Abstract
Approximately 35% of patients fail to attain ideal initial blood glucose control under metformin monotherapy. The objective of this observational study is to simulate the optimal protocol of metformin according to the different renal function.
The population pharmacokinetics of metformin was performed in 125 subjects with type 2 diabetes mellitus. Plasma concentrations of metformin were quantified by high-performance liquid chromatography. A population pharmacokinetic model of metformin was developed using NONMEN (version 7.2, Icon Development Solutions, USA). Monte Carlo simulation was used to simulate the concentration-time profiles for doses of metformin for 1000 times at different stages of renal function.
The mean population pharmacokinetic parameters were apparent clearance 53.0 L/h, apparent volume of distribution 438 L, absorption rate constant 1.4 hour−1 and lag-time 0.91 hour. Covariate analyses revealed that estimated glomerular filtration rate (eGFR) and bodyweight as individual factors influencing the apparent oral clearance: CL/F = 53.0 × ( bodyweight/75)0.688 × (eGFR/102.5)0.914EXP(0.1797). The results of the simulation showed that patients should be prescribed metformin 2550 mg/d (t.i.d.) vs 3000 mg/d (b.i.d.) as the minimum doses for patients with augmented renal clearance.
eGFR had a significant impact on metformin pharmacokinetics. Patients administered metformin twice a day require higher total daily doses than those with a regimen of 3 times a day at each stage of kidney function.
Keywords: gene polymorphism, individualized medicine, metformin, population pharmacokinetics, type 2 diabetes mellitus
1. Introduction
Metformin, a biguanide, is a hypoglycemic drug, which can reduce fasting and postprandial hyperglycemia, and reduce HbA1c by 1% to 2% in patients with type 2 diabetes (T2DM). Its hypoglycemic mechanism is to increase the sensitivity of peripheral tissues to insulin and increase insulin-mediated glucose utilization. Besides, it increases the use of glucose in non-insulin-dependent tissues such as the brain, blood cells, renal medulla, intestine, skin, etc. It can also inhibit liver gluconeogenesis and reduce liver sugar output, and inhibit the uptake of glucose by intestinal wall cells.[1] Different from insulin, this product does not promote fat synthesis and has no obvious hypoglycemia effect on healthy people. It generally does not cause hypoglycemia when used alone for type 2 diabetes. Metformin is also a potent antioxidant, reduces oxidative stress levels, has anti-obesity effects.[2,3] Moreover, metformin is an anti-atherosclerotic and can reduce carotid-intima media thickness.[4] Therefore, metformin has been recommended as the first-line therapy for the prevention, delay and treatment of T2DM during recent years.[5] However, approximately 35% of patients failed to attain ideal initial blood glucose control on metformin monotherapy because of the considerable variations in the clinical response to metformin.[6,7] Several factors influence the pharmacokinetics (PK) of metformin and potentially affect the hypoglycemic response.[8]
Metformin is a highly ionized hydrophilic compound, with very limited passive diffusion through cell membranes. Metformin is excreted almost unchanged through the urine without significant metabolism by cytochrome enzymes or being bounded to plasma proteins.[9] Drug transporters play an important role in the oral absorption, distribution over tissues and the renal excretion of metformin.[10,11] Particularly, organic cation transporters (OCTs) and multidrug and toxin extrusion proteins (MATEs) have been identified as uptake and efflux transporters, which may contribute to the wide variation in the in vivo disposition of metformin.[12–16] The impact of the polymorphisms in these genes on PK of metformin including oral absorption,[6] transportation and elimination was explored a lot in recent years.[17–20] Although previous studies have shown that OCTs and MATEs are associated with the transportation of metformin in vivo,[19,20] these studies have not got any unified conclusion. Therefore, the influence of the polymorphisms in these genes on PK and PD of metformin is still moderately clarified and contradictory.
Another major factor interfering with the PK profiles of metformin is the renal function. Since most of the metformin is excreted through the kidneys in its original form, it is widely stated to be contraindicated in patients with estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2 because of the accumulation of the drug may be associated with lactic acidosis.[21] On the other hand, the use of regular unadjusted doses of renal-eliminated drugs in patients with augmented renal clearance (ARC) might lead to therapy failure,[22] which was defined as eGFR increased by 20% to 50%.[23] Therefore, how to adjust the dosage of metformin in patients with abnormal eGFR is still a problem. FDA has stated that “during controlled clinical trials, which served as the basis of approval for metformin, maximum metformin plasma levels did not exceed 5 μg/mL, even at maximum doses”.[24] Another recommendation from the International Association of Forensic Toxicologists reference list states that serum concentrations of metformin between 1 and 4 μg/mL are therapeutic.[25] According to the literature, the mean plasma concentrations of metformin fluctuate between 0.4 and 1.3 μg/mL with the daily dosage of 2000 mg, and concentrations above 5 μg/mL are considered to be elevated.[9,10] Based on the evidence, in this paper we recommended that the concentration of metformin should be between 0.4 and 5 μg/mL.
Unfortunately, the therapeutic range of plasma concentrations of metformin is not a routine determination in subjects with T2DM, and its PK characteristics in patients at varying stages of renal function are not well described. In the present study, we have developed a population pharmacokinetic model for metformin in Chinese patients with T2DM. Factors potentially influencing the PK, such as eGFR, body weight (WT), BMI, age as well as genetic variants of metformin transporters (OCT1, OCT2 and MATE1) were investigated. Lastly, we enabled time courses of metformin concentration to be simulated over a range of doses and kidney function to determine the dosing regimen of metformin in patients with T2DM.
