Abstract
Background
Receptor tyrosine kinases such as epidermal growth factor receptors (EGFRs) and their downstream signaling pathways such as the Ras-Raf-mitogen-activated protein kinase (MAPK) pathway play important roles in glioblastoma (GBM). This study investigated the safety, pharmacokinetics, and efficacy of sorafenib (Ras/Raf/MAPK inhibitor) in combination with erlotinib (EGFR inhibitor) for treatment of recurrent GBMs.
Methods
Patients with recurrent GBM were eligible. A novel sequential accrual trial design was used, where patients were sequentially accrued into separate treatment arms in phase I and phase II investigations to optimize recruitment efficiency. In phase I, a standard 3 + 3 format was used to identify dose-limiting toxicities (DLTs), determine maximum tolerated dose (MTD), and investigate pharmacokinetics. Phase II followed a 2-stage design with the primary endpoint being 6-month progression-free survival (PFS6).
Results
Sixteen patients were recruited for phase I, and the MTD was determined to be sorafenib 200 mg twice daily and erlotinib 100 mg once daily. DLTs include Grade 3 hypertension, Grade 3 elevated liver transaminases, and Grade 4 elevated lipase. While erlotinib did not affect sorafenib levels, sorafenib reduced erlotinib levels. In phase II, 3 of 19 stage 1 participants were progression free at 6 months. This did not meet the predetermined efficacy endpoint, and the trial was terminated.
Conclusion
This study identified the MTD and DLTs for sorafenib and erlotinib combination therapy for recurrent GBMs; however, efficacy data did not meet the primary endpoint. This study also demonstrates the feasibility of a novel sequential accrual clinical trial design that optimizes patient recruitment for multiarm studies, which is particularly effective for multicenter clinical trials.
Keywords: erlotinib, glioblastoma, molecular therapy, novel trial design, sorafenib
Key Points.
The MTDs for sorafenib and erlotinib are 200 mg twice daily (BID) and 100 mg once daily (QD), respectively.
Sorafenib and erlotinib therapy does not improve PFS6 for recurrent GBM.
A sequential accrual trial design can streamline studies of multiple treatments.
Importance of the Study.
This study provides new data on the toxicities and efficacy of sorafenib and erlotinib combination therapy in recurrent GBM. In contrast with previous studies, this study identified elevated lipase as a dose-limiting toxicity, leading to a lower maximum tolerated dose. Furthermore, this study provides new evidence that sorafenib in combination with erlotinib does not improve progression-free survival at 6 months. Finally, this study demonstrates the feasibility of a novel sequential accrual clinical trial design, which can significantly improve phase I and phase II patient recruitment efficiency for studying multiple experimental agents, particularly in multicenter settings. Detailed documentation of this clinical trial design in this report can serve as a roadmap for investigators seeking to optimize patient accrual across multiple investigational treatments in future clinical trials.
Glioblastoma (GBM) is the most common primary CNS malignancy in adults with an annual incidence of nearly 12,000 cases in the United States.1 Despite multimodality treatment with surgery, chemotherapy, and radiotherapy, 5-year survival is less than 7%.1 After initial therapy, tumor recurrence almost always occurs, often within months following the completion of adjuvant radiotherapy. Once GBM recurs, response to second-line therapies is limited; chemotherapy response rate is below 10% and is often short-lived, and 6-month progression-free survival (PFS) is typically less than 15% for most treatment regimens.2 Thus, novel therapies are desperately needed for GBM patients.
The Ras-Raf-mitogen-activated protein kinase (Ras/Raf/MAPK) pathway is known to play a major role in tumor cell growth and survival, and is responsible for transducing signals from receptor tyrosine kinases (RTKs) that are known to drive GBM growth.3,4 These RTKs include epidermal, vascular endothelial, or platelet-derived growth factor receptors (EGFR, VEGFR, or PDGFR, respectively). Sorafenib, a bi-aryl urea, is an inhibitor of RAF1 and b-RAF kinase family members (which are part of the Ras/Raf/MAPK pathway), and it has been proposed as a promising therapy for GBM. More specifically, sorafenib has been shown to inhibit proangiogenic receptor tyrosine kinases such as VEGFR-2, PDGFR-, and VEGFR-3,5–7 all of which are implicated in the disease process of GBM. However, sorafenib performed poorly in past clinical trials. A previous phase II trial adding sorafenib to standard GBM treatment failed to show an improvement in PFS.8 Furthermore, sorafenib alone does not seem to inhibit the MAPK pathway efficiently, even in systemic solid tumors.9 Thus, given these data, it is unlikely that sorafenib alone would be sufficient to alter the disease course of GBM.
Over past decades, EGFR has become one of the most widely studied therapeutic targets in GBM.10 EGFR is overexpressed in most GBMs,4 and about 30% have EGFR gene rearrangements resulting in the expression of a constitutively active mutant EGFR vIII.11 Various inhibitors of EGFR have been developed (eg, erlotinib); however, results from previous phase II investigations of erlotinib suggest that EGFR blockade alone is unlikely to be effective in GBM.12–16 While the EGFR and Ras/Raf/MAPK pathways are intricately connected insofar that Ras/Raf/MAPK is activated downstream of EGFR activation,17 they likely play significant roles in GBM independent of each other. For example, EGFR activation can lead to activation of other signaling pathways known to promote cell growth (eg, P13K/Akt/mTOR), and the Ras/Raf/MAPK pathway can transduce signals for other growth factor receptors implicated in GBM (eg, VEGFR and PDGFR). Thus, we hypothesize that dual Ras/Raf/MAPK and EGFR blockade may be more effective for GBM compared to monotherapy as it would not only amplify the inhibitory effects on the EGFR/Raf/Ras/MAPK axis but also exert inhibitory effects on other pathways downstream of EGFR as well as other RTKs.
