Abstract
Pore functionalized membranes with appropriate ion exchange/chelate groups allow toxic metal sorption under convective flow conditions. This study explores the sorption capacity of ionic mercury in a polyvinylidene fluoride–poly(acrylic acid) (PVDFs–PAA) functionalized membrane immobilized with cysteamine (MEA). Two methods of MEA immobilization to the PVDF–PAA membrane have been assessed: (i) ion exchange (IE) and (ii) carbodiimide cross-linker chemistry using 1-(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), known as EDC/NHS coupling. The ion exchange method demonstrates that cysteamine (MEA) can be immobilized effectively on PVDF–PAA membranes without covalent attachment. The effectiveness of the MEA immobilized membranes to remove ionic mercury from the water was evaluated by passing a dissolved mercury(II) nitrate solution through the membranes. The sorption capacity of mercury for MEA immobilized membrane prepared by the IE method is 1015 mg/g PAA. On the other hand, the sorption capacity of mercury for MEA immobilized membrane prepared by EDC/NHS chemistry is 2446 mg/g PAA, indicating that membrane functionalization by EDC/NHS coupling enhanced mercury sorption 2.4 times compared to the IE method. The efficiencies of Hg removal are 94.1 ± 1.1 and 99.1 ± 0.1% for the MEA immobilized membranes prepared by IE and EDC/NHS coupling methods, respectively. These results show potential applications of MEA immobilized PVDF–PAA membranes for industrial wastewater treatment specifically from energy and mining industries to remove mercury and other toxic metals.
Graphical Abstract

1. INTRODUCTION
Mercury (Hg) can be present as dissolved, elemental, or solid (e.g., nanoparticle HgS) species in wastewater streams from various industries, including industrial wastewater treatment plants, oil refineries, and ore extraction operations from mining industries. Mercury species present in industrial wastewaters are typically dominated by HgS nanoparticles and soluble Hg2+ complexes.1,2
The affinity between mercury ions and sulfur is well-known. HgS may be present in geologic formations and enter wastewater streams as HgS particles or may be formed in an industrial facility by the reaction between ionic Hg2+ and sulfur. HgS formation and precipitation can occur in the wastewater stream or can take place elsewhere in a facility and migrate to the wastewater. Organic compounds present in soils, natural waters, sediments, and living beings containing thiol (−SH) groups can complex mercury or form mercurythiol species (Hg(SR)x) through physicochemical sorption, ion exchange, and ligand-induced oxidative complexation.2 In the aqueous phase, the soft Lewis acids Ag+ and Hg2+ prefer complexation with the soft base SH− over reactions with hard bases such as Br−, Cl−, and OH−. The mercury–sulfur complex is poorly soluble and very resilient, which allows for effective removal of ionic dissolved mercury species using sulfur groups.3–5 Cysteine (C3H7NO2S, (Cys)), an amino acid that contains a thiol functional group, is known to interact strongly with mercury ions, affecting the metabolism and nucleic acids in the body.6 Various studies, including some from this group, have used immobilized derivatives of cysteine and polythiol compounds onto polyelectrolytes, taking advantage of the Hg/Ag–thiol affinity for removal of Hg2+ and Ag+.3,7–9 Another amino compound with similar characteristics is cysteamine or β-mercaptoethylamine (C2H7NS (MEA)). One industrial application of MEA is as a chelating agent to support gold and silver in biosensors and membranes. MEA is a better candidate than Cys for ion exchange immobilization onto polyelectrolyte-functionalized membranes because it does not contain carboxyl groups. Due to its use for metal sorption, MEA could be an effective adsorptive material for capturing Hg ions from wastewaters.
Polyvinylidene fluoride (PVDF) microfiltration membranes have a distinct advantage for open structure in terms of high internal surface area, and ease of access in the pore domain enables it to separate solids and bacteria.10 Functionalization of this PVDF membrane with suitable responsive (pH, temperature) polymer allows incorporation of charged groups (−COOH, −OH) in membrane pore domain and tuning of pore size and flux.11,12 This functionalized membrane is a very attractive platform to further incorporation of metallic (Pd–Fe) nanoparticles to use this membrane for degradation of chlorinated organic compounds (COCs).13,14 Apart from this, the incorporated carboxylic (−COOH) groups in PVDF functionalized membrane could be replaced by thiol (−SH) groups by either ion-exchange or EDC/NHS chemistry. This thiol incorporated membrane is a suitable platform for heavy metal sorption from industrial wastewater. In this study, incorporation of thiol groups using both Cys and MEA has been assessed for the application to adsorb mercury and silver from water. However, the application could be further extended to capture other metals such as nickel, gold, arsenic from wastewater.
The overall goal of this work is to synthesize thiol functionalized PVDF membranes for removal of mercury from water. Under this broader aspect, specific aims of this work are to (i) prepare and characterize cysteine and cysteamine functionalized PVDF membranes and (ii) evaluate the efficacy of MEA functionalized membrane for removal of Hg2+ from synthetic water for the future application to treat wastewater.