2. Methods
2.1. Participants and study design
The trial was a prospective, open-label pharmacokinetic study. Adult participants with T2DM (diagnosed according to the 1999 World Health Organization criteria) were hospitalized in the department of endocrinology in Shandong Provincial Qianfoshan Hospital and received metformin hydrochloride (Glucophage film-coated tablets, Merck Co. Inc) orally for at least 7 days so that they were at pharmacokinetic steady state. All patients were unrelated. Patients in pregnancy or lactation period, with drug abuse history within a year, with other endocrine diseases or malignant tumors, taking drugs that might interfere with the PK of metformin such as proton pump inhibitors were excluded. The demographic characteristics of the recruited subjects were collected. None of the patients showed a recent alteration of renal function, so they could also be considered to be in a stable concerning renal function. Written informed consent was obtained from all the subjects. This study was approved by the Ethical Committee, Shandong Provincial Qianfoshan Hospital (NO. 2017S010), and adhered to the tenets of the Declaration of Helsinki. It was registered with the Chinese Clinical Trial Registry (ChiCTR1800014273).
2.2. Pharmacokinetic sampling
In this study, a sparse sampling method was employed whereby 1 to 2 blood samples were collected from the patients. Patients were required to report the exact times when they took metformin, compliance with their medications, dosage and frequency. The collecting times of the blood samples were selected to obtain the peak concentration (Cmax; 2–4 hours post-dose) and the trough concentration of metformin (Cmin; 10–12 hours post-dose).[26] Blood samples were centrifuged (5112 × g at 4°C for 10 minutes), and plasma samples were transferred to polyethylene tubes and immediately stored at −70°C for analysis.
2.3. Analysis of metformin concentration
Metformin concentrations in plasma were determined using a reverse-phase high-performance liquid chromatography assay with an Agilent C18 column (250 × 4.6 mm, 5 μm particles) at the temperature of 40°C. The mobile phase consisted of acetonitrile: water (28:72, v: v) at a flow rate of 1.0 mL/min. Quantification was achieved using UV detection (233 nm). The calibration curve was linear from 0.2 to 5 μg/mL (r2 = 0.9999, n = 6). The lower limit of quantification was 0.2 μg/mL. There were no samples below the lower limit of quantitation in this study.
2.4. Genotyping
Patients were genotyped for a total of 4 SNPs in OCT1 (rs 622342), OCT2 (rs 316019) and MATE1 (rs2289669, rs2252281) transporters. Genomic DNA was extracted from the whole blood using the TIAN amp Blood DNA Kit-DP348 (TianGen, Beijing, China). Genotyping of the selected SNPs was performed using polymerase chain reaction with the primers designed specifically to span DNA fragments containing the 4 SNPs.
2.5. Population pharmacokinetic modeling of metformin
Pharmacokinetic analysis was carried out using the nonlinear mixed-effects modeling program (NONMEM V7.2, Icon Development Solutions). The first-order conditional estimation method with interaction was used to estimate the pharmacokinetic parameters and their variability.
The inter-individual variability of the pharmacokinetic parameters was estimated using an exponential model and was expressed as follows: θi = θmean∗eηi, where θi represented the parameter value of the ith subject, θmean the typical value of the parameter in the population, and ηi the variability between subjects which was assumed to follow a normal distribution with a mean of zero and variance ω2. Covariate analysis followed a forward and backward selection process. The likelihood ratio test was used to test the effect of each variable on model parameters. The effects of WT, BMI, age, eGFR and the genotype of the transporters were investigated as potential variables affecting pharmacokinetic parameters of metformin. The eGFR was calculated using the CKD-EPI Scr equation, eGFR = 141 × min (Scr /κ, 1)α × max (Scr /κ, 1)−1.209 × 0.993Age × 1.018 [if female], where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is –0.329 for females and –0.411 for males, min indicates the minimum of Scr /κ or 1, and max indicates the maximum of Scr /κ or 1.[27] During the first step of covariate model building, 1 covariate was included if a significant decrease (P < .05, χ2 distribution with 1 degree of freedom, reduction>3.84) in the objective function value (OFV) from the basic model and a reduction in the variability of the pharmacokinetic parameter were obtained. All of the significant covariates were then added simultaneously into a “full” model. Subsequently, each covariate was independently removed from the full model. If the increase in the OFV was higher than 6.635 (P < .01, χ2 distribution), the covariate was considered as significantly correlated with the PK parameters and was therefore retained in the final model.
Model validation was based on graphical and statistical criteria. Goodness-of-fit plots, including observed versus population prediction (PRED), observed versus individual prediction, conditional weighted residuals (CWRES) versus time, and CWRES versus PRED were initially used for diagnostic purposes.[28] The stability and performance of the final model were also assessed using a nonparametric bootstrap with resampling and replacement. Resampling was repeated 1000 times and the values of estimated parameters from the bootstrap procedure were compared with those estimated from the original data set. The final model was also evaluated graphically and statistically by normalized prediction distribution errors (NPDE).[29] NPDE results were summarised graphically by default as provided by the NPDE R package (v1.2)[30]:
-
(i)
QQ-plot of the NPDE;
-
(ii)
histogram of the NPDE. The NPDE was expected to follow the N (0, 1) distribution.
