Supplemental Digital Content is available in the text
Keywords: magnesium sulfate, renal colic, urolithiasis
Abstract
Background:
Magnesium sulfate (MgSO4) is widely used in analgesia for different conditions. Recent randomized controlled trials (RCTs) have evaluated the effects of MgSO4 on renal colic; however, this new evidence has not been synthesized. Thus, we conducted a systematic review and meta-analysis to assess the efficacy and safety of MgSO4 in comparison with control for renal colic.
Methods:
PubMed, EMBASE, and Scopus databases were searched from inception to February 2020. We included RCTs that evaluated MgSO4 vs control for patients with renal colic. Data were independently extracted by 2 reviewers and synthesized using a random-effects model.
Results:
Four studies with a total of 373 patients were analyzed. Intravenous MgSO4 15 to 50 mg/kg did not significantly reduce renal colic pain severity at 15 minutes (mean difference [MD] = 0.35, 95% confidence interval [CI] −0.51 to 1.21; 2 RCTs), 30 minutes (MD = 0.19, 95% CI −0.74 to 1.13; 4 RCTs), and 60 minutes (MD = −0.28, 95% CI −0.72 to 0.16; 3 RCTs) in comparison with controls. In patients who failed to respond to initial analgesics, intravenous MgSO4 15 mg/kg or 2 ml of 50% solution provided similar pain relief to ketorolac or morphine at 30 minutes (P = .90) and 60 minutes (P = .57). No significant hemodynamic changes were observed with short-term use of MgSO4 in these studies.
Conclusion:
MgSO4 provides no superior therapeutic benefits in comparison with control treatments. MgSO4 may be used as a rescue medication in patients not responding to initial analgesics. The short-term use of MgSO4 did not affect hemodynamic values.
1. Introduction
Renal colic is a common manifestation of nephrolithiasis, accounting for 7.9% of emergency department visits in the United States; it is 1 of the top 10 major medical complaints, costing more than US$4000 per visit.[1] The prevalence of nephrolithiasis ranges from 0.1% to 18.5%.[2] Its incidence increased remarkably from 0.42% to 1.47% within 2 decades in Germany,[3] and an even greater increase was noted in the Middle East.[4] Renal colic arises from partial or complete obstruction caused by urolithiasis, which induces increased intraluminal pressure, stretching forces that stimulate nerve endings, and prostaglandin release.[5] Most patients have acute attacks of renal colic that reaches its peak pain intensity within 2 hours of onset.[6] Patients experiencing unbearable pain from renal colic require prompt pain management.
Primary treatment options include nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids. A systematic review and meta-analysis revealed superior pain-relieving effects of NSAIDs as the first-line treatment compared with those of opioids.[7,8] Although using NSAIDs or opioids or a combination of both soothes renal colic, 16% to 42% of patients were unsatisfied with the effect and required rescue analgesia.[9] Considering the adverse effects of the cumulative usage of NSAIDs and opioids, which include the risks of anaphylaxis, gastrointestinal insult, and renal impairment for NSAIDs and nausea, vomiting, and respiratory suppression for opioids, identifying alternative or add-on medications to reduce pain intensity and analgesia requirements is imperative.
Tocolytic agents are considered an alternative or adjunct medication for the management of acute renal colic. Magnesium sulfate (MgSO4) induces analgesic effects through the antagonism of the N-methyl-D-aspartate receptor.[10] Once the N-methyl-D-aspartate receptor is blocked, the permeability of calcium channel on cell membrane decreases and alters the emission of neurotransmitter that generalizes pain stimuli.[11] Additionally, MgSO4 reportedly relaxes the smooth muscle by reducing the depolarizing effect of acetylcholine on neuromuscular junctions.[12] MgSO4 is also effective in treating acute headaches of various etiologies.[13] Moreover, Ng et al demonstrated that the use of intravenous magnesium as part of multimodal analgesia may reduce postoperative pain.[14] Interest in the use of MgSO4 as an alternative or adjunct medication to reduce pain intensity in patients with renal colic has been increasing among researchers.
Numerous randomized controlled trials (RCTs) have been conducted to investigate the effect of MgSO4 on patients with renal colic.[6,15–17] Verki indicated that MgSO4 did not affect renal colic severity,[15] whereas Majidi considered MgSO4 a safe adjunct pain-control medication.[17] Therefore, evidence for the efficacy of MgSO4 in reducing acute renal colic remains inconclusive. We performed a systematic review and meta-analysis to assess the effects of MgSO4 on pain intensity in patients with acute renal colic.
