Abstract
Background
Statistical data on the burden and relevant risk factors of lung cancer are valuable for policy‐making. This study aimed to compare the mortality of lung cancer attributable to smoking stratified by sex and age among adults in China and the United States (US).
Methods
We extracted age‐standardized mortality rates of lung cancer during 1990‐2017 using the comparative risk assessment framework of the 2017 Global Burden of Disease study. We performed an age‐period‐cohort analysis to estimate time trend of lung cancer mortality attributable to smoking.
Results
During 1990‐2017, the age‐standardized mortality rate of lung cancer was increasing in China but decreasing in the US for both sexes. The mortality attributable to smoking in China showed a generally increasing trend, while a continuous decrease was observed in the US. The age‐period‐cohort analysis showed a similar trend of age effect among adults between China and the US: the mortality substantially increased from the 30‐34 to 80‐84 age group and subsequently decreased in the 90‐94 age group. However, the period effect rapidly increased in Chinese adults during 1990‐2017, while it tended to be stable in the US although it was still slightly increasing in women. The cohort effect generally peaked in the earlier cohort born in 1902‐1906 in the two countries.
Conclusions
During 1990‐2017, the lung cancer mortality attributable to smoking and the period effect are generally increasing in Chinese adults; the mortality attributable to smoking is decreasing in the US adults, but the period effect tends to be stable. The rapid aging and prevalence of smoking may intensify the increasing mortality of lung cancer in China.
Keywords: age‐period‐cohort effect, epidemiology, lung cancer, mortality, smoking, trend
Abbreviations
- ASMR
age‐standardized mortality rate
- CDC
Chinese Center for Disease Control and Prevention
- CI
confidence intervals
- CODEm
Cause of Death Ensemble model
- DSPs
Disease Surveillance Points
- e‐cigarettes
electronic cigarettes
- GBD
Global Burden of Disease
- RR
relative risk
- VA
verbal autopsy
- VR
vital registration
1. BACKGROUND
Lung cancer has been the leading cause of cancer deaths for many years worldwide, with the incidence and mortality markedly varying between countries, and smoking was the main cause of lung cancer [1, 2]. Lung cancer has the highest mortality among all cancers in both the United States (US) and China, where it ranks second and first, respectively [3]. The US and Chinese populations have different smoking exposure levels that contribute to unique trends of lung cancer mortality. Specifically, smoking cessation campaigns were successfully conducted in the US, and the smoking prevalence was decreasing in the US [4]. In China, smoking is still prevalent and tobacco control has remained particularly difficult due to interference by the tobacco industry [5, 6]. Consequently, over the past years, lung cancer mortality has been continuously decreasing in the US but increasing in China [4].
More than 90% of lung cancers have been shown to be attributable to smoking based on a previous study [7]. However, the secular trends in lung cancer mortality attributable to smoking remain unknown in both China and the US, and more epidemiologic evidences on the effectiveness of tobacco control are needed. Smoking rate is rising in China [8], and the smoking‐related economy burden is still substantial in China [9].
Age‐period‐cohort analysis simutaneously eatimates age, period, and cohort effects which influence the incidence and mortality of a disease in specific ways, especially that the period effect on disease represents that complex sets of historical events and environmental factors may affect morbidity and mortality of the disease during a period [10]. The estimated age, period, and birth cohort trends in disease could provide epidemiologic evidence on effective measures to control and prevent lung cancer in China [11]. The present study aimed to estimate the secular trend and age‐period‐cohort effects of lung cancer mortality attributable to smoking among adults during 1990‐2017 in China and the US.
2. MATERIALS AND METHODS
2.1. Data source
This study used the latest data from the 2017 Global Burden of Disease (GBD) study (http://ghdx.healthdata.org/), which provided a comprehensive annual estimation of global, regional and national incidence, prevalence, mortality for causes of death and risk factors in 195 countries and territories [12, 13]. The GBD study summarized the burden of a disease for global populations among different causes, locations, ages, and sexes [14]. All data used in the study were deidentified.
