Abstract
BACKGROUND:
The misuse of opioids stems, in part, from inadequate knowledge of molecular interactions between opioids and opioid receptors. It is still unclear why some opioids are far more addictive than others. The κ-opioid receptor (KOR) plays a critical role in modulating pain, addiction, and many other physiological and pathological processes. Butorphanol, an opioid analgesic, is a less addictive opioid with unique pharmacological profiles. In this study, we investigated the interaction between butorphanol and KOR in order to obtain insights into the safe usage of this medication.
METHODS:
We determined the binding affinity of butorphanol to KOR with a naltrexone competition study. Recombinant KORs expressed in mammalian cell membranes (Chem-1) were used for G protein activation studies, and a HEK-293 cell line stably transfected with the human KOR was used for β-arrestin study as previously described in the literature. The effects of butorphanol on KOR internalization were investigated using N2A-tdT cells over-expressing KOR. The active state KOR crystal structure was used for docking calculation of butorphanol to characterize the ligand binding site. Salvinorin A, a full KOR agonist, was used as a control for comparison.
RESULTS:
The affinity of KOR for butorphanol is characterized by Kd of 0.1 ± 0.02 nM, about twenty-fold higher compared with that of the μ-opioid receptor (MOR, 2.4 ± 1.2 nM). Our data indicate that butorphanol is more potent on KOR than on MOR. In addition, butorphanol acts as a partial agonist of KOR in the G protein activation pathway, and is a full agonist on the β-arrestin recruitment pathway, similar to that of salvinorin A. The activation of the β-arrestin pathway is further confirmed by KOR internalization. The in silico docking model indicates that both salvinorin A and butorphanol share the same binding cavity with the KOR full agonist MP1104. This cavity plays an important role in determining either agonist or antagonist effects of the ligand.
CONCLUSIONS:
In conclusion, butorphanol is a partial KOR agonist in the G protein activation pathway, and a potent KOR full agonist in the β-arrestin recruitment pathway. The structure analysis offers insights into the molecular mechanism of KOR interaction and activation by butorphanol.
A recent review stated that around 4% of the adult US population misuses prescription opioids.1 This misuse and abuse is now an opioid crisis, especially in the US.2 The misuse of opioids comes partially from the mis-prescribing of them, which, in turn, stems from a lack of understanding of the medical indications and the pharmacological profile of opioids. Opioids are medications that include a wide array of compounds interacting with opioid receptors. Individual members of this diverse class of compounds can have unique pharmacological profiles that are suited for a wide range of different clinical situations. In the 2016 guideline from the Centers for Disease Control and Prevention for opioid prescription for pain management, no detailed discussion on specific types of opioids was provided.3 The likely reason for this is that there are limited data available to understand the unique pharmacological profiles of these opioids. More basic studies, especially on the molecular pharmacology of each individual opioid, are needed. Only through a deeper understanding of the pharmacological characteristics of these opioid medications can we develop specific indications and predict side effects for these opioids.
Morphine, fentanyl, hydromorphone, methadone, and oxycodone are all highly potent opioids that are schedule II-controlled substances used for moderate and severe pain control. In preventing opioid dependence and addiction, compounds that have high potency but are placed in a lower scheduling category should be considered. Butorphanol, an opioid assigned to schedule IV, has a much lower potential of abuse and limited physical and psychological dependence compared to morphine.4 It is also almost 6 times as potent as morphine as an analgesic, as indicated in a clinical trial for perioperative pain management.5 Butorphanol is consider as a partial μ-opioid receptor (MOR) agonist, and morphine is a full MOR agonist.6 In a rodent pain model, it was shown that the agonism of butorphanol on the κ-opioid receptor (KOR) plays an important role in its analgesic effects.7
KOR plays a critical role in modulating opioid addiction, dependence, and pain, and the literature suggests that butorphanol generates its pharmacological effects through KORs.8 Therefore, a pharmacological and clinical importance exists to investigate the molecular interactions between butorphanol and KOR. We hypothesized that butorphanol might have unique interactions with KOR different from other KOR agonists. In this study, we explore such interactions using computational and in vitro experiments. The information derived from the study could potentially improve the safe usage of butorphanol and its potential novel clinical indications.
