Abstract
Prediction of aspiration pneumonia development in at-risk patients is vital for implementation of appropriate interventions to reduce morbidity and mortality. Unfortunately, studies utilizing a comprehensive approach to risk assessment are still lacking. The objective of this study was to analyze the clinical features and videofluoroscopic swallowing study (VFSS) findings that predict aspiration pneumonia in patients with suspected dysphagia.
Medical records of 916 patients who underwent VFSS between September 2014 and June 2018 were retrospectively analyzed. Patients were divided into either a pneumonia group or a non-pneumonia group based on diagnosis of aspiration pneumonia. Clinical information and VFSS findings were evaluated.
One hundred seven patients (11.7%) were classified as having pneumonia. Multivariate analysis indicated that aspiration during the 2- cubic centimeter thick-liquid trial of VFSS (odds ratio [OR] = 3.23, 95% confidence interval [CI]: 1.93–5.41), smoking history (OR = 2.63, 95% CI: 1.53–4.53), underweight status (OR = 2.27, 95% CI: 1.31–3.94), abnormal pharyngeal delay time (OR = 1.60, 95% CI: 1.01–2.53), and a Penetration-Aspiration Scale level of 8 (OR = 3.73, 95% CI: 2.11–6.59) were significantly associated with aspiration pneumonia development. Integrated together, these factors were used to develop a predictive model for development of aspiration pneumonia (DAP), with a sensitivity of 82%, specificity of 56%, and an area under the receiver operating characteristic curve of 0.73.
The best predictors for DAP included videofluoroscopic findings of aspiration during a 2-cubic centimeter thick-liquid trial, prolonged pharyngeal delay time, a Penetration-Aspiration Scale level of 8, history of smoking, and underweight status. These 5 proposed determinants and the associated DAP score are relatively simple to assess and may constitute a clinical screening tool that can readily identify and improve the management of patients at risk for aspiration pneumonia.
Keywords: aspiration pneumonia, dysphagia, risk factors, videofluoroscopic swallowing study
1. Introduction
Aspiration pneumonia is an acute lung infection caused by macroaspiration of colonized oropharyngeal or upper gastrointestinal contents.[1] Although this condition has a distinct pathophysiology, it is also regarded as part of a spectrum that includes community and hospital-acquired pneumonias.[1,2] Aspiration pneumonia has been reported to comprise 5 to 15% of cases of community-acquired pneumonia (CAP).[2] According to a single-center study, the proportion of aspiration pneumonia cases in patients with CAP was 14.2% in Korea.[3] In a multicenter study conducted in hospitalized patients in Japan, the incidences of aspiration pneumonia were 60.1% in patients with CAP and 86.7% in patients with hospital-acquired pneumonia.[4] Aspiration pneumonia constituted the majority of pneumonia cases in patients older than 70.[4]
Several studies have shown that aspiration pneumonia is a significant prognostic factor. In a cohort study of 1348 hospitalized patients with pneumonia in the United Kingdom, the subset of patients with aspiration risk had significantly increased 1-year mortality, risk of rehospitalization, and risk of recurrent admissions for pneumonia.[5] In another multicenter cohort study consisting of 637 patients with CAP or healthcare-associated pneumonias, aspiration pneumonia was an independent risk factor for 30-day mortality after adjusting for the type of pneumonia, performance status, and treatment failure due to resistant pathogens.[6] In that study, patients with aspiration pneumonia showed significantly worse survival than those who only had the risk factors of aspiration and/or evidence of gravity-dependent opacities on chest computed tomography imaging. Therefore, it is vital to identify effective interventional strategies to prevent aspiration pneumonia in at-risk patients.
Dysphagia is considered to be a major risk factor for the development of aspiration pneumonia (DAP). A 2011 meta-analysis conducted by van der Maarel-Wierink et al[7] reported a significant association between dysphagia and aspiration pneumonia development in frail older adults; however, their analysis was limited by variations in the definition of dysphagia across studies. The gold-standard technique for evaluating swallowing function and dysphagia is a videofluoroscopic swallowing study (VFSS).[8] This technique allows for an accurate and objective evaluation of dysphagia. Schmidt et al[9] reported that the identification of aspiration using VFSS is associated with an approximately 7-fold increase in the risk of developing pneumonia, regardless of the amount or consistency of the test material. Langmore et al[10] argued, however, that aspiration of food or liquid identified on an instrumental study such as VFSS is not a significant predictor of pneumonia alone; rather, other factors, such as overall health, oral health, and feeding status, should also be considered. In support of this multifactorial viewpoint, aspiration of small amounts of oropharyngeal secretions during sleep is actually common and normal in healthy people. The low virulence of pathogens in secretions and the intrinsic host defense mechanisms interact to prevent parenchymal infections in most people.[1]
Prediction of aspiration pneumonia development in at-risk patients is vital for implementation of appropriate interventions to reduce morbidity and mortality. Recently, Tomita et al[11] have proposed a prognostic VFSS scale to predict aspiration pneumonia in patients with Parkinson disease; however, studies that utilize a comprehensive approach to risk assessment, including a synthesis of clinical risk factors and objective swallowing assessment findings, are still lacking. The aim of this study was to use a large database of patients with suspected dysphagia to identify clinical features and VFSS findings that could predict aspiration pneumonia, thereby forming a standardized approach to the clinical management of these patients.