2. EXPERIMENTAL SECTION
2.1. Materials.
All chemicals used during the laboratory-scale membrane fabrication and other studies were of reagent grade and used without further purification. Acrylic acid (AA), 98% extra pure and stabilized (ACROS ORGANICS, France); N,N′-methylenebisacrylamide (MBA), for electrophoresis, 99+ % (ACROS ORGANICS, Belgium); potassium persulfate (KPS), min 99% (EM SCIENCE, Germany). Fluoraldehyde o-phthaldialdehyde reagent solution (product number (PN) 26025) (Thermo Scientific, Rockford, IL, USA), sodium hydroxide (NaOH) solution (1.0 N), (PN BDH7222) (VWR Analytical, USA), sodium chloride (NaCl) salt, PN BDH9286 (VWR Chemicals, OH, USA), sulfuric acid (H2SO4) solution (1.0 N), PN BDH7232 (VWR Analytical, USA), nitric acid 68.0–70.0%, AR Select ACS for trace metal analysis (Macron Fine Chemicals, Center Valley, PA, USA). Ethanol, 99.5%, (PN EX0276–3) (EMD Millipore Corporation, USA), ammonium persulfate ((NH4)2S2O8), 98+% (Acros Organic, Geel, Belgium), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), ≥98.0% (Sigma-Aldrich, St Louis, MO, USA), and N-hydroxysucinimide (NHS), (C4H5NO3), >98.0% (TCI, Tokyo, Japan). Cysteamine hydrochloride (MEA), ≥98.0% (Sigma-Aldrich, St. Louis, MO, USA), l-cysteine hydrochloride; monohydrate (Cys), (C3H7NO2-HCl-H2O), PN C-6852 (Sigma-Aldrich, St. Louis, MO, USA). Mercury(II) nitrate hydrate (Hg(NO3)2-xH2O, x = 1–2), ACS 98.0% (Alfa Aesar, Ward Hill, MA, USA). Silver nitrate (AgNO3), crystal, 99.8–100.5% (PN JT3429–04), (J. T. Baker, Phillipsburg, NJ, USA). Commercial scale membranes of polyvinylidene fluoride (PVDF, microfiltration 250–400 nm pore size, thickness with backing is around of 174 ± 8 μm (PVDF layer ≈70 μm) and porosity around 36–44%) (PV700 produced in collaboration with Nanostone Water, Inc., USA). Membrane surface area of 13.2 cm2 was used.
2.2. Membrane Functionalization.
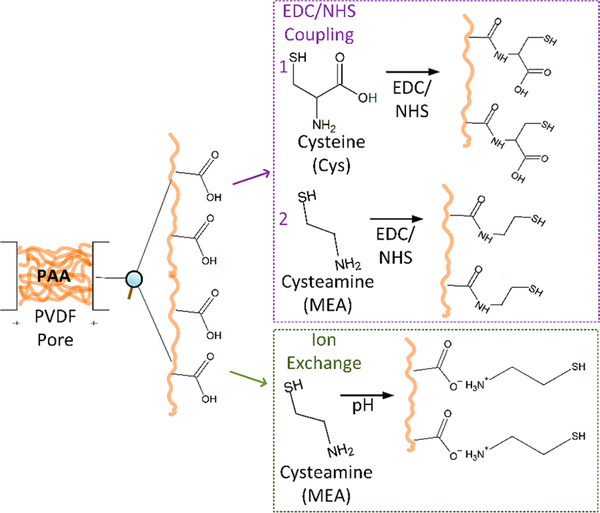
Thiol functionalized membranes using a polyvinylidene fluoride (PVDF) support were used in order to capture mercury from wastewaters. The microfiltration PVDF membrane was functionalized with cross-linked poly(acrylic acid) (PAA). The functionalization of PAA was conducted through a free radical in situ polymerization reaction using a 10 wt % aqueous solution of the AA in the presence of MBA as cross-linker (1.0 mol % of AA) following our previous published works.11,15,16 The thickness of the PAA layer depends on the degree of concentration of the polymer and the cross-linker in solution used during the in situ polymerization reaction.11 Immobilization of thiol functionality to the PVDF–PAA membrane was done by two methods. In the first method, an ion exchange process is proposed for immobilization of MEA on the PVDF–PAA membrane using an alkaline solution (1 g/L of MEA adjusted with 0.1 N NaOH solution to pH ≈ 8.5; T = 22 °C), as depicted in Figure 1. This ion exchange process is partially derived from previous work by this group, using a dead-end cell (Sterlitech HP4750) to pass through the MEA alkaline solution.13,14,17
Figure 1.
General functionalization process of PVDF–PAA membranes (PVDF Nanostone PV700) with cysteine or cysteamine through two routes: EDC/NHS coupling with (1) cysteine (Cys) or (2) cysteamine (MEA), and ion exchange with cysteamine (MEA).
The ion exchange with MEA has advantage over Cys because of its higher affinity to be immobilized by ion exchange to the PVDF–PAA membrane at pH relevant to wastewater treatment. The Cys is a zwitterionic molecule with three pKa values, the carboxyl being the lowest (see Table 1).18–21 Cys is negatively charged at neutral pH (6–8).22 Meanwhile, MEA zwitterion has two high pKa values. Compared to Cys, MEA can adsorb more strongly to a deprotonated PAA (which pKa is weakly acidic) because the MEA zwitterion is usually present in its cationic form (see Table 1).12,23–25
Table 1.
Dissociation Constants of Selected Organic Compounds in Aqueous Solution
In the second method, EDC/NHS coupling is used to functionalize the membranes with Cys or MEA as depicted in Figure 1. Amino compounds (Cys or MEA) can be covalently attached through activation of carboxyl groups with EDC. Since this reaction is performed in solution, the hydrolysis of the active EDC complex has to be avoided by the addition of NHS, forming a more stable intermediate that will react with the amino groups present in Cys and MEA.26,27 An EDC/NHS solution is passed three times through the membrane. The pH of the EDC/NHS solution dropped from 5.5 to 4.9, indicating removal of protons from carboxylic acid groups.
Covalent attachment of Cys or MEA is achieved via bond formation between its primary amino group and the carboxylic acid groups of PAA. An equimolar (5 mM) mixture of EDC and NHS solution was prepared, and pH was adjusted to 5.5–6.5 using NaOH. This solution was passed through the membrane by convective flow mode using the dead-end cell. Once the solution starts passing through the membrane pores, it enables the ion exchange process to activate the carboxyl groups by removing their protons, thereby forming carboxylate ions. Evidence of this reaction is the change in the pH of feed solutions when the EDC/NHS filtrate is recycled. Carboxylate acts as a nucleophile that interacts with the electrophilic nitrogen present in the amino acid. Later, a (1 g/L) Cys or MEA solution was prepared, and pH was adjusted to 7.5 using NaOH. This solution was then passed through the membrane in the same mentioned manner in order to attach Cys or MEA to the membrane to prepare the thiol functionalized membrane. Once Cys or MEA is attached, their thiol functional groups are at the end of a carbon chains originating from the PAA. This thiol incorporated PVDF–PAA membrane has high adsorption capacity to capture mercury ions from aqueous solution once it is passed through the membrane. After each functionalization step (PAA followed by thiols), the membranes were washed several times with DI water and dried afterward.
