Abstract
Background:
There are currently no available standard drugs treating human papillomavirus (HPV) infection, especially for patients with low-grade cervical lesion. Several therapies are explored but the results are inconclusive. The objective of this study was to evaluate the efficacy of reported non-invasive treatments in patients with HPV infection and cervical lesions by meta-analysis.
Methods:
A comprehensive search of prospective and randomized studies published from April 2000 to April 2020 was conducted in electronic databases. The statistical analyses of the pooled risk ratios (RRs) and the corresponding 95% confidence intervals (95% CIs) were performed using the Revman 5.2 software.
Results:
Twelve articles including 12 randomized controlled studies and 1 prospective controlled randomized pilot study were enrolled. Therapeutic medications included biological and herbal regimen, interferon regimen and probiotics. The meta-analysis showed the experimental treatments had a statistically significant improvement in HPV clearance rate compared with the controls (RR = 0.71, 95% CI [0.63, 0.80], P < .00001); subgroup analyses stratified by regimen categories were consistent with results in the overall group. Treatment using biological and herbal regimen, interferon regimen or probiotics also resulted in a beneficial outcome in regression rate of cervical lesions compared with the controls (RR = 0.55, 95% CI [0.39, 0.79], P = .001). The trend was more favorable in the probiotics than that in the biological and herbal regimen (RR 0.48 vs 0.72).
Conclusion:
Treatment of biological and herbal regimen, interferon regimen and probiotics benefit patients who have HPV infection and cervical lesions. Both the clearance of HPV and regression of cervical lesions are significant. More studies with less heterogeneity are needed to draw a concrete conclusion.
Keywords: cervical lesion, herb, human papillomavirus, interferon, probiotics
1. Introduction
Cervical cancer is the fourth most common cancer in women worldwide. According to the most recent Globocan report, there were 569,847 new cases and 311,365 deaths in 2018.[1] Persistent human papillomavirus (HPV) infection, especially the infection of high-risk subtypes, is a confirmed precursor to the development of almost all invasive cervical carcinomas.[2–4] The recent application of virus-like particle prophylactic HPV vaccination offers an effective primary prevention strategy for squamous cervical cancer.[5,6] However, prophylactic vaccines are type-specific (mostly 16 and 18) and mostly target HPV negative populations. They are relatively expensive and inaccessible to many domestic and developing regions and countries.[7,8]
For populations who are positive of HPV but negative of cervical lesion, the chances of self-regression are relatively high, especially if they are under 30 years old, thus close surveillance is recommended.[9] However, for patients with cervical lesions, there is no effective treatment directed towards clearance of HPV till date. HPV infections can be treated through laser, cryotherapy, conization, and so on. The rationale is to cause inflammatory reactions of the local tissue so that the body will be stimulated to mount an antibody response which shows an anti-viral effect.[10–14] However, the invasive therapy is often indicated for those who have high-grade squamous intraepithelial lesion (HSIL) and potential side effects like late abortions and preterm delivery brought by invasive procedures are major concerns.[15,16] For patients who have HPV infection and low-grade lesions (LSIL), the preferred care would be frequent surveillance till they clear the infection or develop cervical neoplasia.[17]
Some noninvasive therapies have been investigated in preclinical and clinical trials for the treatment of HPV infection or cervical lesions. It has been reported that probiotics can contribute to HPV clearance via creating a synergistic environment, enhancing adaptive and innate immunity, and directly demonstrating anti-viral effect via specific metabolites.[18] In addition, interferon and leukocyte extract have been reported as a modulator of the immune response, which up-regulate chemotaxis of natural killer cells and macrophages in tissues infected with fungi, viruses, and parasites.[19] Several in vitro studies also suggest some herbal formulations and their metabolites can be cytotoxic to cervical cancer cells by inhibiting the transcription of HPV E6/E7 oncogene. Such biological and herbal regimens include polyphenon E, curcumin, myrtle, and phytolacca Americana.[20–23] Notably, these formulations can also be found in food and are of low cost. To explore the clinical efficacy of those non-invasive therapies in patients with both HPV infection and cervical lesions, several clinical trials were conducted. However, the regimens studied were diverse and results were inconsistent. In this study, in order to draw a more concrete conclusion, we conducted a meta-analysis of the currently published prospective and randomized trials on non-invasive treatments for patients with both HPV infection and cervical lesions.
2. Materials and methods
2.1. Search strategy
Prospective controlled studies for all possible non-invasive treatments of HPV infections in patients with cervical intraepithelial neoplasia that were published from April 2000 to April 2020 were searched in PubMed, EMBASE, CNKI, OVID, and Cochrane databases. The search terms for the disease included “HPV,” “human papillomavirus,” “cervical lesion,” “atypical squamous cells of undetermined significance (ASCUS),” “LSIL,” “HSIL,” and “cervical intraepithelial neoplasia (CIN).” The search terms for treatment included “non-invasive,” “oral,” “injection,” “herb,” “probiotics,” “interferon” and “leukocyte.” The search strategies for prospective controlled trials included using the corresponding filters for different databases. The search was updated every week until April 20th, 2020. The study is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[24] No ethical approval and patient consent are required because all analyses were based on previously published studies. The analysis is not a registered study.