2. Materials and methods
2.1. Subjects
We conducted a single-center, retrospective, observational cohort study of consecutive inpatients and outpatients with dysphagia who underwent VFSS at a university-affiliated hospital from September 2014 to June 2018. Patient inclusion criteria were as follows:
-
(1)
≥ 20 years of age, and
-
(2)
suspected or diagnosed dysphagia based on physicians’ clinical assessments.
In general, patients were suspected of having a swallowing disorder if they exhibited coughing or suffocating symptoms during meals, had coughing or a wet voice when swallowing water, or were using a gastrointestinal tube. Patient exclusion criteria were as follows:
-
(1)
any structural lesions that could adversely affect swallowing function, including tumors of the mouth and throat or related surgeries except tracheostomies, and
-
(2)
medical records lacking data on study variables.
Patient medical records, including a pulmonologist's consultation, were reviewed to determine whether patients had developed pneumonia during the 3 months before or after the initial VFSS.[12] Initially, pneumonia diagnosis screening was performed by identification of International Classification of Disease-10 codes J10 to J20 or J69. For confirmation, all subjects’ radiological images, including chest radiographs and computed tomography images, were reviewed to detect the presence of pulmonary infiltrates. In addition, medical records of all subjects were reviewed to identify signs of apparent aspiration or any clinically suspicious findings of dysphagia. Patients were classified into a pneumonia group or a non-pneumonia group based on the medical record review.
This study was approved by the Institutional Review Board of Nowon Eulji Medical Center, Eulji University (EMCS 2017-06-001). The need for informed consent was waived due to the retrospective nature of the study.
2.2. Data collection
We collected VFSS results and the following clinical data: age, sex, comorbid medical/neurological conditions, body mass index (BMI), ambulatory status, smoking history, and alcohol intake. We included only initial VFSS findings in patients who underwent multiple VFSSs.
Aspiration pneumonia generally occurs in older patients with negative health-related and functional characteristics, as well as 2 or more chronic comorbidities.[12] In this study, we classified the number of neurological or medical comorbidities for each subject as none, 1, or 2 or more. Neurological comorbidities included cerebral infarction, cerebral hemorrhage, traumatic brain injury, brain tumor, hydrocephalus, Parkinson disease, status epilepticus, Guillain-Barré syndrome, and dementia. Illnesses with similar mechanisms for causing dysphagia, such as cerebral infarctions and cerebral hemorrhages, were considered as 1 comorbidity. Medical comorbidities included hypertension, heart failure, diabetes mellitus, tuberculosis, chronic obstructive pulmonary diseases, hyperlipidemia, and hepatitis.
2.3. VFSS protocol
VFSS was conducted by an experienced physiatrist using our hospital's standard protocol in a videofluoroscopy room. While sitting upright in a chair, swallowing images of the lateral projection were obtained from patients. The evaluator simultaneously recorded fluoroscopic images using a digital camera (HMX-QF30; Samsung, Seoul, Korea). The patients underwent swallowing trials with varying viscosities, including thick-liquids, semisolids, solids, and thin-liquids. All trials were coated or mixed with a solution consisting of 300 g of barium sulfate (Baritop HB powder) and 75 mL of water. For the thin-liquid trial, still water was prepared. The barium solution was prepared immediately before VFSS and was diluted with water to equal a 35% weight per volume. For the thick-liquid trial, a thin rice gruel was prepared with a consistency of a slightly thick liquid, categorized as level 1 on the International Dysphagia Diet Standardisation Initiative flow test.[13] For the semisolid trial, a soft and moist rice porridge was prepared. For the solid trial, a regular textured rice was prepared. The evaluator started with a thick-liquid trial, followed by semisolid, solid, and thin-liquid trials. Per our institution's VFSS protocol, a 2-cubic centimeter (cc) trial preceded the 5-cc trial. If the 2-cc trial indicated aspiration, the 5-cc trial could be skipped and the VFSS discontinued if the evaluator determined there was a high risk for further aspiration. The number of trials administered per viscosity was decided by the evaluator and determined by the patient's known swallowing function from a prior clinical assessment and by the risk of aspiration as determined by VFSS findings.
2.4. VFSS parameters
Airway invasion was expressed as “penetration” when foreign materials did not pass below the vocal folds and as “aspiration” when foreign materials passed below the vocal folds, into the trachea, and sometimes beyond.[14] The following VFSS parameters were assessed:
-
(1)
oral transit time (OTT): a normal value is 1.0 to 1.5 seconds or, at most, 1.75 seconds,
-
(2)
height of laryngeal elevation (LE): a normal value is 2 cm or more,
-
(3)
pharyngeal delay time (PDT): up to 0.5 seconds is acceptable for patients older than 60 years,
-
(4)
pharyngeal transit time (PTT): ≤ 1 second is in normal range, and
- (5)
OTT, LE, PDT, and PTT values were based on the thin-liquid trial. If the thin-liquid trial was not applicable, these values were based on the thick-liquid trial. PAS scores were determined based on the highest level obtained during trials where aspiration or penetration occurred. PAS level 1 signified that material did not enter the airway, while a PAS level of 8 signified that material entered the airway, passed below the vocal folds, and no effort was made to eject it. Based on a previous method of PAS classification,[17] PAS results were grouped into 4 categories:
-
(1)
PAS levels 1, 2, and 4;
-
(2)
PAS levels 3, 5, and 6;
-
(3)
PAS level 7; and
-
(4)
PAS level 8.