2.3. Materials Characterization.
Several characterization techniques were used to support the immobilization of Cys and MEA in the PVDF–PAA membrane. The total organic carbon (TOC) of the Cys/MEA solutions was measured by the TOC-5000A instrument of Shimadzu Corporation. Changes in the concentration of the feed to permeate and feed to retentate were measured to quantify the immobilization of Cys/MEA to the PVDF–PAA membrane. This TOC analyzer was calibrated using in-house prepared carbon standards. The experimental error during analysis was lower than 2%. Ultrahigh purity oxygen was used as carrier gas (pressure of 6.0 bar and 150 mL/min) during TOC measurement.
Attenuated total reflectance Fourier transformation infrared (ATR-FTIR) detector (Varian 7000e) was used to assess the different stages of functionalization of the PVDF membrane with PAA, Cys, and MEA. Analyzing the absorption peaks of fluorocarbons, carboxyl, thiol, and amine groups will ascertain the functionality of each step. The ATR-FTIR spectrum during measurement was set between 500 and 4000 cm−1. Varian Resolution Pro 4.0 software was used with the instrument to set and record the parameters of measurement. The resolution was set to a value of 8 cm−1, sensitivity was set to 1, the number of scans was 32, and speed was set to 5 kHz during FTIR measurement. The samples were placed on a diamond crystal while recording the signal. The detectors used in the ATR-FTIR instrument are specially designed to measure the special interferogram signal. It measures the amount of energy at each frequency which has passed through the sample. This results in a spectrum ultimately generated in a plot of intensity vs frequency. The elemental composition on the surface of functionalized membranes was measured using X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-alpha XPS system). The analysis of the XPS spectrum further backs up the functionalization of Cys/MEA to the PVDF–PAA membrane either by EDC/NHS coupling or by the ion-exchange process. Besides, XPS was used again after metal (Hg/Ag) removal studies using thiol-functionalized membranes to reveal the binding of Hg/Ag to thiol groups. Both survey scan and high-resolution scan were conducted for XPS analysis. The β angles (degrees from vertical) of the XPS instrument were the following: monochromator (crystal) 60°, ion gun 58°, flood gun 58°, and height adjust microscope 45°. No tilt was used. The step size was 1 and 0.1 eV for survey and high-resolution scan. The pass energy was 200 and 50 eV for survey and high-resolution scan. XPS spectra were calibrated (charge correction) to a C 1s peak value of 284.8 eV. Further, the presence of thiol groups in functionalized membranes was also characterized by fluorescence-labeling, using fluoraldehyde in the presence of n-propylamine, using a confocal microscope (Zeiss LSM880 multiphoton microscope).
2.4. Membrane Transport and Removal Studies with Mercury Solutions.
Membrane transport studies were conducted using a 47 mm diameter membrane coupon in a stirred dead-end filtration cell (Sterlitech HP4750). The water permeability of the membrane was determined with pH adjusted Hg2+ and Ag+ solutions (0.16 mg/L (163 ppb) of Hg(NO3)2-xH2O (x = 1–2) and 1 mg/L AgNO3). The pH of the solution was monitored throughout the experiment. A transmembrane pressure of 2.1 bar and ambient temperature (20 ± 2 °C) were maintained during the experiment. Permeance as a function of pH was examined by adjusting the water pH with either HCl or NaOH solution, and it is directly proportional to the applied pressure.
Mercury concentrations of the feed and retentate were determined using a Nippon Instruments Corporation (NIC) MA-3000. Samples were injected into the Nippon MA-3000 without preparation. The instrument thermally decomposes all mercury to elemental mercury vapor. The mercury is captured and concentrated on a gold trap. The mercury is quantitatively desorbed and quantified by an atomic absorption detector. The instrument was calibrated to 1–100 ppb range. Ag+ solutions were used as a control of metal capturing due to its higher affinity to sulfur in membrane coupon replicas. Inductively coupled plasma optical emission spectroscopy (ICP-OES, VARIAN VISTA-PRO) was used to quantify the concentration of silver ions in the feed, and retentate, after acidification with nitric acid (1 v/v%)]. For Ag+ analysis the instrument was calibrated from 5 to 100 ppm.
3. RESULTS AND DISCUSSION
3.1. Membrane Characterization.
The functionalization of the PVDF membranes with PAA, reaction with EDC/NHS, and subsequent functionalization with Cys or MEA were assessed by ATR-FTIR. The FTIR spectra of pristine PVDF membrane are presented in Figure 2. The characteristic peaks for C–F bonds (1170 cm−1), C–F2 bonds (1200 cm−1), and vibration of CH2 bending (1400 cm−1) are shown in Figure 2a. The PVDF membrane spectra are also present in the functionalized membranes. In Figure 2b, the zoomed spectra are shown and some characteristic peaks are identified by comparing to published data.28
Figure 2.
Absorbance FTIR spectra of thiol (cysteine (Cys)/cysteamine (MEA)) functionalization of PVDF membranes. (a) PVDF pristine membrane [spectrum (1)]; PVDF functionalized with poly(acrylic acid) [spectrum (2)]; PVDF functionalized with poly(acrylic acid) and cysteine (Cys) by EDC/NHS coupling [spectrum (3)]; PVDF functionalized with Poly(acrylic acid) and cysteamine (MEA) by EDC/NHS coupling [spectrum (4)]. (b) Zoom-in image of dashed frame in (a) in the spectral range of 1450–3750 cm−1.
