Abstract
STUDY QUESTION
What is the role of the Hippo and PI3K/Akt pathway in follicles during ovarian tissue culture in tissue derived from oncological patients and transgender men?
SUMMARY ANSWER
Results highlight a Hippo pathway driven primordial follicle activation in vitro, predominantly from Day 0 to Day 4.
WHAT IS KNOWN ALREADY
In-vitro ovarian tissue culture aims at activating and maturing primordial follicles for fertility restoration in patients with a threatened ovarian reserve. Not all patients are eligible for ovarian cortex transplantation and therefore several groups are attempting to culture ovarian tissue in-vitro. Cortex fragmentation disrupts the Hippo pathway, leading to increased expression of downstream growth factors and follicle growth. The PI3K/Akt pathway is considered the intracellular pathway to where different extracellular factors involved in primordial follicle activation in-vivo converge. In order to optimise current ovarian tissue culture models, information on progression of these pathways during tissue culture is mandatory.
STUDY DESIGN, SIZE, DURATION
The first step of a multistep cortex culture system was performed using 144 ovarian cortex pieces from a total of six patients. Per patient, 24 cortical strips were cultured for 6 days and six pieces per patient were collected for downstream analysis of follicle development and Hippo and PI3K/Akt pathway targets every second day.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Ovarian tissue was obtained from oncological (N = 3; 28.67 ± 4.51 years) and transgender (N = 3; 23.33 ± 1.53 years) patients. Follicles were analysed using haematoxylin-eosin staining and pathways were studied using immunohistochemistry and precise follicle excision by laser capture micro-dissection for RT-qPCR analysis. MIQE guidelines for RT-qPCR were pursued. Reference gene selection (GAPDH, RPL3A, 18s rRNA) was performed using GeNorm Reference Gene Selection Kit. Statistical analysis was conducted with IBM SPSS Statistics 23 (Poisson regression, negative binomial regression, ANOVA and paired t-test).
MAIN RESULTS AND THE ROLE OF CHANCE
Immunohistochemical analysis confirmed a Hippo pathway driven primordial follicle activation due to mechanical manipulation of the cortical strips. Ovarian tissue preparation and culture induced the inhibitory phosphorylated Yes-associated protein (pYAP) to disappear in granulosa cells of primordial follicles on Day 2. The stimulatory YAP on the contrary appeared in primordial granulosa cells over increasing culture days. Looking at the YAP target connective tissue growth factor (CTGF), a significantly up-regulated CTGF was noted in primordial follicles when comparing Day 2 and Day 4 (ratio Day 2/4 = 0.082; P < 0.05), clearly showing an effect on the Hippo pathway in primordial follicles during tissue culture. Follicle classification showed a significant drop in estimated primordial follicle counts in the oncological cohort (−78%; P = 0.021) on Day 2 and in the transgender cohort on Day 4 (−634%; P = 0.008). Intermediate follicle counts showed a non-significant increasing trend to during culture and this follicle recruitment and growth resulted in a significant rise in estimated primary follicle counts on Day 6 in oncological patients (170%; P = 0.025) and, although limited in absolute numbers, a significant increase in secondary follicles on Day 4 (367%; P = 0.021) in the transgender cohort. Subsequent antral follicle development could not be observed.
LIMITATIONS, REASONS FOR CAUTION
A limitation is the small sample size, inherent to this study subject, especially as a large amount of tissue was needed per patient to reduce inter-patient variation in different downstream analysis techniques. A particular and specific weakness of this study is the inability to include an age-matched control group.
WIDER IMPLICATIONS OF THE FINDINGS
These findings support an adapted tissue preparation for Hippo pathway disruption and a shorter first phase of tissue culture. This work may also have implications for transplantation of cryopreserved tissue as larger strips (and thus slower burnout due to less Hippo pathway disruption) could be a benefit.
STUDY FUNDING/COMPETING INTEREST(S)
This research was financially supported by the Foundation Against Cancer (Stichting tegen Kanker, TBMT001816N), the Flemish Foundation of Scientific Research (FWO Vlaanderen, FWO G0.065.11N10) and the Gender Identity Research and Education Society (GIRES) foundation. The authors declare no competing interests.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: primordial follicle, tissue culture, hippo pathway, PI3K/Akt pathway, laser capture micro-dissection, oncology, gender dysphoria
Introduction
Human ovarian tissue cryopreservation is a fertility preservation technique that has been performed for many years, gradually shifting from an experimental to a clinically implemented fertility preservation approach (De Vos et al. 2014; Donnez et al., 2015). The goal of storing ovarian tissue is to safeguard the primordial follicle pool for destructive influences originating from gonadotoxic treatments. Autologous transplantation of the stored tissue in post-pubertal women most commonly orthotopically (Demeestere et al., 2009; Donnez and Dolmans, 2014), but also heterotopically (Stern et al., 2014), has resulted in more than 130 births worldwide (Donnez and Dolmans, 2017; Ladanyi et al., 2017). However, not all patients are eligible for transplantation and therefore several groups have attempted to culture ovarian tissue in-vitro (Kawamura et al., 2013; Suzuki et al., 2015; Ladanyi et al., 2017). The common goal of both transplantation and in-vitro culturing of ovarian tissue is to activate, mature and use the residing primordial follicle pool for fertility restoration in patients with a threatened ovarian reserve, including cancer patients facing a gonadotoxic treatment, transgender men undergoing a (bilateral) oophorectomy, patients diagnosed with primary ovarian insufficiency and women facing aging-associated subfertility (Donnez and Dolmans, 2013; De Vos et al., 2014).
An efficient use of the cryopreserved cortical follicular reserve remains a challenge. In order to produce meiotically and developmentally competent oocytes through in-vitro tissue culturing, many hurdles still remain (Telfer and Zelinski, 2013). Since the majority of cortical follicles are primordial, the first challenge is an effective, efficient and balanced activation of this dormant follicle pool in-vitro (Telfer and Zelinski, 2013). Transplanting ovarian tissue does indeed successfully activate the dormant follicle pool. The graft lifespan, however, and thus reproductive potential of the grafted tissue, is limited (4–5 years) (Schmidt et al., 2005; Donnez et al., 2011, 2013), due to a very effective, but unbalanced activation of the primordial follicles resulting in an early follicle burnout (Meirow et al., 2015). This delicate equilibrium of controlled activation is the result of the complex combination of inhibitory and stimulatory factors of primordial follicle activation (Hsueh et al., 2015). The promising advances in tissue culturing and transplantations should therefore be fine-tuned according to this balance to increase their efficacy.
In this context, two pathways, the Hippo pathway and the PI3K/Akt pathway, are of interest (Grosbois and Demeestere, 2018). The Hippo pathway mediates a reciprocal relationship between cell–cell contact growth inhibition and mitogenic signalling (Gumbiner and Kim, 2014). Fragmentation of the cortex, thereby disturbing cell–cell contact, and molecular targeting of the pathway, for instance with sphingosine-1-phosphate, were found to disrupt the Hippo signalling, leading to an increased expression of downstream growth factors and follicle growth (Yu et al., 2012; Kawamura et al., 2013, 2016; Hsueh et al., 2015). The PI3K/Akt pathway on the other hand is considered the intracellular pathway to where the different extracellular factors involved in primordial follicle activation in-vivo converge (Hsueh et al., 2015). A number of extracellular ligands involved in primordial follicle growth have been identified, including kit ligand, vascular endothelial growth factor and insulin-like growth factor I (Hsueh et al., 2015). The exact trigger of primordial follicle activation remains unidentified; however, all these factors tend to have a common intracellular pathway, the PI3K/Akt pathway. In order to optimise the current ovarian tissue culture models, detailed information on the progression of these pathways during tissue culture and immature follicle development is needed. Unravelling this progression is mandatory to identify clues to a more effective use of primordial follicles in-vitro.
Another barrier to advancements in follicle research is the simple fact that an ovary consists of various cell types, such as connective tissue, neurons, follicles, stromal cells and blood vessels (Shea et al., 2014). These different cell types possibly contain different pathway molecules, all influencing the bidirectional paracrine communication between the follicle and surrounding somatic cells, making it very difficult to distinguish the inhibitory and stimulatory factors of primordial follicle activation (Woodruff and Shea, 2007; Shea et al., 2014; Hsueh et al., 2015). This heterogeneous ovarian composition limits target-specific analysis of the follicular unit, especially in transcriptome analysis as these methods require isolation of pure cell populations (Fend and Raffeld, 2000). Therefore, laser capture micro-dissection (LCM) for separate collection of follicles and stromal cells can be applied (Markholt et al., 2012). LCM combines visualisation of the cells of interest by light microscopy and targeting of these specific cells with laser beam technology (Fend and Raffeld, 2000).
In order to accurately evaluate the changes in the Hippo and PI3K/Akt pathway, we repeated the first step of the promising multistep cortex culture system developed by the group of E. Telfer (Telfer et al., 2008). Follicle count and classification using haematoxylin/eosin (HE) staining and a follicle-specific analysis of the Hippo and PI3K/Akt pathway using LCM, RT-qPCR and fluorescent immunohistochemistry (IHC) were performed. To be able to implement our findings for further research or for a future clinical setting, ovarian tissue from two target patient populations was used: post-pubertal oncological patients and post-pubertal transgender men.