2.5. Statistical analyses
We conducted independent sample t tests for continuous variables and Chi-squared tests for nominal variables. For 3-way comparisons (e.g., BMI or number of comorbidities), variables were assessed by binary logistic analysis. After verifying significant differences between 2 groups using Chi-squared testing, variables were further evaluated using binary logistic analysis. When divided into 4 categories (e.g., PAS), the variables were evaluated similarly. Continuous variables were converted into dichotomous values based on the discriminating cutoff. When analyzing OTT, LE, PDT, and PTT values, they were coded into 2 categories, either within or outside of the normal range. All relevant covariates with a P value < .05 were entered into the multiple logistic regression analysis. Results are displayed as odds ratio with 95% confidence intervals. Factors identified to be significant using multiple logistic regression analysis were used to make a predictive model for the DAP score.[11,18] The inherent validity of the DAP score was evaluated by receiver operating characteristic (ROC) curve analysis. Analyses were performed using IBM SPSS Statistics version 22.0 for Windows (IBM, Armonk, NY). Significance was set to P < .05.
3. Results
In total, 936 consecutive patients underwent VFSS during the study period. Twenty patients were excluded based on the exclusion criteria. A total of 916 patients were included in the cohort, with 107 patients (11.7%) in the pneumonia group (Fig. 1). Table 1 summarizes the demographic and clinical characteristics of the patients. The mean age of the pneumonia group was 73.44 ± 11.05 years, and the mean age of the non-pneumonia group was 71.13 ± 13.43 years (P = .089). There was a significant difference in the number of medical comorbidities between groups (Table 1). A significantly higher percentage of patients in the pneumonia group had 2 or more medical comorbidities (42.1%) than patients in the non-pneumonia group (35.6%; P = .033). Univariate analysis indicated that a history of smoking, BMI under 18.5 (underweight), and male sex were possible significant risk factors for the DAP (Table 1).
Figure 1.

Patient recruitment flow chart, including number of subjects. VFSS = videofluoroscopic swallowing study.
Table 1.
Demographics and analyzed data of study subjects.
| Variables | Pneumonia group (n = 107) | Non-pneumonia group (n = 809) | P-value | OR | 95% CI |
| Age (yr)a | 73.44 ± 11.05 | 71.13 ± 13.43 | .089 | ||
| Sex-maleb | 67 (62.6) | 419 (51.8) | .036∗ | 1.559 | 1.209–2.361 |
| Etiology-neurologicb | 85 (79.4) | 694 (85.8) | .086 | 0.640 | 0.385–1.065 |
| Past history or current accompanying conditionb | |||||
| Hypertension | 70 (65.4) | 456 (56.4) | .076 | 1.465 | 0.960–2.233 |
| Heart failure | 4 (3.7) | 30 (3.7) | .988 | 1.008 | 0.348–2.920 |
| Diabetes mellitus | 46 (43.0) | 277 (34.2) | .076 | 1.448 | 0.962–2.181 |
| Tuberculosis | 4 (3.7) | 29 (3.6) | .936 | 1.045 | 0.360–3.031 |
| COPD | 8 (7.5) | 49 (6.1) | .569 | 1.253 | 0.577–2.724 |
| Hyperlipidemia | 9 (8.4) | 88 (10.9) | .437 | 0.752 | 0.367–1.542 |
| Hepatitis | 3 (2.8) | 10 (1.2) | .210 | 2.305 | 0.624–8.510 |
| Smoking | 29 (27.1) | 117 (14.5) | .001∗ | 2.199 | 1.376–3.515 |
| Alcohol | 66 (61.7) | 508 (62.8) | .823 | 0.954 | 0.630–1.444 |
| BMI (kg/m2)c | .013∗ | 1.390 | 1.071–1.804 | ||
| 18.5–25.0 | 64 (59.8) | 550 (68.0) | .092 | 0.701 | 0.463–1.060 |
| ≥ 25.0 | 20 (18.7) | 166 (20.5) | .898 | 1.035 | 0.609–1.761 |
| < 18.5 | 23 (21.5) | 93 (11.5) | .005∗∗ | 2.125 | 1.258–3.592 |
| Functional level-ambulationb | 10 (9.3) | 109 (13.5) | .236 | 1.510 | 0.764–2.986 |
| Number of neurologic comorbiditiesc | .667 | 0.917 | 0.618–1.361 | ||
| 0 | 28 (26.2) | 180 (22.2) | .364 | 1.239 | 0.780–1.965 |
| 1 | 71 (66.4) | 580 (71.7) | .309 | 0.784 | 0.491–1.253 |
| ≥ 2 | 8 (7.5) | 49 (6.1) | .835 | 1.094 | 0.468–2.557 |
| Number of medical comorbiditiesc | .041∗ | 1.300 | 1.011–1.672 | ||
| 0 | 23 (21.5) | 262 (32.4) | .060 | 0.637 | 0.397–1.020 |
| 1 | 39 (36.4) | 259 (32.0) | .052 | 1.715 | 0.996–2.953 |
| ≥ 2 | 45 (42.1) | 288 (35.6) | .033∗∗ | 1.780 | 1.048–3.022 |
For VFSS parameters, aspiration during the 2-cc thick-liquid and thin-liquid trials showed significant between-group differences (P < .001 and P = .048, respectively), while aspiration during the 5-cc trials of all consistencies showed no significant between-group differences (Table 2). There were no significant between-group differences in positive penetration findings, regardless of the type or amount of the trial (Table 2).