Functionalization of the pristine PVDF with PAA is supported by the appearance of a carbonyl stretch peak (C=O) between 1600 and 1740 cm−1 wavelength.29,30 The addition of a covalently bonded MEA to the PVDF–PAA membrane is further backed up by the appearance of a deformation peak centered at 1551 cm−1 wavelength that could be attributed to amide II stretching.29,30 However, the signal of amide II stretching peak for Cys (3) is weaker than MEA (4) due to high concentration of −COOH groups present in the membrane and could be overlapped. This amide II stretching peak is absent in PVDF and PVDF–PAA spectra 1 and 2.
The covalent attachment of Cys and MEA by EDC/NHS coupling is further supported by a small absorption peak observed close to the wavelength of 3000 cm−1 in spectrum 3 of Figure 2b, corresponding to the presence of C–H bonds. The peaks that represent the C–S and S–H stretching vibrations of Cys or MEA around 1250 and 2500 cm−1, respectively, are not observed in spectra 3 and 4 in Figure 2b. This could indicate the cleavage of the S–H bond but may also be a result of overlapping by the strong stretches and wide bands in those regions corresponding to the C–F and C–F2 groups from PVDF and O–H groups from PAA. The absorbance FTIR spectra of each functionalization step for Cys immobilized PVDF–PAA membrane is shown in Figure S1. The amide II and amide I bands are visible around wavelength of 1450 cm−1and 1650 cm–1, respectively. In addition, amide II stretching in Cys immobilized PVDF–PAA membrane is clearly visible near wavelength of 3250 cm−1, suggesting covalent attachment of Cys to PVDF–PAA membrane.29,30
The functionalization of the PVDF membranes with PAA and subsequent Cys or MEA was analyzed by measuring the elemental composition of the membrane surfaces before and after Hg2+ or Ag+ capture by XPS instrument. The spectra of C 1s, N 1s, S 2p, Hg 4f, or Ag 3d in each functionalization process using MEA and the subsequent metal capture from synthetic Hg2+ and Ag+ solutions are shown in Figure 3, Figure S2, and Figure S3. Peaks were identified through comparison with published results.31–35 The survey and high resolution XPS spectra of fluorine (F 1s) for MEA immobilized PVDF–PAA membrane is presented in Figure S2. Further, Figure S3 shows the XPS results of Cys immobilized PVDF–PAA membrane used to capture silver for control experiment.
Figure 3.
Cysteamine (MEA) functionalization of PVDF membranes and bonding between thiol and Hg were assessed by X-ray photoelectron spectroscopy (XPS) spectra of carbon (C 1s), nitrogen (N 1s), sulfur (S 2p), and mercury (Hg 4f). Immobilization of cysteamine (MEA) to PVDF–PAA membrane was done by two methods: ion exchange (a, c, e, g) and EDC/NHS coupling (b, d, f, h).
The observation of C 1s spectra supports functionalization of the PVDF–PAA membrane with MEA by ion exchange and EDC/NHS coupling. The C 1s spectra are deconvoluted in five peaks corresponding to C–C (283.2; 283.3 eV), C–N (284.9; 284.8 eV), C–S (286.2; 286.4 eV), C–O and O–C=O (289.3; 289.2 eV), and CF2 (291.4; 290.8 eV) bonds respectively, and are shown in Figure 3a,b. The presence of N 1s spectra has a single peak corresponding to protonated amino groups C–NH3+ of ion exchange (399.5 eV) and C–N–C covalent bonds of EDC/NHS coupling (400.0 eV) (Figure 3c,d). These peaks can be attributed to the amine group from MEA, suggesting lower intensity for the ion exchange than for the EDC/NHS coupling functionalization.
The two XPS peaks of S 2p of ion exchange of MEA in Figure 3e (163.3 eV for C–SH and 167.9 eV for Hg–SH) have less intensity on the metal binding side than the ones from EDC/NHS shown in Figure 3f. These lower intensities are possibly due to differences in charge densities of MEA’s zwitterion in the presence of other competitive charges. This supports the notion that the ion exchange process is weaker than the EDC/NHS chemistry to incorporate thiol groups in PVDF–PAA membrane. For S 2p peak in the functionalization of MEA by EDC/NHS, the two peaks in Figure 3f are related to the C–SH bonds (162.4 eV) and Hg–SH bonds (164.2 eV). They show higher intensity for metal binding than for free MEA trapped within the PAA entanglements. The presence of C-SH bonds indicates attachment of MEA in PVDF–PAA membrane.
The presence of the thiol (−SH) groups was further examined by confocal analysis using a red tag, showing a complete functionalization of the membrane structure by MEA presented in Figure S4. The XPS peaks of Hg 4f7/2 and Hg 4f5/2 at binding energies between 100 and 105 eV for ion exchange (Figure 3g) and between 102 and 106 eV for EDC/NHS (Figure 3h) indicate that Hg2+ was bound to sulfur through chelation (the deconvolutions show two additional peaks that correspond to the same binding energies, as discussed above).
3.2. Permeance of Mercury Solution by Cys and MEA-Functionalized Membranes.
The water permeance (A) was measured for various pH values at each step of functionalization (Figure 4). The goal of this analysis is to show the change of the membrane responsiveness in terms of permeability at different stages of functionalization, including ion exchange and EDC/NHS coupling. Due to its low concentration for the capturing tests, the mercury solution could then be used for pH responsive behavior and for mercury capture simultaneously. The 8 mg/L Hg(NO3)2 stock solution had a pH of 4.5. Diluting the stock solution to 163 ppb of Hg(NO3)2 with DI water increased its pH to 5.3. The pH of the solution increased during the testing of thiol functionalized membranes and decreased for PVDF–PAA membranes. Low pH values increase the A value of the PVDF–PAA membrane while high pH values (≥9.8) reduce A values. At low pH, pore structure is more open due to the lower charge–charge repulsion between the high density of −COOH groups in the PAA. This results in a more open structure. At high pH, the high density of –COO− in the PAA causes a more closed pore structure due to charge–charge repulsion. The repulsion of –COO− groups results in a physical change in the PAA structure causing swelling. This swelling in turn closes the pores of the membrane. However, charge density is not changing in the pore domain. This has been discussed in detail with previous studies on similar PVDF–PAA membranes performed by this group.10
Figure 4.