2.6. Dosing regimen optimization
Monte Carlo simulations were performed using the parameter estimates obtained from the final model to evaluate optimal dosing regimen at varying levels of eGFR (45–59, 60–89, 90–119, ≥120 mL/min /1.73 m2) to ensure that the 95th percentile of metformin Cmax stayed below 5 μg/mL and 5th percentile of metformin Cmin stayed above 0.4 μg/mL. One thousand simulations were performed using the original dataset, and the concentration-time profiles at steady state were calculated for all subjects in each renal function group.
3. Results
3.1. Study population
One hundred and thirty patients were initially included from February to September 2017. All the participants fulfilled the inclusion and exclusion criteria and informed consent was obtained from all participants. Five participants were excluded from the pharmacokinetic analysis because 1 received metformin at 0.5 g 3 times daily (t.i.d.) + 0.25 g before bedtime (q.n.), 1 was administered metformin at 1.0 g antemeridiem (a.m.), 0.5 g post meridiem (p.m.) and 1.0 g q.n., 1 was given metformin 0.5 g a.m. and 1.5 g q.n., and 2 other patients did not take the medicine as prescribed. Finally, 125 participants were included for the population PK analysis, among which there were 55 patients received metformin at 1.0 g twice daily (b.i.d.), 29 at 0.5 g t.i.d., 28 were given metformin 0.5 g b.i.d., 12 received metformin at 0.5 g 4 times daily (q.i.d.) and 1 was administered metformin at 0.85 g 1 time daily (q.d.). No participants discontinued the metformin treatment due to adverse events and no drug-related adverse events were shown to have a causal association with metformin therapy.
The median (range) of ages and WT of the 125 participants at the time of the study were 56 (range 27–83) years and 75 (range 51–113) kilograms, respectively. The median(range) BMI was 26.4 (range18.1–35.3). Ages and WTs were all normally distributed (P = .534 and P = .602, respectively, Kolmogorov–Smirnov test). A summary of the patients’ characteristics at baseline is presented in Table 1 and Table 2.
Table 1.
Demographic information.
| Characteristics | n | Median (range) |
| Total patients | 125 | |
| No.of blood samples | 160 | |
| Sex (male/female) | 85/40 | |
| Age (yr) | 56 (27–83) | |
| Bodyweight (kg) | 75 (51–113) | |
| Body mass index (kg/m2) | 26.4 (18.1–35.3) | |
| Height (cm) | 170 (140–188) | |
| Serum creatinine (μmol/L) | 65.1 (32–138.7) | |
| Glomerular filtration rate (mL/min/1.73m2) | 102.5 (46.9–137.7) | |
| Metformin dosage (mg/d) | 2000 (1000–2000) |
Table 2.
Demographic information of different estimated glomerular filtration rate group.
| Characteristics | eGFR:45–59mL/min/1.73m2 | eGFR:60–89 mL/min/1.73m2 | eGFR:90–120mL/min/1.73m2 | eGFR ≥120mL/ min/1.73m2 |
| Sample size (n) | 5 | 26 | 73 | 10 |
| Sex (male/female) | 3/2 | 19/7 | 51/22 | 6/4 |
| Age (yr) | 61 (52–75) | 67.5 (45–83) | 54 (33–73) | 39 (27–53) |
| Body weight (kg) | 71.5 (62–92) | 75 (60–95) | 75 (51–108) | 87 (65–113) |
| Body mass index (kg/m2) | 25.6 (23.7–27.7) | 25.4 (22.7–31.7) | 26.5 (18.1–35.3) | 29.2 (25.2–33.7) |
| Height (cm) | 170 (158–184) | 170 (157–180) | 170 (140–188) | 169 (158–183) |
| Serum creatinine (μ mol/L) | 114 (100–138.7) | 83.5 (55–114) | 62 (37–81) | 45 (32–73) |
| eGFR (mL/min/1.73m2) | 50.4 (46.9–55.4) | 79.7 (60.6–89) | 105 (90.1–119) | 123.7 (120.4–137.7) |
| Metformin dosage (mg/d) | 1500 (1000–2000) | 1500 (1000–2000) | 2000 (1000–2000) | 1750 (1000–2000) |
3.2. Genetic polymorphisms of metformin transporters
There was no participant or SNP excluded from genetic analyses. Therefore, the effects of 4 SNPs (rs622342, rs316019, rs2289669, and rs2252281) on the PK profile of metformin in 125 participants were investigated. All SNPs followed the Hardy-Weinberg equilibrium (P > .05). The frequency of genotype and allele are described in Table 3. Subjects were categorized into 2 groups based upon their genotypes: variants (homozygous and heterozygous variants) and wild types.
Table 3.
Genotype and allele frequencies of the studied gene variants.
| Gene | SNP | Variant | Allele | frequency | χ2 | P |
| SLC22A1 | rs622342 | A/C | A | 82.4% | 0.37 | .54 |
| C | 17.6% | |||||
| SLC22A2 | rs316019 | G/T | G | 88.4% | 0.67 | .41 |
| T | 11.6% | |||||
| SLC47A1 | rs2289669 | G/A | G | 54.8% | 0.33 | .57 |
| A | 45.2% | |||||
| SLC47A1 | rs2252281 | T/C | T | 75.2% | 0.23 | .63 |
| C | 24.8% |
3.3. Model building
In population modeling, the concentrations of metformin in 160 samples ranged from 0.22 to 4.01 μg/mL. The concentration versus time profile is shown in Figure 1.