METHODS
Materials
L0005RED, a tag-lite receptor red labeled naltrexone (a KOR antagonist) and C1TT1KOP, the tag-lite labeled KOR cells, are from Cisbio (Bedford, MA). Recombinant human KOR expressed in the mammalian cell line (Chem-1) were purchased from Millipore (Billerica, MA, USA). Salvinorin A with a purity greater than 98% was obtained from Apple Pharms Ingredients Inc (Asheville, NC, USA).
Butorphanol and dezocine (greater than 98% purity) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Yangtze River Pharmaceutical group (Jiangsu, China) respectively. All other chemicals used were analytic grade or higher and were used without further purification.
Affinity Determinations with KOR
The affinities of butorphanol, salvinorin A, and dezocine were determined by competing with L0005RED in the tag-lite labeled KOR on HEK293 cells from Cisbio using a similar method reported previously.9,10 Briefly, the cells were dispensed at 3700 cells/10 μL/well with 60 nM of Tag-lite Opioid receptors red antagonist. Series amounts of butorphanol, salvinorin A, or dezocine were added with subsequent incubation for two hours in room temperature, the fluorescence intensity changes are then determined at 665 nm and 620 nm by a POLARstar Omega plate-reader (BMG-Labtech Inc, Cary, NC). Salvinorin A is used as a positive control as a KOR agonist, and dezocine was used as a negative control as a KOR antagonist. Affinities were determined by obtaining a concentration–response association using the GraphPad (version 8.2.1, GraphPad Software, San Diego, CA). Three repeats were performed to ensure reproducibility.
G Protein Activation
The effects of butorphanol on the activation of the KOR expressed in mammalian cell membranes were investigated by measuring G protein activation in vitro. Salvinorin A, a full agonist of the κ-receptor was used as a positive control. The assay reports the initial activation rates of heterotrimeric G proteins (Gαii1β1γ2) on an agonist-bound KOR by measuring the accumulation of [35S]-GTPγS bound to the activated Gαi1 subunit.11 Myristoylated Gαi1 was expressed using a Escherichia coli over-expression system, and purified as described previously11. Purified human β1γ2 subunits of G protein were obtained using baculovirus-infected Sf9 cell over-expression system as previously described 12–20. The G protein activation assay was conducted with series concentrations in 50 μL reaction mixture as indicated parentheses. The membrane with KOR was diluted into 40 ng/μL with 3-(N-morpholino)propanesulfonic acid (MOPS, 10 mM) ice-cold buffer. Ten μL of the diluted dispersion were dispensed into siliconized glass tubes, and mixed with the butorphanol or salvinorin A in MOPS buffer containing 0.1% (w/v) bovine serum albumin. Upon adding a mixture of Gαi1 (100 nM) and Gβ1γ2 (500 nM), the samples were incubated for 30 minutes on ice. Then, MOPS buffer (50 mM, pH 7.5), EDTA (1 mM), MgCl2 (3 mM), GDP (4 μM), NaCl (100 mM), DTT (1 mM), bovine serum albumin (0.3% w/v), and [35S]-GTPγS (5 nM, 1250 Ci/mmol) were added to initiate the activation followed by a water bath at 30 oC for 45 minutes. Ice-cold stop solution (2 mL) containing TNMg (20 mM Tris-HCl pH 8.0, 100 mM NaCl, and 25 mM MgCl2) was used to stop the reaction. The samples were then filtered rapidly through nitrocellulose filters. Filters were washed with 2 mL each of cold TNMg buffer for four times, they were dried and placed in ScintiSafe Econo F scintillation liquid. The radioactivity is counted in each vial for data analysis. The activations were determined by obtaining a concentration–activation association curve using the GraphPad.