Table 2.
Comparison of aspiration and penetration between the 2 groups with and without aspiration pneumonia.
| Variables | Pneumonia group (n = 107) | Non-pneumonia group (n = 809) | P-value | OR | 95% CI |
| 2-cc Aspiration, n (%) | |||||
| Thick-liquid | 34 (31.8) | 83 (10.3) | < .001∗ | 4.074 | 2.556–6.492 |
| Semisolid | 2 (1.9) | 32 (4.0) | .295 | 0.462 | 0.109–1.958 |
| Solid | 0 (0) | 7 (0.9) | .999 | ||
| Thin-liquid | 15 (14.0) | 66 (8.2) | .048∗ | 1.835 | 1.006–3.348 |
| 5-cc aspiration, n (%) | |||||
| Thick-liquid | 11 (10.3) | 58 (7.2) | .255 | 1.484 | 0.753–2.925 |
| Semisolid | 3 (2.8) | 14 (1.7) | .444 | 1.638 | 0.463–5.796 |
| Solid | 0 (0) | 0 (0) | |||
| Thin-liquid | 5 (4.7) | 47 (5.8) | .634 | 0.795 | 0.309–2.044 |
| 2-cc penetration, n (%) | |||||
| Thick-liquid | 4 (3.7) | 18 (2.2) | .342 | 1.707 | 0.567–5.141 |
| Semisolid | 1 (0.9) | 15 (1.9) | .503 | 0.499 | 0.065–3.819 |
| Solid | 0 (0) | 4 (0.5) | .999 | ||
| Thin-liquid | 4 (3.7) | 21 (2.6) | .498 | 1.457 | 0.491–4.329 |
| 5-cc penetration, n (%) | |||||
| Thick-liquid | 6 (5.6) | 22 (2.7) | .111 | 2.125 | 0.842–5.365 |
| Semisolid | 1 (0.9) | 7 (0.9) | .942 | 1.081 | 0.132–8.871 |
| Solid | 0 (0) | 2 (0.2) | .999 | ||
| Thin-liquid | 3 (2.8) | 13 (1.6) | .381 | 1.766 | 0.495–6.301 |
For comparisons of VFSS parameters between the 2 groups, PDT and PTT were significantly prolonged in the pneumonia group (P = .009 and P = .013, respectively). However, OTT and LE values showed no significant differences between the groups. A PAS level of 8 was significantly more common in the pneumonia group (P < .001), while the other PAS levels were not significantly different (Table 3).
Table 3.
Comparison of videofluoroscopic swallowing study parameters between the 2 groups with and without aspiration pneumonia.
| Variables | Pneumonia group (n = 107) | Non-pneumonia group (n = 809) | P-value | OR | 95% CI |
| OTTa | 10 (9.3) | 45 (5.6) | .126 | 1.750 | 0.854–3.585 |
| LEa | 15 (14.0) | 76 (9.4) | .136 | 1.573 | 0.868–2.850 |
| PDTa | 47 (43.9) | 253 (31.3) | .009∗ | 1.721 | 1.143–2.593 |
| PTTa | 38 (35.5) | 196 (24.2) | .013∗ | 1.722 | 1.123–2.641 |
| PASb | < .001∗ | 1.432 | 1.213–1.690 | ||
| 1,2,4 | 57 (53.3) | 563 (69.6) | .065 | 0.676 | 0.446–1.025 |
| 3,5,6 | 6 (0.06) | 49 (0.06) | .676 | 1.209 | 0.496–2.946 |
| 7 | 17 (15.9) | 120 (14.8) | .253 | 1.399 | 0.786–2.490 |
| 8 | 27 (25.2) | 77 (9.5) | < .001∗∗ | 3.463 | 2.067–5.803 |
From the factors identified as significantly associated with aspiration pneumonia, we sought to identify the most significant factors via multiple logistic regression analysis. The multivariate analysis results indicated that aspiration during the 2-cc thick-liquid trial of VFSS (OR = 3.23, 95% CI: 1.93–5.41), history of smoking (OR = 2.63, 95% CI: 1.53–4.53), underweight status (OR = 2.27, 95% CI: 1.31–3.94), abnormal PDT (OR = 1.60, 95% CI: 1.01–2.53), and a PAS level of 8 (OR = 3.73, 95% CI: 2.11–6.59) were significantly associated with the DAP (Table 4). While male sex, number of medical comorbidities, aspiration during the 2-cc thin-liquid trial of VFSS, and abnormal PTT were significant in the univariate analysis, they were not significant in the multiple logistic regression analysis (Table 4).
