1.68 (1.44 – 1.95) | <0.001 |
| Unknown | 1.18 (0.92 – 1.52) | <0.001 | 1.07 (0.83 – 1.38) | 0.614 |
| Overall TNM Stage: | ||||
| Stage I | 1 | 1 | ||
| Stage II | 1.79 (1.61 – 1.98) | <0.001 | 1.85 (1.66 – 2.06) | <0.001 |
| Stage III | 3.29 (2.95 – 3.67) | <0.001 | 3.92 (3.44 – 4.46) | <0.001 |
| Estrogen receptor status: | ||||
| Negative | 1 | 1 | ||
| Positive | 1.03 (0.88 – 1.21) | 0.725 | 1.01 (0.83 – 1.23) | 0.921 |
| Unknown | 1.24 (0.98 – 1.56) | 0.070 | 1.46 (0.74 – 2.86) | 0.273 |
| Progesterone receptor status: | ||||
| Negative | 1 | 1 | ||
| Positive | 0.87 (0.79 – 0.97) | 0.012 | 0.82 (0.72 – 0.93) | 0.002 |
| Unknown | 1.04 (0.86 – 1.26) | 0.705 | 0.59 (0.31 – 1.12) | 0.106 |
| Type of surgery: | ||||
| Breast conserving surgery | 1 | 1 | ||
| Total mastectomy | 1.85 (1.66 – 2.07) | <0.001 | 1.16 (1.02 – 1.31) | 0.020 |
| Radiation: | ||||
| No radiation | 1 | 1 | ||
| Received radiation | 0.77 (0.71 – 0.84) | <0.001 | 0.82 (0.74 – 0.90) | <0.001 |
| Unknown | 0.62 (0.44 – 0.89) | 0.008 | 0.77 (0.53 – 1.13) | 0.184 |
| Chemotherapy: | ||||
| Chemotherapy not administered | 1 | 1 | ||
| Chemotherapy administered | 0.67 (0.62 – 0.73) | <0.001 | 0.67 (0.60 – 0.74) | <0.001 |
| Unknown | 0.80 (0.65 – 0.98) | 0.030 | 0.92 (0.72 – 1.16) | 0.480 |
| Hormone therapy: | ||||
| Hormone therapy not given | 1 | 1 | ||
| Hormone therapy given | 0.69 (0.63 – 0.75) | <0.001 | 0.74 (0.68 – 0.81) | <0.001 |
| Unknown | 0.67 (0.57 – 0.80) | <0.001 | 0.70 (0.58 – 0.84) | <0.001 |
: Analysis restricted to patients who underwent surgery (N=10327);
In order to further clarify the role of chemotherapy and radiation in MBC, we performed separate univariate and multivariate analyses stratified by stage at diagnosis and type of surgery (Supplemental Figure 5-6, Supplemental Table 2–3). Among patients with ER+ tumors treated with surgery and endocrine therapy, chemotherapy was associated with improved overall survival in patients with stage II and III tumors in multivariate analysis (Supplemental Table 2). In multivariate analysis, post breast-conservation radiation therapy was associated with improved overall survival in all stages (Supplemental Table 3). Among 178 older patients (≥70 years) with T1N0 ER+ disease treated with BCS and endocrine therapy, we did not observe an association between radiation and overall survival in univariate analysis (Supplemental Figure 6B). Although there was a trend towards improved survival with radiation in this analysis, the number of cases were small to allow for a multivariate analysis. Post-mastectomy radiation was associated with poor overall survival in the entire mastectomy cohort in univariate analysis (Supplemental Figure 6C). In multivariate cox-proportional hazard regression model adjusting for variables listed in table 3, no statistically significant association was noted with post-mastectomy radiation (HR: 0.95, 95%CI: 0.85 – 1.07, p=0.43, Supplemental Table 4). However, in univariate analysis in a subset of patients with node-positive disease, post-mastectomy radiation was associated with a better overall survival (Supplemental Figure 6D).
Discussion
In one of the largest studies on MBC, we describe treatment patterns of MBC in the United States and identify prognostic factors. Similar to prior studies, our study confirms that in the US, the median age at diagnosis of MBC is in the mid-sixties 6, 17, 18, a majority of patients present with stage I or II disease 18 and most tumors are ER+ 18, 19. The rate of ER positivity in our study was approximately 89% (unknown in 4%), which is lower than two prior studies that applied uniform methods of receptor status assessment and classification across their study participants 6, 18, but similar to other large multi-institutional database-based studies on MBC 19, 20. These differences could be due to the change in the cutoffs for classification of ER positivity in 2010 21 or due to variations in laboratory techniques for assessing ER status across different institutions.