β-Arrestin Recruitment Study
β-arrestin recruitment studies were performed in HTLA cells as described by Kroeze et al.21 HTLA cells are a HEK-293 cell line from the laboratory of Richard Axel. These cells, via the “Tango” assay described by Barnea et al, allow for the reporting of GPCR-arrestin interactions via luciferase gene expression.22 In HTLA cells, GPCRs are expressed as fusion proteins with tTA (a transcription factor). A TEV protease target sequence separates the receptor from tTA. These cells also express a TEV protease-arrestin fusion protein and a tTA dependent luciferase gene. Upon ligand-GPCR interaction and subsequent arrestin recruitment, TEV protease cleaves tTA from the GPCR, allowing luciferase to be expressed.22 Kroeze et al modified this construct for high throughput screening of GPCRs by adding restriction sites to allow for simple cloning of different GPCR coding sequences upstream of the TEV protease signal sequence.21 A KOR-TANGO construct constructed this way was a gift from the lab of Bryan Roth; it was used to transfect HTLA cells in the following assay. Broadly, HTLA cells were transfected with the human KOR TANGO construct and exposed to drug solutions; luminescence was measured the next day and served as a proxy for β-arrestin pathway activation.
First, non-transfected HTLA cells were cultured at 37°C with 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS (fetal bovine serum), 100 μg/ml hygromycin B, and 2 μg/ml puromycin. On the first day of the experiment, 3 × 106 cells were plated in a 10-mm cell culture dish in DMEM with 10% FBS overnight. Cells were transfected the next day using FuGENE (Promega) and 19 μg of plasmid DNA construct. On the third day, transfected cells were transferred at 20,000 cells per well in 100 μl of DMEM medium with 1% dialyzed FBS, streptomycin (100 μg/mL) and penicillin (100 IU/mL). Prior to transfer, 96-well cell culture plates (Greiner Bio-one) were coated with poly-L-lysine and rinsed six times. Drug stimulation solutions were prepared using assay buffer. The assay buffer, which was filter-sterilized, consisted of 20 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) in 1X HBSS (Hanks’ Balanced Salt Solution) at pH 7.4. On day 3, 7 hours after transferring cells into wells, 20 μL of 6X drug stimulation solution was added to each well for a final well volume of 120 μL. Finally, on day 4, the media and the drug solution mixture were aspirated from each well and 48 μl of Bright-Glo solution (Promega) diluted 4-fold in assay buffer was added to each well. Luminescence was measured following incubation for 5 minutes at room temperature and in the absence of light. Data was analyzed by calculating average luminescence for each drug concentration. Background luminescence was calculated by measuring luminescence of Bright-Glo solution (Promega) diluted 4-fold in assay buffer, and average background luminescence was subtracted from the sample values. The data was then normalized and nonlinearly fit with the 4-parameter Hill Equation.
The Effects of Butorphanol on KOR Internalization
Mouse neuroblastoma Neuro2A cells over-expressing KOR as a mKOR-tdTomato fusion protein (N2A-mKOR-tdT) were prepared as described previously.23 N2A-mKOR-tdT cells were cultured in DMEM with 10% FBS, 1% Sodium Pyruvate, 100 U/ml penicillin, 1% GlutaMAX™-1(100×) and 0.2% Geneticin(G418). Cells were maintained in a humidified atmosphere consisting of 95% air and 5% CO2 at 37°C. Cells were synchronized overnight before use. In order to visualize and determine whether butorphanol could induce KOR internalization, cells were treated with various concentrations of butorphanol ranging from 0.1 to 8 μM for 5min,10min,20min,30min,40min and 1h and observed by fluorescence microscopy. To demonstrate the internalization can be blocked by KOR antagonist, 2 μM of dezocine was co-incubated with 0.4 μM of butorphanol for 30 min, and examined under microscope. Cells were examined under the same conditions for at least 3 repeats in each group.