The North American Brain Tumor Consortium (NABTC) 05-02 trial is a phase I/II study to assess the safety and efficacy of sorafenib in combination with other promising therapeutic agents for recurrent GBM. Three combination therapy arms were studied: arm 1 was sorafenib + erlotinib, arm 2 was sorafenib + temsirolimus (an mTOR inhibitor), and arm 3 was sorafenib + tipifarnib (a P13K/Akt/mTOR inhibitor). In this report, results from arm 1 (sorafenib + erlotinib) are presented; results from arms 2 and 3 have been published previously.18,19 To optimize patient accrual efficiency, a novel sequential accrual design was implemented. To our knowledge, NABTC 0502 is the first phase I/II clinical trial to employ a sequential accrual format. Details of this trial design will be discussed in this report.
Methods
Patient Eligibility
Adult patients ≥18 years of age with histologically confirmed intracranial GBM or gliosarcoma and unequivocal tumor progression determined by MRI were eligible. Baseline imaging was required to be within 14 days prior to trial registration, and steroid dose was required to be stable for at least 5 days. Patients were required to have recovered from toxic effects of prior drug therapy: 28 days from any investigational agent, 28 days from prior cytotoxic therapy, 14 days from vincristine, 42 days from nitrosoureas, 21 days from procarbazine administration, and 7 days from noncytotoxic agents. Patients who underwent recent resection of recurrent or progressive tumor were eligible as long as they had recovered from the effects of surgery, regardless of presence of postsurgical residual disease. Patients were required to have failed radiation therapy 42 days or more prior to registration. Furthermore, patients were required to meet the following criteria: Karnofsky performance status (KPS) ≥ 60, adequate bone marrow function (WBC ≥3000/mcl, ANC ≥1500/mm3, platelet count ≥100,000/mm3, and hemoglobin ≥10 gm/dL), total bilirubin within normal limits, liver transaminases (AST/ALT) ≤2.5× upper limit of normal, creatinine < 1.5 mg/dL, INR <1.5 (or < 3.0 for patients on chronic anticoagulation therapy). Patients receiving enzyme inducing antiepileptic drugs (EIAEDs) or any other CYP3A4 inducers (except dexamethasone) were excluded. For women of child-bearing age, contraception was required for trial participation, and pregnant patients were excluded. For the phase I component, patients may have had any number of prior disease relapses and treatments; however, for the phase II component, patients could not have had treatment for more than 2 prior relapses. Finally, patients in arm 1 (sorafenib + erlotinib) could not have received prior sorafenib, AE788, PTK 787, or other EGFR targeting agents.
This study was approved by each participating center’s institutional review board and was conducted in accordance with federal and institutional regulations for clinical trials. All patients signed informed consent indicating that they were aware of the investigational nature of this study. Furthermore, patients also signed authorization for the release of their protected health information and were entered into the NABTC study database prior to therapy initiation.
Patient Accrual and Treatment Plan
The NABTC 05-02 trial included 3 treatment arms testing combinations of signal transduction targeted agents with sorafenib as the backbone agent. In addition to sorafenib, patients in arms 1, 2, and 3 received erlotinib, temsirolimus, and tipifarnib, respectively. Here, we present the data from arm 1—sorafenib and erlotinib—of this trial and discuss the sequential accrual design in detail. Sorafenib and erlotinib were supplied by the Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, under a cooperative agreement with Bayer Healthcare and Genentech. Oral sorafenib was administered twice daily and oral erlotinib once daily. Each treatment cycle was 4 weeks (28 days).
Phase I study
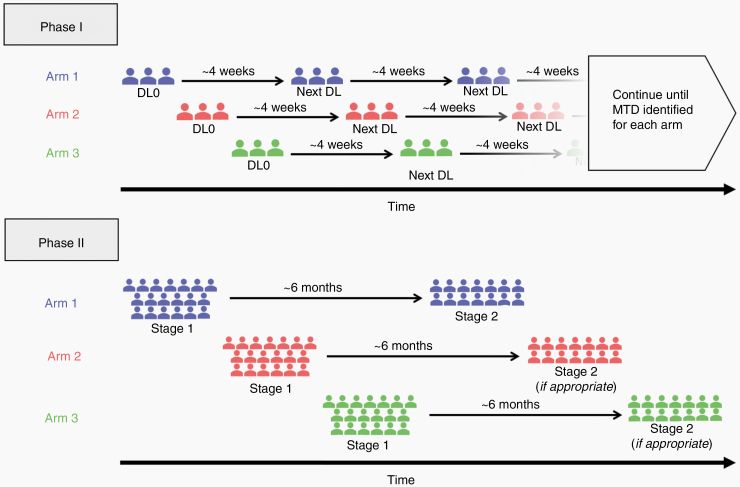
We employed a standard 3 + 3 dose-escalation format, enrolling 3 patients per cohort per arm at a time. Sequential accrual of eligible patients is described in Figure 1, and patients were assigned to treatment arms as follows:
Figure 1.
Schematic of novel sequential accrual clinical trial design.
Patients #1–3: Arm 1 at dose level 0.
Patients #4–6: Arm 2 at dose level 0.
Patients #7–9: Arm 3 at dose level 0.
Patients #10–12: Arm 1 at the next appropriate dose level per standard protocol, after patients #1–3 completed treatment cycles and DLTs are assessed.
Patients #13–15: Arm 2 at the next appropriate dose level per standard protocol, after patients #4–6 completed treatment cycles and DLTs are assessed.
Patients #16–18: Arm 3 at the next appropriate dose level per standard protocol, after patients #7–9 completed treatment cycles and DLTs are assessed.
Patients #19–21: Arm 1 at next appropriate dose.