Methods
Patient inclusion and workflow
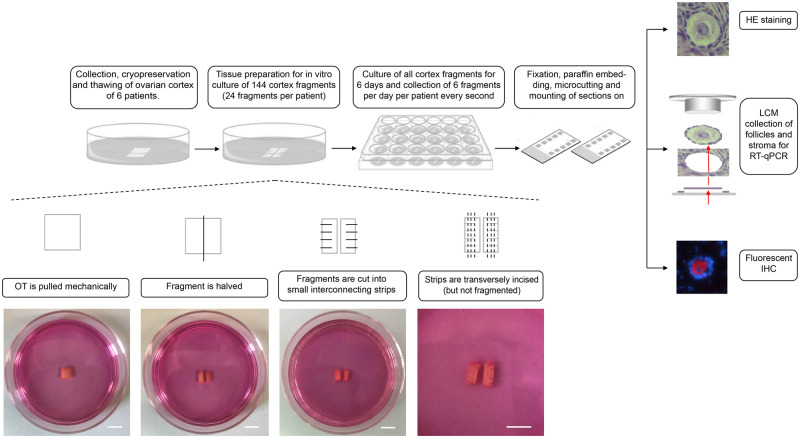
Ethical approval was obtained from the Ghent University hospital institutional review board under reference 2012/780, with Belgian registration number B670201 21 5468. This study was performed in accordance with good clinical practice guidelines and written informed consent was obtained from all patients. In total, 144 ovarian cortex pieces from a total of six patients were used in this study. From these six patients, ovarian tissue was obtained from three deceased oncological patients (28.67 ± 4.51 years), who consented to donate their cryopreserved ovarian tissue to research post-mortem. These patients underwent unilateral oophorectomy for fertility preservation purposes prior to chemotherapy. Oncological indications for fertility preservation comprised acute lymphoblastic lymphoma, breast cancer and acute myeloid leukaemia. None of the patients received chemo- and/or radiotherapy before the oophorectomy. In parallel, three post-pubertal age-matched transgender men (23.33 ± 1.53 years) consented to donate their ovarian tissue upon bilateral oophorectomy for gender-confirming surgery. All transgender men included in this study received intramuscular testosterone undecanoate 1000 mg every 12 weeks during 55.33 ± 1.15 weeks as cross-sex hormone treatment prior to the oophorectomy. Per patient, 24 cortical strips were cultured for 6 days and six pieces per patient were collected for downstream analysis every second day (Fig. 1). Analyses included HE staining for follicle classification, RT-qPCR on LCM obtained follicles for transcriptome analysis and fluorescent IHC to study the localisation of different targets on the Hippo and PI3K/Akt pathway (Fig. 2).
Figure 1.
Study design. In total, 144 cortex fragments (24 fragments per patient, N = 6 patients) were collected, prepared and cultured for 6 days. During tissue preparation, the ovarian cortexwas pulled mechanically using the blunt end of a scalpel to flatten out the tissue and subsequently halvedand cut into small interconnecting strips. These interconnecting comb-like strips were further transversely incised (but not fragmented) per 1 mm (d). Scale bar = 5 mm. Every second day, six fragments per patient were collected for downstream (HE staining, LCM-RT-qPCR or fluorescent IHC) analysis. HE, haematoxylin/eosin; LCM, laser capture micro-dissection; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; IHC, immunohistochemistry.
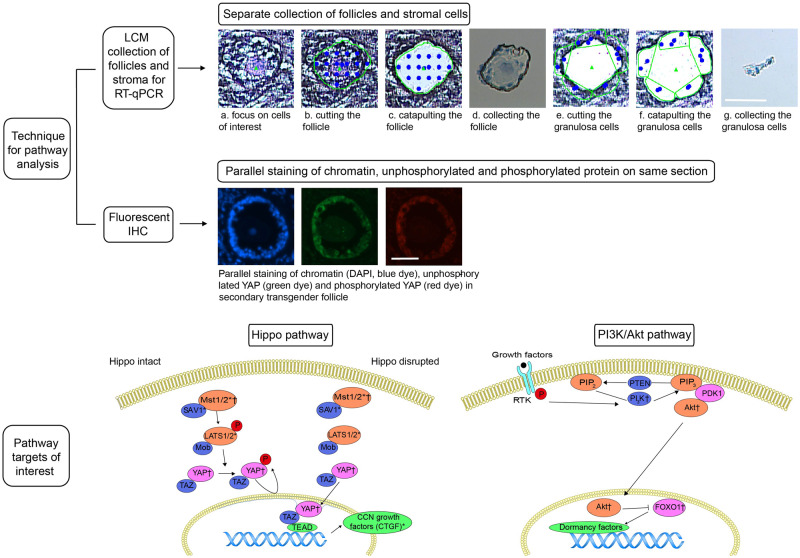
Figure 2.
Analysis techniques and targets of interest on the Hippo and PI3K/Akt pathway. Upper panels: LCM to collect follicle and stromal cells separately by focusing on cells of interest (a), cutting (b), catapulting (c) and collecting (d) of follicles and cutting (e), catapulting (f) and collecting (g) of surrounding stromal cells (illustration shows transgender intermediate follicle on culture Day 0). Underneath, example of IHC for YAP (green dye) and pYAP (red dye) in transgender secondary follicle on culture Day 2, illustrating parallel staining of chromatin (DAPI), unphosphorylated and phosphorylated protein on same section. Lower panels: simplified Hippo and PI3K/Akt pathways with focus on targets of interest. *Pathway targets analysed by RT-qPCR, †Pathway targets analysed by immunohistochemistry. LCM, laser capture micro-dissection; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; IHC, immunohistochemistry; Mst 1/2, mammalian sterile 20-like protein kinase I and II; SAV1, WW domain scaffolding protein Salvador; LATS1/2, large tumour suppressor homologues 1 and 2; CTGF, connective tissue growth factor; YAP, Yes-associated protein; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; FOXO1, Fork head box O1. Scale bar = 50 µm.
Pathway targets analysed by RT-qPCR were mammalian sterile 20-like protein kinase I and II (Mst1 and Mst2), WW domain scaffolding protein Salvador (SAV1), large tumour suppressor homologues 1 and 2 (LATS1 and LATS2) and connective tissue growth factor (CTGF). To study protein phosphorylation as well as potential spatial dynamics of the pathway molecules in the oocyte, granulosa cells and surrounding stroma, IHC for Mst1, Yes-associated protein (YAP) and phosphorylated YAP (pYAP), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), protein kinase B (PKB) also known as Akt and phosphorylated Akt (pAkt), Fork head box O1 (FOXO1) and phosphorylated FOXO1 (pFOXO1) was applied.
Ovarian tissue collection and processing
Immediately after hysterectomy with bilateral oophorectomy, the entire ovaries were transported to the laboratory on ice, in Leibovitz L-15* medium (Life Technologies, Ghent, Belgium), supplemented with 0.45% human serum albumin (Red Cross, Belgium). The ovaries were bisected and the medulla was carefully removed by scraping with a scalpel to prepare the cortical tissue to the required thickness of 1 mm. This cortex was subsequently fragmented in pieces of 5 × 5 mm2. The fragments were cryopreserved by controlled slow-freezing and stored in liquid nitrogen, as described elsewhere (Lierman et al., 2015).
Tissue preparation and culture
The cortical tissue culture system was adapted from the first phase of the two-step culture model as described by E. Telfer (Telfer et al., 2008). The frozen vial containing the cortical fragment was thawed in a warm water bath at 37°C for 2 min. Following three wash steps in Leibovitz L-15* medium (Life Technologies, Ghent, Belgium), supplemented with 0.45% human serum albumin (Red Cross, Belgium) for 5 min, the ovarian cortex (5 × 5 × 1 mm3) was pulled mechanically using the blunt end of a scalpel to flatten out the tissue and to minimise the underlying stroma. To ensure an adequate number of follicles per strip for downstream analysis, larger fragments of ovarian cortex were cultured compared with the protocol by E. Telfer (Telfer et al., 2008). The tissue was halved (2.5 × 5 × 1 mm³) and cut into small interconnecting strips per 1 mm (Fig. 1). These interconnecting strips were further transversely incised (but not fragmented) per 1 mm to mimic the 1 mm³ as described by E. Telfer (Telfer et al., 2008). The cortical comb-like strips were transferred individually in 24-well culture plates (Corning B.V. Life Sciences Europe, Amsterdam, The Netherlands) containing 300 µl of McCoy's 5a medium with bicarbonate supplemented with HEPES (20 mM), HSA (0.1%) (Red Cross, Belgium), glutamine (3 mM) (Thermo Scientific, Merelbeke, Belgium), penicillin G (0.1 mg/ml)-streptomycin (0.1 mg/ml) (Thermo Scientific), transferrin (2.5 µg/ml), selenium (4 ng/ml), insulin (10 ng/ml) and ascorbic acid (50 µg/ml), all obtained from Sigma-Aldrich (Bornem, Belgium), unless stated otherwise. As described by E. Telfer (Telfer et al., 2008), tissue pieces were cultured for 6 days at 37°C in humidified air with 5% CO2 with medium change and collection of six cortical strips per each patient every second day. To serve as a reference on Day 0 in different downstream analyses, six cortical fragments (5 × 5 × 1 mm3) per patient were taken and fixed in 10% buffered formalin immediately after thawing and prior to tissue preparation. On culture Days 2, 4 and 6, the pair of interconnecting strips were considered as one cortical fragment (resulting in twice 2.5 × 5 × 1 mm³) in order to analyse a constant ovarian cortex surface of 5 × 5 × 1 mm. Every second culture day, six pairs of interconnecting strips per patient were fixed in 10% buffered formalin (Sigma-Aldrich, Bornem, Belgium) for 4 h, followed by dehydration and paraffin embedding. These paraffin-embedded tissue cubes were stored at −20°C until further use.
