Abstract
BACKGROUND
Primary ovarian insufficiency (POI) and diminished ovarian reserve are two conditions that affect women’s fertility. Oocyte donation remains an option for these patients; however, the development of certain novel technologies, such as in vitro activation of ovarian cortex (IVA), enables the possibility of activating the pool of resting primordial follicles, increasing the chance of pregnancy.
OBJECTIVE AND RATIONALE
Here, we review the main pathways (PI3K and Hippo signaling) that govern the activation of primordial follicles and its application through the development of culture systems that support ovarian cortex for autologous transplantation. We also review the available data from case reports regarding outcomes of pregnancy and live birth rates with IVA.
SEARCH METHODS
A PubMed search was conducted using the PubMed-NCBI database to identify literature pertinent to the pathways involved in the activation of primordial follicles and the outcomes of IVA techniques from 2013 to the present.
OUTCOMES
Women with POI have around a 5% chance of spontaneous pregnancy. Recently, novel techniques involving the activation of primordial follicles through molecular pathways have been developed, thus increasing the odds of these patients. More recently, the introduction of a drug-free IVA technique has shown to increase the number of antral follicles with successful oocyte maturation after gonadotropin treatment, reaching pregnancy rates over 30%, either through spontaneous conception or by the implementation of assisted reproductive technology.
LIMITATIONS
The evidence of this review is based on a few small series, so data should be interpreted with caution, and only randomized controlled trials could estimate the real magnitude and success of the procedure.
REASONS FOR CAUTION
IVA technique remains an experimental strategy, with limited available data and the requirement of invasive procedures. Moreover, possible carcinogenic effects not yet determined after transplantation require special caution.
WIDER IMPLICATIONS
In view of the results achieved, IVA could provide a promising option for the preservation of fertility in some cancer patients and prepuberal girls where the only alternative is tissue cryopreservation.
STUDY FUNDING/COMPETING INTERESTS
The authors received no specific funding for this work and declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Keywords: invitro activation/ primary ovarian insufficiency/ diminished ovarian reserve/ 38 PI3K pathway/ Hippo pathway/ dormant primordial follicle/ growth factors/ 39 autotransplantation
WHAT DOES THIS MEAN FOR PATIENTS?
This article summarizes the latest evidence on how the development of novel techniques could become an option for achieving pregnancy in patients with early stages of primary ovarian insufficiency (POI) and diminished ovarian reserve (DOR).
Women with POI are those whose ovaries fail early in their functioning with a consequent compromised fertility before the age of 40, while women with DOR are those with lower reproductive potential due to a reduction in their quantity of oocytes without reaching the limit of insufficiency. Until now, the best alternative in both cases has been oocyte donation.
The introduction of certain techniques could allow the activation of some of the remaining ovarian reserve of these patients, through laparoscopic surgery, manipulation of the ovarian tissue and finally autotransplantation. This knowledge arises from the extensive research of molecular mechanisms that were primarily described in animals and then translated into humans using experimental models.
Introduction
In mammals, oocytes are sequestered in primordial follicles before birth and remain quiescent, often for decades, until recruited into the growing pool throughout the reproductive years (Choi and Rajkovic, 2006).
From birth onwards, the cohort of primordial ovarian follicles decreases progressively, from approximately 2 million to just a few thousand by the age of 40, with the subsequent advent of menopause (Ferreri et al., 2020). It is estimated that ‘natural infertility’ occurs at an average age of 41 years, while ovarian endocrine activity continues until menopause, at an average age of 51 years. Even so, histological samples reveal that the pool of follicles will not be completely exhausted until the entry into the eighth decade of life (Griesinger and Fauser, 2020). The main reason for this event is atresia; however, most of them remain in a dormant state caused by certain inactivating factors, while those selected to grow will develop toward the primary stage under control of different signaling pathways (Dolmans et al., 2019).
Therefore, primordial follicles would take one of three alternatives: to remain quiescent and preserve the length of the reproductive age; to enter into apoptosis directly from the dormant state, contributing to female reproductive aging; or to be activated from the recruited pool, constituting primary and subsequent secondary follicles under the influence of paracrine, autocrine and endocrine factors (Zhang and Liu, 2015).
It is estimated that three out of four women with primary ovarian insufficiency (POI) have dormant primordial follicles remaining in their ovaries (Lee and Chang, 2019). The main goal of reproductive medicine is to find a way to activate part of this remaining ovarian reserve, in order to ensure an alternative to pregnancy, for these patients and also for those patients who suffer from diminished ovarian reserve (DOR). This knowledge could then be extended to more complex cases such as oncological patients under surgical treatment or chemotherapy.
The aim of this review is to understand the basis of molecular mechanisms and pathways that control primordial follicular activation, and to analyze the efficacy of in vitro activation (IVA) methods applied in POI and DOR patients, in terms of reproductive outcomes. The latter findings are based on case reports from literature.
In vivo and in vitro follicular growth and development
Follicular growth in humans begins during the fourth month of fetal life, constituting the total population of female germ cells. At birth, it is estimated that the number of follicles is between 266 000 and 472 000; this pool will be exhausted throughout reproductive life, leading to an estimated stock of less than 100–1000 follicles with the advent of menopause (Fabbri et al., 2018).
While some of the developing follicles begin their growth almost immediately during fetal life, most of them will remain in a resting state until certain inhibitory signals are removed or activating signals promote their entry into the developmental phase. Normal development will depend mainly on the communications between the oocyte and the granulosa, produced by regulated cytoplasmic projections that extend from the granulosa membrane, across the pellucid zone and end up near the oolema (Albertini and Barrett, 2003).
The so-called primordial follicles are the earliest, being composed of a quiescent oocyte (arrested in Prophase of Meiosis I), and surrounded by a single layer of flat somatic cells, probably progenitors of granulosa cells. They represent a very important source of germ cells, which will be recruited from rest to growth throughout the woman’s reproductive life (Fabbri et al., 2018). The resting follicles are found in three types (primordial, intermediate and small primary), and it is well known that a large proportion of them will enter into atresia, a hormonally regulated apoptotic process that depends predominantly on the apoptosis of granulosa cells (Gougeon, 1996; Zhou et al., 2019). In addition to hormones, other factors involved in this process have been described in mammalian ovaries, such as FAS ligand (FASLG) and FAS system, tumor necrosis factor-alpha (TNF-α), TNF-α-related apoptosis-inducing ligand (TRAIL) and B cell lymphoma/leukemia 2 (BCL2) family members (Matsuda et al., 2012).