Patients #22–24: Arm 2 at next appropriate dose.
Patients #25–27: Arm 3 at next appropriate dose.
This pattern continued until either the maximum dose level was deemed safe or a DLT and MTD was reached for each arm. In arm 1, patients initially received sorafenib at 200 mg BID and erlotinib at 100 mg QD. Subsequently, doses were escalated or de-escalated according to a predetermined schedule depending on the number of DLTs observed per cohort, with the dosing schedule provided in Table 1. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria version 4 (http://ctep.cancer.gov/reporting/ctc.html). DLTs included any Grade 4 hematologic toxicity; Grade 3 thrombocytopenia lasting ≥7 days; any Grade 3 or 4 nonhematologic toxicity (electrolyte imbalances, diarrhea, nausea, and vomiting are only considered DLTs if they remain Grade 3 or 4 despite maximal medical therapy); any intolerable Grade 2 nonhematological, or Grade 3 hematological toxicity requiring dose reduction during the first treatment cycle (28 days); and any toxicity resulting in a treatment delay of more than 1 week during the first treatment cycle (28 days). MTD was defined by the highest dose level at which less than one third of patients experienced DLTs.
Table 1.
Phase I Dose Levels, Enrollment, and DLTs
| Dose Level | Sorafenib Dose | Erlotinib Dose | Enrolled | DLTs | Replaced |
|---|---|---|---|---|---|
| −2 | 200 mg QD | 75 mg QD | 0 | 0 | 0 |
| −1 | 200 mg QD | 100 mg QD | 0 | 0 | 0 |
| 0 (MTD) | 200 mg BID | 100 mg QD | 6 | 1a | 0 |
| 1 | 400 mg BID | 100 mg QD | 7 | 2b | 1d |
| 2 | 400 mg BID | 150 mg QD | 3 | 2c | 0 |
aGrade 3 Elevated AST/ALT.
bGrade 3 Hypertension and Grade 4 Elevated Lipase.
cGrade 3 and Grade 4 Elevated Lipase.
dPatient received half dose of sorafenib for first 14 days.
Phase II study
Here, we employed a 2-stage design for all treatment arms. Sequential accrual of eligible patients is described in Figure 1 and patients were assigned as follows:
Patients #1–19: Arm 1 stage 1 (sorafenib and erlotinib).
Patients #20–38: Arm 2 stage 1 (sorafenib and temsirolimus).
Patients #39–57: Arm 3 stage 1(sorafenib and tipifarnib).
Patients #58–70: Arm 1 stage 2, if prespecified efficiency endpoint is reached in arm 1 stage 1.
Patients #71–83: Arm 2 stage 2, if prespecified efficiency endpoint is reached in arm 2 stage 1.
Patients #84–96: Arm 3 stage 2, if prespecified efficiency endpoint is reached in arm 3 stage 1.
Nineteen patients were recruited to stage 1 of each arm, and if PFS at 6 months (PFS6, defined radiographically) passed a prespecified efficiency threshold, 14 additional patients were recruited to stage 2 of that arm. Therapy was continued for each patient until completion of 24 cycles or radiographically confirmed disease progression, whichever occurred first. If Grade 3 or 4 toxicities developed, treatment was held until abnormalities are adequately reversed, at which point a lower dose level was administered. Treatment was terminated if therapy was held for longer than 28 days for any reason.
Patient Evaluation
A complete history, physical, and neurological examination were performed at baseline. Electrocardiogram, MRI confirming tumor progression, and laboratory tests to confirm normal organ function including bone marrow, hepatic and renal, were performed within 14 days prior to registration. Serum β-HCG was measured for women of childbearing potential.
During the study, complete neurologic and physical exams were performed prior to every treatment cycle (every 4 weeks). Adverse events were evaluated weekly during the first treatment cycle for phase I patients and after each treatment cycle (once every 4 weeks) for phase II patients. Patients were instructed to monitor and record their blood pressure in a Blood Pressure Diary. For laboratory testing, patient CBC (with differential) and platelet counts were evaluated weekly during the initial treatment cycle for phase I patients and every 2 weeks for phase II patients. Other laboratory tests were evaluated weekly during the initial treatment cycle of all patients and then every 4 weeks (prior to each subsequent cycle) thereafter. MRI was performed prior to every other cycle (every 8 weeks) and at 6 months following initiation of therapy to ensure assessment of primary endpoint. If patient achieved PFS at 6 months or partial/complete response, imaging results were evaluated by central film review at University of California San Francisco. The response was determined by the MacDonald criteria20 to ensure comparability to historical data as this study predated the development of more modern evaluation methods such as the RANO criteria.21
When possible, all patients were followed for overall survival. Survival was evaluated every 3 months for patients who discontinued treatment due to disease progression. Patients who discontinued treatment due to other reasons continued to be followed until disease progression or initiation of new antitumor therapy and were followed for survival thereafter.
Pharmacokinetic Studies
Sample collection
All phase I participants and the first 10 phase II participants were evaluated for pharmacokinetic studies. Whole blood samples (5 mL for erlotinib and 6 mL for sorafenib) were collected in heparinized (sodium or lithium) containing, nonseparator tubes by venipuncture (heparin lock) or by central venous catheter if present. During sampling, the first 1 mL of blood was discarded prior to collection. Samples were serially collected on days 1, 15, and 28 at the following time points: baseline, 1, 2, 4, 6, 8, and 12 h postadministration and at 24 h prior to drug administration. Erlotinib was started on day 1 followed by sorafenib on day 2 after the 24 h post-erlotinib sample. Discontinuation of erlotinib was planned for day 22 as a washout period prior to the evaluation of a single-dose sorafenib on day 28; however, due to a protocol oversight, erlotinib was continued through day 28. Blood samples were centrifuged within 30 min at 3000 rpm for 15 min. Plasma samples were stored at −70°C until analysis.