Follicle classification
From the six cortical strips per patient collected every second day, two cortical strips were fixed in 10% buffered formalin (Sigma-Aldrich, Bornem, Belgium) for a maximum of 24 h and embedded in paraffin (Thermo Scientific, Erembodegem, Belgium). The paraffin-embedded ovarian cortical tissue piece was serially sectioned at 5 µm, resulting in 10 slices of 5 × 5 mm2 (surface) × 5 µm (depth), and stained with (Mayer) haematoxylin (Merck, Overijse, Belgium) and eosin (Thermo Scientific, Merelbeke, Belgium). Follicles were analysed using an inverted microscope with a 40× magnification. Follicles were classified according to the Gougeon classification for human follicles (Gougeon, 1986), as described and illustrated previously (De Roo et al., 2017). Follicles were classified on the section containing the nucleus to avoid double counting. Two independent observers analysed the follicles and the mean of the two observations was used for further analysis. Hence, the follicle count sometimes resulted in decimal numbers.
Laser capture micro-dissection
The paraffin-embedded ovarian cortical tissue piece was serially sectioned at 5 µm using diethylpyrocarbonate (DEPC) water in the microtome bath and mounted on RNAse-free membrane polyethylene naphthalene (PEN) membrane glass slides (Zeiss, Zaventem, Belgium) and immediately processed further. Consecutively, the slides were deparaffinised, dehydrated, stained and rehydrated: xylene (5 min twice), isopropyl alcohol (1 min), disolol (1 min), 70% ethanol (1 min), rinsed in DEPC water, Mayer’s haematoxylin (1 min), rinsed in DEPC water, eosin yellow (10 s), 70% ethanol (20 dips), disolol (15 dips) and isopropyl alcohol (10 dips), all products obtained from ChemLab, Zedelgem, Belgium. All reagents were prepared with DEPC-treated water. Stained samples were immediately used for LCM.
Laser capture micro-dissection was performed using the PALM Micro Beam System (P.A.L.M./Zeiss Micro Laser Technologies, Munich, Germany). Follicles and surrounding stromal cells were separately collected per follicle type resulting in selection of primordial follicles, granulosa cells surrounding the primordial follicle, intermediate follicles, granulosa cells surrounding the intermediate follicle, primary follicles, granulosa cells surrounding the primary follicle, secondary follicles and granulosa cells surrounding the secondary follicles. This resulted in the collection of eight different cell types per patient per culture day. Following visualisation, selection and micro-dissection of the targeted cells, the micro-dissected tissue piece is catapulted against gravity and collected in a collection device. This guarantees contamination-free isolation of pure cell populations (Niyaz et al., 2005). Cells were catapulted using the laser pressure catapulting function and collected in the cap of a 200 µl tube containing 40 µl of RNeasy RLT Lysis Buffer (Qiagen, Antwerp, Belgium) with 1% of β-mercaptoethanol (Life Technologies, Ghent, Belgium).
RNA extraction and cDNA synthesis
Follicles and surrounding stromal cells were separately collected per follicle type. Surrounding stromal cells in this setting were defined as the equal width of the corresponding granulosa cell layer width. Additionally, per culture day, stromal cell at a distance from follicles (equals at least 1 microscope field distance from a follicle at 20× magnification) was collected as well. An overview of the collected material using laser capture micro-dissection, with details on number and total surface of collected follicles and stromal cells can be found in Supplementary File S1. The cells of interest (eight batches: primordial follicles, granulosa cells surrounding the primordial follicle, intermediate follicles, granulosa cells surrounding the intermediate follicle, primary follicles, granulosa cells surrounding the primary follicle, secondary follicles and granulosa cells surrounding the secondary follicles) were collected in vials per follicle type per patient per day, transported on ice and immediately processed further. To minimise handling time during RNA extraction, samples were processed in batches of a maximum of eight vials. All follicles and surrounding cells per data point were collected. Therefore, the number of cells was dependent on the follicle number (9.64 ± 11.28, min 1, max 48) resulting in ranging cell surfaces per vial from 72 666.91 ± 89 618.04 μm2 (min 5530, max 349 190) for follicles to 122 103.50 ± 147 573.48 μm2 (min 7540, max 739 475) for stromal cells (Supplementary File S1). Total RNA was extracted on ice using the RNeasy FFPE kit (Qiagen, Antwerp, Belgium) according to the manufacturer’s protocol. Since the samples were laser captured, total RNA extracted was too low to permit quality assessment (Bustin et al., 2009). To avoid RNA disintegration, conversion into cDNA was performed immediately on ice using the iScript advanced cDNA synthesis kit for RT-qPCR (Biorad, Temse, Belgium). CDNA concentration was determined using Qubit fluorometric quantitation method (Life Technologies, Ghent, Belgium). The obtained cDNA was stored −80°C until use.
Quantitative real-time PCR and reference gene selection
To ensure quality, MIQE guidelines for RT-qPCR were pursued (Vandesompele et al., 2002; Hellemans et al., 2007; Bustin et al., 2009). Primers for Mst1, Mst2, SAV1, LATS1, LATS2 and CTGF were designed using Primer Blast software (http://www.ncbi.nlm.nih.gov/tools/primer-blast) (Taylor and Mrkusich, 2014), checked with Netprimer software (http://www.premierbiosoft.com/netprimer/index.html) and subsequently commercially obtained (Invitrogen, Thermo Fisher Scientific, Belgium). Primers were tested for their optimal annealing temperature and the obtained PCR product was validated in silico (Table I). Reference gene selection was performed using the GeNorm Reference Gene Selection Kit for follicles and stromal cells separately (Primer design, Rownhams Southampton, UK) using qBase+ (BioGazelle, Ghent, Belgium). For normalisation of the target gene expression in follicles, the geometric mean of three reference genes (GAPDH, RPL3A and 18s rRNA) was used. For normalisation of the target gene expression in stromal cells RPL13A, EIF4A2 and B2M were used as reference genes.
Table I.
Target gene primer details.
| Target | Primer sequence | Tm optimal (°C) |
|---|---|---|
| Mst1 | Forward: AGCACCGATTTACGCCAGAA | 54°C |
| Reverse: CCGTACCTTTGGTCTCACCC | ||
| Mst2 | Forward: TCCCTCTTTCCGCAAACCTC | 63.3°C |
| Reverse: GATTTGGAGGGGGTCCGAAG | ||
| SAV1 | Forward: GCGGGGAAAGTTTACGGGAT | 66.5°C |
| Reverse: CAGTCGCTGGTCAGTTCCTT | ||
| LATS1 | Forward: CGACGCTCACGAACGATCAG | 54°C |
| Reverse: ATGTAGCCCACACGAAGGAC | ||
| LATS2 | Forward: CAATGTAGCGAATGTCCCACTT | 57°C |
| Reverse: TGAAGATTATCACTCTCTCCAGGG | ||
| CTGF |
Forward: AAACTGGAACGGTGAAGGTG Reverse: CTCAAGTTGGGGGACAAAAA |
59.9°C |
Primer name, sequence and optimal melting temperature (Tm optimal).
RT-qPCR was performed in technical duplicates on a CFX96 Touch™ Real-Time PCR Detection System (Biorad, Temse, Belgium) using iTaq Universal Sybr Green Supermix (Biorad, Temse, Belgium). Each quantitative PCR (qPCR) reaction included 15 μl master mix and 5 ng cDNA and was performed at optimal annealing temperature in hard-shell low-profile thin-wall 96-well skirted PCR plates, sealed with adhesive film. No template (water) was used as negative control. Thermo-cycling conditions were as follows: 3 min at 95°C followed by 40 cycles of 10 s at 95°C followed by 1 min at optimal annealing temperature (Table I).