On the other hand, the activation of human follicles is also precisely regulated and involves follicular growing from the primordial to the antral stage, which is estimated to naturally take more than six months (McGee and Hsueh, 2000). It consists of complex, regulated, and time-prolonged events that occur sequentially: (i) onset of primordial follicle growth and preantral follicle development; (ii) establishment of the antral follicle, whose expansion to the preovulatory stage (Graaf) is associated with granulosa cell proliferation and accumulation of antral fluid; and (iii) rupture of Graaf follicle releasing the oocyte in response to the LH peak (ovulation) (Telfer and Zelinski, 2013).
In recent years, certain novel technologies have been introduced to promote the growth and maturation of primordial follicles in vitro, becoming a promising alternative approach to fertility restoration (Devos et al., 2020). In order to achieve a complete development of oocytes, a dynamic culture system that adapts to each stage of transition is required. These stages comprise: (i) activation of primordial follicles through culture of ovarian cortex, (ii) isolation and culture of preantral follicles to achieve oocyte development and (iii) removal of the follicular environment and maturation of the cumulus-oocyte complex (McLaughlin et al., 2018). Specifically, regarding autologous transplantation in humans, and in reference to contents of this review, it is vitally important to develop the first of these steps.
The earliest in vitro experiments were performed by culturing the entire ovary (Betteridge et al., 1989); which gave the advantage of maintaining normal cell connections, but proved inconvenient in preserving the perfusion of the organ as well as physical difficulties. Subsequently, the culture of fragments of ovarian cortex was performed. These basically presents three challenges: to avoid the damage produced due to the harvesting process; to promote a complex support system that resembles the ovary itself; and last but not least, to provide the biochemical control pathways capable of triggering the onset of follicular growth (Picton and Gosden, 2000).
Moreover, the greatest challenge is to achieve these goals in a short period of time. Although it is known that under ordinary conditions, most of the primordial follicles are at rest and gradually activated over a long period of time, findings from in vitro cultures for a short period of 2 to 6–10 days suggest that inhibitory mechanisms, which are responsible for maintaining latency, are also disturbed by just the removal of the ovarian cortex from its environment (Bertoldo et al., 2018).
Important factors contributing to the initiation of in vitro follicular growth are tissue size and stromal density: solid cubes and strips 1 mm thick show less growth onset and a high proportion of atretic follicles, compared to cortex culture as flattened ‘sheets’, where most of the underlying stromal is also removed (Telfer and Zelinski, 2013).
Numerous studies have shown that the physical environment of follicles within the ovarian cortex affects their response to either stimulating or inhibiting factors and influences their ability to grow (McLaughlin and McIver, 2009). As mechanisms involved in achieving follicular growth, the molecular pathways that governs follicular activation have been discovered, mainly through the use of genetically modified mouse models (Reddy et al., 2005). These pathways have allowed a better understanding of the physiology of the human ovary for clinical applications.
In vitro activation of ovarian cortex
Primary ovarian insufficiency and diminished ovarian reserve
POI is the depletion of ovarian follicles with cessation of menstruation in women under 40 years of age. Its incidence is estimated at 1% and it is classically defined as amenorrhea of at least 4 months duration, associated with persistently high FSH values (>25 IU/l) (Lee and Chang, 2019). Ninety percent of the etiology is idiopathic, while the remaining cases are associated with genetic, autoimmune, toxic, chemotherapeutic or radiation factors (Cordeiro et al., 2016).
Before POI, the ovarian follicle pool reduction begins at a clinical stage known as DOR, a condition that affects 10% of women pursuing pregnancy (Ferreri et al., 2020).
In recent years, new techniques have emerged with the aim of regenerating, rejuvenating and activating ovarian germ cells; these attempts include: artificial generation of gametes from ovarian stem cells (Woods and Tilly, 2012), intra-ovarian injection of platelet-rich plasma activated with calcium gluconate (Sills et al., 2018), in vitro autologous mitochondrial transfer into oocytes (Labarta et al., 2019) and androgen supplementation to increase the sensitivity of follicles to exogenous gonadotrophin stimulation (Nagels et al., 2015). However, none of these techniques are performed routinely and the current evidence is poor or questionable (Griesinger and Fauser, 2020).
In 2010, based on clinical trials in murine rodents and later in humans, a new method for activating dormant follicles by in vitro culture of ovarian cortex fragments with stimulating agents was introduced, based on the PTEN (phosphatase and tensin homolog deleted on chromosome 10)-PI3K (phosphatidylinositol 3 kinase)-Akt (protein kinase B)-Foxo3 (Forkhead box protein O3 transcription factor) pathway. This technique would allow the rescue of residual follicles at preovulatory stages (Li et al., 2010).
Subsequent studies indicated that even simple fragmentation of the ovarian cortex could interfere with the Hippo pathway and hence restore the growth of ovarian follicles (Fabregues et al., 2018; Ferreri et al., 2020; Kawamura et al., 2020). In summary, the Hippo pathway regulates cell proliferation and apoptosis and thereby organ size by performing a negative growth control. The name comes from tissue growth (a hippo-type phenotype) observed in the context of Hpo gene mutations (Griesinger and Fauser, 2020).
In 2013, a trial combined both methods, and called it ‘in vitro ovarian activation’ (IVA), in order to support patients with POI who are pursuing pregnancy (Kawamura et al., 2013).
Since patients with DOR, or at the initial stages of POI, have spontaneous activation of dormant primordial follicles reaching the secondary stage, subsequent follicular growth could be achieved using simplified in vitro activation, by just performing ovarian tissue fragmentation in a single surgical act, without the requirement of culture with stimulant drugs. For patients with residual secondary follicles, sole fragmentation of the ovarian cortex (causing interruption of the Hippo pathway) is likely to be enough to promote follicular growth (Fabregues et al., 2018).
Molecular mechanisms and pathways underlying the activation of primordial follicles
The molecular mechanisms that govern follicular activation have been extensively studied in animals, mainly from genetically modified mouse ovaries, allowing the development of experimental mammalian models of activation through in vitro culture of ovarian tissue (Castrillon et al., 2003; John et al., 2008; Reddy et al., 2008). This molecular machinery, which has not been fully elucidated in our species, is believed to reflect the follicular activation in women since it has been demonstrated similar performance by in vitro culture of human ovarian follicles (Grosbois and Demeestere, 2018).