Docking calculations
This experiment aims to compare the molecular interactions of butorphanol and salvinorin A, a KOR agonist without pathway selectivity and demonstrate potential binding forces for butorphanol in the KOR binding pocket. The active form structure of the human KOR was obtained from the protein data bank (PDB access code:6B73).24 The docking calculations were carried out using DockingServer (http://www.dockingserver.com) 25 as previously described.26 The molecular structure of butorphanol and salvinorin A were obtained from PyMOL using SMILES string (The PyMOL Molecular Graphics System VS, LLC). The initial position, orientation, and torsions of butorphanol and salvinorin A molecules were set randomly to allow free searching for the final binding mode. The image of the result was analyzed using PyMOL. The orientation of the molecule in the binding pocket was compared with the intrinsic binding molecule in the known crystal structure.
Statistical Analysis
The results for internalization and docking are descriptive. The affinity determination and beta arrestin activation are quantitative. Data are presented in mean ± standard deviation (SD) where statistical analysis was needed. For the affinity estimation and activation determination, the hypothesis is that there is no concentration response observed if no significant binding or activation occurs. The outcome on the affinity determination is the changes in the fluorescence signal, the outcome on the activation is either the radioactive signal change in the G protein activation studies or the fluoresce signal changes in the β-arrestin recruitment studies which is further confirmed with receptor internalization study. Both the reference and the outcome variables are tested. For the affinity study, HTRF signal was calculated as a two-wavelength signal ratio: [intensity (665 nm)/intensity (620 nm)]. ΔF is used for the comparison of different runs of the same assay which reflects the signal to background of the assay. ΔF=[(Ratiosample-Ratiobackgroud)/Ratiobackgroud](%). For the β-arrestin recruitment Study, the receptor activation induced fluorescence changes (%) were plotted against log concentration of the added ligands. In each set of experiments, three to four repeats were repeated depending on the available of the samples to ensure reproducibility. When the results became equivocal (i.e., large variance of standard errors or only a subtle change is observed, or the chosen concentration range failed to reveal full concentration-response curve for the efficacy estimation), additional experiments were performed by other lab staff to verify the results. 95% of the confidence interval (CI) is calculation. All the analysis was reported previously without deviation. GraphPad (version 8.1.2.1 (411)) was used for data analysis. No p values were compared in this biological study.
RESULTS
Affinity Determination of Butorphanol with KOR
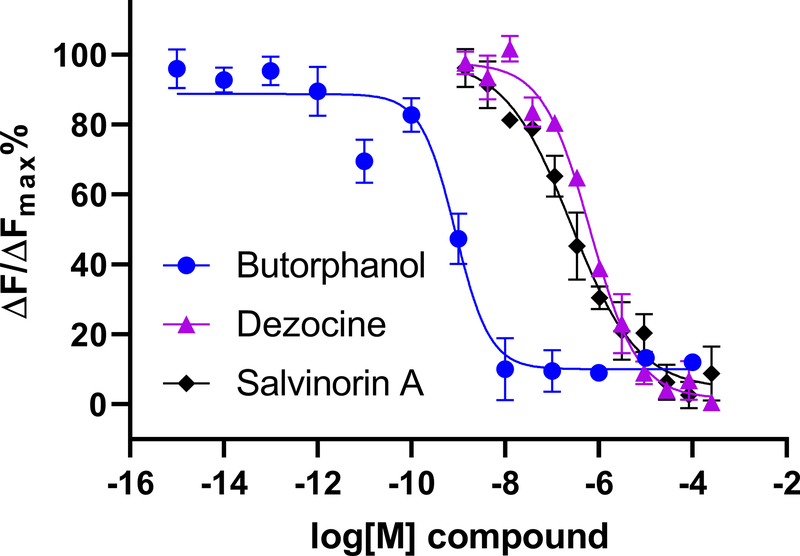
As indicated in Figure 1, butorphanol demonstrated strong affinity for KOR compared to salvinorin A and dezocine, with the respective Ki values being 0.1 ± 0.02 nM (95% CI [0.077, 0.123]) for butorphanol, 56.0 ± 2.0 nM (95% CI [53.74, 58.26]), for dezocine, and 60.0 ± 3.0 nM (95% CI [53.74, 58.26]) for salvinorin A. The affinity of butorphanol for KOR is about twenty-fold higher compared to its affinity for the μ-opioid receptor (2.4 ± 1.2 nM) (95% CI [1.042, 3.758]).