Figure 1.
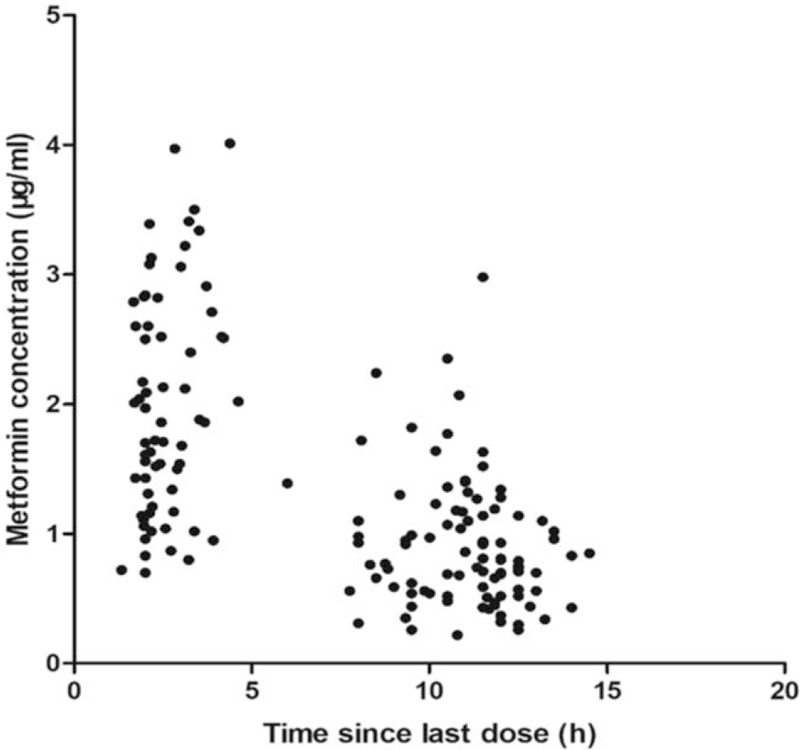
Concentrations of metformin versus time profile. The concentration of metformin versus time profile is shown in this figure. In population modeling, the concentrations of metformin in 160 samples ranged from 0.22 μg/mL to 4.01 μg/mL.
The data in the concentration-time curve of metformin were adequately described by a 1-compartment model with first-order absorption. The final model was parameterized in terms of the volume of distribution (V/F), the clearance (CL/F), the rate constant of absorption (ka), and the lag time for absorption (tlag) of metformin. Inter-individual variability was best described by an exponential model and was then estimated for CL/F. An exponential model best described residual variability.
3.4. Covariate analysis
WT was identified as the covariate on CL/F, associated with a drop in the OFV of 13.87 units. A further decrease in the OFV of 38.15 points was achieved by implementing eGFR on clearance. However, BMI, age, and the polymorphisms of the genes of metformin transporters were not retained into the final model in the forward selection process because of the value of ΔOFV by less than 3.84. The ε-shrinkage was 12.48%. As a result, the final population PK model:
CL/F (L/h) = 53.0 × (WT/75)0.688 × (eGFR/102.5)0.914 EXP (0.1797)
V/F (L) = 438
Data in Table 4 summarizes parameter estimates of the final pharmacokinetic model. The median (range) of estimated weight-normalized CL/F was 0.71 (0.31–0.99) L/h/kg. The AUC0–24 at steady-state for the evaluated dose regimen ranged from 12.35 to 71.23 mg/h/L. Data showed that the CL/F of metformin increased proportionally with the increase of eGFR (Fig. 2).
Table 4.
Population pharmacokinetic parameters of metformin and bootstrap results.
| Full dataset | Bootstrap (n = 1000) | |||
| Parameters | Final estimate | RSE (%) | Median | 5th–95th |
| V/F(L) | 438 | 15.0 | 438 | 303.6–565.6 |
| ka (h−1) | 1.4 | 51.5 | 1.47 | 0.6058–4.736 |
| t lag (h) | 0.914 | 30.3 | 1 | 0.4042–1.43 |
| CL/F (L/h) = θ1 × (WT/75) θ2 × (eGFR/102.5) θ3. EXP (0.1797) | ||||
| θ1 | 53.0 | 4.6 | 52.7 | 48.8–57.28 |
| θ2 | 0.688 | 24.6 | 0.714 | 0.4606–1.01 |
| θ3 | 0.914 | 19.9 | 0.939 | 0.6332–1.258 |
| Inter-individual variability (%) | ||||
| CL/F | 18.0 | 51.1 | 16.25 | 8.49–24.18 |
| Residual variability (%) | 35.07 | 19.3 | 34.64 | 29.49–39.72 |
Figure 2.