In this cohort, more than two-thirds of the patients underwent mastectomy, similar to prior reports on MBC 6, 22. This is in contrast to female breast cancer, for which approximately two-thirds of patients undergo BCS and one-third undergo mastectomy 23, 24. The observed difference in the rates of BCS and mastectomy between men and women is likely due to concern in men that all breast tissue at risk cannot be removed with adequate margins due to small breast size, and possibly due to differences in cosmetic goals between the sexes. In addition, MBCs are often centrally located and involve the nipple, necessitating removal of the nipple-areolar complex and limiting the potential aesthetic benefits of BCS 25. Similar to other recent studies 22, 26–28, our study suggests that BCS is likely a safe and effective option in select patients with MBC compared to total mastectomy. Despite this, we noted a slight decrease in the rates of BCS between 2004 and 2014. MBC care may have mirrored female breast cancer care, with more opting for mastectomy and potentially avoiding radiation despite the option to have BCS 24, 29. We found an association between total mastectomy and poor overall survival, which could be due to overrepresentation of patients with larger tumors and/or node-positive disease in this group, and this association remained significant on multivariate analysis. These findings require further evaluation. Surgical evaluation of the axilla (sentinel lymph node surgery and/or axillary dissection) was performed in >90% of the patients who underwent local surgery, similar to the rates reported by Cardoso et al. 6.
The magnitude of overall survival benefit of adjuvant radiotherapy observed in MBC was greater than that expected from studies of female breast cancer 30. Even among patients with stage 1 disease, the survival advantage was pronounced (Supplemental table 3). However, just 70% of patients that underwent breast conserving surgery were recorded as having received adjuvant radiation. Our findings suggest that radiation therapy should be considered for MBC patients who undergo BCS irrespective of the stage, which is the current standard of care for most women with invasive breast cancer. Considering this association with improved overall survival, it is encouraging to note the increasing trend in post-BCS radiation among MBC patients.
In older women with early stage ER+ tumors treated with BCS and hormonal therapy, additional radiation therapy may not improve overall survival 31–34, but in older men with similar disease, we noted a non-significant trend towards improved survival with radiation (Supplemental Figure 6B). It is possible that radiation may be more beneficial in this sub-group of men compared to women, due to poor compliance with endocrine therapy 35, 36 or due to unique tumor biology in men37. In terms of the post-mastectomy setting, we observed that radiation was associated with poor overall survival in the entire cohort in univariate analysis, likely due to higher risk disease features in those patients as this difference was not noted in multivariate analysis adjusting for stage, reflecting appropriate use of post-mastectomy radiation in patients with more advanced tumors. In addition, post-mastectomy radiation was associated with an improved overall survival in a sub-set of patients with node-positive disease, similar to prior studies 38.
The association of adjuvant endocrine therapy use and better overall survival illustrates its importance in the treatment of ER+ MBC, similar to prior studies in men 39, 40. While there was an overall increase in the use of adjuvant endocrine therapy during the study period, almost a third of men with ER+ breast cancer did not receive any endocrine therapy. Furthermore, we are unable to assess long-term compliance or duration of use in this study, both of which may be an issue in men 35, 36. Further studies evaluating factors influencing the decision to use or not use adjuvant endocrine therapy—the most effective form of systemic therapy for ER+ breast cancer—are needed. We also observed that chemotherapy was associated with better overall survival in patients with stage II or III MBC, but not in patients with stage I disease. Although prospective evidence to support their use is lacking, gene expression profiles such as the OncotypeDx recurrence score may be helpful in patients with MBC to decide regarding use of chemotherapy. The results from our study and other studies 41–43 suggest that the distribution of Oncotype Dx scores is similar between men and women and that Oncotype Dx is already increasingly being used in management of MBC. Long-term outcomes data are awaited to fully understand the implications of this practice.
Apart from treatment-related variables, we also identified several demographic and tumor-related parameters that have significant prognostic value (Table 3). Factors such as older age at diagnosis, higher T and N category and higher Charlson comorbidity index are associated with worse prognosis in several malignancies including female breast cancer 44, 45. Black race has been previously reported to be associated with poor prognosis in MBC 46, 47 and in female breast cancer 48, 49. Multiple factors may contribute to poor outcomes in black patients, including presence of multiple associated comorbidities, poor access to health care, and aggressive tumor biology 46, 50, 51. Higher income level has also been known to be associated with better outcomes in female breast cancer, although this is mostly true for whites 52.
In contrast to one of the seminal prior studies on MBC 6, we noted that higher grade of tumor is associated with poor overall survival, perhaps due to greater power from a larger sample size. Positive ER status was not found to be prognostic in our study, which may be due to the small number of ER negative patients in this cohort. In addition, our ER negative cohort may have included patients with low ER expression due to changes in the classification overtime 21. Our observation that PR negativity is associated with worse prognosis is consistent with findings in female breast cancer 53.