Immunohistochemistry
Ten paraffin-embedded, sectioned ovarian tissue slices were mounted on glass slides (1 slide per target protein pair (=protein + phosphorylated protein)), deparaffinised and hydrated. Sections were permeabilised and antigens were retrieved in 0.05% Tween 20 (Sigma-Aldrich, Bornem, Belgium) in sodium citrate buffer at 95–100°C for 20 min. After beaker temperature decreased below 35°C, slides were washed in PBS for 2 × 3 min. Primary antibodies (monoclonal rabbit anti-human Mst1 (clone EPR6207, Abcam, Cambridge, UK), monoclonal mouse anti-human YAP (clone number not available, Abcam, Cambridge, UK), monoclonal rabbit anti-human pYAP (clone EP1675Y, Abcam, Cambridge, UK), monoclonal rabbit anti-human PI3K p110α (clone C73F8, Bioke, Leiden, The Netherlands), monoclonal mouse anti-human Akt (clone 9Q7, Thermo Scientific, Merelbeke, Belgium), monoclonal rabbit anti-human pAkt (clone 14-6, Thermo Scientific, Merelbeke, Belgium), monoclonal mouse anti-human FOXO1 (clone 7H3, Thermo Scientific, Merelbeke, Belgium) and polyclonal rabbit anti-human pFOXO1 (Thermo Scientific, Merelbeke, Belgium)) were diluted in PBS with 1% BSA (1:100) and slides were incubated at 4°C overnight. The next day, the slides were washed with 1× PBS two times for 5 min. Secondary antibodies (polyclonal goat anti-mouse IgG Alexa fluor 488 (Abcam, Cambridge, UK) and polyclonal goat anti-rabbit IgG Alexa fluor 594 (Abcam, Cambridge, UK)) were diluted in PBS + 1% BSA (1:200) and slides were incubated for 1 h at room temperature. Finally, slides were washed three times in 1× PBS for 3 min and mounted in Vectashield with 4ʹ,6-diamidino-2-phenylindole (DAPI) (Lab consult SPRL, Brussels, Belgium). Staining without primary antibody was used as negative control for all targets analysed. As a positive control, human ovarian granulosa tumour cell line COV434 (Sigma-Aldrich, Bornem, Belgium) (positive for Mst1, YAP, pYAP, PI3K, FOXO1 and pFOXO1) and tissue sections of mouse liver (positive for YAP, Akt and pAkt) were used. Follicles were analysed using a digital, inverted microscope for fluorescence applications with light cubes for DAPI, GFP and Texas red (EVOS™ FL Color Imaging System, Thermo Scientific, Merelbeke, Belgium). For immunostaining, positive and negative controls were included per batch of eight slides to be stained and microscope settings were adapted to these controls prior to analysis of the corresponding slides. Per patient, per culture day, all follicles in 10 stained ovarian tissue slices were classified and per follicle stage, target protein presence and localisation were described in oocyte, granulosa cells and surrounding stromal cells. Conclusions were drawn on general protein patterns per follicle per culture day, resulting in descriptive non-quantitative results.
Statistical analysis
Statistical analysis was conducted with IBM SPSS Statistics 23 (IBM Corp., New York, USA). The inter-observer variation in HE follicle classification was verified according to Bland-Altman’s limits of agreement. If the difference between the two observations exceeded a single standard deviation, the section was counted again.
For the follicle counts, outcome variables were all count data. The fits for a Poisson regression and a negative binomial regression were compared. Based on Akaike Information Criterion (AIC, it was decided to perform a negative binomial regression. Follicle counts were analysed using a negative binomial regression. For the outcomes follicles classification, a negative binomial regression was performed with culture day, patient group (oncological/transgender) and their interaction as fixed factors, and individual patients as random factor (model 1). A negative binomial regression with the culture day as fixed factor and individual patients as random factor was applied on the whole dataset (if the interaction patient group * culture day was not significant) on the subset with oncological patients and on the subset with transgender men (model 2). For each day, counts for oncological patients were compared to counts for transgender men using a model containing group as fixed factor and individual patients as random factor.
For the RT-qPCR experiments, Cq values were normalised to the three identified reference genes and analysed using the ANOVA and paired t-test on fold change gene expression using the inbuilt statistical software in qBase+ (Primer design, Rownhams Southampton, UK). Gene expression was studied comparing different culture days, different follicle classifications, overall and per patient type (transgender and oncological patients). P < 0.05 was considered to be statistically significant.
Results
In-vitro fragmentation of ovarian tissue activates primordial follicles
Follicles originating from transgender ovarian tissue
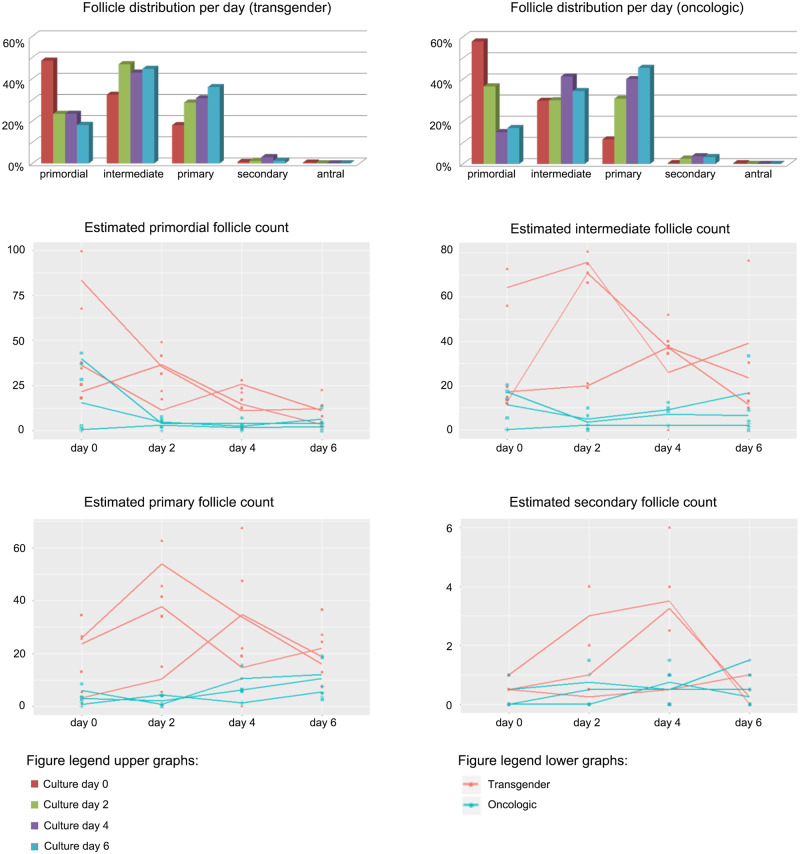
In the ovarian cortex originating from transgender men, a total of 2099 follicles with a mean of 699.67 ± 242.06 follicles per patient and 524.75 ± 166.04 follicles per culture day were counted (Supplementary File S2). On Day 0, the follicle distribution on a total of 584 follicles was: 283 (48.46%) primordial, 188.5 (32.28%) intermediate, 106 (18.15%) primary, 4 (0.68%) secondary and 2.5 (0.43%) antral follicles (Fig. 3). During culture, the counts for primordial follicles on Day 4 were estimated to be 64% lower when compared to Day 0 (95% CI −83% to −23%; P = 0.008) (Fig. 3). Estimated counts for intermediate and primary follicles did not change significantly during culture (Fig. 3). In contrast, the estimated counts for secondary follicles were significantly higher on Day 4 (compared to Day 0, an estimated increase of 367%; 95% CI +26% to +1630%; P = 0.021); however, this finding has to be interpreted with caution due to a limited absolute number of secondary follicles (Fig. 3).
Figure 3.
Follicle distribution per culture day. Follicle distribution as a percentage of the total amount of follicles per culture day in transgender men and oncological patients. Estimated follicle counts in primordial, intermediate, primary and secondary follicles in transgender men (red) and oncological patients (green). Significant lower estimated primordial follicle counts were found on Days 2, 4 and 6 in the oncological group (−78%; 95% CI −94% to −21%; P = 0.02 on Day 2) and on Day 4 and 6 in the transgender cohort (−64%; 95% CI −83% to −23%; P = 0.008 on Day 4). Primary follicle counts were estimated to increase significantly in oncological patients on Day 6 (estimated increase of 170%; 5% CI +14% to +589%; P = 0.025). Significant higher estimated counts of secondary follicles were observed in transgender men on Day 4 (+367%; 95% CI +26% to +1630%; P = 0.021). All follicle counts are compared to the counts on Day 0. *Statistically significant in comparison to Day 0 (P < 0.05).
Follicles originating from oncological ovarian tissue
In the ovarian cortex originating from oncological patients, a total of 481.5 follicles with a mean of 160.50 ± 92.00 follicles per patient and 120.38 ± 56.47 follicles per culture day were counted (Supplementary File S2). The follicle classification on Day 0 showed 112 (58.64%) primordial, 58 (30.37%) intermediate, 19.5 (10.21%) primary, 1 (0.52%) secondary and 0.5 (0.26%) antral follicles (Fig. 3). On Day 0, primary follicle counts were estimated to be 80% lower (95% CI −94% to −26%; P = 0.016) in the oncological group compared to the transgender follicle counts on Day 0 whereas no significant difference in primordial, intermediate or secondary follicle counts was observed.