2.2. Inclusion and exclusion criteria
The following criteria determined the eligible studies:
-
(1)
women should be diagnosed with cervical or vaginal HPV infection regardless of subtypes;
-
(2)
cervical lesions were confirmed by Pap smear (ASCUS, LSI, and HSIL) or colonoscopy (CIN) in some, if not all of these women;
-
(3)
experimental treatments were non-invasive, including oral administration and subcutaneous or intramuscular injection;
-
(4)
control arm used placebo; active controls with proved effective ingredient were allowed if the schemes were significantly different in frequency or dose compared with experimental treatments;
-
(5)
prospective and randomized trials;
-
(6)
numbers of patients who were HPV positive or cervical lesion positive before and after treatments should be clearly described.
The exclusion criteria were trials that
-
(1)
studied women with cervicitis only or cervical cancer;
-
(2)
used invasive treatments such as conization or laser;
-
(3)
did not have the full text available;
-
(4)
failed to report the required numeric results.
2.3. Data extraction and quality assessment
Two authors independently searched and retrieve the literature. The third author would adjudicate the discrepancy if there were any. Data extracted as study characteristics included first author, published year, study type, HPV type, HPV testing methods, cervical lesion type, cervical lesion testing methods, treatment regimen, and matched parameters. The data collected for statistical analysis demonstrated dichotomous outcomes, as presented by numbers of HPV and lesion positive patients before and after treatment in both arms. When different efficacy criteria (such as partial response based on the area of lesion affected) were defined in individual trials, only those who had complete HPV clearance and normal Pap smear or colonoscopic findings (including inflammatory manifestations) would be considered as HPV-free and lesion-free. If different follow-ups were conducted, the outcomes would be collected for the latest follow up.
The modified Jadad scale was used to investigate the methodological quality of included studies. Four aspects of random sequence production, allocation concealment, blinding method, and withdrawals and dropouts were scored. Studies were rated as either low quality or high quality under scores of 1 to 3 and 4 to 7, respectively.[25]
2.4. Statistical analysis
Heterogeneity assumptions were tested using Higgins’ I2 test and the I2 value was a quantitative parameter of inconsistency across studies.[26,27] When the I2 value was less than 50%, low heterogeneity was considered and a fixed-effects model was applied otherwise a random-effects model was used. The risk ratio (RR) was the efficacy estimate for the pooled data (RR = risk of event in experimental group/risk of event in control group = (SE/NE)/(SC/NC)).[28] The presence of publication bias was evaluated by inspecting the asymmetry in funnel plots visually. When the funnel plots indicated visible asymmetry, Egger test was performed to further measure the bias, which was considered as existing when P was less than .05.
Statistical analyses were conducted using Review Manager (Version 5.2; Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark) and Stata (Version 14.0; StataCorp LLC, College Station, TX).
3. Results
3.1. Search results
One hundred seventeen studies were preliminarily retrieved according to the developed retrieval strategy. After the removal of duplicates, 102 articles were screened. Sixty-two articles were further excluded based on titles and abstracts. For the remaining 40 articles, 12 were pre-clinical studies; 4 studied other diseases in cervix like cervicitis and cervical cancer; 6 were single arm studies with no controls, 2 did not provide numeric dichotomous outcomes; 4 were non-English publications (Fig. 1). Thus, 12 articles were included in the meta-analysis (Table 1).
Figure 1.

Flow chart of study selection.
Table 1.
Characteristics of included studies.
| Author | Year | Country | Study Type | Patient No. | HPV type | HPV testing | Cervical lesion type | Cervical lesion testing | Treatment | Matched parameters | Study quality (Jadad scale) |
| Acosta-Rios | 2017 | Mexico | RCT | 54 | Not known | Not known | CIN 1 | Colposcopy | Dialyzable Leukocyte Extract | Not known | 3 |
| Ahn | 2003 | South Korea | RCT | 90 | Not known | DNA hybrid capture assay | ASCUS/LSIL/HSIL | Pap smear and colposcopy | Polyphenon E and EGCG | Not known | 3 |
| Basu | 2013 | India | RCT | 126 | Type 16 or 18 | PCR | LSIL | Colposcopy | Basant cream (polyherbal) | Age, parity, menopausal status, HPV type | 5 |
| Basu | 2013 | India | RCT | 161 | Type 16 or 18 | PCR | LSIL | Colposcopy | Curcumin capsule | Age, parity, menopausal status, HPV type | 5 |
| Garcia | 2014 | US | RCT | 98 | High risk | DNA hybrid capture | CIN1 | Colposcopy | Polyphenon E | Age, weight, height, race, ethnicity, smoking status, current sexual activity, age at 1st intercourse, total lifetime partners | 6 |
| Gonzalez-Sanchez | 2001 | Mexico | RCT | 122 | Not known | Not known | CIN1-3 | Pap smear and colposcopy | Interferon beta | Age, beginning of sexual relationships, number of pregnancies, use of contraceptives, types of previous CIN treatments | 4 |