Our study is limited by its reliance on a retrospective database. For NCDB-based research, major limitations include the non-population based dataset with geographical and sociodemographic disparities in case coverage 54 and lack of recurrence data. Issues with under-ascertainment of treatment related variables have also been reported for similar datasets 55–57. In addition, we did not take the effect of changes in treatment over time into account when evaluating factors associated with overall survival. The small number of patients in some sub-groups, e.g. ER negative patients, also limits generalization of our findings to all MBC.
Conclusions:
In this large NCDB cohort, we identified several factors associated with prognosis in MBC and demonstrated that the treatment of MBC has evolved over the past decade, with increases in the rates of total mastectomy, post-BCS radiation, ordering of Oncotype Dx testing, and use of endocrine therapy. Our study highlights unique practice patterns and factors associated with prognosis in MBC, furthering our understanding of the treatment and prognosis of MBC and identifying unanswered questions for future research in MBC.
Supplementary Material
Key Message:
Male breast cancer is a rare disease for which our understanding of treatment patterns and prognostic factors is limited. In this analysis of 10, 873 male breast cancer cases in the National Cancer Database, the authors demonstrate the changes in treatment patterns in the United States over a 10-year period and identify several prognostic factors associated with this disease.
Acknowledgements:
This study was supported in part by the NCI Specialized Program of Research Excellence (SPORE) in Breast Cancer awarded to Mayo Clinic (P50 CA116201)
Footnotes
Conflict of Interest Disclosure: All other authors have nothing to disclose. Karthik V. Giridhar has received travel costs to his institution from Menarini Silicon Biosystems to attend an investigators meeting for work performed outside of the current study. Tina J. Hieken has received grants from Genentech for work performed outside of the current study. Roberto A. Leon-Ferre has received travel support from Immunomedics for work performed outside of the current study. Kathryn J. Ruddy inherited and then sold stock from Pfizer and Merck in February 2018. The other authors made no disclosures.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Duma N, Hoversten KP, Ruddy KJ. Exclusion of Male Patients in Breast Cancer Clinical Trials. JNCI Cancer Spectrum. 2018;2: pky018-pky018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015. Available from URL: https://seer.cancer.gov/csr/1975_2015/ [accessed December 4, 2018. [Google Scholar]
- 4.Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115: 429–430. [DOI] [PubMed] [Google Scholar]
- 5.Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83: 77–86. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29: 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pritzlaff M, Summerour P, McFarland R, et al. Male breast cancer in a multi-gene panel testing cohort: insights and unexpected results. Breast Cancer Res Treat. 2017;161: 575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans DB, Crichlow RW. Carcinoma of the male breast and Klinefelter’s syndrome: is there an association? CA Cancer J Clin. 1987;37: 246–251. [DOI] [PubMed] [Google Scholar]
- 9.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351: 2817–2826. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Jama. 2011;305: 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaidya JS, Massarut S, Vaidya HJ, et al. Rethinking neoadjuvant chemotherapy for breast cancer. Bmj. 2018;360: j5913. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 13.Njeh CF, Saunders MW, Langton CM. Accelerated Partial Breast Irradiation (APBI): A review of available techniques. Radiat Oncol. 2010;5: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Database (NCDB). Available from URL: https://www.facs.org/quality-programs/cancer/ncdb.
- 15.Joinpoint Trend Analysis Software Available from URL: https://surveillance.cancer.gov/joinpoint/.
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19: 335–351. [DOI] [PubMed] [Google Scholar]
- 17.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101: 51–57. [DOI] [PubMed] [Google Scholar]
- 18.Masci G, Caruso M, Caruso F, et al. Clinicopathological and Immunohistochemical Characteristics in Male Breast Cancer: A Retrospective Case Series. Oncologist. 2015;20: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male Breast Cancer: A Population-Based Comparison With Female Breast Cancer. Journal of Clinical Oncology. 2010;28: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Johnson KJ, Ma CX. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin Breast Cancer. 2018;18: e997-e1002. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134: 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fields EC, DeWitt P, Fisher CM, Rabinovitch R. Management of male breast cancer in the United States: a surveillance, epidemiology and end results analysis. Int J Radiat Oncol Biol Phys. 2013;87: 747–752. [DOI] [PubMed] [Google Scholar]
- 23.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. 2009;16: 2682–2690. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood U, Hanlon AL, Koshy M, et al. Increasing national mastectomy rates for the treatment of early stage breast cancer. Ann Surg Oncol. 2013;20: 1436–1443. [DOI] [PubMed] [Google Scholar]