Figure 1.
Homogeneous Time-Resolved Fluorescence (HTRF) Based Binding Assay. Binding studies for butorphanol, dezocine, and salvinorin A with κ-opioid receptor. Butorphanol demonstrates much stronger binding with the kappa opioid receptor as compared to that of dezocine and salvinorin A. HTRF signal ratio was calculated as a two-wavelength signal ratio: [intensity (665 nm)/intensity (620 nm)]. ΔF is used for the comparison of different runs of the same assay which reflects the signal to background of the assay. ΔF=[(Ratiosample-Ratiobackgroud)/Ratiobackgroud](%). ΔFMAX is the largest ratio for the background. X axis is the log concentration of each tested ligand. Data are presented as mean ± SD, n=3.
G protein Activation and β-arrestin Recruitment on KOR by Butorphanol
We tested G protein activation of the recombinant human KOR by butorphanol. The assay measures the initial rates of nucleotide exchange on the G protein in response to activation of the receptor with specific ligands (Figure 2A). Salvinorin A was used as a positive control. It resulted in a full activation of the receptor at concentrations above 1 μM. The EC50 for Salvinorin A was about 41 nM. On the other hand, butorphanol only partially activated the receptor (at about 50% of that of salvinorin A), with the maximal activation achieved at low nanomolar concentrations of the ligand. Butorphanol’s EC50 was determined to be 2.8 nM. Therefore, butorphanol acts as a partial, high affinity agonist at the KOR for the G protein pathway. Both butorphanol and salvinorin A induce the arrestin recruitment response of KOR in a similar manner. The concentration-response curve for the response of KOR in the β-arrestin pathway to butorphanol and salvinorin A is shown in Figure 2B. It indicates that butorphanol is a full agonist in the β-arrestin recruitment pathway, similar to salvinorin A.
Figure 2.
Kappa opioid receptor (KOR) activation study. A): G protein activation pathway for butorphanol as compared to salvinorin A, Salvinorin A is a full agonist on the kappa opioid receptor, butorphanol only demonstrated partial agonist effects on the G protein pathway. B): Both butorphanol and salvinorin A demonstrate full agonist effects on the β-arrestin pathway.
The Effects of Butorphanol on KOR Internalization in N2A-mKOR-tdT Cells
Under normal conditions, most KORs were located on the cell surface. However, KOR internalization was observed at as early as 5 min with a final concentration of butorphanol from 0.1μM to 8μM. Figure 3 demonstrates that there is no significant KOR internalization in the control condition, KOR internalization was observed in the presence of butorphanol. Dezocine was able to abolish the KOR internalization induced by butorphanol.
Figure 3.
Butorphanol induced KOR internalization in the N2A-mKOR-tdT cell was confirmed by confocal microscopy. N2A-mKOR-tdT cells were either untreated (A) or treated with butorphanol (0.1 μM) for 1hr followed by fixation and immunostaining (B). A. Control without butorphanol, white arrows point to the cell membrane with KOR over-expression. B. Cells in the presence of butorphanol (0.1 μM). White arrows point to dense dot inside cells, indicating KOR internalization.
Molecular interaction in the binding pocket
Burtorphanol and Salvinorin A shared the same orthosteric binding pocket as the agonist MP1104 (6B73) in the active site (Figure 4) 27. Both ligands make contact with the residues from transmembrane domains (TM) of TM3, TM5, and TM7 in the KOR orthosteric site. It is noteworthy that both butorphanol and salvinorin A form a salt bridge with D138 in a transmembrane region of KOR (Figure 4). However, the tetracyclo group of butorphanol extends deeper into a hydrophobic pocket located at the bottom of the orthosteric site than the furan group of salvinorin A, which is similar to the cyclopropylmethyl group of M1104. The tetracyclo group of butorphanol and the furan group of salvinorin A are engaged in the π-π interaction with Y320 of TM5 in order to stabilize themselves at the bottom of binding cavity.
Figure 4.