| Iljazović | 2006 | Bosnia | RCT | 55 | Not known | DNA hybrid capture | ASCUS/LSIL mainly | Pap smear | Interferon and aloe vera | Not known | 3 |
| Mutombo | 2019 | Congo | RCT | 59 | Not known | PCR | ASCUS and LSIL mainly | Pap smear | AV2 (carvone, eugenol, geraniol, nerolidol) | Age, marital status, education, profession, menopausal status, abortion, miscarriage, lifetime sexual partners, oral contraceptives use, condoms use, use of traditional herbs | 6 |
| Nikakhtar | 2018 | Iran | RCT | 60 | Not known | DNA hybrid capture assay | Lesion not specified | Colposcopy | Myrtle | Age, marriage duration, parity, detection time of disease, HPV typing, mean affected area, external genital wart, vaginal itching and burning | 5 |
| Ou | 2019 | China | RCT | 121 | High risk | DNA hybrid capture assay | ASCUS/LSIL | Pap smear | Probiotics | Age, menopausal status, parity, IUD, educational level, history of TH, HR-HPV load, HR-HPV clearance, ASCUS/LSIL | 5 |
| Palma | 2018 | Italy | Prospective controlled pilot study | 117 | Not known | PCR | Pap smear abnormality | Pap smear and colposcopy | Probiotics | Age, contraceptive methods, regular menses, symptoms prevalence, smoking, multiple partners, bacterial vaginosis, yeast vaginitis, pap smear alterations | 3 |
| Verhoeven | 2013 | Belgium | Prospective controlled pilot study | 51 | Not known | PCR | LSIL | Pap smear | Probiotics | Age, smoking status, level of stress, alcohol use, hormonal contraception, tampon use, condom use, lifetime partners, cancer history, physical exercise. | 3 |
| Yang | 2019 | China | RCT | 79 | High risk | DNA hybrid capture | CIN1 | Colposcopy | REBACIN (Phytolacca americana) | Not known | 4 |
3.2. Characteristics of included studies
Among the 12 included articles, 1 study included 2 different treatment regimens and had separate controls for both treatments thus were considered as 2 studies. For the 13 studies, 8 were of high quality (Jadad score of 4–7) and 5 were of low quality (Jadad score of 1–3). Meanwhile, 11 studies were randomized controlled studies (RCTs) and 2 studies were a prospective controlled randomized pilot study. One study used active control (short-term probiotics) while the rest were placebo controlled. Most of the studies did not specify HPV subtypes but 5 studies only included high-risk subtypes of type 16 and 18. Not all patients with HPV infection had cervical lesions. For those who were confirmed as having both HPV infection and cervical lesion, most of lesions were of low grade (ASCUS, LSIL, CIN1) and 2 studies did not mention specific grades by generally describing as abnormality. Treatments included biological and herbal regimens (polyphenon E, curcumin, basant cream, myrtle, phytolacca americana, and multi-herbal regimens), interferon related regimens (interferon and leukocyte extract), and probiotics. Nine studies listed matched parameters between the experimental and control arms. Most frequently reported matched parameters included age, menopausal status, contraceptive use, smoking status, parity, and sexual activity (Table 1).[29–40]
3.3. Meta-analysis of efficacy
3.3.1. The clearance of HPV
A total of 12 studies including 548 patients in the experimental arm and 526 patients in the control arm were analyzed. In the experimental arm, 60.22% (330/548) of patients had HPV cleared, while in the control arm, 43.35% (228/526) were HPV negative. Under a fixed-effects model (I2 = 48%), the pooled results showed treatments in the experimental arm had a statistically significant difference in HPV clearance compared with the control arm (RR = 0.71, 95% CI [0.63, 0.80], P < .00001). In summary, treatments of biological and herbal regimen, interferon regimen, and probiotics benefited those patients who had HPV infection (Table 2 and Fig. 2).
Table 2.
Numeric distributions between experimental arms and control arms for HPV clearance and cervical lesion regression.
| Experimental | Control | Experimental | Control | ||||||||
| Group | Author | Year | Patient No. | HPV+ | Total | HPV+ | Total | Lesion+ | Total | Lesion+ | Total |
| Biological and Herbal | Ahn | 2003 | 79 | 15 | 41 | 21 | 38 | 10 | 40 | 5 | 13 |
| Basu | 2013 | 113 | 8 | 65 | 12 | 48 | 3 | 7 | 2 | 2 | |
| Basu | 2013 | 155 | 14 | 75 | 22 | 80 | 4 | 6 | 0 | 0 | |
| Garcia | 2014 | 82 | 31 | 41 | 29 | 41 | 34 | 41 | 35 | 41 | |
| Mutombo | 2019 | 59 | 13 | 27 | 21 | 32 | 6 | 17 | 13 | 24 | |
| Nikakhtar | 2018 | 52 | 2 | 27 | 8 | 25 | 16 | 27 | 21 | 25 | |
| Yang | 2019 | 79 | 15 | 39 | 32 | 40 | 16 | 32 | 31 | 35 | |
| Interferon related | Acosta-Rios | 2017 | 54 | Not known | Not known | Not known | Not known | 3 | 27 | 27 | 27 |
| Gonzalez-Sanchez | 2001 | 122 | 18 | 61 | 35 | 61 | Not known | 18 | Not known | 9 | |
| Iljazović | 2006 | 55 | 25 | 35 | 18 | 20 | Not known | 35 | Not known | 20 | |
| Probiotics | Ou | 2019 | 121 | 26 | 62 | 27 | 59 | 4 | 13 | 6 | 9 |
| Palma | 2018 | 106 | 33 | 51 | 48 | 55 | 8 | 39 | 25 | 40 | |
| Verhoeven | 2013 | 51 | 18 | 24 | 25 | 27 | 12 | 24 | 19 | 27 | |
Figure 2.