2. Methods
We conducted our systematic review and meta-analysis according to the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines.[18] Ethical approval or patient consent was not required because the present study was a review of published articles. We have registered our protocol on PROSPERO (PROSPERO ID: CRD42020173718).
2.1. Search strategy and study selection
We searched PubMed, EMBASE, Scopus, and the Cochrane Library from inception to April 2020 (Supplementary Table 1). We used the following search keywords to identify eligible studies: magnesium, magnesium sulfate renal colic, ureteral stone, ureteral calculi, and urolithiasis. No language restrictions were applied. We scrutinized the references of identified studies for other potentially eligible articles and collected unpublished studies from the ClinicalTrials.gov registry (http://clinicaltrials.gov/). Subsequently, we combined results and removed duplicates by using EndNote X8 reference manager (Thompson ISI Research-Soft, Philadelphia, PA). Then, the titles, abstracts, and full-text articles were independently examined by 2 researchers (CHY and TYL) to identify potentially eligible studies. We included all published human RCTs that evaluated the effects of intravenous MgSO4 on renal colic pain management. We excluded animal studies, retrospective cohort studies, case series, and case reports. Studies in which patients received shock wave lithotripsy were also excluded. In included studies, MgSO4 could be administered in any dose and by any route for analgesia in acute renal colic. Our primary outcomes of interest were pain severity measured using the visual analog scale (VAS) after the administration of MgSO4 or control. Secondary outcomes were hemodynamic parameter changes, such as blood pressure, heart rate, respiratory rate, and oxygen saturation.
2.2. Data extraction and management
Two reviewers (PJP and KCWC) independently extracted the following data: the general characteristics of the study (author, year of publication, study location, and sample size), study design, study population characteristics, inclusion and exclusion criteria, procedures, intervention route, and outcomes of interest parameters. Disagreements regarding recorded data were resolved through discussion between the aforementioned reviewers or by referral to a third reviewer (YPH).
2.3. Assessment of the risk of bias in included studies
Two authors (CHY and TYL) independently appraised the methodological quality of each study by using the revised Cochrane risk of bias tool for parallel-group RCTs. The tool includes the following 6 domains: bias arising from the randomization process, bias due to deviations from the intended intervention, bias due to missing outcome data, bias in measurements of outcomes, bias in the selection of reported results, and other biases. Discrepancies were resolved by consensus and arbitration (YPH).
2.4. Statistical analysis
We analyzed continuous outcomes by using mean differences (MDs) and 95% confidence intervals (CIs). We used the DerSimonian and Laird random-effects model to synthesize results. The I2 statistic was used to evaluate heterogeneity among studies with predetermined thresholds for low (25%–49%), moderate (50%–74%), and high (>75%) levels.[19] We performed a subgroup analysis if patients failed to respond to initial analgesics and if MgSO4 was added to the first-line analgesic. We assessed publication bias by detecting asymmetry in funnel plots. Data analysis was performed using RevMan 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Copenhagen, Denmark). A 2-sided P value of <.05 was considered statistically significant.
3. Results
3.1. Search results
The flowchart of the article selection process is illustrated in Figure 1. In addition to consulting PubMed, EMBASE, Scopus, the Cochrane Library, and clinicaltrials.gov, we also hand-searched for relevant articles; this process yielded 243 records. We removed 44 duplicate articles and excluded 192 studies after reading titles and abstracts. Two full-text studies were excluded for being ongoing and another was excluded because no target population was specified. Finally, 4 RCTs were included for qualitative and quantitative synthesis.
Figure 1.
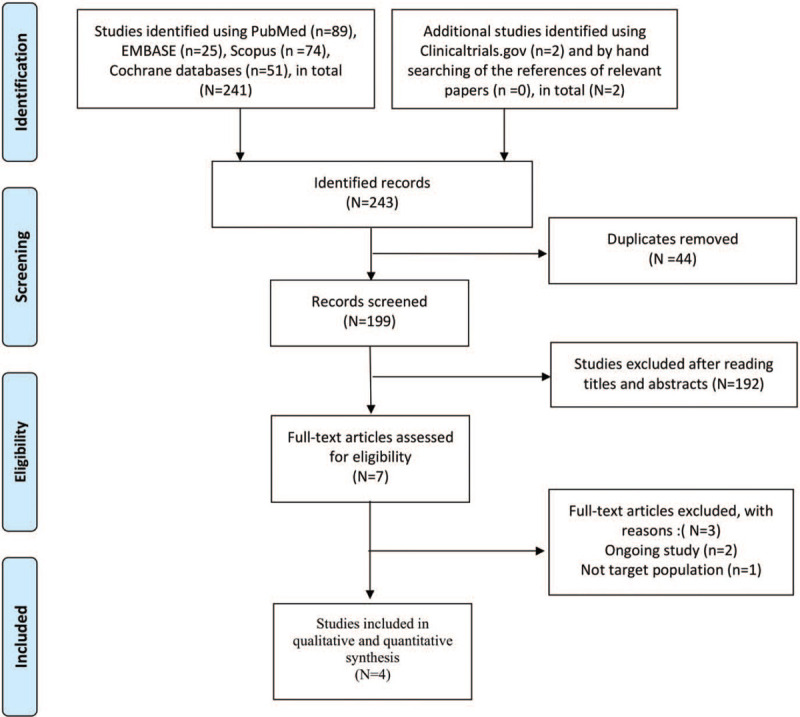
Flowchart of the article selection process.