Table 4.
Results of multivariate logistic regression model for predicting high risk of aspiration pneumonia.
| Variables | Coefficient | OR (95% CI) | P-value |
| Sex | 0.03 | 1.027 (0.638–1.654) | .912 |
| Smoking | 0.97 | 2.632 (1.531–4.526) | < .001∗ |
| Underweight | 0.82 | 2.272 (1.312–3.935) | .003∗ |
| ≥ 2 Medical comorbidities | 0.33 | 1.389 (0.894–2.157) | .144 |
| Thick-liquid 2-cc aspiration | 1.17 | 3.231 (1.930–5.406) | < .001∗ |
| Thin-liquid 2-cc aspiration | 0.18 | 1.202 (0.617–2.342) | .589 |
| PDT | 0.47 | 1.598 (1.009–2.531) | .046∗ |
| PTT | 0.14 | 1.150 (0.700–1.889) | .581 |
| PAS level 8 | 1.32 | 3.728 (2.109–6.588) | < .001∗ |
3.1. Prediction model for the DAP
We integrated these 5 factors to form a prediction model for DAP. The score of each factor was given after considering the coefficients. The final points for each factor were rounded to the nearest integer and then adjusted to give a DAP score of 20. The DAP score ranged from 0 to 20, with 0 representing normal function and higher scores indicating worse function (Table 5). The area under the ROC curve for the DAP score was 0.73 (95% CI: 0.67–0.78). With a cutoff value of 1, sensitivity was 0.82 and specificity was 0.56 (Fig. 2).
Table 5.
Prediction model for the development of aspiration pneumonia.
| Variables | Definitions | Coded value | Score |
| Thick-liquid 2-cc aspiration | Aspiration during the 2-cc thick-liquid trial | Absent | 0 |
| Present | 5 | ||
| Smoking | Current or past smoker | Absent | 0 |
| Present | 4 | ||
| Underweight | Body mass index under 18.5 kg/m2 | ≥ 18.5 | 0 |
| < 18.5 | 3 | ||
| PDT[15] | Duration (seconds) from when the bolus head arrives at the point where the lower edge of the mandible crosses the tongue base to when laryngeal elevation starts | ≤ 0.5 | 0 |
| > 0.5 | 2 | ||
| PAS level 8[14] | Material enters the airway, passes below the vocal folds, and no effort is made to eject | Absent | 0 |
| Present | 6 | ||
| Total | 20 |
Figure 2.
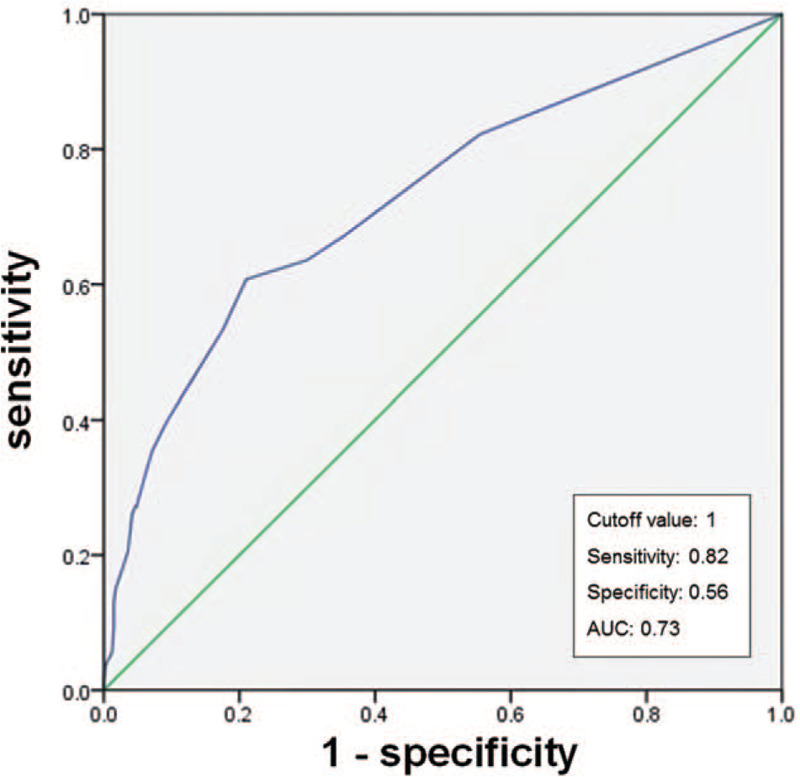
Receiver operating characteristic curve for risk of developing aspiration pneumonia according to the development of aspiration pneumonia score. AUC = area under the curve.