The PI3K pathway
This pathway involves a group of molecules (such as kinases, phosphatases and transcription factors) which regulate cell proliferation, survival, migration and metabolism in the mammalian ovaries (Fig. 1). Its role was demonstrated by using ovaries and isolated oocytes from postnatal mice and rats (Reddy et al., 2005), and from there it has been actively studied in the context of cancer development (Cantley, 2002; Stokoe, 2005). Special mention is required for a human experimental study in which the use of an IVA protocol that targets this pathway in cryopreserved ovarian cortex from cancer patients increased the pool of viable activated primordial follicles without deleterious effects, providing a useful instrument for fertility preservation (Novella-Maestre et al., 2015).
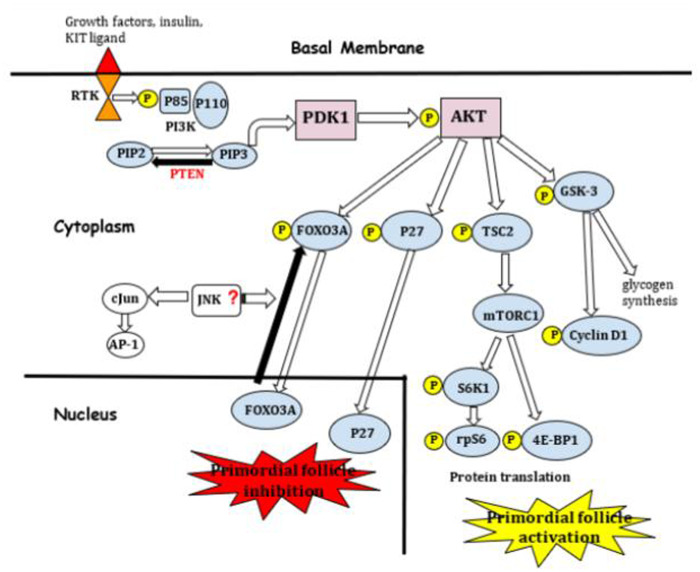
Figure 1.
The PI3K pathway. 4E-BP1, eukaryotic translation initiation factor 4E-binding protein 1; AKT, protein kinase B; AP-1, activating protein-1; Cjun, proto-oncogene; FOXO3A, forkhead box protein O3 transcription factor; GSK-3, glycogen synthase kinase 3; JNK, c-Jun N-terminal kinase; mTORC1, rapamycin-sensitive mTOR complex 1; P, phosphorylation; P110, PI3K catalytic subunit; P27, cyclin-dependent kinase inhibitor; P85, PI3K regulatory subunit; PDK1, 3´-phosphoinositide-dependent kinase-1; PI3K, phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5-trisphosphate; PTEN, phosphatase and tensin homolog deleted on chromosome 10; rpS6, ribosomal protein S6; RTK, tyrosine kinase receptor; S6K1, P70 S6 kinase 1-ribosomal protein S6; TSC2, tuberous sclerosis complex 2 or tuberin.
The critical role in this pathway is played by PI3K (phosphatidylinositol 3-kinase), which is differentiated into three classes (I, II and III), based on its structure and specific substrates. In mammals, PI3K IA is activated by cell membrane tyrosine kinase receptors (RTK), which are expressed on the oocyte surface of postnatal ovaries of mice, rats, and humans; such as insulin and growth factor receptors. PI3K IA members are heterodimers composed of a regulatory subunit (with isoforms p85α, p85β and p55γ) and a catalytic subunit (with isoforms p110α, p110β and p110δ) (Adhikari and Liu, 2009).
Kit ligand (KL) is a stem cell factor produced by granulosa cells which has been shown to stimulate mouse oocyte growth when functional FSH receptors are not yet expressed (Packer et al., 1994; Adhikari and Liu, 2009); an increase in this factor in follicular fluid from women undergoing IVF, has been correlated with successful pregnancies (Smikle et al., 1998). KL binds to its RTK, so the receptor is dimerized and thus it phosphorylates its own tyrosine residues, which are capable of binding to Src homology 2 (SH2) domain-containing molecules of the p85 regulatory subunit of PI3K. This allows the p110 catalytic subunit to phosphorylate the 3′-OH group of the inositol ring of inositol phospholipids. Recruitment of PI3K from the cytoplasm to the inner cell membrane, enables the conversion of PIP2 (phosphatidylinositol-4,5-bisphosphate) to PIP3 (phosphatidylinositol-3,4,5-trisphosphate). PIP3 then activates PDK1 (3′-phosphoinositide-dependent kinase-1), resulting in the activation of AKT (protein kinase B). AKT within the nucleus, among other functions, phosphorylates FOXO3 (member of forkhead transcription factors) and p27 (cyclin-dependent kinase inhibitor). Both factors are involved in the avoidance of primordial follicle activation and are exported out of the oocyte nucleus and thereby the primordial follicles are activated (Cordeiro et al., 2016). In addition, AKT phosphorylates and inactivates GSK-3 (glycogen synthase kinase 3), which leads to increased glycogen synthesis, and also prevents phosphorylation and degradation of cyclin D1 (Adhikari and Liu, 2009), a proto-oncogene whose amplification and protein overexpression are often exhibited in tumor cells (Bates and Peters, 1995).
The major effectors of this pathway, FOXO3 and p27, would likely suppress follicular activation independently one from the other. The FOXO family is part of a large group of transcription factors, and in mammals consists of three members: FOXO1, FOXO 3a and FOXO4; all of them providing common sites for AKT phosphorylation. Suppression of the FOXO3a gene in a female mouse model caused age-dependent infertility and abnormal development of the ovarian follicle as a result of immediate global activation, which led to ovarian hyperplasia, follicle exhaustion, premature ovarian failure and infertility (Castrillon et al., 2003).
p27 belongs to the CIP/KIP family, inhibitors of CDK (cyclin-dependent kinase); it is a negative regulator of cell cycle and growth, and operates through inhibiting CDK2 kinase activity (Adhikari and Liu, 2009). A study in which wild-type mice model was used, demonstrated that p27 suppresses follicle activation and induces follicle atresia before sexual maturity, and furthermore showed that in case of p-27 deficient ovaries, there would be a depletion of follicles in early adulthood leading to POI (Rajareddy et al., 2007).