3.2. Study characteristics
The characteristics of the included studies are summarized in Table 1. Of the studies, 3 were conducted in Iran and 1 in Egypt. All studies were conducted within the past decade, ranging from 2017 to 2020. All studies were performed in emergency departments. The mean age of participants ranged from 32.0 to 39.4 years. All studies recruited more male than female patients, with the proportion of female participants ranging from 11.1% to 42%. Three studies excluded patients who had received calcium channel blockers (CCBs) and one excluded those who had received α-blockers. All the studies made the diagnosis of acute renal colic based on clinical symptoms and only one study confirmed the presence of stone by imaging tools including x-ray, sonography, and computed tomography. Two of the studies compared MgSO4 with normal saline, ketorolac, or ketorolac plus morphine as comedication. The other 2 studies compared MgSO4 with ketorolac or morphine. All studies used the VAS to assess pain severity at various time points after the administration of the intervention or control. The results of the risk of bias assessment are displayed in Figure 2. One study had a high risk of bias because of the randomization process, bias in the measurement of outcome, and unclear risk of bias in selection of the reported result. Another study had a high risk of bias in the measurement of outcome and unclear risk of bias because of the randomization process and in the selection of reported results. The remaining 2 studies were rated as having a low risk of bias.
Table 1.
Characteristics of the included studies.
| Author, publication year | Country/ setting | Inclusion criteria | Exclusion criteria | Diagnosis of renal colic | Sample size, N | Age, mean (SD) | Sex, F (%) | Intervention/ control | Comedication | Outcome measure |
| Jokar et al,[15] 2017 | Iran/ED | Renal colic; age 18-55 | CCB use | Clinical judgement by symptoms | MgSO4: 50 | MgSO4: 33.6 (8.6) | MgSO4: 40.0% | MgSO4 15mg/kg/ saline | 0.1 mg/kg morphine sulfate + 30 mg ketorolac | VAS at baseline, 30 min, 60 min |
| Control: 50 | Control: 35.2 (9.0) | Control: 42.0% | ||||||||
| Maleki Verki et al,[14] 2018 | Iran/ED | Renal colic; age 18-65 | α-blocker use | Clinical judgement and x-ray, ultrasound or computed tomography | MgSO4: 44 | MgSO4: 39.4 (12.1) | MgSO4: 11.4% | MgSO4 50mg/kg IV/ saline | Ketorolac 30 mg IV | VAS at baseline, 15 min, 30 min |
| Control: 43 | Control: 37.2 (10) | Control: 20.9% | ||||||||
| Sayel et al,[6] 2019 | Egypt/ ED | Renal colic; age 18–60; no response to initial 30 mg IV ketorolac | CCB use | Clinical judgement by symptoms | MgSO4: 48 | MgSO4: 32.0 (8.3) | MgSO4: 43.8% | MgSO4 15mg/kg/30 mg ketorolac IV + saline | N/A | VAS at baseline, 30 min, 60 min |
| Control: 48 | Control: 32.0 (8.1) | Control: 39.6% | ||||||||
| Majidi et al,[16] 2020 | Iran/ED | Renal colic; age 18–60; no response to initial morphine 0.1 mg/kg | CCB use | Clinical judgement by symptoms | MgSO4: 45 | MgSO4: 35.6 (10.8) | MgSO4: 40.0% | MgSO4 50% solution 2mL/ morphine 0.1 mg/kg | N/A | VAS at baseline, 20, 30, 60, 120, 180 min |
| Control: 45 | Control: 39.1 (13.2) | Control: 28.9% |
Figure 2.