4. Discussion
In this retrospective study of a patient cohort with suspected dysphagia, several factors were found to increase the risk of aspiration pneumonia, including aspiration of a 2-cc thick liquid during VFSS, previous or current history of smoking, BMI < 18.5, PDT > 0.5 sec, and a PAS level of 8. We used a multivariate logistic regression model to predict the DAP with a sensitivity of 82%, specificity of 56%, and an area under the ROC curve of 0.73.
In this study, aspiration during the 2-cc thick-liquid trial and a PAS level of 8 were the most significant predictors for the DAP in the regression model. When considering the PAS level, the most influential factor for the DAP was whether or not effort was made to eject the bolus when there was aspiration. A PAS level of 8 reflects impairment of effective cough responses to aspiration and thus implies impairment of sensory circuits that are typically expected to trigger protective cough reflexes.[17] The resultant aspiration of pathogens from the oral cavity is believed to be the most important mechanism for DAP.[1,7] Since it is difficult to detect silent aspiration with clinical observation or bedside swallowing tests alone, the results of the present study illustrate the importance of VFSS for swallowing function evaluations, particularly in patients with high risk for silent aspiration and aspiration pneumonia.
The significant association between aspiration of a thick-liquid during the 2-cc trial and aspiration pneumonia may be due to the fact that the thick-liquid trial was the first-order test performed in all patients. It is therefore possible that the results may have been different if all patients were tested for all diets with each consistency, which is a limitation of our study. Due to safety considerations, however, it was not possible to proceed further with VFSS in patients who showed significant aspiration. Therefore, we added PAS to confirm the degree of aspiration for each tested diet. Additionally, Park et al[19] demonstrated that, in patients with stroke, aspirators had significantly longer PTTs for nectar-thick liquids and purees than for thin liquids. Consequently, aspiration of thick liquids in small amounts during the initial steps of VFSS is important to note.
Current or ex-smoking status was identified as another significant risk factor for the occurrence of aspiration pneumonia in our study. Langmore et al[10] reported that smoking is one of the best predictors of aspiration pneumonia. A recent meta-analysis also revealed that smoking is a significant risk factor for the development of CAP.[20] Traditionally, smoking has been considered to impair pulmonary clearance due to reductions in coughing ability and mucociliary clearance.[21] Smoking may also alter the cellular and humoral immune systems, resulting in increases in the incidence and severity of lower respiratory tract infections.[22] In addition, it has been shown that smoking diminishes the airway's protective mechanism against aspiration by weakening the triggering of the pharyngoglottal closure reflex, pharyngo-upper esophageal sphincter contractile reflex, and reflexive pharyngeal swallow. Therefore, it is important to recommend smoking cessation to patients with dysphagia.[22]
BMI under 18.5, which reflects an underweight status, was also identified as a predictor of the DAP in our study. Underweight status is generally thought to represent a phenotype of malnutrition, sarcopenia, and frailty. Underweight can be considered both a cause and a result of dysphagia since inadequate oral intake due to dysphagia may result in an underweight status. In comparison with patients without pneumonia, patients with aspiration pneumonia were found to be older and frailer, with more severe diseases and lower BMIs.[23] Phung et al[24] conducted a meta-analysis to evaluate the relationship between BMI and the risk of pneumonia. They found that patients with an underweight BMI had a higher risk of developing CAP and speculated that these patients had a higher risk due to malnutrition or undiagnosed subclinical underlying chronic conditions.[24] Therefore, the nutritional status of patients with dysphagia should be promptly evaluated and managed.
Prolonged PDT on VFSS was found to be a strong predictor for developing aspiration pneumonia in this study. Power et al[25] reported that PDT (expressed as swallow response time in their study) and PTT are significantly delayed in aspirators compared to non-aspirators. They proposed a discriminant model that combined these 2 variables and laryngeal closure duration to predict aspiration in patients with stroke. Prolongation of PDT implies that triggering of swallowing movement, as demonstrated by LE, is delayed even when the bolus head passes the base of the tongue.[15] Adequate, well-timed hyolaryngeal elevation is critical for the prevention of premature spillage of the bolus into the laryngeal vestibule and for enabling the opening of the upper esopharyngeal sphincter for bolus passage.[26] A delay in the triggering of the reflexive pharyngeal swallow hampers the intrinsic protective mechanism that closes the airway during swallowing.[27] Thus, any delay in the initiation of pharyngeal swallowing increases the risk of aspiration. Lin et al[27] reported that, in patients with dysphagia and Parkinson disease, a history of aspiration pneumonia reflects delayed onset of pharyngeal swallowing, which is equivalent to PDT.