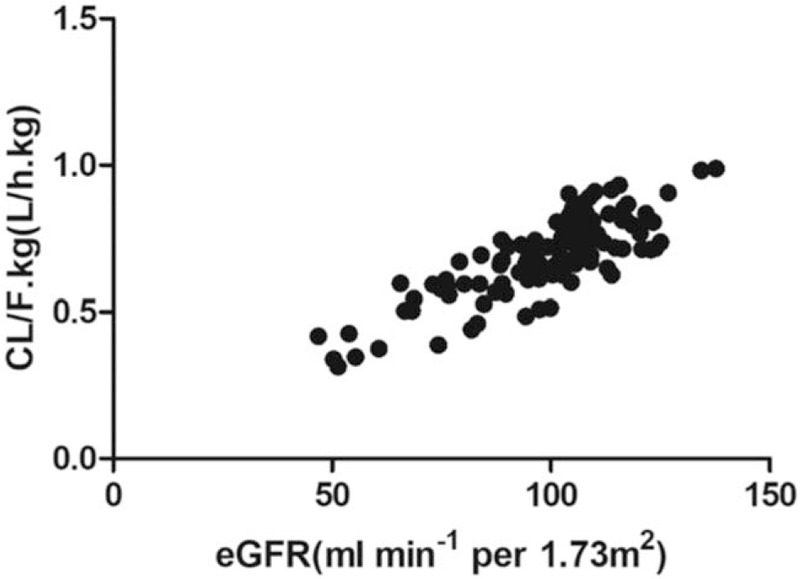
The relationship between CL/F.kg (L/h.kg) and estimated glomerular filtration rate. Data showed that the CL/F of metformin increased proportionally with the increase of estimated glomerular filtration rate.
3.5. Model evaluation
Model diagnostics showed acceptable goodness-of-fit for the final model of metformin. As shown in Figure 3A-B, predictions were unbiased. In the diagnostic plots of CWRES versus time and PRED, no trends were observed (Fig. 3C-D). In addition, the median parameter estimates resulting from the bootstrap procedure closely agreed with the respective values from the final population model, indicating that the final model was stable and could redetermine the estimates of population pharmacokinetic parameters in Table 4. The NPDEs were presented in Figure 3E-F. The distribution and histogram NPDE met well the theoretical N (0, 1) distribution and density, indicating a good fit of the model to the individual data. The mean and variance of NPDE were –0.0391 (Wilcoxon signed-rank test P = .74) and 1.08 (Fisher variance test P = .483), respectively.
Figure 3.
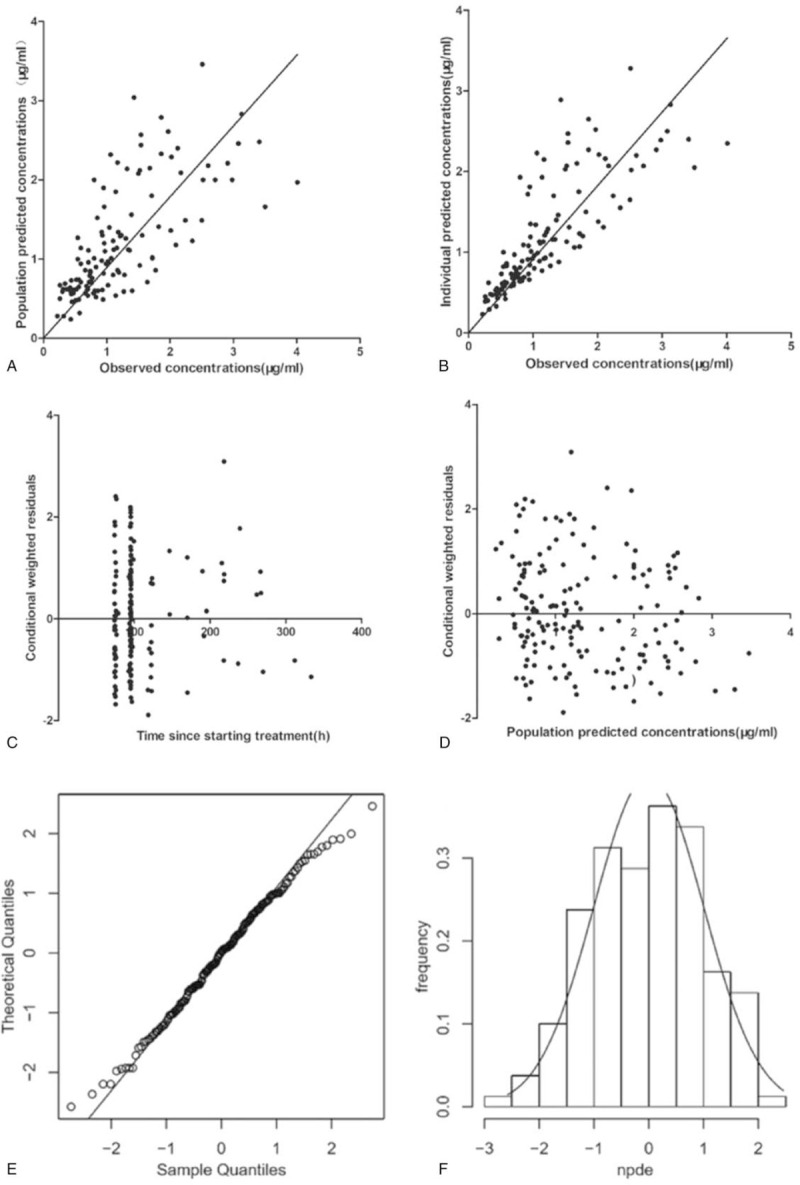
Model evaluation for metformin. (A) Population predicted concentrations versus observed concentrations; (B) individual predicted concentrations versus observed concentrations; (C) conditional weighted residuals versus time; (D) conditional weighted residuals versus population predicted concentrations; (E) QQ-plot of the distribution of the normalized prediction distribution errors versus the theoretical N (0, 1) distribution; (F) histogram of the distribution of the normalized prediction distribution errors, with the density of the standard Gaussian distribution overlaid.