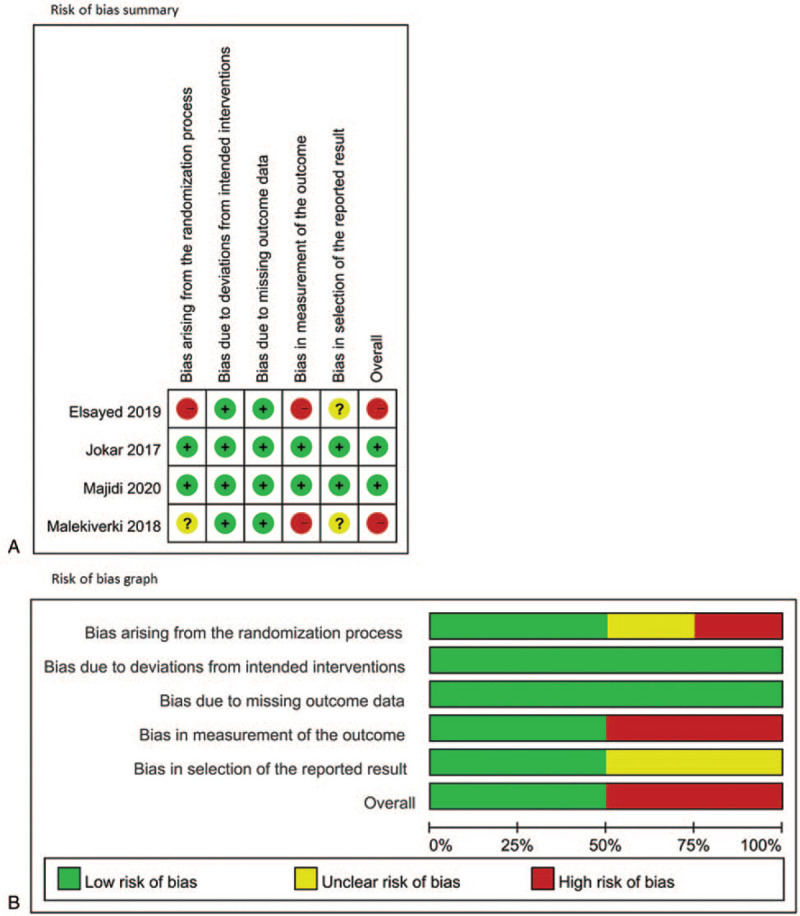
Risk of bias assessment of the included studies.
3.3. Primary outcome: pain severity
All RCTs evaluated the pain severity of renal colic by comparing intravenous MgSO4 with the control. The results of the meta-analysis are presented in Figure 3. Compared with control, using MgSO4 to treat renal colic did not significantly reduce pain severity at 15 minutes (n = 177, MD = 0.35, 95% CI −0.51 to 1.21; Fig. 3A, 2 RCTs), 30 minutes (n = 373, MD = 0.19, 95% CI −0.74 to 1.13; Fig. 3B, 4 RCTs) and 60 minutes (n = 286, MD = −0.28, 95% CI −0.72 to 0.16; Fig. 3C, 3 RCTs). The limited data from the included studies regarding patients who did not respond to the initial analgesic dose or who received intravenous MgSO4 as an add-on treatment were pooled. For patients who did not respond to the initial analgesic dose, those receiving intravenous MgSO4 did not report significantly decreased pain severity at 30 minutes (n = 186, MD = −0.05, 95% CI −0.74 to 0.65; Fig. 4A, 2 RCTs) and 60 minutes (n = 186, MD = −0.16, 95% CI −0.70 to 0.39; Fig. 4B, 2 RCTs) than did those treated with secondary analgesics. Using intravenous MgSO4 as an add-on treatment was not superior to using analgesics alone in reducing pain severity at 30 minutes (n = 187, MD = 0.50, 95% CI −2.31 to 3.31; Fig. 4C, 2 RCTs). Publication bias was disregarded because no asymmetry was detected in funnel plots. (Supplementary Fig. 1)
Figure 3.