Analytical methods
Concentrations of erlotinib and its O-demethylated isomeric metabolites (OSI-420/OSI-413 collectively called OSI-420) in plasma were analyzed using a validated liquid chromatography-mass spectrometry (LC/MS) method with atmospheric pressure chemical ionization (APCI) in the positive ion mode as previously described.22 The lower limit of quantitation of erlotinib and OSI-420 was 1 ng/mL. Analytical grade erlotinib, OSI-420 and CP-396,059 (IS) were obtained from OSI Pharmaceuticals. Analytical standards for sorafenib and sorafenib n-oxide were obtained from Toronto Research Chemicals, Inc. Tolnaflate (IS) was obtained from Sigma–Aldrich. A validated LC/MS method using APCI was initially developed for the detection of sorafenib and its n-oxide metabolite (Bayer 673472). Because of the dynamic range (1–1000 ng/mL) for the assay, significant plasma dilutions were required for quantitation. Therefore, a previously published HPLC assay was implemented and validated.23 Briefly, duplicate calibration standards, quality control (QC), or patient samples were spiked with the IS followed by acetonitrile protein precipitation then double extracted with diethyl ether. The absolute recoveries of sorafenib and sorafenib n-oxide were 70% and 75%, respectively. After evaporation, the residue was reconstituted with methyl alcohol and subjected to linear gradient elution on a reverse-phase C18 column with UV detection (254 mm). Calibration curves (7 points) were linear (R2 > 0.99) from 0.5 μg/mL (LLQ) to 12 μg/mL for sorafenib and 0.08 μg/mL to 4 μg/mL for the n-oxide metabolite. The interday precision for sorafenib/n-oxide was 7.1%/7.5%, 7.5%/11%, and 8.5%/7.3% for the low, medium, and high OC samples, respectively.
The pharmacokinetic parameters for erlotinib, sorafenib, and their respective metabolites were analyzed by noncompartmental analysis. Peak concentrations (Cpmax) were determined by inspection of each individual’s concentration–time curve. The area under the concentration–time curve was calculated using the linear trapezoidal rule up to the last measurable time point (AUC0-t). Differences between the kinetic variables were evaluated using an unpaired 2-tailed t-test. P values less than 0.05 were considered statistically significant.
Statistical Considerations
The primary endpoints for the phase I component were the determination of the MTD of sorafenib + erlotinib, characterization of toxicities, and evaluation of drug interactions via pharmacokinetics studies. The primary endpoint for the phase II component was 6-month PFS (PFS6), and this study was designed to have 90% power to detect an increase of PFS6 to 35% from 15% (historical data from 8 consecutive negative phase II trials for GBM2). Thus, it was determined that 19 patients were to be assigned to stage 1 and 14 patients to stage 2. If 4 of the 19 patients in stage I were progression free at 6 months, the arm would continue to stage 2 accrual for a total recruitment of 33 patients. Overall, if more than 7 patients were progression free at 6 months, the treatment would be considered effective (α < 0.1 with a 1-tailed binomial test of a single proportion).
RESULTS
Phase I Component
Patient characteristics
Sixteen eligible patients were enrolled into the phase I component of the sorafenib + erlotinib treatment arm, and characteristics are given in Table 2. In brief, there were 9 men and 7 women. The median age was 53 years, with a range of 36–70. Median KPS was 90 (range: 70–100), and patients had a median of 2 prior chemotherapy regimens. Two patients had previously received bevacizumab.
Table 2.
Patient Characteristics
| Patient Characteristics | Phase I | Phase II |
|---|---|---|
| Number of evaluable patients | 16 | 19 |
| Sex | ||
| Male | 9 (56.3%) | 9 (47.4%) |
| Female | 7 (43.8%) | 10 (52.6%) |
| Age (y) | ||
| Median | 53 | 52 |
| Range | 36–70 | 30–76 |
| KPS | ||
| Median | 90 | 90 |
| 100 | 4 (25.0%) | 2 (10.5%) |
| 90 | 6 (37.5%) | 8 (42.1%) |
| 80 | 5 (31.3%) | 5 (26.3%) |
| 70 | 1 (6.3%) | 3 (15.8%) |
| 60 | 0 (0.0%) | 1 (5.3%) |
| Histology | ||
| Glioblastoma | 16 (100%) | 19 (100%) |
| Prior chemotherapy regimens | ||
| Median | 2 | 2 |
| 1 | 7 (38.9%) | 7 (36.8%) |
| 2 | 8 (50.0%) | 12 (63.2%) |
| 3 | 1 (6.3%) | 0 (0.0%) |
MTDs and toxicities
The number of patients and events at dose levels 0, 1, and 2 are given in Table 1. At the initial sorafenib 200 mg twice daily (BID) and erlotinib 100 mg once daily (QD) dose level (DL0), 1 of 6 patients developed DLT (Grade 3 elevated AST/ALT). At the sorafenib 400 mg BID and erlotinib 100 mg QD dose level (DL1), 2 of 6 patients developed DLT (Grade 4 elevated lipase and Grade 3 hypertension). At the sorafenib 400 mg BID and erlotinib 150mg QD dose level (DL2), 2 of 3 patients developed DLT (Grades 3 and 4 elevated lipase). Of note, 1 patient in the first DL1 cohort experienced Grade 4 elevated lipase, and at the time of data review in August 2007, Grade 4 elevated lipase was not considered a DLT as it was deemed unlikely related to study drugs. However, in the second DL1 cohort (March 2008), a Grade 3 hypertension event was observed. This prompted a re-evaluation of the first DL1 cohort, resulting in the reclassification of the elevated lipase event as a DLT and making DL0 the MTD.