Within the oncological tissue experiment, the counts for primordial follicles were estimated to be significantly lower from culture Day 2 onwards (−78%, 95% CI −94% to −21%; P = 0.021 on Day 2) when compared to Day 0 (Fig. 3). Intermediate follicle counts did not change during culture (Fig. 3). Primary follicle counts were estimated to increase significantly on Day 6 (compared to Day 0) with 170% higher counts (95% CI +14% to +589%; P = 0.025) (Fig. 3).
The Hippo pathway
Based on the assumption that tissue fragmentation promotes follicle activation and development through the Hippo pathway, different Hippo pathway proteins were studied. RNA and protein expression is discussed in oocyte, granulosa cells and stromal cells with emphasis on the expression dynamics during culture and follicle development, including attention for expression differences between cortex of transgender and oncological patients (Fig. 2). Analysis of the pathway in secondary follicles was limited due to the low number of secondary follicles. Results are reported per target under analysis and not by experiment.
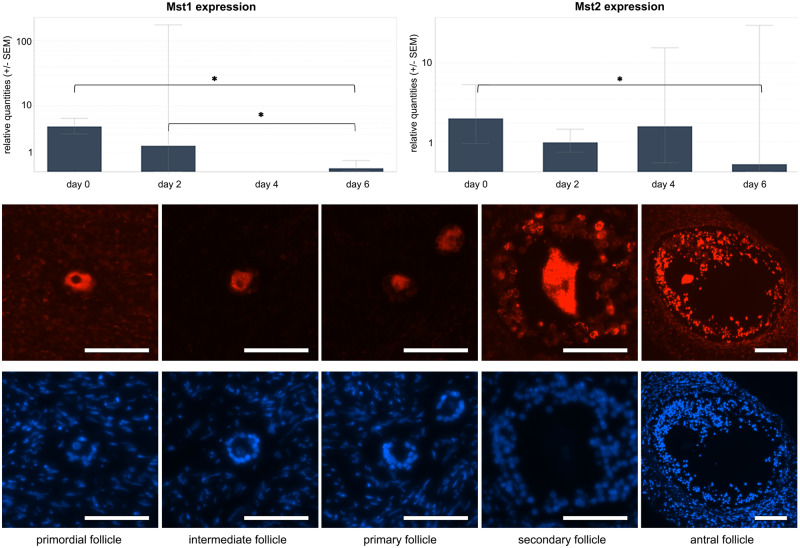
Mammalian sterile 20-like protein kinase 1 and 2 (Mst1 and Mst2) form a complex with Salvador1 to phosphorylate (and thus activate) LATS1/2 (Badouel and McNeill, 2011). RT-qPCR analysis of follicles derived from transgender ovarian cortex revealed a significantly down-regulated expression when comparing Mst1 expression over different culture days (P = 0.009; r2 = 0.977) with significant subgroup ratios between culture Day 0 and Day 6 (ratio Day 0/6 = 30.797; 95% CI 11.649– 81.242; P < 0.05) and between culture Day 2 and Day 6 (ratio Day 2/6 = 11.738; 95% CI 2.623–52.538; P < 0.05). Mst2 appeared to be significantly down-regulated when comparing culture Day 0 and Day 6 (ratio Day 0/6 = 12.019; 95% CI 1.167–123.798, P < 0.05) (Fig. 4). Sub-analysis of the oncological data did not reveal any differences (data not shown).
Figure 4.
Mst analysis. Upper panels: Mst1 expression over different culture days with significant downregulated subgroup ratios between culture Day 0 and Day 6 and between culture Day 2 and Day 6 in transgender follicles (left panel). Mst2 expression over different culture in transgender follicles with significant down-regulated subgroup ratios between culture Day 0 and day (right panel). *Statistically significant. Lower panels: Representative images of Mst1 expression (red dye) in primordial, intermediate, primary, secondary and antral follicle; underneath the same follicles stained with DAPI as a reference (in this example pictures: all transgender follicles on culture Day 6). Mst1 is present in the entire oocyte cytoplasm of the primordial and intermediate follicle, followed by clustering around the nucleus in the secondary follicle and resulting in a nuclear localisation in the antral oocytes. Scale bar = 50 µm.
Immunohistochemical study of Mst1 showed no difference in localisation when comparing tissue derived from transgender and oncological patients. Regardless of the follicle stage or culture day, Mst1 was always observed in the oocyte. The localisation within the oocyte was, however, dynamic during follicle development. Mst1 was present in the entire oocyte cytoplasm of the primordial, intermediate and primary follicle, followed by clustering around the nucleus in the secondary follicle and resulting in a nuclear localisation in the antral oocytes (Fig. 4). Mst1 could also be stained in granulosa cells in follicles of the later stages independent of the culture days. Mst1 was also present in the stromal cells, where no specific clustering around the follicle was seen, nor was there a change in expression of MST1/2 or a change in localisation of the MST1 staining pattern during culture or in different stages of follicle development.
Salvador 1 (Sav1), the regulatory protein of Mst1/2, showed a stable expression in follicles and stromal cells over different follicle types and different culture days in tissue derived from both transgender and oncological patients (data not shown).
Large tumour suppressor homologues 1 and 2 (LATS1 and LATS2), phosphorylated and activated by Mst1/2, in turn phosphorylates and inhibits co-activators YAP and TAZ (Badouel and McNeill, 2011). As for Sav1, RT-qPCR analysis did not reveal altered gene expression of LATS1 and LATS2 during culture nor in the different follicle classes. Also in stromal cells, no difference in gene expression could be detected (data not shown).
The core of the Hippo pathway is the phosphorylation and subsequent localisation of YAP. YAP accumulates in the nucleus and activates the transcription of several growth-promoting genes (Fan et al., 2013). pYAP by Lats1/2 results in cytoplasmic translocation and, therefore, inactivation of YAP (Zhao et al., 2008). The localisation and phosphorylation of YAP are often taken as a measure of the activity of the Hippo pathway; therefore, YAP and pYAP were studied using IHC (Gumbiner and Kim, 2014).
Overall, phosphorylated YAP (pYAP), the inactive form of YAP, was predominantly expressed in the granulosa cells of the follicles whereas YAP was predominantly, but not exclusively, located in the oocyte. On Day 0, follicles of all maturation stages were dominated by a pYAP expression and the presence of YAP in the oocyte gradually diminished in the more mature follicles compared to primordial follicles. Induced by ovarian tissue fragmentation, the presence of pYAP diminished while YAP expression was induced in the oocyte and granulosa cells of maturing follicle stages and in later culture days (Fig. 5).
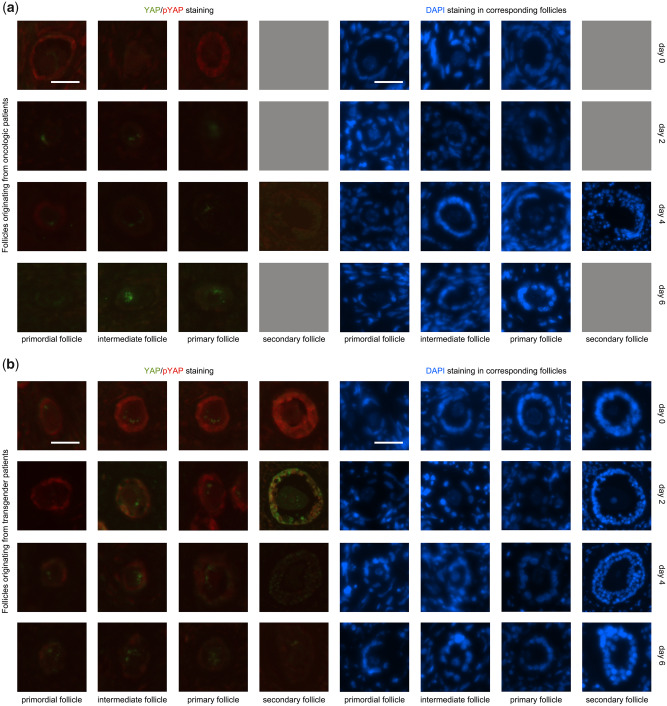
Figure 5.
YAP/pYAP presence in follicles: Representative images of YAP and pYAP expression in primordial, intermediate, primary, secondary and antral follicles. From left to right, increasing follicle stages and from top to bottom increasing culture days. The left four columns illustrate the stainings of interest (green dye = YAP and red dye = pYAP). The right four columns are the same follicles stained with DAPI as a reference. The top four rows (a) are follicles derived from transgender ovaries, the bottom four rows from oncological patients (b). Grey squares = follicles missing for analysis. Scale bar = 25 µm. Induced by ovarian tissue fragmentation, the gradual disappearance of YAP in the oocyte as on Day 0 could not be observed in the maturing follicle stages on culture Days 2, 4 and 6. In granulosa cells, YAP appears in increasing follicle stages and in increasing culture days. During culture, YAP appeared earlier in transgender granulosa cells in all follicle development stages compared to oncological granulosa cells. Phosphorylated YAP (pYAP) was observed in granulosa cells of follicles of every stage, independent of the culture days, except for the disappearance of pYAP in primordial granulosa cells on Day 2 in both patient groups and the absence on culture Day 6 in almost all granulosa cells of oncological patients, independent of the maturation stage.