Forest plot of HPV clearance: overall group treatment vs. control. ∗Basu 2013 studied 2 regimes with separate controls and was considered as 2 trials. HPV = human papillomavirus.
Further subgroup analysis explored the different categories of the regimens in clearing HPV infection. For biological and herbal regimen, 7 studies with 619 patients included. After treatments, 31.11% and 47.70% of patients in the experimental and control arms remained HPV positive, respectively. Six out of the 7 studies showed favorable trends (RR <1) for biological and herbal regimen; the further meta-analysis showed that the difference in the clearance rates of HPV between the 2 arms were statistically significant (RR = 0.66, 95% CI [0.47, 0.92], P = .01). Only 2 studies described interferon treatment for HPV infection. With heterogeneity observed (I2 = 76%), the pooled data showed a favorable but insignificant benefit of interferon over the control (RR = 0.66, 95% CI [0.39, 1.10], P = .11). Three studies compared probiotics and controls in HPV clearance. Notably, 1 study (Palma et al) used active control, comparing standard treatment plus short-term (3 months) vaginal Lactobacillus implementation versus the same standard treatment plus long-lasting (6 months) vaginal Lactobacillus administration. The meta-analysis of the 278 patients showed no heterogeneity (I2 = 0%) and statistically significant difference between probiotics and the control (RR = 0.81, 95% CI [0.68, 0.96], P = .01) (Table 2 and Fig. 3).
Figure 3.
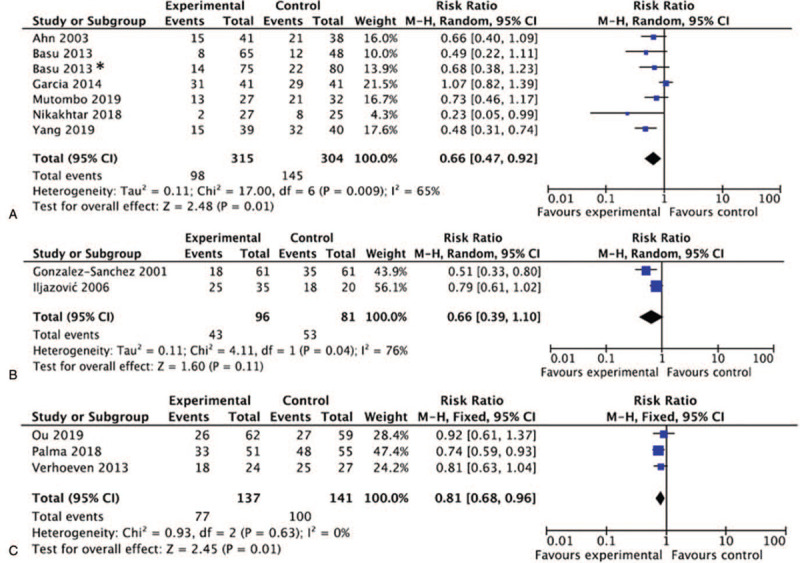
Forest plots of HPV clearance. (A) Biological and herbal regimen vs. control; (B) Interferon vs. control; (C) Probiotics vs. control. ∗Basu 2013 studied 2 regimes with separate controls and was considered as 2 trials. HPV = human papillomavirus.
3.3.2. Regression of cervical lesion
Among all the included studies, 11 studies reported the numbers of patients who had cervical lesion before and after treatments. In addition, 1 study had no patients with cervical lesion in the control arm, thus the regression of lesion would not be estimable. Regardless of regimens, the meta-analysis included 273 patients and 243 patients in the experimental and control arms, respectively. All individual studies reported decreased percentages of patients who were still lesion positive after treatments, with 3 studies showed significant difference between the experimental and the control arm. The pooled results showed that treatment using biological and herbal regimen, interferon regimen or probiotics resulted in a beneficial outcome in regression rate of cervical lesions compared with the controls (RR = 0.55, 95% CI [0.39, 0.79], P = .001) (Table 2 and Fig. 4).
Figure 4.
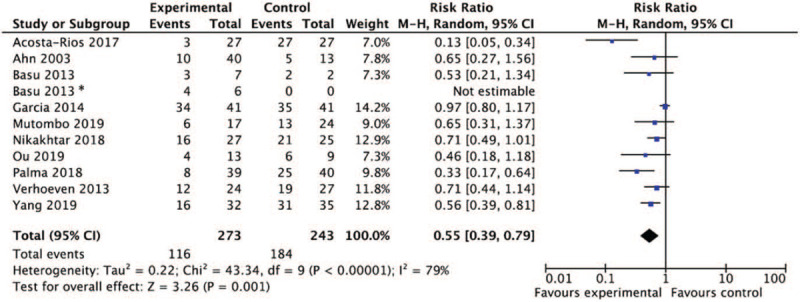
Forest plot of cervical lesion regression: overall group treatment versus control. ∗Basu 2013 studied 2 regimes with separate controls and was considered as 2 trials.