Pharmacokinetic results
Samples from 18 patients treated with erlotinib 100 mg daily plus sorafenib 200 mg or 400 mg BID were available for pharmacokinetic (PK) analyses. Table 3 shows the PK parameters for the daily administered erlotinib 100 mg for all patients and for 6 patients who had a complete set of blood samples available for analysis on days 1, 15, and 28. No statistical difference was observed between the day 1 PK parameters and the day 15/28 steady-state parameters. With continuous dosing of erlotinib, exposure did not increase over time. The accumulation ratio was 1.2 which is in contrast to the accumulation ratio of 2.8 with single-agent erlotinib.15,24
Table 3.
Pharmacokinetic Values of Erlotinib and Its Metabolite OSI-420
| Erlotinib 200 mg BID | |||
|---|---|---|---|
| C pmax (ng/mL) | |||
| All Patients | Day 1 (n = 14) | Day 15 (n = 16) | Day 28 (n = 10) |
| Erlotinib (mean ± SD) | 443 ± 155 | 662 ± 373 | 653 ±653 |
| OSI-420 (mean ± SD) | 31 ± 14 | 62 ± 44 | 66 ± 52 |
| Matched Patients | Day 1 (n = 6) | Day 15 (n = 6) | Day 28 (n = 6) |
| Erlotinib (mean ± SD) | 483 ± 197 | 729 ± 385 | 806 ± 54a |
| OSI-420 (mean ± SD) | 28 ± 19.4 | 58 ± 35.1 | 88 ± 54 |
| Comparison with other trials (mean) | Day 1 | Day 28 | |
| Current Trial | 443 | 653 | |
| Yamamoto et al.24 | 571 | 1023 | |
| AUC0-4 (μg × h/mL) | |||
| All Patients | Day 1 (n = 14) | Day 15 (n = 16) | Day 28 (n = 10) |
| Erlotinib (mean ± SD) | 6.3 ± 2.61 | 6.9 ± 4.59 | 7.7 ± 4.05 |
| OSI-420 (mean ± SD) | 0.34 ± 0.16 | 0.62 ± 0.46 | 0.86 ± 0.46 |
| Matched Patients | Day 1 (n = 6) | Day 15 (n = 6) | Day 28 (n = 6) |
| Erlotinib (mean ± SD) | 6.8 ± 3.13 | 8.2 ± 4.95 | 9.0 ± 4.03b |
| OSI-420 (mean ± SD) | 0.32 ± 0.15 | 0.61 ± 0.46 | 0.95 ± 0.45 |
| Comparison with other trials (mean) | Day 1 | Day 28 | |
| Current Trial | 443 | 653 | |
| Yamamoto et al.24 | 571 | 1023 |
a P value = .20 (Day 1 vs Day 28).
b P value = .20 (Day 1 vs Day 28).
The following patient illustrates our observed influence of sorafenib on the PK of erlotinib: patient was started on erlotinib 150 mg continuously days 1 through 28 plus sorafenib 400 mg BID days 2 through 7. Sorafenib was held for 6 days as a result of Grade 3 elevation in lipase and restarted on day 15 at a reduced dose of 200 mg BID. Day 1 erlotinib Cpmax and AUC were within the reported range for a 150 mg dose (538 ng/mL and 8.6 µg × h/mL, respectively). On day 15, prior to resuming sorafenib, we observed increased Cpmax and AUC (1349 ng/mL and 16 µg × h/mL, respectively) as well as an expected accumulation of erlotinib (accumulation ratio of 2.5). However, on day 28 (13 days after resuming sorafenib), Cpmax and AUC were significantly reduced (506 ng/mL and 10.7 µg × h/mL, respectively). This case highlights the effects of sorafenib on erlotinib’s PK parameters and also provides insight into the rapidity of the onset/offset.
Pharmacokinetic results for sorafenib—the arithmetic mean steady-state peak concentrations (Cpmax) and area under the plasma time curve (AUC0–12) for sorafenib and its n-oxide metabolite for the dose levels of 200 mg and 400 mg on days 15 and 28—are summarized in Table 4. Overall, there were no observed differences in the kinetic parameters for sorafenib in the presence of erlotinib compared to prior reports.25,26
Table 4.