3.6. Dosing regimen optimization
Proposed metformin doses were made according to different levels of renal function, that was to say, calculate the 8- and 12-hour maintenance dose required to achieve the effective therapeutic concentration in T2DM patients at eGFR of 45 to 59, 60 to 89, 90 to 119, and ≥120 mL/min /1.73 m2. The optimal dose of metformin for patients with varying degrees of renal function is presented in Table 5.
Table 5.
The optimal dose of metformin for patients with varying degrees of renal function.
| Dosing interval of 8 hours | Dosing interval of 12 hours | ||
| Different renal function stages | eGFR(mL/min/1.73m2) | Dose(mg) | Dose(mg) |
| G3a | 45–59 | 750 | 1000 |
| G2 | 60–89 | 1275 | 1700 |
| G1 | 90–119 | 1500 | 2000 |
| Augmented renal clearance | ≥120 | 2550 | 3000 |
4. Discussion
This is the population pharmacokinetic study of metformin conducted in a cohort of Chinese T2DM patients. It was undertaken to estimate the pharmacokinetic parameters of metformin and to evaluate the impact of demographic, genetic polymorphism factors on metformin disposition. Although metformin is uptaken into the red blood cells after absorption, resulting in a prolonged efficacy maintenance time, previous research suggested that a 1-compartment model is sufficient for the immediate-release formulation of metformin.[8,11] Our results also show that the plasma concentration profiles of metformin versus time can be perfectly described by a 1-compartment model with first-order absorption and lag time.
Data showed that eGFR had a significantly greater influence on the CL/F of metformin than WT. As a key pharmacokinetic parameter, CL/F of metformin in our study was similar to that reported previously in adult patients (53 L/h vs 56 L/h).[8] Data also showed that CL/F was proportional to eGFR values, which was consistent with the previous researches and the “intact nephron hypothesis”, whereby clearance by filtration was proportional to any clearance by secretion.[31] In contrast to the results by Bardin et al,[8,32] we found that age was not a covariate for CL/F. No gender differences were observed, and no analysis of age differences was carried out due to the small proportion of patients over 65-year-old in the study population.
Few tests have focused on the relationship in Chinese T2DM patients. Therefore, the current study investigates whether metformin transporters (OCT1 rs622342, OCT2 rs316019, MATE1 rs2289669, rs2252281) have significant effects on the PK of metformin in Chinese T2DM patients. Previous studies have been previously reported that genetic variants of SLC22A2 (rs316019, GT, and TT) encoding OCT2 were associated with slightly higher peak plasma concentrations and lower renal clearance of metformin than the GG.[33,34] However, we found no significant effect of these SNPs on the CL/F of metformin. Consistent with our results, Christensen found that OCT1 (rs12208357, rs461473, rs34104736, rs34130495, rs72552763, rs622342, rs34059508), OCT2 (rs316019) and MATE1 (rs2289669 or rs2252281) genetic variants affected neither the renal nor the secretory clearance of metformin.[18,35] Consequently, since the SNPs in the genes encoding the transporters of metformin did not have an observable effect on the CL/F of metformin, we did not include the genotypes for modifying dosage.
Administering metformin to individuals with different renal functions remains a challenge for physicians. And there is no detailed and precise protocol for adjusting the dose of metformin according to eGFR in Chinese patients. Since metformin is largely excretion through the kidney, it is widely stated to be contraindicated in patients with renal impairment eGFR < 30 mL/min/1.73m2.[36] The guidelines for the prevention and treatment of T2DM in China (2017 edition) recommends that metformin should not be administered to Chinese patients with eGFR values lower than 45 mL/min/1.73 m2, and the dose of metformin be decreased in the patients with eGFR<60 mL/min/1.73 m2.[21] Besides, metformin is frequently prescribed to patients with renal impairment without dose reduction. Contrary to renal dysfunction, ARC is also a commonly occurring clinical situation, which could be identified easily by renal function measurement. Some researchers have defined ARC as patients with creatinine clearance > 120 mL/min/1.73 m2.[23,37] In the patients of diabetes mellitus, ARC may occur at the early stage of diabetic nephropathy.[19] In this research, 10 T2DM patients with eGFR > 120 mL/min/1.73 m2 were included. Since the results indicated that eGFR is proportional to CL/F, we speculated that there was a significant difference in CL/F of metformin between the patients at different renal function stages. Therefore, modifying the dosage of metformin based on renal function is necessary.
Researches have clarified the pharmacokinetic parameters of metformin when administered with a single-dose, while it is not sufficient to describe the conditions of multiple-dose administration.[32] This study described the pharmacokinetic parameters of metformin at conditions of a steady state. Previous studies have shown that the maximum recommended daily dose of metformin is 3000 mg for the immediate-release formulation of metformin and 2000 mg for the extended-release formulation of metformin.[10] Our experiments have different results, patients should be prescribed 2550 mg/d (t.i.d.) vs 3000 mg/d (b.i.d.) as the minimum doses for patients with ARC. The simulations show that 2550 mg/d is the minimum dose for patients with ARC when administered with metformin at the interval of 8 hours, which is the maximum recommended dose for metformin hydrochloride tablets by FDA (Glucophage label, FDA). However, in the interval of 12 hours regimen, the minimum dose for patients with ARC patients is 3000 mg/d, which is above the maximum recommended dose by FDA.[24]
Due to the long course of T2DM disease, the short-term efficacy is not obvious, so most patients blindly increase the drug dose, resulting in poor efficacy and adverse reactions. In order to prevent various complications caused by hyperglycemia, it is often necessary to combine many other drugs. Polypharmacy may lead to drug interactions that reduce efficacy and increase adverse reactions. Clinically, effective pharmaceutical interventions and individual dose calculation should be carried out to improve the patient's medication compliance.