With only 1 study reported the dichotomous outcomes among interferon related regimens, subgroup analysis was conducted for biological and herbal regimen and probiotics. Compared with 140 patients in the control arm, 164 patients who were treated with biological and herbal regimens showed a significantly decreased rate of lesion positivity after treatments under a random-effects model (RR = 0.72, 95% CI [.54, 0.94], P = .02, I2 = 55%). Three studies with 152 patients showed the lesion regression rate was 68.42% (52/72) in probiotics arm and 36.11% (26/72) in the control arm, the meta-analysis displayed a significant deference between the 2 arms (RR = 0.48, 95% CI ([0.34, 0.70)], P < .0001) and the trend was more favorable compared with the biological and herbal regimen (RR = 0.72) (Table 2 and Fig. 5).
Figure 5.

Forest plots of cervical lesion regression. (A) Biological and herbal regimen versus control; (B) Probiotics versus control.
3.3.3. Publication bias and sensitivity analysis
The funnel plot demonstrated a symmetrical shape for overall group HPV clearance studies as well as subgroup analysis for probiotics in both HPV clearance and lesion regression, suggesting no significant publication bias. Despite the interferon subgroup analysis with only 2 studies included, the distribution was imbalanced for overall group cervical lesion regression studies and analysis for biological and herbal subgroups (Fig. 6). Further Egger test also demonstrated significant bias in biological and herbal subgroups (P < .05).
Figure 6.
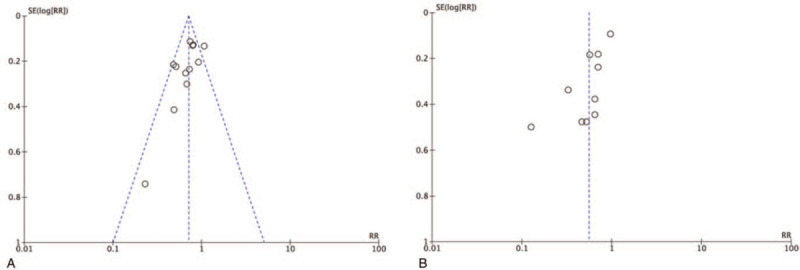
Representative funnel plots. (A) Overall group analysis in HPV clearance; (B) Overall group analysis in cervical lesion regression. HPV = human papillomavirus.
Sensitivity analysis was conducted by checking the consistency of the overall effect estimate using the leave-one-out method. Studies with significantly smaller sample sizes were removed but the value of I2 was similar (data not shown). The exclusion of any single study did not materially change the summary result. We believe that the heterogeneity may arise from studies that used biological and herbal regimens, which were complex in nature. Also, different follow-up periods among different studies and self-regression in low-graded patients might also contribute the heterogeneity.[41,42]
4. Discussion
Cervical malignancies, the commonest infection-associated neoplasm, and its premalignant precursor cervical intraepithelial neoplasia are relative with strains of HPV. Although the virus is highly prevalent, only a few women have a persistent HPV infection and subsequently develop significant disease that requires clinical intervention.[43] Currently, there is no standard of care for HPV infection, especially for patients with low-grade cervical lesions.
The mechanism of persistent infection was not definite but there is emerging evidence that indicates cervicovaginal microbiota plays an important role in the persistence infection and subsequent disease. [44–47] The acidic microbial environment in vagina can prohibit pathogen growth through decreasing oxidative stress, modulating immune responses, and expressing bacteriocins.[48,49] Women with unbalanced composition of the cervicovaginal microbiota, including low Lactobacillus abundance and high diversity, are more likely to acquire infections of HIV, HPV, et al. It has been approved in preclinical studies that the presence of certain species of Lactobacillus spp. was a protective factor by maintaining the “cervical epithelial barrier” function that prevents entry of HPV to the basal keratinocytes.[50] Thus, by replenishing the depleted pool of Lactobacillus spp., probiotics, which is defined as “live microorganisms that, when administered in adequate amounts, deliver a health benefit on the host” by the World Health Organization, has been suggested recently as an interventional method to promote HPV clearance.[51] In a recent trial, Palma et al reported that HPV clearance was higher in the treatment of metronidazole plus 6 months vaginal Lactobacillus implementation than that with 3 months use.[38] However, in another RCT conducted by Verhoeven et al, the probiotics of oral Lactobacillus on HPV clearance was observed in women with HPV-related LSILs but the result was not significant compared with controls.[39]
Despite of probiotics, several other non-invasive treatments are also studied in patient with HPV infection and cervical lesions, the most popular ones are biological and herbal regimens which are of low cost and can serve as food adjuvant. For example, polyphenon E, a decaffeinated, enriched, and defined mixture of green tea catechins, was proved in vitro to prohibit the growth of HPV-positive cervical cancer cells in a dose dependent mode.[52] In a randomized study by Ahn et al, treatment with green tea extracts (polyphenon E; poly E and (–) -epigallocatechin-3-gallate) in 51 patients with HPV infected cervical lesions demonstrated a response rate of 69% (35/51), which was significantly higher as compared with 10% (4/39) in untreated controls.[30] Another example is myrtle. It is the common name of M. communis and is from the family of Myrtaceae. Therapeutic effects of myrtle include antiviral, antibacterial, antioxidant, antifungal, anti-inflammatory, and proapoptotic activities.[53] Nikakhtar et al published an RCT to compare the efficacy of herbal vaginal suppository (10% of myrtle aqueous extract and 0.5% of myrtle essential oil) and placebo in women with cervicovaginal HPV infection. The interventional group benefited significantly from myrtle, with HPV clearance rate as 92.6% and the change in cervical lesion size as 71.4%.[36]