Pharmacokinetic Values of Sorafenib and Its Metabolite N-oxide
| Sorafenib 200 mg BID | |||
|---|---|---|---|
| C pmax (µg/mL) | |||
| All Patients | Day 15 (n = 9) | Day 28 (n = 7) | P value |
| Sorafenib (mean ± SD) | 5.51 ± 2.68 | 4.67 ± 2.10 | .51 |
| N-oxide (mean ± SD) | 1.5 ± 1.51 | 0.8 ± 0.38 | .33 |
| Comparisons with other trials (geometric mean) | Day 14–15 | Day 28 | |
| Current Trial | 4.4 | 4.0 | |
| Furuse et al. 26 | 3.4 | 4.2 | |
| Strumberg et al. 25 | — | 4.0 | |
| AUC0-12 (µg × h/mL) | |||
| All Patients | Day 15 (n = 9) | Day 28 (n = 7) | P value |
| Sorafenib (mean ± SD) | 45.85 ± 21.51 | 40.29 ± 18.6 | .60 |
| N-oxide (mean ± SD) | 11 ± 11.1 | 6.1 ± 3.28 | .26 |
| Comparisons with other trials (geometric mean) | Day 14–15 | Day 28 | |
| Current Trial | 38 | 35 | |
| Furuse et al. 26 | 26 | 32 | |
| Strumberg et al. 25 | — | 35 | |
| Sorafenib 400 mg BID | |||
| C pmax (µg/mL) | |||
| All Patients | Day 15 (n = 6) | Day 28 (n = 3) | P value |
| Sorafenib (mean ± SD) | 8.4 ± 5.18 | 4.1 ± 0.56 | .20 |
| N-oxide (mean ± SD) | 2.0 ± 1.75 | 0.9 ± 0.55 | .37 |
| Comparisons with other trials (geometric mean) | Day 14–15 | Day 28 | |
| Current Trial | 6.2 | 4.1 | |
| Furuse et al. 26 | 4.7 | 3.3 | |
| Strumberg et al. 25 | — | 5.4 | |
| AUC0-12 (µg × h/mL) | |||
| All Patients | Day 15 (n = 6) | Day 28 (n = 3) | P value |
| Sorafenib (mean ± SD) | 62.4 ± 38 | 38.7 ± 9.61 | .34 |
| N-oxide (mean ± SD) | 15 ± 14.7 | 9.7 ± 6.54 | .57 |
| Comparisons with other trials (geometric mean) | Day 14–15 | Day 28 | |
| Current Trial | 43 | 38 | |
| Furuse et al. 26 | 34 | 29 | |
| Strumberg et al. 25 | — | 48 |
Phase II Component
Patient characteristics
Nineteen eligible patients were enrolled into the phase II component (Table 2). Among participants, 9 were men and 10 women. Median age was 52 years (range: 30–76), and median KPS was 90 (range: 60–100). Patients had a median of 2 prior chemotherapy treatments, and 6 patients had received prior bevacizumab therapy. All patients received the MTD of the study agent identified in the phase I component, which was sorafenib 200 mg BID and erlotinib 100 mg QD. Treatment was terminated for 4 patients prior to confirmed disease progression. One patient experienced leakage at surgical site, requiring re-operation; postoperative course was complicated with further leakage and treatment was held for longer than 28 days and ultimately terminated. One patient terminated treatment due to adverse events. Two patients withdrew from the trial following initiation therapy.
Efficacy data
Fifteen patients were evaluable for radiological response. No patient showed complete or partial response. Three patients remained progression free at 6 months and 3 patients’ time of disease progression was unknown. Among the 12 patients with known time of disease progression, PFS rate at 6 months (PFS6) was 18.8%, and median PFS was 1.8 months. Six patients were alive at 12 months, representing a 12-month overall survival (OS12) of 31.6%; median OS was 5 months (Supplementary Figure 1). Median number of 4-week treatment cycles was 2 (range: 1–9). As fewer than 4 patients were progression free at 6 months, the sorafenib + erlotinib treatment arm was terminated and did not progress to stage 2 recruitment.
Toxicity data
All patients from phase I and phase II components (35 total) of this trial were evaluated for treatment toxicity. Overall, sorafenib and erlotinib were well tolerated at the MTD. All Grade 3 and 4 events deemed related (possible or higher) to sorafenib + erlotinib combination therapy are detailed in Table 5.
Table 5.
Grade 3 or 4 Adverse Events with Relationship (possible or higher) to Sorafenib + Erlotinib
| Adverse Event | DL0 (MTD, N = 25) | DL1 (N = 7) | DL2 (N = 3) | Total (N = 35) |
|---|---|---|---|---|
| Alanine aminotransferase increased | 1 (4%) | 1 (3%) | ||
| Aspartate aminotransferase increased | 1 (4%) | 1 (3%) | ||
| Diarrhea | 1 (4%) | 1 (3%) | ||
| Generalized muscle weakness | 5 (20%) | 5 (14%) | ||
| Fatigue (aethenia, lethargy, malaise) | 1 (4%) | 1 (3%) | ||
| Hypertension | 2 (8%) | 1 (14%) | 3 (9%) | |
| Hypophosphatemia | 3 (12%) | 2 (29%) | 1 (33%) | 6 (17%) |
| Lipase increased | 1 (4%) | 2 (67%) | 3 (9%) | |
| Lymphocyte count decreased | 1 (4%) | 1 (3%) | ||
| Rash (maculo-papular) | 1 (4%) | 1 (3%) |
Discussion
This phase I/II study of sorafenib and erlotinib identified the MTD of sorafenib as 200 mg BID and of erlotinib as 100 mg QD and demonstrated that while erlotinib did not affect the PK of sorafenib, sorafenib reduced erlotinib levels. Furthermore, this study shows that while sorafenib and erlotinib are well tolerated at the MTD, this combination therapy does not improve PFS6 for recurrent GBM compared to historical controls.
In the phase I component, MTD was established at sorafenib 200 mg BID and erlotinib 100 mg QD after observing 2 DLTs (1 Grade 4 elevated lipase and 1 Grade 3 hypertension) among 6 patients at the next higher dose (DL1). This resulted in an MTD that is lower than previously reported for sorafenib and erlotinib for treatment in GBM and other cancers.27–30 Notably, the NABTT 0502 trial, conducted at the same time as the current study, defined the MTD of sorafenib as 400 mg BID and of erlotinib as 150 mg QD. The difference in MTD between the NABTT trial and the current trial is largely because in this study, Grade 3 and 4 elevated lipase are classified as DLTs, while in the NABTT trial, Grade 3 and 4 elevated lipase events are not (unless there are associated elevations in amylase or symptoms of pancreatitis). Elevated lipase is a common toxicity of sorafenib,26 however, clinical significance of isolated and asymptomatic Grade 3 or 4 elevated lipase due to sorafenib is unclear. Thus, further investigations are needed to determine the significance of isolated and asymptomatic Grades 3 or 4 elevated lipase in GBM patients treated with combination therapies that include sorafenib.