This research has several limitations. The pharmacokinetic model of metformin was developed and internally validated, yet external validation was not performed because of the limited sample size. Ultimately, patient-tailored dose based on modeling and simulation has to be evaluated in clinical practice to confirm its clinical benefits. The blood glucose is affected by many factors. The patients in this trial take different hypoglycemic drugs at the same time, which will affect blood glucose. On the other hand, the sample size is still relatively small for the analysis of genetic polymorphism and the dose adjustment for those with decreased renal functions. Hence, it is necessary to carry out further studies with larger sample sizes to stratify the age and gender analysis, and pharmacodynamic evaluations.
5. Conclusions
Our study showed that eGFR had a significantly greater influence on the CL/F of metformin than WT. Patients administered metformin twice a day require higher total daily doses than those with a regimen of 3 times a day at each stage of kidney function.
Acknowledgments
The authors thank all 125 patients for their valuable contribution to the study. The authors also thank all the physicians, nurses, and technicians involved for their assistance.
Author contributions
Yan Li and Wei Zhao designed the study, all authors contributed to the final version of the protocol. Ling Li developed the eCRF and designed SOP, Yuedong Xu, Lin Liao, Huanjun Wang, Li Gao, and Yuxia Gao organized the trial and recruited patients, Rui Li, Yan Yan, and Kunrong Wu set up the analytical method, Guoxiang Hao performed the population pharmacokinetic analysis, Ling Li and Ziwan Guan drafted the manuscript. Significant contributions to the final version were from Yan Li. All authors approved the final version of the manuscript.
Ziwan Guan is the co-first author. Ling Li and Ziwan Guan contributed equally to this work. Please note this in the author information.
Conceptualization: Yan Li.
Data curation: Rui Li, Yan Yan, Li Gao.
Formal analysis: Yan Li.
Funding acquisition: Yan Li.
Investigation: Kunrong Wu, Yuxia Gao.
Methodology: Wei Zhao, Guoxiang Hao.
Project administration: Yuedong Xu, Lin Liao.
Resources: Huanjun Wang.
Software: Wei Zhao, Guoxiang Hao.
Supervision: Yan Li.
Validation: Yan Li.
Writing – original draft: Ling Li.
Writing – review & editing: Ziwan Guan.
Footnotes
Abbreviations: ARC = augmented renal clearance, CWRES = conditional weighted residuals, eGFR = estimated glomerular filtration rate, MATEs = multidrug and toxin extrusion proteins, NPDE = normalized prediction distribution errors, OCTs = organic cation transporters, OFV = objective function value, PK = pharmacokinetics, PRED = prediction, T2DM = type 2 diabetes mellitus, WT = bodyweight.
How to cite this article: Li L, Guan Z, Li R, Zhao W, Hao G, Yan Y, Xu Y, Liao L, Wang H, Gao L, Wu K, Gao Y, Li Y. Population pharmacokinetics and dosing optimization of metformin in Chinese patients with type 2 diabetes mellitus. Medicine. 2020;99:46(e23212).
ZG is the co-first author.
LL and ZG contributed equally to this work.
This work was supported by the National key research project precision special medical integrated application demonstration system construction project (2017YFC0910004) and Jinan Science and Technology Bureau Project (201602171).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The data were shown as median and range.
eGFR = estimated glomerular filtration rate was calculated using the CKD-Epi formula.
The data were shown as median and range.
All SNPs followed the Hardy-Weinberg equilibrium (P > .05).
θ = pharmacokinetic parameter, CL/F = clearance, eGFR = estimated glomerular filtration rate mL/min/1.73m2, ka = first-order absorption = prop proportional, RSE = percentage calculated as standard error divided by mean estimate, t lag = lag time for absorption, V/F = volume of distribution, WT = body weight kilograms.
G1 = Normal or high, G2 = Mildly decreased, G3a = Mildly to moderately decreased.