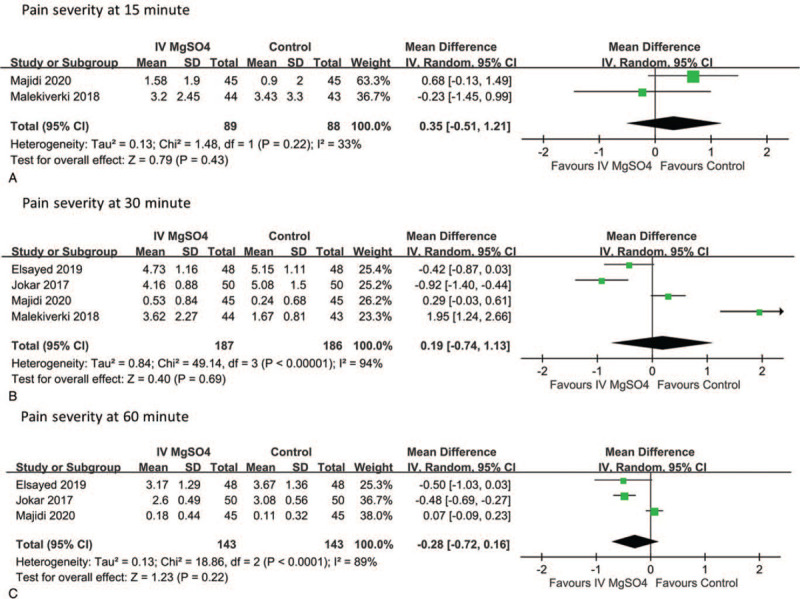
Meta-analysis evaluating the pain severity of renal colic after MgSO4 administration (A) at 15 minutes, (B) at 30 minutes, and (C) at 60 minutes.
Figure 4.
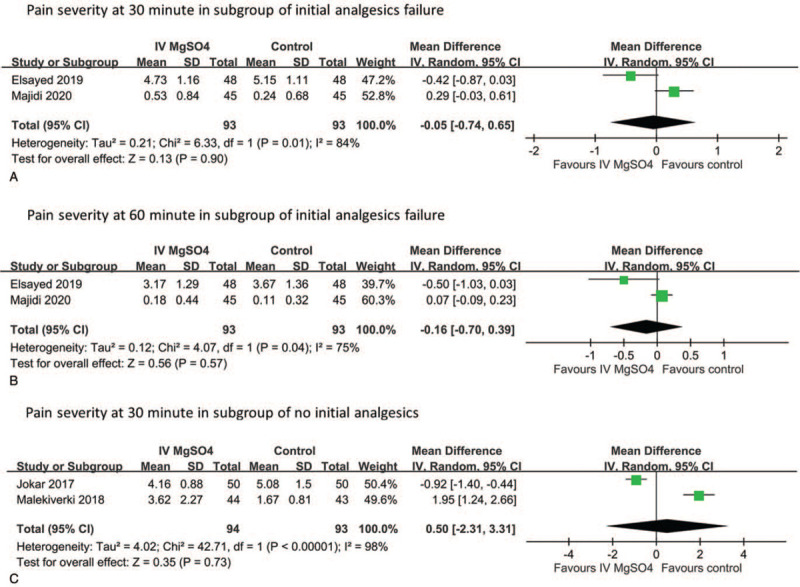
Meta-analysis evaluating the pain severity of renal colic after MgSO4 administration in the subgroup with initial analgesic failure (A) at 30 minutes and (B) 60 minutes and in the subgroup with no initial analgesic (C) at 30 minutes.
3.4. Secondary outcome: hemodynamics and vital signs monitoring
The meta-analysis results of secondary outcomes are summarized in Table 2. Of the 4 studies, 3 considered the change in hemodynamics and other vital signs at 30 and 60 minutes following treatment. No significant changes were observed in blood pressure, respiratory rate, heart rate, oxygen saturation, or body temperature between the MgSO4 and control groups.
Table 2.
Meta-analysis of the secondary outcome.
| Outcome of interest | No. of trials | No. of patients | PMD [95% CI] | P value | I2 (%) |
| Systolic blood pressure at baseline | 2 | 196 | 0.04 [−0.24, 0.32] | .78 | 0 |
| Systolic blood pressure at 30 min | 2 | 196 | −0.39 [−2.43, 1.64] | .71 | 0 |
| Systolic blood pressure at 60 min | 2 | 196 | −0.13 [−2.00, 1.75] | .89 | 0 |
| Pulse rate at baseline | 2 | 196 | 0.20 [−0.24, 0.64] | .38 | 0 |
| Pulse rate at 30 min | 2 | 196 | −1.61 [−4.94, 1.71] | .34 | 0 |
| Pulse rate at 60 min | 2 | 196 | −1.95 [−5.92, 2.03] | .34 | 0 |
| Respiratory rate at baseline | 2 | 196 | 0.21 [−0.14, 0.55] | .25 | 0 |
| Respiratory rate at 30 min | 2 | 196 | −0.38 [−1.16, 0.41] | .35 | 0 |
| Respiratory rate at 60 min | 2 | 196 | −0.18 [−0.60, 0.25] | .42 | 0 |
| O2 saturation at baseline | 2 | 196 | 0.01 [−0.26, 0.27] | .96 | 0 |
| O2 saturation at 30 min | 2 | 196 | −0.00 [−0.27, 0.26] | .98 | 0 |
| O2 saturation at 60 min | 2 | 196 | −0.04 [−0.31, 0.22] | .74 | 0 |
4. Discussion
To the best of our knowledge, this is the first meta-analysis to compare the effects of MgSO4 with those of control for renal colic. In this meta-analysis of 4 clinical trials, using MgSO4 failed to reveal superior effects in comparison with control at 15, 30, and 60 minutes. The results of the subgroup analysis indicated that in patients who were unsatisfied with the initial ketorolac and opioid, MgSO4 provided similar pain relief as other analgesics at 30 and 60 minutes. Using MgSO4 as an add-on treatment when patients received analgesics did not provide additional pain reduction at 30 minutes. The short-term use of MgSO4 did not affect hemodynamic or respiratory status.