Permeance results of cysteamine (MEA) membranes (Nanostone PV700) functionalized by (a) ion exchange and (b) EDC/NHS coupling. Transmembrane pressure = 2.1 bar. The EDC/NHS coupling membrane MEA/COOH ratio is 1 molar for the membrane tested at pH = 5.0. The ion exchanged MEA/COOH ratio is 0.4 molar for the membrane tested at pH = 6.3. Error bars indicate the confidence interval of the permeability (95%) in the different pH values tested.
The water permeance (A) of the ion exchange membrane with MEA groups is sensitive to pH. During a single experiment using DIUF water and solutions of HCl and NaOH at pH values of 5.3, 3.6 and 8.0, respectively, the A values increased from 296.3 ± 39.4 to 349.9 L/(m2-h-bar) (LMH/bar) as pH decreases and then decreased to 4.7 (LMH/bar) as pH increases. For Hg2+ capture, the Hg(NO3)2 solution pH decreased from 5.3 to 4.5 during filtration while the A value decreased to 168.2 LMH/bar as pH decreased from 5.3 to 4.5. It is possible that the uptake of Hg by −SH groups decreases the charge–charge repulsion of these groups within the membrane. Alternatively, the decrease in pH may have caused the removal of MEA by ion exchange due to proton recovery by –COO− groups. It has been observed that a decrease of pH from 6.3 to 4.5 decreases the loading of MEA functionalization by ion exchange from 1.7 to 0.4 thiol/AA molar ratio. Having a ratio higher than the stoichiometric (1.7 in thiol/AA) could be related to partial immobilization of MEA, more than 1:1, due to higher charge density of carboxylic groups in the vicinity. The decrease in MEA immobilization may then increase the A value.
The immobilization of MEA on PVDF–PAA membrane by EDC/NHS coupling resulting in covalent attachment of MEA to PAA reduces the variability in the responsive behavior compared with the PVDF–PAA membrane at similar pH; see Figure 4. The A value decreases from 317.5 LMH/bar to 78.2 LMH/bar as pH is increased from 5.3 to 9.8.
3.3. Mercury Capture Analysis.
Mercury capture in membranes functionalized with MEA through ion exchange is significantly affected by pH. The breakthrough of Hg through the membrane is observed at <50 L/m2 of treating a 162 ppb Hg(NO3)2 solution at pH 4.5. At pH 6.3, no significant breakthrough was observed during the 400 L/m2 experiment. The removal of mercury was 6.9% and 20.2% with respect to the feed solution at the end of the pH 4.5 and 6.3 runs, respectively. The breakthrough curve in of the experiments are shown in Figure 5.
Figure 5.
Sorption of Hg2+ (solution volume per area of the membrane) by PVDF–PAA–MEA membranes (Nanostone PV700) functionalized by ion exchange and EDC/NHS coupling. Pressure is 2.1 bar. Initial Hg concentration is ~160 ppb.
The poor breakthrough performance of the membrane functionalized with MEA through ion exchange at low pH may be caused by multiple mechanisms. The permeance increased during each experiment due to the decreasing pH within each experiment as described earlier. This increase in permeance results less residence time in pore domain, ultimately causeing less mercury sorption by thiol groups immobilized at the end of the PAA chain.
The high mercury capacity of the membrane functionalized with MEA through ion exchange was not achieved, possibly due to the low pH. Previous results in this study show a decrease of more than 50% of MEA in the membrane as solution pH is lowered from 6.3 to 4.5 due to protonation of carboxylic groups present in the PAA. The concentration of Hg2+ in permeate fluctuated significantly due to pH responsiveness of PAA at pH 6.3; see Figure 5. Once pH of the membrane permeance was stabilized, the Hg2+ concentration did not increase with time as volume treated, as in the beginning.
Mercury capture with membrane functionalized with MEA through EDC/NHS coupling achieved higher Hg loadings than membranes functionalized through ion exchange at lower pH values. In the membrane functionalized by EDC/NHS coupling, the concentration of Hg2+ in permeate is also constant compared to the membrane immobilized by ion exchange, due to almost complete functionalization with MEA to the PAA groups. As shown previously, MEA was immobilized in almost a stoichiometric ratio. This result allowed a constant Hg2+ capture of 98.6% of the feed solution and prevents the loss of MEA due to variations in pH, which may occur with ion-exchange membranes and affect their sorption efficiencies. The variability of the Hg2+ capture by MEA immobilized by EDC/NHS coupling shown in Figure 5 could be due to a small pH responsiveness from the free −COO− groups present.
The efficient capture of Hg2+ under mild conditions for all membranes tested is shown in Figure 6. The diagonal line indicates the theoretical complete sorption of all Hg2+ passed through the membranes. The efficiencies of Hg capture by MEA functionalized membranes are 94.1 ± 1.1 and 99.1 ± 0.1% for ion exchange and by EDC/NHS coupling, respectively. The idea of pore functionalization is to functionalize the whole pore domain and not only the pore wall. This leads to passing of the fluid through the functionalized hydrogel when an external pressure is applied. This allows the wastewater (in this case Hg water) to pass through the thiol functionalized polymer domain resulting high encounters with thiol groups to allow adsorption of heavy metals. This ultimately results high metal capture leading to high adsorption efficiency. However, sometimes pore channels result due to poor functionalization during fabrication of membrane. The sorption capacity of the EDC/NHS functionalized membrane is more stable than for the ion exchange functionalized membrane, possibly due to the covalent grafting of MEA that may make the sorption sites more available and the avoidance of losing MEA due to changes in pH. Similar results for Ag+ were found during functionalization with Cys through EDC/NHS coupling for control experiments of Ag+ capture, indicating that functionalization is reproducible; see Figure S3. This EDC/NHS coupling of MEA is comparable to the control experiments using Cys for silver capture shown in Figure S5.
Figure 6.