Both study groups included patients with a high mean age and with more than 1 neurological condition (74% in the pneumonia group, 78% in the non-pneumonia group). Medical comorbidities were also common. Hence, neurologic and/or sarcopenic causes of dysphagia may have affected all parameters of VFSS.[28] Therefore, it is vital to determine the most appropriate and safest bolus consistencies for patients during VFSS. We should also apply adequate interventions to improve oropharyngeal muscle strength and coordination. If pharyngeal time is prolonged in patients without a history of airway compromise, close monitoring is necessary.[19]
There were several strengths to this study. First, we included a relatively large number of patients (n = 916) and recruited all indicated patients who had undergone VFSS during the study period. Also, the proposed DAP score is relatively easy to assess and could constitute the basis of a clinical screening tool that readily identifies patients at high risk for developing aspiration pneumonia. The present study also had some important limitations. First, all subjects were not evaluated with all test diets of different consistencies and sizes. In some cases, there were massive aspirations during the initial trials of VFSS, and additional examinations could not be performed due to risk. Therefore, the PAS was assessed to confirm the degree of aspiration for the tested diet. Second, the study design was retrospective and data were collected from a single center. As a result, limitations in our collected data may have biased the outcomes of this study. Third, for those patients who developed pneumonia before initial VFSS, the decision to perform this evaluation may have been influenced by the presence of pneumonia itself, which was the endpoint of this study; thus, the predictive value of the DAP score may have been overestimated. However, a VFSS is usually conducted to evaluate the swallowing function for those patients who have been suspected or diagnosed dysphagia based on a physician's clinical assessment. Accordingly, it is generally not conducted based on the presence of pneumonia alone. In addition, in this observational cohort study we collected the data of only consecutive patients with dysphagia who underwent VFSS. Therefore, our results did not eliminate the possibility of overstatement caused by the nature of the retrospective study design. Fourth, we were unable to include some possible associated risk factors, including oral health status, feeding method, cognitive status, and laboratory data. To validate and expand our findings, further prospective multicenter studies are needed.
In conclusion, our study showed that the best predictors for the development of aspiration in patients with dysphagia include videofluoroscopic findings of aspiration of an initial 2cc thick liquid, prolonged PDT, a PAS level of 8, current or former smoking status, and underweight status. These results highlight that aspiration pneumonia is a multifactorial problem. We emphasize the importance of detecting aspiration of even small amounts of thick liquid on VFSS and, moreover, detecting whether effort is made by patients to eject aspirate. Using these variables, we propose the use of a DAP score, a newly derived predictive model for the DAP. When used adequately, this model is easily applied in clinical setting to screen individuals at risk. Modifiable risk factors should be managed appropriately including improving nutritional status, supporting smoking cessation, and rehabilitating swallowing function. Therefore, in line with previous reports, we recommend first screening frail patients with dysphagia for swallowing function using an instrumental evaluation and, subsequently, managing important risk factors.[9,10] Our results may be helpful for preventing aspiration pneumonia and for improving the management of patients with suspected dysphagia.
Acknowledgments
The authors are thankful to Dr Youbin Yi for the valuable support to this research. The authors would also like to sincerely thank Prof Mira Park from the Department of Preventive Medicine at Eulji University for her statistical consultation related to this research.
Author contributions
Conceptualization: Hyun Jung Kim.
Data curation: Joon Woo Kim, Jisang Jung.
Formal analysis: Joon Woo Kim, Hyoseon Choi.
Investigation: Joon Woo Kim, Jisang Jung.
Supervision: Hyoseon Choi, Hyun Jung Kim.
Writing – original draft: Joon Woo Kim.
Writing – review & editing: Hyoseon Choi, Hyun Jung Kim.
Footnotes
Abbreviations: BMI = body mass index, CAP = community-acquired pneumonia, cc = cubic centimeter, CI = confidence interval, DAP = development of aspiration pneumonia, LE = laryngeal elevation, OR = odds ratio, OTT = oral transit time, PAS = Penetration-Aspiration Scale, PDT = pharyngeal delay time, PTT = pharyngeal transit time, ROC = receiver operating characteristic, VFSS = videofluoroscopic swallowing study.
How to cite this article: Kim JW, Choi H, Jung J, Kim HJ. Risk factors for aspiration pneumonia in patients with dysphagia undergoing videofluoroscopic swallowing studies: a retrospective cohort study. Medicine. 2020;99:46(e23177).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
BMI = body mass index, CI = confidence interval, COPD = chronic obstructive pulmonary disease, OR = odds ratio.
P < .05 Chi-squared test.
P < .05 Binary logistic analysis.
Data are presented as the mean ± standard deviation.
Data are presented as the number (%) of patients with each condition.
Data are presented as the number (%) of patients in each category.
CI = confidence interval, OR = odds ratio.
P < .05 Chi-squared test.
CI = confidence interval, LE = laryngeal elevation, OR = odds ratio, OTT = oral transit time, PAS = Penetration-Aspiration Scale, PDT = pharyngeal delay time, PTT = pharyngeal transit time.
P < .05 Chi-squared test.
P < .05 Binary logistic analysis.
Data are presented as the number (%) of patients with results in the abnormal range.
Data are presented as the number (%) of patients in each category.
cc = cubic centimeter, CI = confidence interval, OR = odds ratio, PAS = Penetration-Aspiration Scale, PDT = pharyngeal delay time, PTT = pharyngeal transit time.
P < .05 based on multiple logistic analysis.
cc = cubic centimeter, PAS = Penetration-Aspiration Scale, PDT = pharyngeal delay time.