Another important role attributed to AKT is the inactivation of TSC2 (tuberous sclerosis complex 2 or tuberin) by phosphorylation, leading to lack of suppression of mTOR (mammalian target of rapamycin). mTOR is a serine/threonine kinase that regulates tissue proliferation by diverse signals and is part of two protein complexes, mTORC1 and mTORC2. mTORC1 promotes protein translation and ribosomal biogenesis in oocytes, by phosphorylating S6K1-rpS6 (P70 S6 kinase 1-ribosomal protein S6) and 4E-BP1 (eukaryotic translation initiation factor 4E-binding protein 1) (Adhikari and Liu, 2010). Studies performed in mice and human models suggest that transient culture of ovarian tissue with mTORC1 inhibitors, such as everolimus, partially prevents spontaneous activation by limiting growing follicle counts, without impairing the ability of growing follicles to develop and synthesize hormones (Grosbois and Demeestere, 2018; Grosbois et al., 2019; Devos et al., 2020).
PTEN (phosphatase and tensin homolog deleted on chromosome 10), a tumor suppressor gene, is mainly described as the negative regulation mechanism of this pathway; it acts by dephosphorylating PIP3 and converting it into PIP2, which results in the suppression of PI3K signaling (Cordeiro et al., 2016). It has been demonstrated from bovine, swine and human-based models in vitro, that inhibition of PTEN with BpV(HOpic) affects ovarian follicle development by promoting the initiation of follicle growth and development, similar to that in rodent species (McLaughlin et al., 2014; Maidarti et al., 2019; Raffel et al., 2019), but this results in increased DNA damage and impaired DNA repair capacity (Maidarti et al., 2019).
Germinal mutations of PTEN gene are related to the development of certain diseases, such as Cowden disease (Liaw et al., 1997), an autosomal dominant syndrome characterized by multiple hamartomas of the skin, intestine, breast and thyroid, with an increased risk of breast, thyroid or brain tumors (Eng, 1998). On the other hand, mice PTEN deletion has been associated with overgrowth of immature oocytes and premature activation of the entire pool of primordial follicle (Reddy et al., 2008).
Another regulator, although less studied, is the c-Jun proto-oncogene, whose activity is regulated by the JNK (c-Jun N-terminal kinase). Part of the role of c-Jun is to activate the transcription factor AP-1 (activating protein-1), which plays a key function in cell proliferation, survival and apoptosis. It has also been reported that inhibition of the JNK pathway suppresses the translocation of FOXO3a from nucleus to cytoplasm in muscle cells; which, extrapolated to primordial follicles, would result in the permanence of high levels of FOXO3a within the oocyte, with the consequent inhibition of follicular activation (Clavel et al., 2010) (Fig. 1).
Many growth factors, hormones and cytokines have also been reported to participate in the activation of primordial follicles (Adhikari and Liu, 2009). Among them, the Foxl2 gene (Forkhead box L2), expressed in granulosa cells of primordial and growing follicles, deserves particular interest. Mutations in this gene cause Blepharophimosis, Ptosis and Epicanthus inversus Syndrome, a dominant autosomal disorder with typical characteristics of eyelid dysplasia; it has also been associated with risk of developed POI (De Baere et al., 2003). There is evidence that Foxl2 has a significant role in the differentiation of granulosa from pre-granulosa cells during follicular activation; and that in case of its absence, the primordial oocyte pool is activated prematurely and in an non-synchronized way with granulosa cell division and differentiation, a process that culminates in follicular atresia (Schmidt et al., 2004; Uda et al., 2004). Such transcription factor would therefore provide an inhibitory mechanism, keeping the primordial follicle in a resting state.
The set of mechanisms described suggest that suppression of the AKT pathway allows to primordial follicles to be kept in a quiescent state, necessary to preserve the pool during the reproductive years, avoiding early exhaustion. Likewise, our knowledge allows the implementation of certain novel techniques designed to trigger oocyte activation.
The incubation of small fragments of ovarian cortex (100 cubes of 1–2 mm2) from POI patients with PTEN suppressor drugs, such as bpV(HOpic) and AKT stimulants, such as 740YP, for a short period of time (usually 2 days), has been proposed as a mechanism of activation of dormant follicles, after transplantation of these fragments beneath the serosa of the fallopian tubes (Cordeiro et al., 2016).
However, to date, only a small number of pregnancies and births have been reported in literature by using this method (Kawamura et al., 2013; Suzuki et al., 2015; Zhai et al., 2016).
The Hippo pathway
This pathway is essential for maintaining optimal organ size, it is present in all mammals and was first discovered in Drosophila (Lee and Chang, 2019). Based on the hypothesis that ovarian tissue damage with the subsequent autotransplantation of its fragments could promote follicular growth, several investigations in various species, mainly based on mice models, have been developed (Kawamura et al., 2013). This concept has emerged as early as in 1930, when wedge resection of the ovary of polycystic ovary syndrome patients (Stein and Leventhal, 1935) followed by ovarian drilling, was employed to induce follicular development (Farquhar et al., 2012); an original strategy that was later introduced for the preservation of fertility in cancer patients through freezing and grafting of cortical fragments (Donnez et al., 2011).
Hippo signaling consists of several negative cell growth regulators that operate in a kinase cascade, inactivating key effectors (Fig. 2). These effectors, which are down-regulated by phosphorylation, belong to the oncogenes YAP (YES-associated protein)/TAZ (transcriptional coactivator with PDZ-binding motif) (Cordeiro et al., 2016).
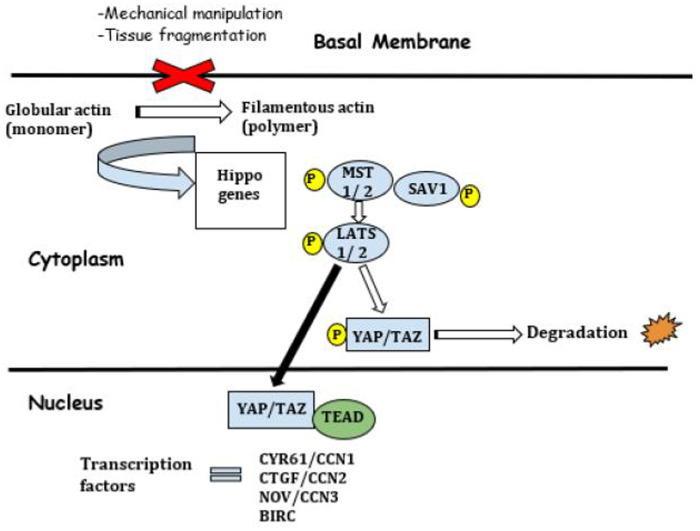
Figure 2.