Binding site for butorphanol and salvinorin A in the active-state KOR with ribbon representation (green, PDB: 6B73) determined by docking prediction. The binding site is enlarged in the square box as a figure insert. Both butorphanol (cyan) and salvinorin A (purple) are sharing the same binding cavity with agonist MP1104 (yellow). The salt bridge interaction between butorphanol/salvinorin A and D138 are shown, with hydrogen bonds depicted as dashed lines (yellow) in the figure insert. In addition, the tetracyclo group of butorphanol and the furan group of salvinorin A are engaged in the π-π interaction with Y320.
DISCUSSION
The key novel finding in this study is that butorphanol has different levels of activation on different KOR specific signaling pathways. Butorphanol is a synthetic opioid analgesic used for pain management. While it is a known MOR partial agonist, its potency is about 5–7 fold stronger than morphine, a MOR full agonist. These drugs have analgesic effects on pain pathways and primarily act through KOR. KOR plays a major role in modulating mood, pain and hemostasis through diuretic effects. The molecular characterization of the butorphanol-KOR interaction can help in better utilizing this medication in a proper manner, and likely guide novel clinical indications. We used both in silico and parallel in vitro assay to investigate the butorphanol binding affinity.
Binding, β-arrestin Recruitment and G Protein Activation
In vitro binding data demonstrate that butorphanol binds KOR with nanomolar affinity. Moreover, butorphanol possesses higher affinity for KOR than salvinorin A. Butorphanol displayed low efficacy as measured by an [35S] GTPγS binding assay using the cell line Chem-1 expressing KOR. Due to its low efficacy, it acts as a partial KOR agonist, which is consistent with results reported for a C6 glioma cell line.28 Unlike salvinorin A, butorphanol is thought to be a partial agonist in both KOR and MOR in vivo assays in nonhuman primates without specification on specific activation pathways. 29 Salvinorin A displays high potency in both G protein and arrestin pathways, which is consistent with the previous studies.30 It equally stimulates G protein and arrestin pathways. Although butorphanol and JDTic are similar in structure to benzomorphan, JDTic behaves as antagonist, which has no agonistic activity in either G-protein activation or arrestin recruitment assays.
KOR Activation and Internalization
Butorphanol induces KOR internalization. KOR internalization or re-distribution is important for the receptor signal transduction, resulting in relevant pharmacological changes. Such changes strongly related to the ligand therapeutic indications, efficacies and side effects. It is reported that phosphorylation of a specific serine-threonine motif in multiple positions is required for receptor internalization as well as β-arrestin recruitment.31 Butorphanol induced KOR internalization is consistent with the β-arrestin recruitment assay, indicating that butorphanol is a full agonist in the β-arrestin signaling pathway. However, the subcellular distribution of KOR can’t be determined by the method presented in this study.
Binding Site Characteristics and Its Relation to Function
The G coupled protein receptor (GPCR) structural studies suggest that the GPCR adopts multiple conformations upon binding the ligands, which leads to diverse signaling profiles.32 We sought to identify the butorphanol binding mode with the KOR-full agonist MP1104 crystal structure. The orientation of butorphanol in the pocket was driven by the receptor subtype-specific interaction and the hybridization of intramolecular bonds forming the functional modules. In the KOR-ligand crystal structures, the salt bridges between morphinans’ respective amine moieties and D138 of the receptor were observed. Salt bridge also contributes to butorphanol binding. Such interaction acts as an anchor for these compounds’ binding process and is critical for KOR activation. The mutation study of D138 diminishes or abolishes β-arrestin recruitment mediated by MP1104.24,27 Thus, such molecular interaction mode could explain why butorphanol has β-arrestin recruitment properties on KOR. The tetracyclo group of butorphanol extends deep into the hydrophobic pocket, indicating hydrophobic interactions play an important role in a strong affinity in addition to the observed π-π interaction and salt bridge.