Intriguingly, comparing transgender and oncological patient populations, YAP expression was slightly different. On Day 0, YAP was not expressed in granulosa cells derived from oncological patients, in contrast to the transgender men cohort where YAP could be found in the granulosa cells of primary and secondary follicles on Day 0. During culture, YAP appeared in transgender granulosa cells in all follicle development stages while YAP was absent in oncological granulosa cells (Fig. 5).
In the surrounding stromal cells, YAP expression appeared discretely on Day 4, in contrast to the YAP absence on the other culture days. In the stromal cells of both oncological and transgender samples, pYAP was diffusely expressed on Day 0 and disappeared gradually until absence on Day 6.
CCN growth factors such as connective tissue growing factor (CTGF) are up-regulated as increased nuclear YAP interacts with TEAD (transcription factors containing the TEA/ATTS DNA-binding domain) (Hsueh et al., 2015). This increased expression ultimately results in cell growth and proliferation. CTGF mRNA in primordial follicles appeared to be significantly up-regulated when comparing Day 2 to Day 4 (ratio Day 4/2 = 0.082; 95% CI 0.007– 0.921; P < 0.05), but not in other day to day comparisons (Fig. 6).
Figure 6.
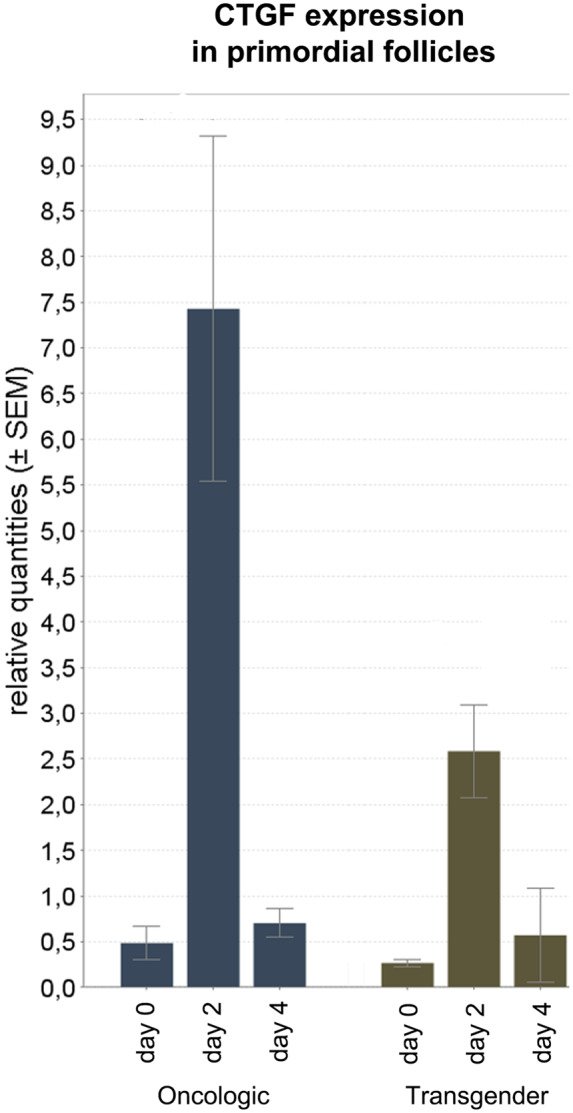
CTGF expression in primordial follicles: RT-qPCR results: CTGF mRNA in primordial follicles appeared to be significantly up-regulated when comparing Day 2 to Day 4 (ratio Day 4/2 = 0.082; 95% CI 0.007– 0.921; P < 0.05), but not in other day-to-day comparisons. *Statistically significant. P < 0.05 was considered to be statistically significant.
The PI3K/Akt pathway
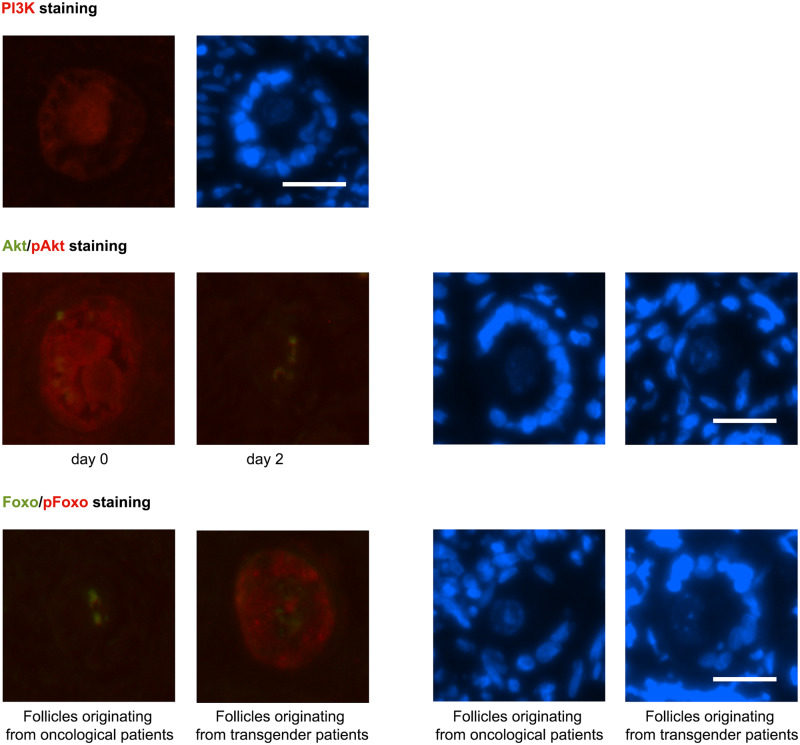
Phosphatidylinositol-3-kinase (PI3K) is stimulated by activated receptor tyrosine kinases (RTK), following the interaction of diverse extracellular factors with their respective RTK (Hsueh et al., 2015). From culture Day 0 until Day 4, PI3K was seen in the cytoplasm of primordial, intermediate and primary oocytes and in all granulosa cells, without change over the culture days. On Day 6, PI3K expression disappeared. PI3K was present in the stromal cells, where no clustering around the follicle could be observed. As in the granulosa cells, PI3K expression disappeared in stromal cells on culture Day 6 (Fig. 7, upper panel).
Figure 7.
IHC PI3K Akt pathway. Upper panel: PI3K was seen in the cytoplasm of primordial, intermediate and primary oocytes and in all granulosa cells (red dye, oncological primary follicle culture Day 4). Middle panel: Akt (green dye) and pAkt (red dye) (oncological intermediate follicles on culture Day 0 and 2). Akt was localised as a hotspot in the oocytes cytoplasm of primordial, intermediate and primary follicles from Day 0 until Day 6. The active counterpart, phosphorylated Akt (pAkt), was diffusely located in the entire oocyte cytoplasm on Day 0 in primordial, intermediate and primary follicles. This diffuse localisation reorganised into a cytoplasmic hotspot on later culture days, independent of the follicle stage. Lower panel: FOXO1 (green dye) and pFOXO1 (red dye) (intermediate follicles culture Day 2 from oncological patients and transgender men). FOXO1 was seen as a hotspot in the oocyte in primordial, intermediate and primary follicles, from Day 0 until Day 4. The inactive phosphorylated 1 (pFOXO1) was observed in both oocyte and granulosa cells. In granulosa cells, pFOXO1 could not be found in from oncological patients derived primordial granulosa cells on every culture day and in intermediate granulosa cells on Day 0 and 2 compared to pFOXO presence in all other conditions. The right panel of the picture pairs represents the same follicles stained with DAPI as a reference. Scale bar = 25 µM.
Akt phosphorylation and thus activation occurs after PI3K induced conversion of phosphatidylinositol-(4,5)-bisposphate (PIP2) into phosphatidylinositol-(3,4,5)-trisposphate (PIP3) and recruitment and activation of phosphatidylinositol-dependent kinases (Hsueh et al., 2015). Akt was localised as a spot in the oocytes cytoplasm of primordial, intermediate and primary follicles from Day 0 until Day 6. Secondary follicles were too limited in number to evaluate the presence of Akt. Akt could not be observed in the granulosa cells in any of the follicle stages, nor in the stromal cells. There was no difference in the findings between oncological and transgender patients (Fig. 7, middle panel).
The active counterpart, pAkt, was diffusely located in the entire oocyte cytoplasm on Day 0 in primordial, intermediate and primary follicles. This diffuse localisation reorganised into a cytoplasmic spot on later culture days, independent of the follicle stage. Again, secondary follicles could not be evaluated due to the limited numbers. In granulosa and stromal cells, pAkt expression was limited in oncological ovarian tissue on Day 0 and completely absent in all other conditions (Fig. 7, middle panel).