Relationship between Hg passed through the membrane and Hg captured, in PVDF–PAA-MEA membranes (Nanostone PV700) functionalized by ion exchange and EDC/NHS coupling. Tests pressure = 2.1 bar.
The measured Hg2+ sorption capacity is 1015 mg/g PAA for MEA immobilized PVDF–PAA membrane prepared by ion exchange. On the other hand, the measured Hg2+ sorption capacity is 2446 mg/g PAA for MEA immobilized PVDF–PAA membrane prepared by EDC/NHS chemistry. On the basis of this result, capacity of a membrane module has been assessed to treat wastewater. An 8040-commercial membrane module was used as a basis with a total surface area of 31.6 m2 (340 ft2) per module. Further considering an operating pressure of 2.0 bar, the calculation reveals, with a Hg2+ concentration of 1.0 mg/L in water, the potential volume of water that could be treated is 212 and 512 m3/module by thiol functionalized membrane prepared by ion exchange and EDC/NHS chemistry. In Table 2 comparison of the adsorption capacity of thiol functionalized membrane with other commercial and reported materials is shown. However, the adsorption capacity of MEA immobilized PVDF–PAA membrane could be increased by incorporating more thiol groups in the membrane. This could be done by increasing the concentration of PAA and MBA during initial in situ polymerization of PVDF membrane but might result in a flux drop instead.11
Table 2.
Comparison of Adsorption Capacity of Thiol Functionalized Membrane with Other Commercial and Reported Materials
| Sl no. |
type of material | functional group |
capacity | ref |
|---|---|---|---|---|
| 1 | PVDF–PAA–MEA membrane (by ion-exchange) (with a functional layer thickness of 70 μm) | Thiol | 96 g/L memb, 1015 mg/g PAA, 55 mg/g membrane | This work |
| 2 | PVDF–PAA–MEA membrane (by EDC/NHS chemistry) (with a functional layer thickness of 70 μm) | Thiol | 232 g/L memb, 2446 mg/g PAA, 133 mg/g membrane | This work |
| 3 | Commercial AMBERSEP GT74 chelating resin | Thiol | ≥1.4 equiv/L, 358 mg/ga | 36 |
| 4 | Magnetic porous organic polymers (MOP-SH) | Thiol | 703 mg/g | 37 |
| 5 | Azo-linked magnetic porous organic polymers (AzoMOP-SH) | Thiol | 910 mg/g | 37 |
| 6 | Thiol-/thioether-functionalized porous organic polymers (POP-SH/SMe) | Thiol | 180 mg/g | 38 |
| 7 | Layered double hydroxide intercalated with the MoS42− ion (MoS4-LDH) | (MoS4)2− | 500 mg/g | 39 |
| 8 | Functionalized porous organic polymer (POP-SH) | Thiol | 1216 mg/g | 40 |
| 9 | Molybdenum disulfide (MoS2) | Sulfide | 2506 mg/g | 41 |
| 10 | Single walled carbon nanotube (SWCNT-SH) | Thiol | 131 mg/g | 42 |
| 11 | Sulfur/reduced GO nanohybrid (SRGO) | Sulfur | 908 mg/g | 43 |
| 12 | Luminescent metal–organic frameworks (LMOF-263) | Thiol | 380 mg/g | 44 |
This value wawws calculated based on the bulk density (784 g/L) of the material given in product data sheet.
4. CONCLUSIONS
This work demonstrated the incorporation of thiol groups (using both cysteamine, MEA, and cysteine, Cys) in PAA functionalized PVDF microfiltration membrane by ion-exchange and EDC/NHS coupling route. This thiol functionalized PVDF–PAA membrane has high adsorption capacity to remove mercury from water. The mercury adsorption capacity of MEA immobilized PVDF–PAA membrane is of 1015 mg/g PAA and 2446 mg/g PAA, respectively, for the membranes prepared by the IE and the EDC/NHS coupling methods. With this capacity, a commercial membrane module with a surface area of 31.6 m2 could theoretically treat approximately 512 m3 of industrial wastewater with a 1 mg/L of dissolved mercury. The efficiencies of Hg removal are 94.1 ± 1.1 and 99.1 ± 0.1%, respectively, for the MEA immobilized membranes prepared by the IE and the EDC/NHS coupling methods. On the basis of these experimental results, this MEA immobilized PVDF–PAA membranes could be an alternative new platform for continuous removal of heavy metals from industrial wastewater.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by Chevron Energy Technology Company, Richmond, CA It is also partially supported by the National Institutes of Environmental Health Sciences (NIEHS-SRC) (Grant P42ES007380) and by NSF-EPSCoR (Grant 1355438). We thank Michael J. Detisch of Department of Chemical and Materials Engineering for his support during XPS analysis. We also acknowledge the support of the Environmental Research Training Laboratory (ERTL) and the Light Microscopy Core at the University of Kentucky for use of the facilities.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.iecr.9b03761.