Implication and limitation
It is critical to understand the specific signaling pathways of the opioids we are using. This will not only improve safe usage, but also help us find potential new indications of certain opioids. This will help us to predict or to understand potential side effects of certain medications used in clinical practice. It is not surprising that there is abuse liability for butorphanol due to its MOR activation activities. KOR plays a critical role in modulating visceral pain.33 Thus, there is increasing interest in developing non-addictive KOR agonists that have no effects on the central nervous system for treatment of visceral pain. Until such medications are available for clinical practice, butorphanol is a potential alternative medication because of its high potency at KOR. Well controlled clinical studies may be needed. A limitation of this study is that the structural analysis is based on computational docking experiments, the information derived is more hypothesis driven and it only provides a partial explanation for the functional assay we observed in this study. Further structural experimental studies are needed to confirm these findings.
In conclusion, butorphanol is a partial KOR agonist in the G protein activation pathway, and a potent KOR full agonist in the β-arrestin recruitment pathway. The structure analysis offers insights into the molecular mechanism of KOR interaction and activation by butorphanol. The clinical implication of such findings has yet to be fully explored.
KEY POINTS.
Question:
How does butorphanol bind with KOR to fulfill its analgesic effects in pain management?
Findings:
Butorphanol is a partial KOR agonist in the G protein activation pathway, and a potent KOR full agonist in the β-arrestin recruitment pathway.
Meaning:
The details of the molecular interaction between butorphanol and KOR would help to understand its clinical indication and potential side effects.
Acknowledgments
Funding: This study is supported by National Institute of Health (Grant No: 1R01GM111421) (to R.L.) and the Intramural Research Program of the NIAAA, NIH (to A.Y.).
A Glossary of Terms
- KOR
κ-opioid receptor
- MOR
μ-opioid receptor
- HEK-293
Human embryonic kidney-293
- DMEM
modified Eagle’s medium
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- FBS
fetal bovine serum
- HBSS
Hanks’ balanced salt solution
- PDB
protein data bank
- TM
transmembrane domains
- GPCR
G coupled protein receptor
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Skolnick P: The opioid epidemic: crisis and solutions. Annu. Rev. Pharmacol. Toxicol 2018; 58: 143–159 [DOI] [PubMed] [Google Scholar]
- 2.Cobaugh DJ, Gainor C, Gaston CL, Kwong TC, Magnani B, McPherson ML, Painter JT, Krenzelok EP: The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm 2014; 71: 1539–54 [DOI] [PubMed] [Google Scholar]
- 3.Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA 2016; 315: 1624–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pachter IJ, Evens RP: Butorphanol. Drug Alcohol Depend 1985; 14: 325–38 [DOI] [PubMed] [Google Scholar]
- 5.Tavakoli M, Corssen G, Caruso FS: Butorphanol and morphine: a double-blind comparison of their parenteral analgesic activity. Anesth. Analg 1976; 55: 394–401 [DOI] [PubMed] [Google Scholar]
- 6.Hoskin PJ, Hanks GW: Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 1991; 41: 326–44 [DOI] [PubMed] [Google Scholar]
- 7.Patrick CA, Holden Ko MC, Woods JH: Comparison of antinociceptive effects induced by kappa opioid agonists in male and female mice. Analgesia (Elmsford N Y) 1999; 4: 397–404 [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka S, Fan LW, Tien LT, Park Y, Liu-Chen LY, Rockhold RW, Ho IK: Butorphanol dependence increases hippocampal kappa-opioid receptor gene expression. J. Neurosci. Res 2005; 82: 255–63 [DOI] [PubMed] [Google Scholar]
- 9.Xi J, Xiao J, Perez-Aguilar JM, Ping J, Johnson ATC, Jr., Saven JG, Liu R: Characterization of an engineered water-soluble variant of the full-length human mu opioid receptor. J Biomol Struct Dyn 2019: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez-Aguilar JM, Xi J, Matsunaga F, Cui X, Selling B, Saven JG, Liu R: A computationally designed water-soluble variant of a G-protein-coupled receptor: the human mu opioid receptor. PLoS One 2013; 8: e66009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mumby SM, Linder ME: Myristoylation of G-protein alpha subunits. Methods Enzymol 1994; 237: 254–68 [DOI] [PubMed] [Google Scholar]