Forkhead box protein O1 (FOXO1) is suppressed by nuclear translocation of Akt and subsequent phosphorylation of transcription factor FOXO1 resulting in nuclear extrusion (Hsueh et al., 2015). In the activated form FOXO1 interacts with dormancy factors, inhibiting follicles from activation and growth.
FOXO1 was predominantly expressed in the oocyte compared to the granulosa cells and could not be observed in the stromal cells. FOXO1 was seen as a hotspot in the oocyte in primordial, intermediate and primary follicles, from Day 0 until Day 4 (Fig. 7, lower panel). On Day 6, FOXO1 could not be detected by IHC in the primordial oocyte. In the intermediate and primary oocytes, FOXO1 could still be seen after 6 days of tissue culture. Again, secondary follicles could not be evaluated because of their low number.
The inactive phosphorylated 1 (pFOXO1) was observed in both oocyte and granulosa cells. In the oocyte, pFOXO1 was observed in all oocytes on every culture day, except in primordial follicles on Day 6. In granulosa cells, pFOXO1 could not be identified by IHC in primordial granulosa cells on culture Day 6 and in derived primordial granulosa cells from oncological patients on every culture day and in intermediate granulosa cells on Day 0 and 2, in contrast to the presence of pFOXO in all other conditions (Fig. 7, lower panel). Phosphorylated FOXO1 was not expressed or very limited in stromal cells (data not shown).
Discussion
This work maps a detailed progression in follicle development and the changes in the Hippo and PI3K/Akt pathway during a 6 days in-vitro ovarian tissue culture in order to optimise the use of primordial follicles in-vitro. For this purpose, ovarian tissue from transgender and oncological patient populations was used. Previous work in in-vitro tissue culture optimisation was performed using tissue was obtained during caesarean section (Telfer et al., 2008) or from patients diagnosed with premature ovarian insufficiency (POI) (Kawamura et al., 2016). Perturbation of the growing follicle pool might be expected due to high levels of hCG and oestradiol at the time of caesarean section, however, effects on the dormant follicle pool during pregnancy remain undescribed so far. Harvesting ovarian tissue after a prolonged exposure to FSH and LH in POI patients might affect the residing follicle pool as pre-antral follicles growth are proven to be FSH responsive (Hsueh et al., 2015). In our current setting, one may question the effect of testosterone on folliculogenesis and on the studied pathways, as ovarian tissue was obtained from transgender persons after a period of exogenous testosterone exposure. On the other side, oncological disease might influence follicle growth and development as well. Hence, a strength of this work is the use of ovarian tissue of the target population groups. An optimised ovarian tissue culture model for both target populations, including an individualised approach in both groups depending on the defined needs, is therefore clinically relevant.
First, follicle classification and progression was studied by HE-based follicle counting and grading. Sections on Day 0 enabled us to assess the follicular cohort at baseline level (before tissue preparation and in-vitro culture effects) and were concordant with previous reports (Schmidt et al., 2003; Telfer and Zelinski, 2013; De Roo et al., 2017). The disparity between follicle numbers can be explained by the innate heterogeneity of follicle distribution in the ovary. The cortical follicle count was then used in analysing the shift in follicles during in-vitro culture. A significant drop in primordial follicles was seen in the oncological cohort on Day 2 and in transgender cohort on Day 4. Intermediate follicle counts showed a non-significant trend to increase during culture and this follicle recruitment and growth resulted in a significant rise in primary follicles on Day 6 in oncological patients and although limited in absolute numbers, a significant increase in secondary follicles on Day 4 in the transgender cohort. These results confirm primordial follicle activation by mechanical manipulation of the cortical strips (Telfer et al., 2008; Telfer and Zelinski, 2013). Subsequent antral follicle development could not be observed in the current in-vitro culture setup.
Subsequently, the effect of ovarian tissue fragmentation and culture on the Hippo and PI3K/Akt pathway was investigated, confirming the primordial follicle activation. Ovarian tissue preparation and culture induced the inhibitory pYAP to disappear in granulosa cells of primordial follicles on Day 2. The stimulatory YAP on the contrary appeared in primordial granulosa cells over increasing culture days. Looking at the YAP target CTGF, a significantly up-regulated CTGF was noted in primordial follicles when comparing Day 2 and Day 4, clearly showing an effect on the Hippo pathway in primordial follicles during tissue culture. During culture, YAP expression in the oocyte did not decrease over increasing follicle stages as on Day 0, illustrating that Hippo pathway disruption also influences later follicle stages. This is in line with previous work by Kawamura et al. (2013, 2016), where cortex fragmentation was applied for as a technique for in-vitro activation of ovarian follicle growth.
A remarkable difference is the limited YAP expression in oncological granulosa cells over all culture days and follicle types, when compared to transgender samples. This might indicate a possible effect of either the oncological disease or an influence of testosterone priming prior to surgery. In a previously published study (De Roo et al., 2017), we could not reveal a quantitative difference in follicle counts and distributions, however, testosterone might influence the surrounding matrix as well. Androgen receptor expression is described in stromal cells (Horie et al., 1992; Chadha et al., 1994; Gervásio et al., 2014) and testosterone administration is known to cause a thicker ovarian cortex with ovarian stromal hyperplasia (Ikeda et al., 2013). Matrix stiffening is furthermore known to enhance YAP activation in cancer-associated fibroblasts (Calvo et al., 2013). Hence, these testosterone-induced changes could result in a stiffer cortical ‘niche’, favouring Hippo pathway signalling where disruption might cause a more pronounced effect (Halder et al., 2012; Hsueh et al., 2015).
Following the direct effects on YAP localisation and subsequent up-regulation of CTGF, a down-regulated expression of Mst1 and Mst2 was observed. These findings are in line with previous work of Xiang et al. where a decreased expression of negative regulators of the Hippo pathway had similarly been observed in an 8-day mouse ovary culture. This can be part of the feedback suppression allowing it to be expressed (Xiang et al., 2015).
In this setting, Mst1 has a striking spatiotemporal evolution in the maturing oocyte, reflected by a cytoplasmic localisation in the primordial, intermediate and primary follicle, gradually reorganising through clustering around the secondary oocyte nucleus (regardless of the culture duration) towards a nuclear localisation in the antral follicle. Mst1/2 proteins are involved in stress-induced apoptosis, and if activated in the apoptosis pathway, Mst1/2 translocates to the nucleus (Pan, 2010). This most probably indicates inadequate support of the more mature follicle stages. Because of the scarcity of this valuable material at the different time points, apoptosis was not further investigated.
In addition to the Hippo pathway, the involvement of the PI3K-Akt pathway in primordial follicle activation was studied. Staining on pathway targets showed a stable expression pattern of PI3K, Akt and FOXO1/pFOXO1 from culture Day 0 to Day 4 in primordial, intermediate and primary follicles. This could indicate a rather limited role for the PI3K-Akt pathway in follicle development during tissue culture. Another hypothesis is the possible later involvement of the PI3K-Akt pathway in follicle development during tissue culture (Masciangelo et al., 2020). This descriptive analysis is a valuable start for research. However, interfering with this signalling pathway using pathway stimulators and/or inhibitors, with bpv (hopic) as a PTEN inhibitor and 740YP as an PI3K stimulator, would enrich our knowledge on the specific role of the PI3K-Akt pathway in primordial follicle activation in tissue culture (Kawamura et al., 2013).
The most important changes in primordial follicle activation and Hippo signalling appeared to happen from Day 0 to Day 4 in vitro. These findings lead to recommending a shorter first phase of tissue culture, preferably tissue source specific, being 2 days for oncological and 4 days for transgender cortex. An earlier change to pre-antral follicle medium conditions can be of interest to increase tissue culture efficacy. Further research is needed to evaluate if follicles at this point are competent to be removed from the tissue and to continue growth and maturation after isolation.
In the stromal cells, Hippo pathway targets were either absent (Mst) or diffusely present (YAP-pYAP). Apart from a diminished YAP-pYAP expression on culture Day 6, other changes in expression (IHC) or differential expression (RT-qPCR) over the different culture days could not be described. We can hypothesise that, although there are clearly changed by stretching and cutting the ovarian cortex, these changes probably do not affect the studied pathways in the stromal cells. Changes in the extracellular matrix and cell adhesion seem to be detected by the follicular unit itself.
The massive activation of primordial follicles based on Hippo interruption may also have implications for tissue cryopreservation for transplantation. If transplantation is the intended technique upon thawing, the primordial follicle pool might benefit from larger strips (and thus less Hippo pathway disruption) where less recruitment (and thus burnout) could be a huge benefit. Hippo pathway disruption does not selectively recruit primordial follicles, but might also regulate growth in more advanced follicle stages (Kawamura et al., 2013; Hsueh et al., 2015). Tissue preparation for cryopreservation includes the removal of the medulla by scraping with a scalpel to prepare the cortical tissue to the required thickness of 1 mm. This most probably results physical removal of secondary and early pre-antral follicles, mostly situated on the dissection plane as previously demonstrated (De Roo et al., 2017; Herraiz et al., 2020; Lunding et al., 2020), but also in an unintended activation and burnout of residing follicles of all maturation stages in the cortex fragments. If so, ovarian tissue preparation for cryopreservation might benefit by keeping the medulla (partially) intact. However, cryopreserving larger cortical strips with or without medulla is challenged by an adequate and equal diffusion of cryoprotectants through the tissue without harming the tissue by prolonged exposure to the cryoprotectant (Martinez-Madrid et al., 2004). Another option would be pre-treatment of smaller cortex to limit Hippo pathway disruption effects, for example using Diaphanous (DIAPH) formins that suppress actin depolymerisation (Hsueh et al., 2015).