Renal colic often manifests in waves lasting 20 to 60 minutes, requiring immediate pain relief. The European Association of Urology guidelines suggests NSAIDs as the first-line treatment for renal colic, with opioids being the second choice.[8] Combining drugs as a the first-line treatment was not mentioned. We found that patients who had received NSAIDs or morphine for renal colic may not benefit from additional MgSO4 administration. Moreover, the aforementioned guidelines did not address the management of patients who do not respond to NSAIDs, opioids, or both. The literature describing the management of renal colic refractory to standard therapy is also limited. Our subgroup analysis suggested that MgSO4 may be an option for rescue treatment.
MgSO4 was first used as an antieclampsia drug in the early 20th century, and interest in its anesthetic abilities developed in the 1990s.[20] A meta-analysis of 21 RCTs reported that intravenous MgSO4 reduced migraine severity within 15 to 45 minutes, 120 minutes, and 24 hours after initial infusion, and oral MgSO4 alleviated the frequency and intensity of migraine as well.[21] Moreover, another meta-analysis, which included 20 RCTs, indicated that systemic administration of MgSO4 could reduce early (0–4 hours) and late (24 hours) postoperative pain and morphine consumption.[22] These results support our findings that MgSO4 exerts analgesic effects on patients with renal colic; these effects could be attributed to the relaxation effects of MgSO4 on the smooth muscle.
Nonetheless, a previous guideline recommended NSAIDs and paracetamol as the standards of care for initial treatment on renal colic pain management.[8] A wide variety of NSAIDs had been tried in the attempt of relieving renal colic pain.[23] A systematic review and network meta-analysis had demonstrated that diclofenac and ketorolac provided comparable pain-relief effect.[24] Paracetamol in intravenous form had similar effect of pain-relief when compared with diclofenac, according to a large RCT.[25] However, in our study, none of the 4 RCTs compared MgSO4 with NSAIDs (except ketorolac) and acetaminophen. Therefore, more robust evidence is warranted before any generalization in favor or against the use of MgSO4.
Medical expulsive therapy (MET) is widely used as an effective treatment option to facilitate the expulsion of distal ureteral stones because the rate of spontaneous passage is low when stone size exceeds 5 mm.[26] The latest guidelines recommend treating these patients with MET for ureteral stones of >5 mm instead of immediate surgical intervention.[8] α-Blockers and CCBs are commonly used in MET.[27] An increasing number of patients have ureteral stones of >5 mm in width, and they may benefit from CCBs or α-blockers. However, when undergoing MET, patients may experience episodic colic before the stone is completely expelled.[26] These patients may experience severe and recurrent renal colic and consequently return to the emergency department. In our review, 1 study excluded patients receiving α-blockers and 3 studies excluded patients receiving CCBs. Therefore, the beneficial effect of intravenous MgSO4 cannot be generalized to those with ureteral stones treated with MET using α-blockers or CCBs.
Concerns regarding the safety of MgSO4 administration mainly refer to hemodynamic instability and respiratory distress risk.[28] For patients who underwent surgery and received MgSO4 for postoperative analgesia, no significant hypotension was noted in comparison with the morphine group.[22] Compared with controls, the incidence of bradycardia was also not significantly different in patients who received MgSO4 for postoperative analgesia after noncardiac surgery.[14] No other fatal side effects have been identified in these meta-analyses in comparison with controls. We assessed similar adverse outcomes in our meta-analysis. We found that short-term MgSO4 use in limited doses did not affect patients’ hemodynamics and breathing status.