- 12.Wildman DE, Tamir H, Leberer E, Northup JK, Dennis M: Prenyl modification of guanine nucleotide regulatory protein gamma 2 subunits is not required for interaction with the transducin alpha subunit or rhodopsin. Proc Natl Acad Sci 1993; 90: 794–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikadi Z, Hazai E: Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J Cheminform 2009; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, Perez-Aguilar JM, Liang D, Saven JG: Binding site and affinity prediction of general anesthetics to protein targets using docking. Anesth Analg 2012; 114: 947–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S: Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012; 485: 321–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris GM, Goodsell DS, Huey R, Olson AJ: Distributed automated docking of flexible ligands to proteins: parallel applications of AutoDock 2.4. J Comput Aided Mol Des 1996; 10: 293–304 [DOI] [PubMed] [Google Scholar]
- 17.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ: AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 2009; 30: 2785–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth BL, Laskowski MB, Coscia CJ: Evidence for distinct subcellular sites of opiate receptors. Demonstration of opiate receptors in smooth microsomal fractions isolated from rat brain. J Biol Chem 1981; 256: 10017–23 [PubMed] [Google Scholar]
- 19.Solis FJ, Wets RJB: Minimization by random search techniques. Math Oper Res 1981; 6: 19–30 [Google Scholar]
- 20.Stewart JJ: MOPAC: a semiempirical molecular orbital program. J Comput Aided Mol Des 1990; 4: 1–105 [DOI] [PubMed] [Google Scholar]
- 21.Kroeze W, Sassano M, Huang X, Lansu K, McCorvy J, Giguere P, Sciaky N, Roth B: PRESTO-TANGO: an open-source resource for interrogation of the druggable human GPCR-ome. Nat Struct Mol Biol 2015; 22: 362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ: The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci 2008; 105: 64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi C, Liang X, Chen C, Babazada H, Li T, Liu R: Hypoxia induces internalization of kappa-opioid receptor. Anesthesiology 2017; 126: 842–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manglik A, Cruse A, Kobilka T, Thian F, Mathiesen J, Sunahara R, Pardo L, Weis W, Kobilka B, Granier S: Crystal structure of the mu-opioid receptor bound to a morphinan antagonist. Nature 2012; 485: 321–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikadi Z, Hazai E: Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J Cheminform 2009; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Perez-Aguilar J, Liang D, Saven J: Binding site and affinity prediction of general anesthetics to protein targets using docking. Anesth Analg 2012; 114: 947–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Wacker D, Mileni M, Katritch V, Han G, Vardy E, Liu W, Thompson A, Huang X, Carroll F, Mascarella S, Westkaemper R, Mosier P, Roth B, Cherezov V, Stevens R: Structure of the human kappa-opoid receptor in complex with JDTic. Nature 2012; 485: 327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remmers A, Clark M, Mansour A, Akil H, Woods J, Medzihradsky F: Opioid efficacy in a C6 glioma cell line stably expressing the human kappa opioid receptor. J Pharmacol Exp Thera 1999; 288: 827–833 [PubMed] [Google Scholar]
- 29.Butelman ER, Winger G, Zernig G, & Woods JH: Butorphanol: characterization of agonist and antagonist effects in rhesus monkeys. J Pharmacol Exp Thera 1995; 272: 845–853 [PubMed] [Google Scholar]
- 30.White K, Scopton A, Rives M: Identification of novel functionally selective κ-opioid receptor scaffolds. Mol Pharmacol. 2014; 85: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruchas M, Yang T, Schreiber S, Defino M, Kwan S, Li S, Chavkin C: Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Bio Chem 2007; 282: 29803–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D, Zhao Q, Wu B: Structural studies of G protein-coupled receptors. Mol Cells 2015; 38: 836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rech RH, Mokler DJ, Briggs SL: Effects of combined opioids on pain and mood in mammals. Pain Res Treat 2012; 2012: 145965. [DOI] [PMC free article] [PubMed] [Google Scholar]