The Hippo pathway. BIRC, baculoviral IAP repeat containing proteins; CTGF/CCN2, connective tissue growth factor; CYR61/CCN, cysteine-rich protein 61; LATS 1/2, large tumor suppressor; MST 1/2, serine/threonine mammalian Ste-20 like kinases; NOV/CCN3, nephroblastoma overexpressed; SAV1, protein salvador homolog 1; TAZ, transcriptional coactivator with PDZ-binding motif; TEAD, transcriptional enhanced associate domain; YAP, YES-associated protein.
YAP/TAZ are related to factors that regulate cell contractility, such as Rho GTPase and ROCK (Rho-associated protein kinase). An increase in the internal mechanical stress in the cells will probably result in the expression of negative growth factors, that are expressed in murine and human ovaries, such as: serine/threonine mammalian Ste-20 like kinases (MST 1/2), protein salvador homolog 1 (SAV 1) and the large tumor suppressor 1 and 2 (LATS 1/2), through phosphorylation and consequent inhibition of nuclear translocation of YAP/TAZ, under the influence of Rho GTPase and ROCK. Nuclear location of YAP is necessary to bind TEAD transcription factors and activate transcription of growth factors such as: cysteine-rich protein 61 (CYR61/CCN1), connective tissue growth factor (CTGF/CCN2), nephroblastoma overexpressed (NOV/CCN3) and baculoviral IAP repeat containing proteins (BIRC) (Shah et al., 2018) (Fig. 2).
Therefore, the Hippo signaling pathway seems to employ internal mechanical stress as a cellular response to the external environment, and causes signaling in order to keep the follicle in a dormant state, inhibiting follicular activation.
In contrast, studies on mammalian ovaries showed that manipulation of ovarian cortex by cutting tissue into small fragments interrupt this signaling pathway, since it modifies intercellular tension, transforming globular actin (monomer) into filamentous (polymer), thus altering the cascade of involved genes (Reddy et al., 2013). The consequent dephosphorylation of YAP increases this protein in the cell nucleus, which finally stimulates the expression of cell growth factors and apoptosis inhibitors, previously described (Cordeiro et al., 2016). The presence of YAP has been confirmed in the nucleus of human granulosa cells as well as in their cytoplasm in the growing follicles (Grosbois and Demeestere, 2018).
Based on these findings, drugs have been developed to enhance actin polymerization, such as JASP (Jasplakinolide) and S1P (Sphingosine-1-phosphate), with promising outcomes in mice and humans (Cheng et al., 2015).
Other regulatory agents
Other factors involved in regulating the activation of primordial follicles are: (i) growth factors and hormones that activate RTK such as NT (neurotropins), insulin, bFGF (basic fibroblast growth factor), KGF (keratinocyte growth factor) and PDGF (platelet-derived growth factor); (ii) TGF-B and Smads family members such as AMH (anti-Müllerian hormone), BMP-7 and 4 (bone morphogenetic proteins), GDF-9 (growth differentiation factor-9) and Smads 2 and 3; (iii) ligands that activate cytokine receptors type 1 such as GH (growth hormone) and LIF (leukemia inhibiting factor); (iv) ligands that activate GPCR (G-protein coupled receptor) such as SDF-1 (stromal cell-derived factor-1); (v) steroid hormones such as estradiol and progesterone; and (vi) specific transcription factors such as Nobox genes (newborn ovary homeobox-encoding gene).
Drug-free IVA technique
The drug-free IVA technique is supported by the disruption of the Hippo pathway to promote secondary follicle growth through ovarian biopsying, fragmentation and autografting. It was first described in mice, achieving promising results in oocytes retrieved and pregnancy rates (Kawamura et al., 2013). Later, human studies were conducted in patients with POI and DOR. Two case reports that described the performance of this novel technique on 11 patients with DOR (Kawamura et al., 2020) and 14 patients with early stages of idiopathic POI (Ferreri et al., 2020), showed an increase in antral follicle number in multiple growth waves following FSH or HMG treatment and reported successful oocyte retrieval and pregnancy rates of 36% and 28%, respectively.
Conversely, a prospective cohort study conducted in 20 patients with DOR in which one ovary was biopsied and fragmented, keeping the other ovary as intra-individual control, failed to obtain differences in the number of antral follicles from the ovary that was biopsied versus the contra-lateral after 10 weeks of the procedure. Furthermore, only 15% of the patients (3/20) showed ultrasound signs of ectopic follicular growth on the graft side. However, a high percentage of patients (60%; 12/20) achieved pregnancy (either spontaneously or with assisted reproduction treatment) at an average of 177 days of follow-up (Lunding et al., 2019).
In accordance with available evidence and in order to unify concepts, the following is a brief description of the technique carried out on 11 poor responder patients with DOR, adapted from one published case report (Kawamura et al., 2020).
Prior to the procedure, hormone dosage was performed on each patient, assessing FSH, estradiol and AMH levels. Hormone replacement therapy with estrogens was prescribed to maintain serum estradiol values between 40 and 90 pg/ml, in order to suppress circulating FSH and LH; followed by the administration of estrogen and progesterone for 10–14 days. Prior to surgery, LH values were monitored, ensuring levels below 10 mIU/ml. Dual replacement therapy was withdrawn before surgery with the objective of inducing deprivation a few days following the intervention.
The initial surgery was introduced in mice in 2010 and it consisted in the removal of the complete ovary in order to prepare small fragments before vitrification, followed by its fragmentation into cubes and culture with PI3K stimulant drugs during 2 days. Subsequently, the heterologous transplant was carried out (Li et al., 2010). This in vitro technique was then used in a clinical trial in humans.
The most recently employed drug-free IVA technique consisted of a laparoscopic surgery for partial resection of the cortex of one or both ovaries, performed under general anesthesia. The cortical fragments were immediately transferred to the laboratory in an incubator at 37°C. For disruption of Hippo signaling, the ovarian cortex was dissected to remove residual marrow tissue prior to fragmentation into strips (10 × 10 mm, 1–2 mm thick). After reservation of a small part of the strips for histology to evaluate the presence of residual follicles (5 × 1 × 1 mm3 per strip), the remaining fragment was divided into small cubes (1 × 1 × 1 mm3) for transplant into the contralateral ovary through a tunnel between the cortex and the marrow, or into a peritoneal pocket, just below both fallopian tubes. This technique might be facilitated through the use of an applicator designed for laparoscopy.