Absorbance FTIR spectra of pristine and functionalized PVDF membranes, survey and high-resolution spectra from X-ray photoelectron spectroscopy (XPS) of fluorine (F 1s) of cysteamine-functionalized PVDF membranes by two methods of ion exchange and EDC/NHS coupling, survey and high-resolution spectra from X-ray photoelectron spectroscopy of carbon (C 1s), fluorine (F 1s), nitrogen (N 1s), sulfur (S 2p), and silver (Ag 3d) of cysteine (Cys) functionalized PVDF membranes by EDC/NHS coupling, confocal microscopy results of PVDF–PAA–MEA membrane, and control results of silver capture (Ag+) in PVDF–PAA–cysteine microfiltration membranes functionalized by EDC/NHS coupling (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Meeks ND; Davis E; Jain M; Skandan G; Bhattacharyya D Mercury Removal by Thiol-functionalized Metal Oxide–Carbon Black Sorbent and Mixed-Matrix Membranes. Environ. Prog. Sustainable Energy 2013, 32 (3), 705–714. [Google Scholar]
- (2).Avellan A; Stegemeier JP; Gai K; Dale J; Hsu-Kim H; Levard C; O’Rear D; Hoelen TP; Lowry GV Speciation of Mercury in Selected Areas of the Petroleum Value Chain. Environ. Sci. Technol. 2018, 52 (3), 1655–1664. [DOI] [PubMed] [Google Scholar]
- (3).Smuleac V; Butterfield DA; Sikdar SK; Varma RS; Bhattacharyya D Polythiol-Functionalized Alumina Membranes for Mercury Capture. J. Membr. Sci. 2005, 251 (1), 169–178. [Google Scholar]
- (4).Gu B; Bian Y; Miller CL; Dong W; Jiang X; Liang L Mercury Reduction and Complexation by Natural Organic Matter in Anoxic Environments. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (4), 1479–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Schuster E The Behavior of Mercury in the Soil with Special Emphasis on Complexation and Adsorption Processes - A Review of the Literature. Water, Air, Soil Pollut. 1991, 56 (1), 667–680. [Google Scholar]
- (6).Wagner-döbler I Pilot Plant for Bioremediation of Mercury-containing Industrial Wastewater. Appl. Microbiol. Biotechnol. 2003, 62 (2–3), 124–33. [DOI] [PubMed] [Google Scholar]
- (7).Ritchie SMC; Kissick KE; Bachas LG; Sikdar SK; Parikh C; Bhattacharyya D Polycysteine and Other Polyamino Acid Functionalized Microfiltration Membranes for Heavy Metal Capture. Environ. Sci. Technol. 2001, 35 (15), 3252–3258. [DOI] [PubMed] [Google Scholar]
- (8).Ritchie SMC; Bachas LG; Olin T; Sikdar SK; Bhattacharyya D Surface Modification of Silica- and Cellulose-Based Microfiltration Membranes with Functional Polyamino Acids for Heavy Metal Sorption. Langmuir 1999, 15 (19), 6346–6357. [Google Scholar]
- (9).Ladhe AR; Frailie P; Hua D; Darsillo M; Bhattacharyya D Thiol-Functionalized Silica–Mixed Matrix Membranes for Silver Capture from Aqueous Solutions: Experimental Results and Modeling. J. Membr. Sci. 2009, 326 (2), 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hernández S; Papp JK; Bhattacharyya D Iron-Based Redox Polymerization of Acrylic Acid for Direct Synthesis of Hydrogel/Membranes and Metal Nanoparticles for Water Treatment. Ind. Eng. Chem. Res. 2014, 53 (3), 1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Islam MS; Hernández S; Wan H; Ormsbee L; Bhattacharyya D Role of Membrane Pore Polymerization Conditions for pH Responsive Behavior, Catalytic Metal Nanoparticle Synthesis, and PCB Degradation. J. Membr. Sci. 2018, 555, 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Xiao L; Isner A; Waldrop K; Saad A; Takigawa D; Bhattacharyya D Development of Bench and Full-Scale Temperature and pH Responsive Functionalized PVDF Membranes with Tunable Properties. J. Membr. Sci. 2014, 457, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Wan H; Briot NJ; Saad A; Ormsbee L; Bhattacharyya D Pore Functionalized PVDF Membranes with In-Situ Synthesized Metal Nanoparticles: Material Characterization, and Toxic Organic Degradation. J. Membr. Sci. 2017, 530, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Gui M; Ormsbee LE; Bhattacharyya D Reactive Functionalized Membranes for Polychlorinated Biphenyl Degradation. Ind. Eng. Chem. Res. 2013, 52 (31), 10430–10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hernández S; Lei S; Rong W; Ormsbee L; Bhattacharyya D Functionalization of Flat Sheet and Hollow Fiber Microfiltration Membranes for Water Applications. ACS Sustainable Chem. Eng. 2016, 4 (3), 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Xiao L; Davenport DM; Ormsbee L; Bhattacharyya D Polymerization and Functionalization of Membrane Pores for Water Related Applications. Ind. Eng. Chem. Res. 2015, 54 (16), 4174–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Gui M; Papp JK; Colburn AS; Meeks ND; Weaver B; Wilf I; Bhattacharyya D Engineered Iron/Iron Oxide Functionalized Membranes for Selenium and Other Toxic Metal Removal from Power Plant Scrubber Water. J. Membr. Sci. 2015, 488, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Boethling RS; Mackay D Handbook of Property Estimation Methods for Chemicals: Environmental Health Sciences; CRC Press: Boca Raton, FL, 2000. [Google Scholar]
- (19).O’Neil M The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; Royal Society of Chemistry: Cambridge, U.K, 2013. [Google Scholar]
- (20).Riauba L; Niaura G; Eicher-Lorka O; Butkus E A Study of Cysteamine Ionization in Solution by Raman Spectroscopy and Theoretical Modeling. J. Phys. Chem. A 2006, 110 (50), 13394–13404. [DOI] [PubMed] [Google Scholar]
- (21).Perrin DD; Dempsey B; Serjeant EP pKa Prediction for Organic Acids and Bases; Springer: The Netherlands, 1981. [Google Scholar]