The PK parameters for sorafenib are consistent with published reports and not influenced by the coadministration of erlotinib.31,32 Definitive conclusions regarding sorafenib’s effect on erlotinib PKs could not be determined due to the relatively infrequent sampling schedule. However, from our study, sorafenib appears to alter the PK of erlotinib. With continuous dosing, the exposure of erlotinib did not increase over time. Similarly, past studies of sorafenib and erlotinib combinations also reported that sorafenib seems to affect erlotinib PK, lowering its plasma levels. This phenomenon was also observed in the NABTT trial.27 Interestingly, combination of sorafenib plus gefitinib, another EGFR inhibitor, shows a similar PK pattern.33 Erlotinib and gefitinib share the same structural backbone and are metabolized by CYP3A4/5. Increased activation of CYP3A4 enzyme velocity rather than induction is one of the suggested mechanisms.34–36 In this 2-substrate allosteric binding model, sorafenib and erlotinib could bind simultaneously to CYP3A4 active sites. Sorafenib acting as an actuator or effector would either enhance erlotinib’s affinity for CYP3A4 or induce conformational changes in CYP3A4 that regulate its enzyme activity. Whatever the mechanism for the interaction, the clinical relevance of reduced erlotinib accumulation by sorafenib is unknown.
In the phase II component, only 3 patients in the stage 1 cohort remained progression free at 6 months. As this was below the predetermined efficacy threshold of at least 4 progression-free patients, the trial was terminated. The lack of efficacy may be explained by multiple factors. First, the dose of sorafenib and erlotinib is lower than the dose used in trials for other solid tumors. As discussed previously, this is mainly due to the classification of Grade 3 and 4 elevated lipase as a DLT in the current trial. Second, it is possible that this combination therapy is not sufficient to overcome the multitude of redundant growth signal activations in GBM. Finally, the ability of sorafenib and erlotinib to penetrate the blood–brain barrier is limited, as studies have shown that both drugs can be transported out of the CNS via ABCG2 efflux pumps.37,38 Additionally, the pharmacokinetic interaction between erlotinib and sorafenib leading to reduced levels of erlotinib may have also contributed to the lack of efficacy. Despite these postulations, it is possible that sorafenib and erlotinib combination therapy may simply be ineffective as a treatment for recurrent GBM; thus, further studies of this combination therapy are not warranted.
While all 3 treatment arms of NABTC 05-02 were negative, this trial was able to demonstrate the feasibility of a novel sequential accrual study design of multiple experimental treatments. Traditionally, each combination therapy studied in NABTC 05-02 would have been structured as its own phase I/II trial and coordinating patient recruitment for each of the 3 trials would have been difficult, especially in multicenter settings. In essence, with NABTC 05-02, we were able to combine 3 clinical trials into 1 trial with a systematically streamlined patient accrual process to significantly increase the possibility that patients can be enrolled in a study arm at any given time. This trial design benefits not only patients and their families but also reduces the time needed to investigate multiple drugs as well as help mitigate concerns over available protocol slots in a multicenter study. However, to maximize the utility of this novel sequential accrual design, treatment arms must share similar inclusion/exclusion criteria, therapy cycle lengths, and cohort sizes. Thus, this trial design may be best suited for investigating multiple combination therapies sharing one common agent as in the case of NABCT 05-02.
Supplementary Material
Acknowledgments
We gratefully acknowledge the help of the NABTC Data Management Center at MD Anderson Cancer Center and the ABTC Data Management Center at the Johns Hopkins Oncology Center. We thank our research staffs for their outstanding work and our patients and their families for their participation in this study.
Funding
This study was sponsored by National Institutes of Health [grant number U01 CA06239].
Conflict of interest statement. H.C., J.K., K.L., F.L., H.I.R., S.C., J.D., V.A.L., K.A., J.E.D., J.J.W., M.D.P., and M.R.G. report no COI. L.E.A. is a full time employee and stock holder in Novartis; L.M.D. is on a Scientific advisory board for Sapience Therapeutics; W.K.A.Y. is on the Scientific Advisory Boards of DNAtrix, Quadriga, ILCT; M.P.M. is a consultant for Karyopharm, Tocagen, Astra-Zeneca, Blue Earth Diagnostics, Celgene, Abbvie and on the Board of Directors of Oncoceutics; T.F.C. reports personal fees from Roche, Trizel, Medscape, Bayer, Amgen, personal fees from Odonate Therapeutics, Pascal Biosciences, Del Mar, Tocagen, Karyopharm, GW Pharma, Kiyatec, AbbVie, Boehinger Ingelheim, VBI, Dicephera, VBL, Agios, Merck, Genocea, Puma, Lilly, BMS, Cortice, Wellcome Trust, a patent 62/819,322 with royalties paid to Katmai and Member of the board for the 501c3 Global Coalition for Adaptive Research; and PYW reports consulting/advisory board agreements with Agios, Astra Zeneca, Bayer, Blue Earth Diagnostics, Boston Pharmaceuticals, Immunomic Therapeutics, Karyopharm, Integral Health, Vascular Biogenics, VBI Vaccines, Tocagen, Voyager, Novocure, QED, and Imvax and clinical trial support from Agios, Astra Zeneca/Medimmune, Bayer, Beigene, Celgene, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Oncoceutics, Vascular Biogenics, and VBI Vaccines.
Authorship Statement. Experimental design: J.K., K.R.L., J.E.D., J.J.W., T.F.C., P.Y.W., and M.R.G. Study implementation: J.K., K.R.L., L.E.A., L.M.D., F.L., H.I.R., S.M.C., W.K.A.Y., J.D., M.D.M., V.A.L., K.A., J.E.D., J.J.W., M.D.P., T.F.C., P.Y.W., and M.R.G. Analysis and interpretation: H.C., J.K., K.R.L., L.E.A., L.M.D., F.L., H.I.R., S.M.C., W.K.A.Y., J.D., M.D.M., V.A.L., K.A., J.E.D., J.J.W., M.D.P., T.F.C., P.Y.W., and M.R.G. Manuscript writing: H.C., J.K., K.R.L., L.E.A., L.M.D., F.L., H.I.R., S.M.C., W.K.A.Y., J.D., M.D.M., V.A.L., K.A., J.E.D., J.J.W., M.D.P., T.F.C., P.Y.W., and M.R.G.