References
- [1].DiBardino DM, Wunderink RG. Aspiration pneumonia: a review of modern trends. J Crit Care 2015;30:40–8. [DOI] [PubMed] [Google Scholar]
- [2].Mandell LA, Niederman MS. Aspiration Pneumonia. N Engl J Med 2019;380:651–63. [DOI] [PubMed] [Google Scholar]
- [3].Jeon I, Jung GP, Seo HG, et al. Proportion of aspiration pneumonia cases among patients with community-acquired pneumonia: a single-center study in Korea. Ann Rehabil Med 2019;43:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Teramoto S, Fukuchi Y, Sasaki H, et al. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc 2008;56:577–9. [DOI] [PubMed] [Google Scholar]
- [5].Taylor JK, Fleming GB, Singanayagam A, et al. Risk factors for aspiration in community-acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med 2013;126:995–1001. [DOI] [PubMed] [Google Scholar]
- [6].Komiya K, Ishii H, Umeki K, et al. Impact of aspiration pneumonia in patients with community-acquired pneumonia and healthcare-associated pneumonia: a multicenter retrospective cohort study. Respirology 2013;18:514–21. [DOI] [PubMed] [Google Scholar]
- [7].van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, et al. Meta-analysis of dysphagia and aspiration pneumonia in frail elders. J Dent Res 2011;90:1398–404. [DOI] [PubMed] [Google Scholar]
- [8].Costa M. Videofluoroscopy: the gold standard exam for studying swallowing and its dysfunction. Arq Gastroenterol 2010;47:327–8. [DOI] [PubMed] [Google Scholar]
- [9].Schmidt J, Holas M, Halvorson K, et al. Videofluoroscopic evidence of aspiration predicts pneumonia and death but not dehydration following stroke. Dysphagia 1994;9:7–11. [DOI] [PubMed] [Google Scholar]
- [10].Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia 1998;13:69–81. [DOI] [PubMed] [Google Scholar]
- [11].Tomita S, Oeda T, Umemura A, et al. Video-fluoroscopic swallowing study scale for predicting aspiration pneumonia in Parkinson's disease. PLoS One 2018;13:e0197608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Manabe T, Teramoto S, Tamiya N, et al. Risk factors for aspiration pneumonia in older adults. PLoS One 2015;10:e0140060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cichero JA, Lam P, Steele CM, et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the IDDSI framework. Dysphagia 2017;32:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- [15].Logemann JA. Evaluation and treatment of swallowing disorders. 2nd edAustin, TX: PRO-ED Inc; 1998. [Google Scholar]
- [16].Yang H, Yi Y, Han Y, et al. Characteristics of cricopharyngeal dysphagia after ischemic stroke. Ann Rehabil Med 2018;42:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Steele CM, Grace-Martin K. Reflections on clinical and statistical use of the penetration-aspiration scale. Dysphagia 2017;32:601–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Han K, Song K, Choi BW. How to develop, validate, and compare clinical prediction models involving radiological parameters: study design and statistical methods. Korean J Radiol 2016;17:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Park T, Kim Y, McCullough G. Oropharyngeal transition of the bolus in post-stroke patients. Am J Phys Med Rehabil 2013;92:320–6. [DOI] [PubMed] [Google Scholar]
- [20].Baskaran V, Murray RL, Hunter A, et al. Effect of tobacco smoking on the risk of developing community acquired pneumonia: a systematic review and meta-analysis. PLoS One 2019;14:e0220204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Skerrett SJ. Host defenses against respiratory infection. Med Clin North Am 1994;78:941–66. [DOI] [PubMed] [Google Scholar]
- [22].Dua K, Bardan E, Ren J, et al. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut 2002;51:771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hayashi M, Iwasaki T, Yamazaki Y, et al. Clinical features and outcomes of aspiration pneumonia compared with non-aspiration pneumonia: a retrospective cohort study. J Infect Chemother 2014;20:436–42. [DOI] [PubMed] [Google Scholar]
- [24].Phung DT, Wang Z, Rutherford S, et al. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev 2013;14:839–57. [DOI] [PubMed] [Google Scholar]
- [25].Power ML, Hamdy S, Goulermas JY, et al. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia 2009;24:257–64. [DOI] [PubMed] [Google Scholar]
- [26].Kim Y, McCullough GH, Asp CW. Temporal measurements of pharyngeal swallowing in normal populations. Dysphagia 2005;20:290–6. [DOI] [PubMed] [Google Scholar]
- [27].Lin C-W, Chang Y-C, Chen W-S, et al. Prolonged swallowing time in dysphagic Parkinsonism patients with aspiration pneumonia. Arch Phys Med Rehabil 2012;93:2080–4. [DOI] [PubMed] [Google Scholar]
- [28].Sasaki K, Sakuma K. Sarcopenic dysphagia as a new concept. In: Dionyssiotis Y, ed. Frailty and Sarcopenia - Onset, Development and Clinical Challenges . Rijeka, Croatia: IntechOpen; 2017:81-102. Accessed December 28, 2019. [DOI] [Google Scholar]