For the last decade, intramuscular administration of interferon beta has been used to treat HPV either alone or with other treatments. Both interferon and leukocyte extracts are considered as a modulator of the immune response, which up-regulate the chemotaxis and activation of natural killer cells and macrophages. Although the safety profile has been proved acceptable, efficacy results are varied and elucidation especially for patients with both HPV infection and cervical lesions is limited.[54]
Our study is the first meta-analysis to explore the efficacy of available non-invasive treatments in patients with HPV infection and cervical lesions. The meta-analysis showed the experimental treatments had a statistically significant improvement in HPV clearance rate compared with the controls (RR = 0.71, 95% CI [0.63, 0.80], P < .0001); subgroup analyses stratified by regimen categories (biological and herbal regimen, interferon regimen or probiotics) were consistent with results in the overall group. Included treatments also resulted in a beneficial outcome in regression rate of cervical lesions compared with the controls (RR = 0.55, 95% CI [0.39, 0.79], P = .001). The trend was more favorable in the probiotics than that in the biological and herbal regimen (RR 0.48 vs 0.72). Individually, almost all included studies reported a beneficial trend of using experimental treatment but the majority of them displayed an insignificant difference compared with controls. By conducting the meta-analysis, the pooled results showed statistically significant results in both overall group analysis and subgroup analysis (except interferon related regimen in HPV clearance). This proves the importance of this study to draw a more concrete conclusion in those inconclusive trials. In addition, despite the diversity of regimens used in different trials, this study reported not only the overall group results but also subgroup analysis by grouping individual regimens according to anatomical, therapeutic, chemical class. Such analysis would be more interpretable and can potentially facilitate the development of novel medications with similar chemical structure and mechanisms.
Despite our efforts to include all available publications, several limitations to our meta-analysis should not be ignored. First, heterogeneity was noted among individual studies, especially for biological and herbal regimens. Sensitivity analysis using the leave-one-out method presented similar value of I2. We believe that the heterogeneity may arise from studies that used biological and herbal regimens, which were complex in nature. Herbal therapy is associated with cultures, while some herbs are popularly used in some countries, they might be never tested in other regions. Also, even though the herbs are under 1 anatomical, therapeutic, chemical class, their mechanisms might differ from each other, thus future analysis is needed to explain the anti-viral effect in a more specific manner.[55] Meanwhile, different follow-up periods among different studies and self-regression in low-graded patients might also contribute the heterogeneity. Whereas, the exclusion of any single study did not materially change the summary result, the benefit shown in current meta-analysis can still be concluded cautiously. Second, not all of the studies reported matched parameters between experimental and controls. Baseline characteristics such as ethnicity and sexual activity are intrinsic factors known to be significantly associated with microbial stability. For example, it was reported that Caucasian and Asian women displayed a significantly greater prevalence of Lactobacillus spp. dominant microbiota, compared to Black and Hispanic women.[56] Therefore, unreported, and potentially imbalanced factors might lead to an inaccurate explanation of pooled data. Third, although the number of pooled participants was so far the largest, the number of several subgroup analyses was still relatively limited, especially for interferon related regimen. Thus, cautions must be paid when interpreting such subgroup analysis.
5. Conclusion
Treatment of biological and herbal regimen, interferon regimen, and probiotics exhibit beneficial effects in patients who had HPV infection and cervical lesions. However, more studies with less heterogeneity are needed to draw a concrete conclusion.
Author contributions
Conceptualization: Ying Xiong, Xiaoli Wang.
Data curation: Liuyang Cui.
Formal analysis: Ying Xiong, Ce Bian.
Methodology: Ying Xiong, Xia Zhao, Ce Bian.
Supervision: Xiaoli Wang, Xia Zhao.
Writing – original draft: Ying Xiong, Xiaoli Wang, Xia Zhao, Ce Bian, Liuyang Cui.
Writing – review & editing: Ying Xiong, Xiaoli Wang, Xia Zhao, Ce Bian, Liuyang Cui.
Footnotes
Abbreviations: ASCUS = atypical squamous cells of undetermined significance, CIN = cervical intraepithelial neoplasia, HPV = human papillomavirus, HSIL = high-grade squamous intraepithelial lesion, ISIL = low-grade squamous intraepithelial lesion, RCT = randomized controlled trial.
How to cite this article: Xiong Y, Cui L, Bian C, Zhao X, Wang X. Clearance of human papillomavirus infection in patients with cervical intraepithelial neoplasia: a systemic review and meta-analysis. Medicine. 2020;99:46(e23155).
This study was supported by the Key Research Projects of Science and Technology Department Foundation of Sichuan Province (Grant No: 2017SZ0141).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
ASCUS: atypical squamous cells of undetermined significance, CIN = cervical intraepithelial neoplasia, HPV = human papillomavirus, HSIL = high-grade squamous intraepithelial lesion, LSIL = low-grade lesions, RCT = randomized controlled trial.