- (22).Laughlin RG Fundamentals of the Zwitterionic Hydrophilic Group. Langmuir 1991, 7 (5), 842–847. [Google Scholar]
- (23).Hernández S; Saad A; Ormsbee L; Bhattacharyya D Nanocomposite and Responsive Membranes for Water Treatment. In Emerging Membrane Technology for Sustainable Water Treatment; Hankins NP, Singh R, Eds.; Elsevier Science: Cambridge, MA, U.S., 2016; pp 389–431. [Google Scholar]
- (24).Kadłubowski S; Henke A; Ulański P; Rosiak JM; Bromberg L; Hatton TA Hydrogels of Polyvinylpyrrolidone (PVP) and Poly(acrylic acid) (PAA) Synthesized by Photoinduced Crosslinking of Homopolymers. Polymer 2007, 48 (17), 4974–4981. [Google Scholar]
- (25).Datta S; Cecil C; Bhattacharyya D Functionalized Membranes by Layer-By-Layer Assembly of Polyelectrolytes and In Situ Polymerization of Acrylic Acid for Applications in Enzymatic Catalysis. Ind. Eng. Chem. Res. 2008, 47 (14), 4586–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Riccardi D; Guo H-B; Parks JM; Gu B; Summers AO; Miller SM; Liang L; Smith JC Why Mercury Prefers Soft Ligands. J. Phys. Chem. Lett. 2013, 4 (14), 2317–2322. [Google Scholar]
- (27).Davenport D; Gui M; Ormsbee L; Bhattacharyya D Development of PVDF Membrane Nanocomposites via Various Functionalization Approaches for Environmental Applications. Polymers 2016, 8 (2), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Aina V; Ghigo D; Marchis T; Cerrato G; Laurenti E; Morterra C; Malavasi G; Lusvardi G; Menabue L; Bergandi L Novel Bio-Conjugate Materials: Soybean Peroxidase Immobilized on Bioactive Glasses Containing Au Nanoparticles. J. Mater. Chem. 2011, 21 (29), 10970–10981. [Google Scholar]
- (29).Sarma R; Islam MS; Miller A-F; Bhattacharyya D Layer-by-Layer-Assembled Laccase Enzyme on Stimuli-Responsive Membranes for Chloro-Organics Degradation. ACS Appl. Mater. Interfaces 2017, 9 (17), 14858–14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sarma R; Islam MS; Running M; Bhattacharyya D Multienzyme Immobilized Polymeric Membrane Reactor for the Transformation of a Lignin Model Compound. Polymers 2018, 10 (4), 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wang W; Lu Y-C; Huang H; Wang A-J; Chen J-R; Feng J-J Solvent-free Synthesis of Sulfur- and Nitrogen-co-doped Fluorescent Carbon Nanoparticles from Glutathione for Highly Selective and Sensitive Detection of Mercury(II) Ions. Sens. Actuators, B 2014, 202, 741–747. [Google Scholar]
- (32).Lan M; Zhang J; Chui Y-S; Wang H; Yang Q; Zhu X; Wei H; Liu W; Ge J; Wang P; Chen X; Lee C-S; Zhang W A Recyclable Carbon Nanoparticle-based Fluorescent Probe for Highly Selective and Sensitive Detection of Mercapto Biomolecules. J. Mater. Chem. B 2015, 3 (1), 127–134. [DOI] [PubMed] [Google Scholar]
- (33).Zhang X; Wu T; Zhang Y; Ng DHL; Zhao H; Wang G Adsorption of Hg2+ by Thiol Functionalized Hollow Mesoporous Silica Microspheres with Magnetic Cores. RSC Adv 2015, 5 (63), 51446–51453. [Google Scholar]
- (34).Mostegel FH; Ducker RE; Rieger PH; El Zubir O; Xia S; Radl SV; Edler M; Cartron ML; Hunter CN; Leggett GJ; Griesser T Versatile Thiol-based Reactions for Micrometer- and Nanometer-scale Photopatterning of Polymers and Biomolecules. J. Mater. Chem. B 2015, 3 (21), 4431–4438. [DOI] [PubMed] [Google Scholar]
- (35).Jalani G; Cerruti M Nano Graphene Oxide-wrapped Gold Nanostars as Ultrasensitive and Stable SERS Nanoprobes. Nanoscale 2015, 7 (22), 9990–9997. [DOI] [PubMed] [Google Scholar]
- (36).AMBERSEP GT74. Product Data Sheet. https://www.dupont.com/content/dam/Dupont2.0/Products/water/literature/177-03106.pdf.
- (37).Huang L; Peng C; Cheng Q; He M; Chen B; Hu B Thiol-Functionalized Magnetic Porous Organic Polymers for Highly Efficient Removal of Mercury. Ind. Eng. Chem. Res. 2017, 56 (46), 13696–13703. [Google Scholar]
- (38).Cheng J; Li Y; Li L; Lu P; Wang Q; He C Thiol-/Thioether-Functionalized Porous Organic Polymers for Simultaneous Removal of Mercury(II) Ion and Aromatic Pollutants in Water. New J. Chem. 2019, 43 (20), 7683–7693. [Google Scholar]
- (39).Ma L; Wang Q; Islam SM; Liu Y; Ma S; Kanatzidis MG Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42− Ion. J. Am. Chem. Soc. 2016, 138 (8), 2858–2866. [DOI] [PubMed] [Google Scholar]
- (40).Aguila B; Sun Q; Perman JA; Earl LD; Abney CW; Elzein R; Schlaf R; Ma S Efficient Mercury Capture Using Functionalized Porous Organic Polymer. Adv. Mater. 2017, 29 (31), 1700665. [DOI] [PubMed] [Google Scholar]
- (41).Ai K; Ruan C; Shen M; Lu L MoS2 Nanosheets with Widened Interlayer Spacing for High-Efficiency Removal of Mercury in Aquatic Systems. Adv. Funct. Mater. 2016, 26 (30), 5542–5549. [Google Scholar]
- (42).Bandaru NM; Reta N; Dalal H; Ellis AV; Shapter J; Voelcker NH Enhanced Adsorption of Mercury Ions on Thiol Derivatized Single Wall Carbon Nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [DOI] [PubMed] [Google Scholar]
- (43).Thakur S; Das G; Raul PK; Karak N Green One-Step Approach to Prepare Sulfur/Reduced Graphene Oxide Nanohybrid for Effective Mercury Ions Removal. J. Phys. Chem. C 2013, 117 (15), 7636–7642. [Google Scholar]
- (44).Rudd ND; Wang H; Fuentes-Fernandez EMA; Teat SJ; Chen F; Hall G; Chabal YJ; Li J Highly Efficient Luminescent Metal–Organic Framework for the Simultaneous Detection and Removal of Heavy Metals from Water. ACS Appl. Mater. Interfaces 2016, 8 (44), 30294–30303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.