References
- [1].Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chakraborty A, Chowdhury S, Bhattacharyya M. Effect of metformin on oxidative stress, nitrosative stress and inflammatory biomarkers in type 2 diabetes patients. Diabetes Res Clin Pract 2011;93:56–62. [DOI] [PubMed] [Google Scholar]
- [3].Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes 2013;121:27–31. [DOI] [PubMed] [Google Scholar]
- [4].Chen Y, Li H, Ye Z, et al. The effect of metformin on carotid intima-media thickness (CIMT): a systematic review and meta-analysis of randomized clinical trials. Eur J Pharmacol 2020;886:173458. [DOI] [PubMed] [Google Scholar]
- [5].Gong L, Goswami S, Giacomini KM, et al. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics 2012;22:820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cook MN, Girman CJ, Stein PP, et al. Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with type 2 diabetes in UK primary care. Diabet Med 2007;24:350–8. [DOI] [PubMed] [Google Scholar]
- [7].Pawlyk AC, Giacomini KM, McKeon C, et al. Metformin pharmacogenomics: current status and future directions. Diabetes 2014;63:2590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bardin C, Nobecourt E, Larger E, et al. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol 2012;68:961–8. [DOI] [PubMed] [Google Scholar]
- [9].Graham GGPJ, Arora M. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 2011;50:81–98. [DOI] [PubMed] [Google Scholar]
- [10].Duong JK, Kumar SS, Kirkpatrick CM, et al. Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet 2013;52:373–84. [DOI] [PubMed] [Google Scholar]
- [11].Yoon H, Cho HY, Yoo HD, et al. Influences of organic cation transporter polymorphisms on the population pharmacokinetics of metformin in healthy subjects. AAPS J 2013;15:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Becker ML, Visser LE, van Schaik RH, et al. Interaction between polymorphisms in the OCT1 and MATE1 transporter and metformin response. Pharmacogenet Genomics 2010;20:38–44. [DOI] [PubMed] [Google Scholar]
- [13].Motohashi H, Inui K. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J 2013;15:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goswami S, Gong L, Giacomini K, et al. PharmGKB summary: very important pharmacogene information for SLC22A1. Pharmacogenet Genomics 2014;24:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goswami S, Yee SW, Stocker S, et al. Genetic variants in transcription factors are associated with the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther 2014;96:370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Todd JN, Florez JC. An update on the pharmacogenomics of metformin: progress, problems and potential. Pharmacogenomics 2014;15:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shu Y, Brown C, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther 2008;83:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Christensen MM, Pedersen RS, Stage TB, et al. A gene-gene interaction between polymorphisms in the OCT2 and MATE1 genes influences the renal clearance of metformin. Pharmacogenet Genomics 2013;23:526–34. [DOI] [PubMed] [Google Scholar]
- [19].Stocker SL, Morrissey KM, Yee SW, et al. The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin Pharmacol Ther 2013;93:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hou W, Zhang D, Lu W, et al. Polymorphism of organic cation transporter 2 improves glucose-lowering effect of metformin via influencing its pharmacokinetics in Chinese type 2 diabetic patients. Mol Diagn Ther 2015;19:25–33. [DOI] [PubMed] [Google Scholar]
- [21].Chinese Endocrinologist Association, Chinese Medical Doctor Association. Application principle for oral glucose-lowering drugs in T2DM patients with chronic kidney disease: Chinese experts consensus. Chin J Diabetes 2013;21:865–70. [Google Scholar]
- [22].Mahmoud SH, Shen C. Augmented renal clearance in critical illness: an important consideration in drug dosing. Pharmaceutics 2017;9:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mogensen CE. Microalbuminuria, blood pressure and diabetic renal disease: origin and development of ideas. Diabetologia 1999;42:263–85. [DOI] [PubMed] [Google Scholar]
- [24].The U.S. Food and Drug Administration (FDA). FDA label approved (revised) on 07/19/2013 for metformin hydrochloride. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/091664Orig1s000lbl.pdf. Published 2013. Accessed Aug 11, 2019. [Google Scholar]
- [25].The International Association of Forensic Toxicologists (TIAFT). Reference blood level list of therapeutic and toxic substances. http://www.tiaft.org. Published 2010. Accessed Apr 9, 2019. [Google Scholar]
- [26].Duong JK, Kumar SS, Furlong TJ, et al. The pharmacokinetics of metformin and concentrations of haemoglobin A1C and lactate in indigenous and non-indigenous Australians with type 2 diabetes mellitus. Br J Clin Pharmacol 2015;79:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 2007;24:2187–97. [DOI] [PubMed] [Google Scholar]
- [29].Brendel K, Comets E, Laffont C, et al. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res 2006;23:2036–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Comets E, Brendel K, Mentre F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 2008;90:154–66. [DOI] [PubMed] [Google Scholar]
- [31].Bricker NS, Morrin PAF, Kime SW. The pathologic physiology of chronic Bright's disease. An exposition of the “intact nephron hypothesis”. Am J Med 1960;28:77–98. [DOI] [PubMed] [Google Scholar]
- [32].Sambol NC, Lin CJ, Anita ET, et al. Kidney function and age are both predictors of pharmacokinetics of merformin. J CIin Pharmacol 1995;35:1094–102. [DOI] [PubMed] [Google Scholar]
- [33].Song IS, Shin HJ, Shim EJ, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther 2008;84:559–62. [DOI] [PubMed] [Google Scholar]
- [34].Takane HSE, Otsubo K, Higuchi S. Ieiri I polymorphism in human organic cation transporters and metformin action. Pharmacogenomics 2008;9:415–22. [DOI] [PubMed] [Google Scholar]
- [35].Christensen MMH, Hojlund K, Hother-Nielsen O, et al. Steady-state pharmacokinetics of metformin is independent of the OCT1 genotype in healthy volunteers. Eur J Clin Pharmacol 2015;71:691–7. [DOI] [PubMed] [Google Scholar]
- [36].American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2017;40: Suppl 1: S1–35.27979885 [Google Scholar]
- [37].Grootaert V, Willems L, Debaveye Y, et al. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother 2012;46:952–9. [DOI] [PubMed] [Google Scholar]