Our meta-analysis has some limitations. The results were based on a limited number of included RCTs with relatively small sample sizes. Most of the cases involved in the RCTs were diagnosed to have renal colic by mere clinical symptoms without imaging proof, which may bring to potential risk of biases. All 4 studies measure outcome in subjective VAS grading, which may vary in different populations and thus measurement bias cannot be completely avoided. We also found substantial heterogeneities in the primary outcome. These heterogeneities may be related to diverse MgSO4 dosages and the cointervention used to treat renal colic. Although no publication bias was detected, the finding was not strong because of few RCTs included in the review. Furthermore, all 4 RCTs were conducted in the Middle East and North Africa, which may limit the generalization of findings to the general population. Additional studies including larger samples in diverse regions are recommended to further clarify the effects observed in the current review.
5. Conclusion
Our meta-analysis revealed that MgSO4 did not provide superior therapeutic benefits in comparison with control treatments. MgSO4 may be used as a rescue medication in patients not responding to initial analgesics. Short-term use of MgSO4 did not affect hemodynamic parameters. However, because of the low quality, the small number and the heterogeneity of studies were identified, these findings are inconclusive and cannot be generalized to the general population. Additional well-designed, large RCTs are warranted to clarify the effect.
Author contributions
Conceptualization: Karen Chia-Wen Chu, Yuan-Pin Hsu.
Data curation: Liang-Fu Chen, Chih-Hao Yang, Ting-Yi Lin, Po-Jia Pao, Karen Chia-Wen Chu, Yuan-Pin Hsu.
Formal analysis: Liang-Fu Chen, Chih-Hao Yang, Ting-Yi Lin, Chyi-Huey Bai, Yuan-Pin Hsu.
Funding acquisition: Liang-Fu Chen, Yuan Pin Hsu.
Investigation: Liang-Fu Chen, Chih-Hao Yang, Ting-Yi Lin, Yuan-Pin Hsu.
Methodology: Chih-Hao Yang, Ting-Yi Lin, Po-Jia Pao, Karen Chia-Wen Chu, Yuan-Pin Hsu.
Resources: Chin-Wang Hsu.
Software: Yuan-Pin Hsu.
Supervision: Chin-Wang Hsu, Chyi-Huey Bai, Ming-Hai Du, Yuan-Pin Hsu.
Validation: Liang-Fu Chen.
Visualization: Liang-Fu Chen.
Writing – original draft: Liang-Fu Chen, Chih-Hao Yang.
Writing – review & editing: Liang-Fu Chen, Yuan-Pin Hsu.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CCB = calcium channel blocker, CI = confidence interval, MD = mean difference, MET = medical expulsive therapy, MgSO4 = magnesium sulfate, NSAID = nonsteroidal anti-inflammatory drug, RCT = randomized controlled trial, VAS = visual analog scale.
How to cite this article: Chen LF, Yang CH, Lin TY, Pao PJ, Chu KW, Hsu CW, Bai CH, Du MH, Hsu YP. Effect of magnesium sulfate on renal colic pain: a PRISMA-compliant meta-analysis. Medicine. 2020;99:46(e23279).
M-HD and Y-H contributed equally to this work.
This manuscript was edited by Wallace Academic Editing.
The authors are very grateful for the financial support by the project No. 109-wf-eva-25 of Wan Fang Hospital, Taipei Medical University, Taiwan.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
C = control group, CCB = calcium channel blocker, ED = emergency department, F = female, I = intervention group, IV = intravenous, MgSO4 = magnesium sulfate, N/A = not applicable, SD = standard deviation, VAS = visual analogue scale.
CI = confidence interval, PMD = pooled mean difference.