References
- 1. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Suppl. 5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. [DOI] [PubMed] [Google Scholar]
- 3. Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2(2):120–129. [DOI] [PubMed] [Google Scholar]
- 4. Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. [DOI] [PubMed] [Google Scholar]
- 5. Dy GK, Adjei AA. Novel targets for lung cancer therapy: part II. J Clin Oncol. 2002;20(13):3016–3028. [DOI] [PubMed] [Google Scholar]
- 6. Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22(22):4442–4445. [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8(25):2255–2257. [DOI] [PubMed] [Google Scholar]
- 8. Hainsworth JD, Ervin T, Friedman E, et al. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116(15):3663–3669. [DOI] [PubMed] [Google Scholar]
- 9. Davies MA, Fox PS, Papadopoulos NE, et al. Phase I study of the combination of sorafenib and temsirolimus in patients with metastatic melanoma. Clin Cancer Res. 2012;18(4):1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An Z, Aksoy O, Zheng T, Fan QW, Weiss WA. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wikstrand CJ, Hale LP, Batra SK, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55(14):3140–3148. [PubMed] [Google Scholar]
- 12. Brown PD, Krishnan S, Sarkaria JN, et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J Clin Oncol. 2008;26(34):5603–5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010; 98(1):93–99. [DOI] [PubMed] [Google Scholar]
- 15. Raizer JJ, Abrey LE, Lassman AB, et al. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao Q, Lei T, Ye F. Therapeutic targeting of EGFR-activated metabolic pathways in glioblastoma. Expert Opin Investig Drugs. 2013;22(8):1023–1040. [DOI] [PubMed] [Google Scholar]
- 18. Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol. 2012;14(12):1511–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nghiemphu PL, Ebiana VA, Wen P, et al. Phase I study of sorafenib and tipifarnib for recurrent glioblastoma: NABTC 05-02. J Neurooncol. 2018;136(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 21. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11(21):7841–7850. [DOI] [PubMed] [Google Scholar]
- 23. Afify S, Rapp UR, Högger P. Validation of a liquid chromatography assay for the quantification of the Raf kinase inhibitor BAY 43-9006 in small volumes of mouse serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;809(1):99–103. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto N, Horiike A, Fujisaka Y, et al. Phase I dose-finding and pharmacokinetic study of the oral epidermal growth factor receptor tyrosine kinase inhibitor Ro50-8231 (erlotinib) in Japanese patients with solid tumors. Cancer Chemother Pharmacol. 2008;61(3):489–496. [DOI] [PubMed] [Google Scholar]
- 25. Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12(4):426–437. [DOI] [PubMed] [Google Scholar]
- 26. Furuse J, Ishii H, Nakachi K, Suzuki E, Shimizu S, Nakajima K. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci. 2008;99(1):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peereboom DM, Ahluwalia MS, Ye X, et al. NABTT 0502: a phase II and pharmacokinetic study of erlotinib and sorafenib for patients with progressive or recurrent glioblastoma multiforme. Neuro Oncol. 2013;15(4):490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gridelli C, Morgillo F, Favaretto A, et al. Sorafenib in combination with erlotinib or with gemcitabine in elderly patients with advanced non-small-cell lung cancer: a randomized phase II study. Ann Oncol. 2011;22(7):1528–1534. [DOI] [PubMed] [Google Scholar]
- 29. Spigel DR, Burris HA 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(18):2582–2589. [DOI] [PubMed] [Google Scholar]
- 30. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. [DOI] [PubMed] [Google Scholar]
- 31. Duran I, Hotté SJ, Hirte H, et al. Phase I targeted combination trial of sorafenib and erlotinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(16):4849–4857. [DOI] [PubMed] [Google Scholar]
- 32. Quintela-Fandino M, Le Tourneau C, Duran I, et al. Phase I combination of sorafenib and erlotinib therapy in solid tumors: safety, pharmacokinetic, and pharmacodynamic evaluation from an expansion cohort. Mol Cancer Ther. 2010;9(3):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adjei AA, Molina JR, Mandrekar SJ, et al. Phase I trial of sorafenib in combination with gefitinib in patients with refractory or recurrent non-small cell lung cancer. Clin Cancer Res. 2007;13(9):2684–2691. [DOI] [PubMed] [Google Scholar]
- 34. Ekroos M, Sjögren T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc Natl Acad Sci USA. 2006;103(37):13682–13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korzekwa KR, Krishnamachary N, Shou M, et al. Evaluation of atypical cytochrome P450 kinetics with two-substrate models: evidence that multiple substrates can simultaneously bind to cytochrome P450 active sites. Biochemistry. 1998;37(12):4137–4147. [DOI] [PubMed] [Google Scholar]
- 36. Ludwig E, Schmid J, Beschke K, Ebner T. Activation of human cytochrome P-450 3A4-catalyzed meloxicam 5’-methylhydroxylation by quinidine and hydroquinidine in vitro. J Pharmacol Exp Ther. 1999;290(1):1–8. [PubMed] [Google Scholar]
- 37. Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336(1):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tournier N, Goutal S, Auvity S, et al. Strategies to inhibit ABCB1- and ABCG2-mediated efflux transport of erlotinib at the blood-brain barrier: a PET study on nonhuman primates. J Nucl Med. 2017;58(1):117–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.