After surgery, and once deprivation has been produced, hormone therapy with a GnRH agonist (through nasal spray, such as buserelin) was introduced to maintain LH concentrations below 10 mIU/ml, together with daily injections of recombinant FSH or purified urinary FSH (at doses of 225–300 IU/day). Higher doses of HCG (10 000–20 000 IU) were employed for the discharge once follicles reached 14–18 mm of diameter, and then either natural conception or artificial insemination or oocyte retrieval for IVF (IVF) was chosen, according to certain factors such as age. The administration of GnRH and FSH was interrupted in the absence of increased levels of estradiol, and then repeated in subsequent cycles. Generally, the stimulation is prolonged compared to the usual protocol, given the poor vascularization of the graft.
Serum concentrations of FSH, LH and estradiol were recorded over a year, complemented by ultrasound monitoring, during which time it is estimated that the waves of follicular growth may continue. If follicular puncture was performed for IVF, the embryos that were obtained after several cycles of stimulation were cryopreserved at cleavage stage (Day 2 or 3) and subsequently transferred under optimal uterine conditions.
Outcomes
The evidence about the outcomes of these techniques applied in humans is limited and consists of a few uncontrolled series of cases, so they should be interpreted with caution (Table I).
Table I.
Pregnancy outcomes from case reports.
| Study | Procedure | Patients (n) | Inclusion | Patients with residual follicles/total | Patients with follicular growth/total | Patients with mature oocytes (MII) /total | Pregnancy/ total | Live birth rate: total |
|---|---|---|---|---|---|---|---|---|
| Kawamura et al. (2013) | IVA with drugs | 27 | POI | 13/27 | 8/27 | 5/27 | 2/27 (IVF) | 1:27 |
| Suzuki et al. (2015) | IVA with drugs | 10 | POI | 7/10 | 1/10 | 1/10 | 1/10 (IVF) | 1:10 |
| Zhai et al. (2016) | IVA with drugs | 14 | POI | 7/14 | 6/14 | 4/14 | 1/14 (IVF) | 1:14 |
| Fabregues et al. (2018) | Drug-free IVA | 1 | POI | 1/1 | 1/1 | 1/1 | 1/1 (IVF) | 1 ongoing pregnancy |
| Kawamura et al. (2020) | Drug-free IVA | 11 | POR | 9/11 | 9/11 | 11/11 | 4/11 (3 IVF, 1 SC) |
1:11 2 ongoing pregnancy |
| Ferreri et al. (2020) | Drug-free IVA | 14 | POI | 3/14 | 7/14 | 5/14 | 4/14 (IVF) | 4:14 |
| Lunding et al. (2019) | Drug-free IVA | 20 | DOR | 18/20 | 3/20 | 1/20 |
0/20 (within 10 weeks of surgery) 12/20 (until the year of surgery) |
0:20 (within 10 weeks of surgery) 10:20 (until the year of surgery): 3/20 SC 5/20 IVF 2/20 IUI |
DOR, diminished ovarian reserve; IUI, intrauterine insemination; POI, primary ovarian insufficiency; POR, poor ovarian response; SC, spontaneous conception.
According to reports, pregnancy rates could increase with the implementation of these procedures, especially considering drug-free IVA, when compared with a 5% chance of spontaneous conception of patients with POI, as reported in the literature (Nelson, 2009).
Discussion
Patients with POI are considered infertile due to the lack of follicular growth and ovulation. Although the menstrual cycles of such patients may cease, they still have dormant primordial follicles, which do not produce enough circulating estrogen and progesterone to modulate uterine functions. The chance of pregnancy that has been reported in several reviews vary from 5% to 10%, although controlled trials found only a 1.5% chance (van Kasteren and Schoemaker, 1999). Therefore, the best alternative in these patients remains oocyte donation.
Follicle growth in patients with POI and DOR, who usually present spontaneous activation of primordial follicles toward the secondary stage, can be promoted through the application of in vitro ovarian activation technology. The conventional IVA technique that targets PI3K pathway has yielded some progress but no quite enough, achieving pregnancy rates of 7.8% with own oocytes, according to the series reported (4 of 51 patients) (Kawamura et al., 2013; Suzuki et al., 2015; Zhai et al., 2016). Moreover, its applicability has been criticized in different aspects. First, it has been associated with a deleterious effect on the ovarian reserve because of a massive and premature follicular activation compromising the development of growing follicles and leading to atresia (‘burn out effect’) (Grosbois and Demeestere, 2018; Dolmans et al., 2019). Second, the pathway that becomes activated from this technique has been found altered in several cancers (Luongo et al., 2019), and also showed an increase in DNA damage with a difficult repair when it was applied in bovine follicles culture (Maidarti et al., 2019). Third, it is believed that the 2-day in vitro culture of ovarian cortex may trigger tissue damage, necrosis and thus programmed cell death (Griesinger and Fauser, 2020). So, in order to optimize culture systems, some strategies in the area of fertility preservation have been proposed, such as a transient incubation with mTORC1 inhibitors to delay in vitro massive follicular activation while maintaining the functional integrity of the follicle (Grosbois and Demeestere, 2018; Grosbois et al., 2019).
Subsequently, a novel technique based on mechanobiology through the disruption of the Hippo pathway has been introduced, in which fragmentation of ovarian cortex induced by F-actin (filamentous) leads to the nuclear translocation of YAP, developing in a cascade of activation of growth factors and antiapoptotic ones, ending in the resumption of follicular growth. This procedure, which does not require the culture of ovarian tissue, could offer a less invasive treatment with a single surgical act and without the need of in vitro chemical activation. According to our review, the chance of pregnancy was 34.6% (9 out of 26 patients) (Fabregues et al., 2018; Ferreri et al., 2020; Kawamura et al., 2020). Recently, an uncontrolled trial conducted in 20 women under 39 years with DOR failed to detect increased activity of the fragmented ovarian tissue. In that study, the patients underwent laparoscopic biopsy of an ovary and transplantation of cortex fragments into a peritoneal pocket, while the contralateral ovary was used as control (Lunding et al., 2019). A later editorial suggested abandoning this procedure (Steiner, 2019), although the long-term results (after 10 weeks and within one year of transplantation) found a 60% chance of pregnancy (12 of 20 patients) (Lunding et al., 2019). The main limitations, we highlight of these studies are the small sample sizes, the lack of untreated control groups, and the requirement of a randomized controlled trial to be able to establish a real estimation of such effect. Furthermore, the last study (Lunding et al., 2019) found no direct relationship between the procedure and the number of mature follicles in the biopsied ovary or at the transplant site after ovarian stimulation; whilst the first ones showed an increase in the number of oocytes retrieved but without an impact on the oocyte quality (Fabregues et al., 2018; Ferreri et al., 2020; Kawamura et al., 2020).