HPV = human papillomavirus.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–41. [DOI] [PubMed] [Google Scholar]
- [3].Phumaphi J, Gautam KC, Mason E. Increased production and comprehensive guidelines needed for HPV vaccine. Lancet 2020;395:319–21. [DOI] [PubMed] [Google Scholar]
- [4].Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169–82. [DOI] [PubMed] [Google Scholar]
- [5].Rodriguez AM, Zeybek B, Vaughn M, et al. Comparison of the long-term impact and clinical outcomes of fewer doses and standard doses of human papillomavirus vaccine in the United States: a database study. Cancer 2020;126:1656–67. [DOI] [PubMed] [Google Scholar]
- [6].McClung NM, Gargano JW, Park IU, et al. Estimated number of cases of high-grade cervical lesions diagnosed among women - United States, 2008 and 2016. MMWR Morb Mortal Wkly Rep 2019;68:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sundaram N, Voo TC, Tam CC. Adolescent HPV vaccination: empowerment, equity and ethics. Hum Vaccin Immunother 2020;16:1835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jiang Y, Ni W, Wu J. Cost-effectiveness and value-based prices of the 9-valent human papillomavirus vaccine for the prevention of cervical cancer in China: an economic modelling analysis. BMJ Open 2019;9:e031186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang S, Batur P. Human papillomavirus in 2019: an update on cervical cancer prevention and screening guidelines. Cleve Clin J Med 2019;86:173–8. [DOI] [PubMed] [Google Scholar]
- [10].Gurumurthy M, Cruickshank ME. Management of vaginal intraepithelial neoplasia. J Low Genit Tract Dis 2012;16:306–12. [DOI] [PubMed] [Google Scholar]
- [11].Lince-Deroche N, van Rensburg C, Roseleur J, et al. Costs and cost-effectiveness of LEEP versus cryotherapy for treating cervical dysplasia among HIV-positive women in Johannesburg. South Africa PLoS One 2018;13:e0203921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pinder LF, Parham GP, Basu P, et al. Thermal ablation versus cryotherapy or loop excision to treat women positive for cervical precancer on visual inspection with acetic acid test: pilot phase of a randomised controlled trial. Lancet Oncol 2020;21:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Echelman D, Feldman S. Management of cervical precancers: a global perspective. Hematol Oncol Clin North Am 2012;26:31–44. [DOI] [PubMed] [Google Scholar]
- [14].Gage JC, Rodriguez AC, Schiffman M, et al. An evaluation by midwives and gynecologists of treatability of cervical lesions by cryotherapy among human papillomavirus-positive women. Int J Gynecol Cancer 2009;19:728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ 2008;337:a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ 2016;354:i3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Boardman LA, Kennedy CM. Management of atypical squamous cells, low-grade squamous intraepithelial lesions, and cervical intraepithelial neoplasia. Obstet Gynecol Clin North Am 2008;35:599–614. [DOI] [PubMed] [Google Scholar]
- [18].Mitra A, MacIntyre DA, Marchesi JR, et al. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pierangeli A, Degener AM, Ferreri ML, et al. Interferon-induced gene expression in cervical mucosa during human papillomavirus infection. Int J Immunopathol Pharmacol 2011;24:217–23. [DOI] [PubMed] [Google Scholar]
- [20].Gao H, Kuroyanagi M, Wu L, et al. Antitumor-promoting constituents from Dioscorea bulbifera L. in JB6 mouse epidermal cells. Biol Pharm Bull 2002;25:1241–3. [DOI] [PubMed] [Google Scholar]
- [21].Maher DM, Bell MC, O’Donnell EA, et al. Curcumin suppresses human papillomavirus oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits benzo a,pyrene-induced upregulation of HPV E7. Mol Carcinog 2011;50:47–57. [DOI] [PubMed] [Google Scholar]
- [22].Aleksic V, Knezevic P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol Res 2014;169:240–54. [DOI] [PubMed] [Google Scholar]
- [23].Martinez G, Mutombo BA, Fernandez V, et al. A preliminary study for the treatment of cervical colposcopic lesions with the biological compound AV2. Eur J Gynaecol Oncol 2017;38:342–5. [PubMed] [Google Scholar]
- [24].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang H, Liu B, Tang Y, et al. Improvement of sepsis prognosis by ulinastatin: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 2019;10:1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang Z, He Y, Zheng Y. Probiotics for the treatment of bacterial vaginosis: a meta-analysis. Res Public Health 2019;16:3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yang F, Mu X, Bian C, et al. Association of excision repair cross-complimentary group 1 gene polymorphisms with breast and ovarian cancer susceptibility. J Cell Biochem 2019;120:15635–47. [DOI] [PubMed] [Google Scholar]
- [28].Li C, Wang T, Li Y, et al. Probiotics for the treatment of women with bacterial vaginosis: a systematic review and meta-analysis of randomized clinical trials. Eur J Pharmacol 2019;864:172660. [DOI] [PubMed] [Google Scholar]
- [29].Acosta-Rios MP, Sauer-Ramirez E, Castro-Munoz LJ, et al. Effect of dialyzable leukocyte extract on chronic cervicitis in patients with HPV infection. J Med Life 2017;10:237–43. [PMC free article] [PubMed] [Google Scholar]
- [30].Ahn WS, Yoo J, Huh SW, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev 2003;12:383–90. [DOI] [PubMed] [Google Scholar]
- [31].Basu P, Dutta S, Begum R, et al. Clearance of cervical human papillomavirus infection by topical application of curcumin and curcumin containing polyherbal cream: a phase II randomized controlled study. Asian Pac J Cancer Prev 2013;14:5753–9. [DOI] [PubMed] [Google Scholar]