References
- [1].Caldwell N, Srebotnjak T, Wang T, et al. How much will I get charged for this?” Patient charges for top ten diagnoses in the emergency department. PLoS One 2013;8:e55491–155491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- [3].Hesse A, Brändle E, Wilbert D, et al. Study on the prevalence and incidence of urolithiasis in germany comparing the years 1979 vs. 2000. Eur Urol 2004;44:709–13. [DOI] [PubMed] [Google Scholar]
- [4].Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol 2000;13: Suppl 3: S45–50. [PubMed] [Google Scholar]
- [5].Shokeir AA. Renal colic: pathophysiology, diagnosis and treatment. Eur Urol 2001;39:241–9. [DOI] [PubMed] [Google Scholar]
- [6].Ahmed E, Abouzeid MDZMESMD I, Abu El Sood MSAML. Evaluating effectiveness of intravenous magnesium sulfate as a treatment in acute renal colic patients attending suez canal university hospital emergency department. Med J Cairo Univ 2019;87(September):4021–5. [Google Scholar]
- [7].Pathan SA, Mitra B, Bhutta ZA, et al. A comparative, epidemiological study of acute renal colic presentations to emergency departments in Doha, Qatar, and Melbourne, Australia. Int J Emerg Med 2018;11:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Türk C, Petrik A, Seitz C, et al. EAU Guidelines on Urolithiasis. Arnhem. The Netherlands: EAU Guidelines Office; 2019. [Google Scholar]
- [9].Safdar B, Degutis LC, Landry K, et al. Intravenous morphine plus ketorolac is superior to either drug alone for treatment of acute renal colic. Ann Emerg Med 2006;48:173–81. 181.e171. [DOI] [PubMed] [Google Scholar]
- [10].Ruppersberg JP, Kitzing Ev, Schoepfer R. The mechanism of magnesium block of NMDA receptors. Semin Neurosci 1994;6:87–96. [Google Scholar]
- [11].Fisher K, Coderre TJ, Hagen NA. Targeting the N-Methyl-d-Aspartate receptor for chronic pain management: preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manag 2000;20:358–73. [DOI] [PubMed] [Google Scholar]
- [12].Gilardi E, Marsiliani D, Nicolo R, et al. Magnesium sulphate in the Emergency Department: an old, new friend. Eur Rev Med Pharmacol Sci 2019;23:4052–63. [DOI] [PubMed] [Google Scholar]
- [13].Miller AC, B KP., Lawson MR, et al. Intravenous magnesium sulfate to treat acute headaches in the emergency department: a systematic review. Headache 2019;59:1674–86. [DOI] [PubMed] [Google Scholar]
- [14].Ng KT, Yap JLL, Izham IN, et al. The effect of intravenous magnesium on postoperative morphine consumption in noncardiac surgery: A systematic review and meta-analysis with trial sequential analysis. Eur J Anaesthesiol 2020;37:212–23. [DOI] [PubMed] [Google Scholar]
- [15].Maleki Verki M, Porozan S, Motamed H, et al. Comparison the analgesic effect of magnesium sulphate and Ketorolac in the treatment of renal colic patients: double-blind clinical trial study. Am J Emerg Med 2019;37:1033–6. [DOI] [PubMed] [Google Scholar]
- [16].Jokar A, Cyrus A, Babaei M, et al. The effect of magnesium sulfate on renal colic pain relief; a randomized clinical trial. Emerg (Tehran) 2017;5:e25–125. [PMC free article] [PubMed] [Google Scholar]
- [17].Majidi A, Derakhshani F. Intravenous magnesium sulfate for pain management in patients with acute renal colic; a randomized clinical trial. Arch Acad Emerg Med 2019;8:e5–15. [PMC free article] [PubMed] [Google Scholar]
- [18].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tramer MR, Schneider J, Marti RA, et al. Role of magnesium sulfate in postoperative analgesia. Anesthesiology 1996;84:340–7. [DOI] [PubMed] [Google Scholar]
- [21].Chiu HY, Yeh TH, Huang YC, et al. Effects of intravenous and oral magnesium on reducing migraine: a meta-analysis of randomized controlled trials. Pain Phys 2016;19:E97–112. [PubMed] [Google Scholar]
- [22].De Oliveira GS, Jr, Castro-Alves LJ, Khan JH, et al. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology 2013;119:178–90. [DOI] [PubMed] [Google Scholar]
- [23].Pathan SA, Mitra B, Cameron PA. A systematic review and meta-analysis comparing the efficacy of nonsteroidal anti-inflammatory drugs, opioids, and paracetamol in the treatment of acute renal colic. Eur Urol 2018;73:583–95. [DOI] [PubMed] [Google Scholar]
- [24].2017;Arianto E, Rodjani A, Wahyudi I. Comparison of ketorolac versus diclofenac as treatment for acute renal colic: a systematic review and a network meta-analysis. [Google Scholar]
- [25].Pathan SA, Mitra B, Straney LD, et al. Delivering safe and effective analgesia for management of renal colic in the emergency department: a double-blind, multigroup, randomised controlled trial. Lancet (London, England) 2016;387:1999–2007. [DOI] [PubMed] [Google Scholar]
- [26].Ordon M, Andonian S, Blew B, et al. CUA guideline: management of ureteral calculi. Can Urol Assoc J 2015;9:E837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hollingsworth JM, Rogers MA, Kaufman SR, et al. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet (London, England) 2006;368:1171–9. [DOI] [PubMed] [Google Scholar]
- [28].Hunter LA, Gibbins KJ. Magnesium sulfate: past, present, and future. J Midwifery Women's Heal 2011;56:566–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