Recently, a study conducted on human ovaries could not prove the relevance of drug-free IVA technique, since it did not find a relationship between the fragmentation of the ovarian tissue and actin polymerization, nor between the fragmentation and the effectors of the Hippo pathway; also it did not show an increase in the number of growing follicles from the fragmented tissue (Lunding et al., 2020). However, this trial was limited to nine women and does not rule out a transient effect on this pathway what is difficult to assay, as demonstrated by other authors (Grosbois and Demeestere, 2018).
Prior to fragmentation technique, the effect of ovarian biopsy per se or ‘scratching’ on the Hippo pathway has been described, suggesting that possible ‘tissue damage and removal’ may induce an increase in FSH levels that finally triggers follicular growth (Lunding et al., 2019). One large trial that performed this strategy in 80 patients with POI, showed less efficacy in terms of follicular activation (13.75%) and live birth rates (1.25%) (Zhang et al., 2019).
In view of the results achieved, the IVA technique could also provide not only a promising option for DOR and early stage of POI patients, but also for fertility preservation in cancer patients and prepuberal girls, where the alternative appears limited to tissue cryopreservation.
Since the evidence to date is based on small series of cases with limited numbers of patients, data should be interpreted with caution, and only randomized controlled trials could estimate the real magnitude and success of the treatment. Therefore, its application continues to be experimental, with limited available data and the requirement of invasive procedures.
In the future, a better comprehension of mechanisms underlying the activation of the primordial follicle, the introduction of trials testing new drugs or types of physical damage (laser drilling, scratching, wedge resection, fragmentation, etc.) along with improvements in tissue culture and transplantation technology, may help to optimize this novel technique, potentially providing better clinical outcomes for patients.
Acknowledgements
We would like to thank Delia Maunas for checking our manuscript for linguistic accuracy.
Authors’ roles
R.Q. designed the study and performed critical reading and editing. L.D. conducted literature analysis and drafting of the article. T.Q. critically revised the first manuscript. All authors approved the final version.
Funding
The authors received no specific funding for this work.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 2009;30:438–464. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle 2010;9:1673–1674. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Barrett SL. Oocyte-somatic cell communication. Reprod Suppl 2003;61:49–54. [PubMed] [Google Scholar]
- Bates S, Peters G. Cyclin D1 as a cellular proto-oncogene. Semin Cancer Biol 1995;6:73–82. [DOI] [PubMed] [Google Scholar]
- Bertoldo MJ, Walters KA, Ledger WL, Gilchrist RB, Mermillod P, Locatelli Y. In vitro regulation of primordial follicle activation: challenges for fertility preservation strategies. Reprod Biomed Online 2018;36:491–499. [DOI] [PubMed] [Google Scholar]
- Betteridge KJ, Smith C, Stubbings RB, Xu KP, King WA. Potential genetic improvement of cattle by fertilization of fetal oocytes in vitro. J Reprod Fertil Suppl 1989;38:87–98. [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002;296:1655–1657. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003;301:215–218. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Feng Y, Jansson L, Sato Y, Deguchi M, Kawamura K, Hsueh AJ. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J 2015;29:2423–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Rajkovic A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci 2006;63:579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Dérijard BT. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol 2010;30:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro CN, Christianson MS, Selter JH, Segars JH., Jr. In vitro activation: a possible new frontier for treatment of primary ovarian insufficiency. Reprod Sci 2016;23:429–438. [DOI] [PubMed] [Google Scholar]
- De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, Devriendt K, Dixon M, Fellous M, Fryns J-P. et al. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet 2003;72:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos M, Grosbois J, Demeestere I. Interaction between PI3K/AKT and Hippo pathways during in vitro follicular activation and response to fragmentation and chemotherapy exposure using a mouse immature ovary model. Biol Reprod 2020;102:717–729. [DOI] [PubMed] [Google Scholar]
- Dolmans M-M, Cordier F, Amorim CA, Donnez J, Vander Linden C. In vitro activation prior to transplantation of human ovarian tissue: is it truly effective? Front Endocrinol 2019;10:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans M-M. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med 2011;43:437–450. [DOI] [PubMed] [Google Scholar]
- Eng C. Genetics of Cowden syndrome: through the looking glass of oncology. Int J Oncol 1998;12:701–710. [DOI] [PubMed] [Google Scholar]
- Fabbri R, Zamboni C, Vicenti R, Macciocca M, Paradisi R, Seracchioli R. Update on oogenesis in vitro. Minerva Ginecol 2018;70:588–608. [DOI] [PubMed] [Google Scholar]
- Fabregues F, Ferreri J, Calafell JM, Moreno V, Borrás A, Manau D, Carmona F. Pregnancy after drug-free in vitro activation of follicles and fresh tissue autotransplantation in primary ovarian insufficiency patient: a case report and literature review. J Ovarian Res 2018;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev 2012;6:CD001122. [DOI] [PubMed] [Google Scholar]
- Ferreri J, Fàbregues F, Calafell JM, Solernou R, Borrás A, Saco A, Manau D, Carmona F. Drug-free in-vitro activation of follicles and fresh tissue autotransplantation as a therapeutic option in patients with primary ovarian insufficiency. Reprod Biomed Online 2020;40:254–260. [DOI] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996;17:121–155. [DOI] [PubMed] [Google Scholar]
- Griesinger G, Fauser BCJM. Drug-free in-vitro activation of ovarian cortex; can it really activate the ‘ovarian gold reserve’? Reprod Biomed Online 2020;40:187–189. [DOI] [PubMed] [Google Scholar]
- Grosbois J, Demeestere I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum Reprod 2018;33:1705–1714. [DOI] [PubMed] [Google Scholar]
- Grosbois J, Vermeersch M, Devos M, Clarke HJ, Demeestere I. Ultrastructure and intercellular contact-mediated communication in cultured human early stage follicles exposed to mTORC1 inhibitor. Mol Hum Reprod 2019;25:706–716. [DOI] [PubMed] [Google Scholar]