- [32].Garcia FA, Cornelison T, Nuno T, et al. Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Gynecol Oncol 2014;132:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gonzalez-Sanchez JL, Martinez-Chequer JC, Hernandez-Celaya ME, et al. Randomized placebo-controlled evaluation of intramuscular interferon beta treatment of recurrent human papillomavirus. Obstet Gynecol 2001;97:621–4. [DOI] [PubMed] [Google Scholar]
- [34].Iljazovic E, Ljuca D, Sahimpasic A, et al. Efficacy in treatment of cervical HRHPV infection by combination of beta interferon, and herbal therapy in woman with different cervical lesions. Bosn J Basic Med Sci 2006;6:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Baleka Mutombo A, Tozin R, Kanyiki H, et al. Impact of antiviral AV2 in the topical treatment of HPV-associated lesions of the cervix: results of a phase III randomized placebo-controlled trial. Contemp Clin Trials Commun 2019;15:100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nikakhtar Z, Hasanzadeh M, Hamedi SS, et al. The efficacy of vaginal suppository based on myrtle in patients with cervicovaginal human papillomavirus infection: a randomized, double-blind, placebo trial. Phytother Res 2018;32:2002–8. [DOI] [PubMed] [Google Scholar]
- [37].Ou YC, Fu HC, Tseng CW, et al. The influence of probiotics on genital high-risk human papilloma virus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Womens Health 2019;19:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Palma E, Recine N, Domenici L, et al. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect Dis 2018;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Verhoeven V, Renard N, Makar A, et al. Probiotics enhance the clearance of human papillomavirus-related cervical lesions: a prospective controlled pilot study. Eur J Cancer Prev 2013;22:46–51. [DOI] [PubMed] [Google Scholar]
- [40].Yang Y, Meng YL, Duan SM, et al. REBACIN(R) as a noninvasive clinical intervention for high-risk human papillomavirus persistent infection. Int J Cancer 2019;145:2712–9. [DOI] [PubMed] [Google Scholar]
- [41].Syrjanen K. Mechanisms and predictors of high-risk human papillomavirus (HPV) clearance in the uterine cervix. Eur J Gynaecol Oncol 2007;28:337–51. [PubMed] [Google Scholar]
- [42].de Villiers EM, Fauquet C, Broker TR, et al. Classification of papillomaviruses. Virology 2004;324:17–27. [DOI] [PubMed] [Google Scholar]
- [43].Braaten KP, Laufer MR. Human papillomavirus (HPV), HPV-related disease, and the HPV vaccine. Rev Obstet Gynecol 2008;1:2–10. [PMC free article] [PubMed] [Google Scholar]
- [44].Shrestha T, Choi W, Kim GE, et al. Human papilloma virus identification in ocular surface squamous neoplasia by p16 immunohistochemistry and DNA chip test: a strobe-compliant article. Medicine 2019;98:e13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang W, Zhang A, Sun W, et al. Efficacy and safety of photodynamic therapy for cervical intraepithelial neoplasia and human papilloma virus infection: a systematic review and meta-analysis of randomized clinical trials. Medicine 2018;97:e10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang F, Li H, Qi X, et al. Post-hysterectomy rare collision vulva tumor with long-term human papilloma virus infection composed of squamous cell carcinoma of the labia major and adenosquamous carcinoma of bartholin gland: a case report. Medicine 2019;98:e17043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Eshetu Lemma H, Simoens C, Benoy I, et al. HPV testing on vaginal/cervical nurse-assisted self-samples versus clinician-taken specimens and the HPV prevalence, in Adama Town, Ethiopia. Medicine 2019;98:e16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Reid G. Probiotic agents to protect the urogenital tract against infection. Am J Clin Nutr 2001;73:437s–43s. [DOI] [PubMed] [Google Scholar]
- [49].Ho M, Chang YY, Chang WC, et al. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: a randomized controlled trial. Taiwan J Obstet Gynecol 2016;55:515–8. [DOI] [PubMed] [Google Scholar]
- [50].Borgdorff H, Gautam R, Armstrong SD, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol 2016;9:621–33. [DOI] [PubMed] [Google Scholar]
- [51].Vujic G, Jajac Knez A, Despot Stefanovic V, et al. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol 2013;168:75–9. [DOI] [PubMed] [Google Scholar]
- [52].Zou C, Liu H, Feugang JM, et al. Green tea compound in chemoprevention of cervical cancer. Int J Gynecol Cancer 2010;20:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hedayati A, Khosropanah H, Bazargani A, et al. Assessing the antimicrobial effect of the essential oil of myrtus communis on the clinical isolates of porphyromonas gingivalis: an in vitro study. Jundishapur J Nat Pharm Prod 2013;8:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shi HJ, Song HB, Zhao QY, et al. Efficacy and safety of combined high-dose interferon and red light therapy for the treatment of human papillomavirus and associated vaginitis and cervicitis: a prospective and randomized clinical study. Medicine (Baltimore) 2018;97:e12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jafari S, Abdollahi M, Saeidnia S. Personalized medicine: a confluence of traditional and contemporary medicine. Altern Ther Health Med 2014;20:31–40. [PubMed] [Google Scholar]
- [56].Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108: Suppl 1: 4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