A limitation of this work is the small sample size, which is inherent to this type of study subject. Cryopreserved tissue for fertility preservation has only limited availability for research, and large amounts of tissue are needed to reduce inter-patient variation in different downstream analysis techniques. A particular and specific weakness of this study is the inability to include an age-matched control group. In the described age group, oophorectomy and cryopreservation of ovarian tissue in healthy fertile women rarely occur, making a comparative tissue culture and downstream pathway analysis not feasible. The findings in this work remain however, valuable as previously suggested pathways are now extensively documented in the target groups of this tissue culture technique.
In conclusion, this work describes the detailed evolution of follicle development and a follicle-specific Hippo pathway involvement during 6 days of in-vitro tissue culture. The results highlight a Hippo pathway driven primordial follicle activation in a culture system where the culture efficiency could be increased by an earlier change to optimal pre-antral follicle conditions. Moreover, tissue preparation for culturing might benefit from adaptations for different target patients, in this study illustrated by a different follicle activation following Hippo pathway disruption in the cortex derived from transgender men compared with that from oncological patients.
Supplementary data
Supplementary data are available at Human Reproduction Open online.
Supplementary Material
Acknowledgements
We would like to express our gratitude to the patients who donated their ovarian tissue for scientific purposes. This research has been conducted through collaboration with the Bimetra biobank, a high-quality bio-repository for Ghent University Hospital and Ghent University. We are grateful to L. Pieters, Department of Basic Medical Science, for her help in the histological preparation of the samples, to T. Lepez, for her support in using the PALM Micro Beam System, to L. Dhaenens for her help in RT-qPCR experiments and use of qBase software, and to R. Colman for the statistical support.
Authors’ roles
C.D.R. participated in the study design, execution of the experiments, analysis of the results, manuscript drafting and critical discussion. S.L. participated in the execution of the experiments and analysis of the results. K.T. participated in the study design, execution of the experiments, analysis of the results, manuscript drafting and critical discussion. P.D.S. participated in the study design and analysis of the results and critical discussion. All authors reviewed the manuscript and approved the final version.
Funding
This research was financially supported by the Foundation Against Cancer (Stichting tegen Kanker, TBMT001816N), the Flemish Foundation of Scientific Research (FWO Vlaanderen, FWO G0.065.11N10) and the Gender Identity Research and Education Society (GIRES) foundation.
Conflict of interest
The authors declare no competing interests.
Contributor Information
C De Roo, Department of Reproductive Medicine, Ghent-Fertility and Stem Cell Team (G-FaST), Ghent University Hospital, 9000 Ghent, Belgium.
S Lierman, Department of Reproductive Medicine, Ghent-Fertility and Stem Cell Team (G-FaST), Ghent University Hospital, 9000 Ghent, Belgium.
K Tilleman, Department of Reproductive Medicine, Ghent-Fertility and Stem Cell Team (G-FaST), Ghent University Hospital, 9000 Ghent, Belgium.
P De Sutter, Department of Reproductive Medicine, Ghent-Fertility and Stem Cell Team (G-FaST), Ghent University Hospital, 9000 Ghent, Belgium.
References
- Badouel C, McNeill H. SnapShot: the hippo signaling pathway. Cell 2011;145:484–484.e1. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009;55:611–622. [DOI] [PubMed] [Google Scholar]
- Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 2013;15:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven JM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long-term androgen-treated transsexual women. Hum Pathol 1994;25:1198–1204. [DOI] [PubMed] [Google Scholar]
- De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T'Sjoen G, Cornelissen R. et al. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online 2017;34:557–566. [DOI] [PubMed] [Google Scholar]
- De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet 2014;384:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update 2009;15:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol 2013;9:735–749. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol 2014;28:1188–1197. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med 2017;377:1657–1665. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril 2015;104:1097–1098. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 2013;99:1503–1513. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, Dolmans MM. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril 2011;95:1787.e1–4. [DOI] [PubMed] [Google Scholar]
- Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A 2013;110:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fend F, Raffeld M. Laser capture microdissection in pathology. J Clin Pathol 2000;53:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervásio CG, Bernuci MP, Silva-de-Sá MF, Rosa-E-Silva AC. The role of androgen hormones in early follicular development. ISRN Obstet Gynecol 2014;2014:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod 1986;1:81–87. [DOI] [PubMed] [Google Scholar]
- Grosbois J, Demeestere I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum Reprod 2018;33:1705–1714. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, Kim NG. The Hippo-YAP signaling pathway and contact inhibition of growth. J Cell Sci 2014;127:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 2012;13:591–600. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 2007;8:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herraiz S, Monzó S, Gómez-Giménez B, Pellicer A, Díaz-García C. Optimizing ovarian tissue quality before cryopreservation: comparing outcomes of three decortication methods on stromal and follicular viability. Fertil Steril 2020;113:609–617.e3. [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Hum Reprod 1992;7:1461–1466. [DOI] [PubMed] [Google Scholar]
- Hsueh AJW, Kawamura K, Cheng Y, Fauser B. Intraovarian control of early folliculogenesis. Endocr Rev 2015;36:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, Saito T. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod 2013;28:453–461. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S. et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A 2013;110:17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kawamura N, Hsueh AJW. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol 2016;28:217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladanyi C, Mor A, Christianson MS, Dhillon N, Segars JH. Recent advances in the field of ovarian tissue cryopreservation and opportunities for research. J Assist Reprod Genet 2017;34:709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lierman S, Tilleman K, Cornelissen M, De Vos WH, Weyers S, T'Sjoen G, Cuvelier CA, De Sutter P. Follicles of various maturation stages react differently to enzymatic isolation: a comparison of different isolation protocols. Reprod Biomed Online 2015;30:181–190. [DOI] [PubMed] [Google Scholar]
- Lunding SA, Andersen AN, Hardardottir L, Olesen H, Kristensen SG, Andersen CY, Pors SE. Hippo signaling, actin polymerization, and follicle activation in fragmented human ovarian cortex. Mol Reprod Dev 2020;87:711–719. [DOI] [PubMed] [Google Scholar]
- Markholt S, Grøndahl ML, Ernst EH, Andersen CY, Ernst E, Lykke-Hartmann K. Global gene analysis of oocytes from early stages in human folliculogenesis shows high expression of novel genes in reproduction. Mol Hum Reprod 2012;18:96–110. [DOI] [PubMed] [Google Scholar]
- Martinez-Madrid B, Dolmans MM, Van Langendonckt A, Defrère S, Donnez J. Freeze-thawing intact human ovary with its vascular pedicle with a passive cooling device. Fertil Steril 2004;82:1390–1394. [DOI] [PubMed] [Google Scholar]
- Masciangelo R, Hossay C, Chiti MC, Manavella DD, Amorim CA, Donnez J, Dolmans MM. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J Assist Reprod Genet 2020;37:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirow D, Roness H, Kristensen SG, Andersen CY. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum Reprod 2015;30:2453–2456. [DOI] [PubMed] [Google Scholar]
- Niyaz Y, Stich M, Sägmüller B, Burgemeister R, Friedemann G, Sauer U, Gangnus R, Schütze K. Noncontact laser microdissection and pressure catapulting: sample preparation for genomic, transcriptomic, and proteomic analysis. Methods Mol Med 2005;114:1–24. [DOI] [PubMed] [Google Scholar]
- Pan DJ. The Hippo signaling pathway in development and cancer. Dev Cell 2010;19:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod 2005;20:3539–3546. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, Byskov AG, Nyboe AA, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod 2003;18:1158–1164. [DOI] [PubMed] [Google Scholar]
- Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng 2014;16:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, Jobling T. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod 2014;29:1828. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod 2015;30:608–615. [DOI] [PubMed] [Google Scholar]
- Taylor SC, Mrkusich EM. The state of RT-quantitative PCR: firsthand observations of implementation of minimum information for the publication of quantitative real-time PCR experiments (MIQE). J Mol Microbiol Biotechnol 2014;24:46–52. [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008;23:1151–1158. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013;99:1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002;3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci 2007;14:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Li J, Hu LL, Huang J, Luo T, Zhong ZS, Zheng YH, Zheng LP. Hippo signaling pathway reveals a spatio-temporal correlation with the size of primordial follicle pool in mice. Cell Physiol Biochem 2015;35:957–968. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao JG, Yuan HX, Tumaneng K, Li HR. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012;150:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu JD, Li L, Li WQ, Li SM, Yu JJ, Lin JD, Wang CY, Chinnaiyan AM. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 2008;22:1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.