- 25.Leon-Ferre RA, Giridhar KV, Hieken TJ, et al. A contemporary review of male breast cancer: current evidence and unanswered questions. Cancer Metastasis Rev. 2018. [DOI] [PubMed] [Google Scholar]
- 26.Cloyd JM, Hernandez-Boussard T, Wapnir IL. Outcomes of partial mastectomy in male breast cancer patients: analysis of SEER, 1983–2009. Ann Surg Oncol. 2013;20: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 27.Golshan M, Rusby J, Dominguez F, Smith BL. Breast conservation for male breast carcinoma. Breast. 2007;16: 653–656. [DOI] [PubMed] [Google Scholar]
- 28.Leone JP, Leone J, Zwenger AO, Iturbe J, Leone BA, Vallejo CT. Locoregional treatment and overall survival of men with T1a,b,cN0M0 breast cancer: A population-based study. Eur J Cancer. 2017;71: 7–14. [DOI] [PubMed] [Google Scholar]
- 29.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150: 9–16. [DOI] [PubMed] [Google Scholar]
- 30.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378: 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matuschek C, Bölke E, Haussmann J, et al. The benefit of adjuvant radiotherapy after breast conserving surgery in older patients with low risk breast cancer- a meta-analysis of randomized trials. Radiation Oncology. 2017;12: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31: 2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351: 971–977. [DOI] [PubMed] [Google Scholar]
- 34.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16: 266–273. [DOI] [PubMed] [Google Scholar]
- 35.Oke O, Niu J, Chavez-MacGregor M, Zhao H, Giordano SH. Adjuvant tamoxifen adherence in men with early stage breast cancer. Journal of Clinical Oncology. 2018;36: 550–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu S, Yang Y, Tao W, et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Res Treat. 2012;136: 495–502. [DOI] [PubMed] [Google Scholar]
- 37.Piscuoglio S, Ng CK, Murray MP, et al. The Genomic Landscape of Male Breast Cancers. Clin Cancer Res. 2016;22: 4045–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrams MJ, Koffer PP, Wazer DE, Hepel JT. Postmastectomy Radiation Therapy Is Associated With Improved Survival in Node-Positive Male Breast Cancer: A Population Analysis. Int J Radiat Oncol Biol Phys. 2017;98: 384–391. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro G, Swindell R. Adjuvant tamoxifen for male breast cancer (MBC). Br J Cancer. 1992;65: 252–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giordano SH, Perkins GH, Broglio K, et al. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104: 2359–2364. [DOI] [PubMed] [Google Scholar]
- 41.Peethambaram PP, Hoskin TL, Day CN, Goetz MP, Habermann EB, Boughey JC. Use of 21-gene recurrence score assay to individualize adjuvant chemotherapy recommendations in ER+/HER2- node positive breast cancer-A National Cancer Database study. NPJ Breast Cancer. 2017;3: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shak S, Palmer G, Baehner FL, Millward C, Watson D Jr., GWS. Molecular characterization of male breast cancer by standardized quantitative RT-PCR analysis: First large genomic study of 347 male breast cancers compared to 82,434 female breast cancers. Journal of Clinical Oncology. 2009;27: 549–549. [Google Scholar]
- 43.Giordano SH. Breast Cancer in Men. N Engl J Med. 2018;379: 1385–1386. [DOI] [PubMed] [Google Scholar]
- 44.Braithwaite D, Moore DH, Satariano WA, et al. Prognostic impact of comorbidity among long-term breast cancer survivors: results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012;21: 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen HL, Zhou MQ, Tian W, Meng KX, He HF. Effect of Age on Breast Cancer Patient Prognoses: A Population-Based Study Using the SEER 18 Database. PLoS One. 2016;11: e0165409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sineshaw HM, Freedman RA, Ward EM, Flanders WD, Jemal A. Black/White Disparities in Receipt of Treatment and Survival Among Men With Early-Stage Breast Cancer. J Clin Oncol. 2015;33: 2337–2344. [DOI] [PubMed] [Google Scholar]
- 47.Crew KD, Neugut AI, Wang X, et al. Racial disparities in treatment and survival of male breast cancer. J Clin Oncol. 2007;25: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 48.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24: 1342–1349. [DOI] [PubMed] [Google Scholar]
- 49.Du W, Simon MS. Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91: 243–248. [DOI] [PubMed] [Google Scholar]
- 50.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. Jama. 2005;294: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 51.Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66: 8327–8330. [DOI] [PubMed] [Google Scholar]
- 52.Lehrer S, Green S, Rosenzweig KE. Affluence and Breast Cancer. Breast J. 2016;22: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inic Z, Zegarac M, Inic M, et al. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin Med Insights Oncol. 2014;8: 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3: 1722–1728. [DOI] [PubMed] [Google Scholar]
- 55.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Healy MA, Morris AM, Abrahamse P, Ward KC, Kato I, Veenstra CM. The accuracy of chemotherapy ascertainment among colorectal cancer patients in the surveillance, epidemiology, and end results registry program. BMC Cancer. 2018;18: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker GV, Giordano SH, Williams M, et al. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. Int J Radiat Oncol Biol Phys. 2013;86: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.