- John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol 2008;321:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho C-h, Kawamura N, Tamura M, Hashimoto S. et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Ishizuka B, Hsueh AJW. Drug-free in-vitro activation of follicles for infertility treatment in poor ovarian response patients with decreased ovarian reserve. Reprod Biomed Online 2020;40:245–253. [DOI] [PubMed] [Google Scholar]
- Labarta E, de los Santos MJ, Herraiz S, Escribá MJ, Marzal A, Buigues A, Pellicer A. Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil Steril 2019;111:86–96. [DOI] [PubMed] [Google Scholar]
- Lee HN, Chang EM. Primordial follicle activation as new treatment for primary ovarian insufficiency. Clin Exp Reprod Med 2019;46:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan E-K, Hsueh AJW. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 2010;107:10280–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PLM, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacoke M. et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 1997;16:64–67. [DOI] [PubMed] [Google Scholar]
- Lunding SA, Andersen AN, Hardardottir L, Olesen HØ, Kristensen SG, Andersen CY, Pors SE. Hippo signaling, actin polymerization, and follicle activation in fragmented human ovarian cortex. Mol Reprod Dev 2020;87:711–719. [DOI] [PubMed] [Google Scholar]
- Lunding SA, Pors SE, Kristensen SG, Landersoe SK, Jeppesen JV, Flachs EM, Pinborg A, Macklon KT, Pedersen AT, Andersen CY. et al. Biopsying, fragmentation and autotransplantation of fresh ovarian cortical tissue in infertile women with diminished ovarian reserve. Hum Reprod 2019;34:1924–1936. [DOI] [PubMed] [Google Scholar]
- Luongo F, Colonna F, Calapà F, Vitale S, Fiori ME, De Maria R. PTEN tumor-suppressor: the dam of stemness in cancer. Cancers 2019;11:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidarti M, Clarkson YL, McLaughlin M, Anderson RA, Telfer EE. Inhibition of PTEN activates bovine non-growing follicles in vitro but increases DNA damage and reduces DNA repair response. Hum Reprod 2019;34:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev 2012;58:44–50. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000;21:200–214. [DOI] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction 2009;137:1–11. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE. Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Mol Hum Reprod 2018;24:135–142. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod 2014;20:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels HE, Rishworth JR, Siristatidis CS, Kroon B. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev 2015;11:CD009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LM. Primary ovarian insufficiency. N Engl J Med 2009;360:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novella-Maestre E, Herraiz S, Rodríguez-Iglesias B, Díaz-García C, Pellicer A. Short-term PTEN inhibition improves in vitro activation of primordial follicles, preserves follicular viability, and restores AMH levels in cryopreserved ovarian tissue from cancer patients. PLoS One 2015;10:e0127786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol 1994;161:194–205. [DOI] [PubMed] [Google Scholar]
- Picton HM, Gosden RG. In vitro growth of human primordial follicles from frozen-banked ovarian tissue. Mol Cell Endocrinol 2000;166:27–35. [DOI] [PubMed] [Google Scholar]
- Raffel N, Klemm K, Dittrich R, Hoffmann I, Söder S, Beckmann MW, Lotz L. The effect of bpV(HOpic) on in vitro activation of primordial follicles in cultured swine ovarian cortical strips. Reprod Dom Anim 2019;54:1057–1063. [DOI] [PubMed] [Google Scholar]
- Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, Shen Y, Berthet C, Peng SL, Kaldis P. et al. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol 2007;21:2189–2202. [DOI] [PubMed] [Google Scholar]
- Reddy P, Deguchi M, Cheng Y, Hsueh AJW. Actin cytoskeleton regulates Hippo signaling. PLoS One 2013;8:e73763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Ha Ma la Inen T, Peng SL. et al. Oocyte-specific deletion of PTEN causes premature activation of the primordial follicle pool. Science 2008;319:611–613. [DOI] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu Y-X, Sun Q-Y, Liu K. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev Biol 2005;281:160–170. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier A-C, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004;131:933–942. [DOI] [PubMed] [Google Scholar]
- Shah JS, Sabouni R, Cayton Vaught KC, Owen CM, Albertini DF, Segars JH. Biomechanics and mechanical signaling in the ovary: a systematic review. J Assist Reprod Genet 2018;35:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills ES, Rickers NS, Li X, Palermo GD. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol 2018;34:756–760. [DOI] [PubMed] [Google Scholar]
- Smikle CB, Dandekar PV, Schriock ED, Givens CR. Elevated ovarian follicular fluid stem cell factor concentrations are associated with improved pregnancy rates in in-vitro fertilization cycles. Fertil Steril 1998;69:70–72. [DOI] [PubMed] [Google Scholar]
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935;29:181–186. [Google Scholar]
- Steiner AZ. Evidence that biopsying, fragmentation and auto-transplantation of ovarian tissue should be abandoned as a treatment of diminished ovarian reserve. Hum Reprod 2019;34:1853–1854. [DOI] [PubMed] [Google Scholar]
- Stokoe D. The phosphoinositide 3-kinase pathway and cancer. Expert Rev Mol Med 2005;7:1–22. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod 2015;30:608–615. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013;99:1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 2004;13:1171–1181. [DOI] [PubMed] [Google Scholar]
- van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update 1999;5:483–492. [DOI] [PubMed] [Google Scholar]
- Woods DC, Tilly JL. The next (re)generation of ovarian biology and fertility in women: is current science tomorrow’s practice? Fertil Steril 2012;98:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, Hu L, Zhang Y, Wang J, Dai S. et al. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab 2016;101:4405–4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update 2015;21:779–786. [DOI] [PubMed] [Google Scholar]
- Zhang X, Han T, Yan L, Jiao X, Qin Y, Chen Z-J. Resumption of ovarian function after ovarian biopsy/scratch in patients with premature ovarian insufficiency. Reprod Sci 2019;26:207–213. [DOI] [PubMed] [Google Scholar]
- Zhou J, Peng X, Mei S. Autophagy in ovarian follicular development and atresia. Int J Biol Sci 2019;15:726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]