The mortality data of lung cancer and its attributable burden related to smoking were obtained from the 2017 GBD study. The age‐standardized mortality rates (ASMRs) were calculated by using the GBD 2017 global age‐standard population [15]. The mortality database is composed of vital registration (VR), verbal autopsy (VA), registry, survey, policy and surveillance data, and the statistical modeling tool (DisMod‐MR 2.1), based on available data sources such as the Cause of Death Ensemble model (CODEm), which was used to generate cause fractions and cause‐specific death rates for each location, year, age, and sex [15]. The original data of lung cancer mortality in China were mainly from the Cause of Death Reporting System of the Chinese Center for Disease Control and Prevention (CDC), Disease Surveillance Points (DSPs), and the Maternal and Child Surveillance System, which are considered to be nationally representative [16]. In the following analysis, data of the age groups under 30 years old were excluded because no data of lung cancer diagnosed in younger populations were reported in the GBD study, and the age groups above 95 years old were also excluded because they did not meet the age‐period‐cohort model fitting conditions. For comparison, we also collected the mortality data of lung cancer in the US during 1990‐2017.
According to the GBD study, a systematic literature search through PubMed was performed to find evidence for risk factors of lung cancer deaths. For each included study, the proportions of lung cancer cases induced by specific risk factors were calculated. The proportion data obtained from the systematic literature review were inputted into DisMod‐MR2.1, a Bayesian meta‐regression tool, as the main method for estimation on incidence and prevalence of 354 diseases and injuries in 195 countries and territories [15]. Based on comparative risk assessments in the 2017 GBD study, smoking was defined as current and former use of any tobacco products (among current smokers, cigarette equivalents smoked per smoker per day, and cumulative pack‐years of exposure; among former smokers, number of years since quitting) [17]. Current use of any chewing tobacco products, second‐hand smoke (average daily exposure to air particulate matter from second‐hand smoke with an aerodynamic diameter smaller than 2.5 μm among non‐smokers), and hubble‐bubble (hookah) smoking were excluded.
2.2. Statistical analysis
Age effects represent the increase in mortality risk with the process of aging. Period effects represent influential factors of disease simultaneously affecting all age groups during a same period, and these factors mainly include complex sets of historical events and environmental factors. Cohort effects represent variations across groups of individuals born in the same year or years. In the age‐period‐cohort analysis, age‐specific rates of lung cancer mortality attributable to smoking were classified into consecutive 5‐year periods from 1990 to 2017, successive 5‐year age groups from 30‐34 years to 90‐94 years, and consecutive 5‐year birth cohort groups from 1902‐1906 to 1987‐1991 birth cohort. For the calculation of RR, we chose the mean level in age, period and cohort as reference groups, and we then calculated the difference between other groups and reference groups, respectively. The exponential value of difference denoted the mortality RR of a particular age, period and birth cohort relative to reference groups. The goodness of fit was evaluated using the Akaike information criterion, Bayesian information criterion, and degree of freedom for the estimated models. This analysis was performed using the Stata 14.0 software (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Descriptive analysis of lung cancer ASMRs
Figure 1 shows the ASMRs of lung cancer in China and the US for both sexes at all ages during 1990‐2017. According to the 2017 GBD study, the ASMR of lung cancer increased from 40.47/100,000 in 1990 to 51.92/100,000 in 2017 in men and increased from 17.54/100,000 in 1990 to 21.98/100,000 in 2017 in women in China; in the US, it decreased in both men (from 69.94/100,000 in 1990 to 42.32/100,000 in 2017) and women (from 30.30/100,000 in 1990 to 27.99/100,000 in 2017). Overall, the ASMR of lung cancer was increasing in China for both sexes, while it was decreasing and tended to be stable in recent years in the US, suggesting that the risk factors for lung cancer may differently impact the mortality in the Chinese and US populations.
FIGURE 1.
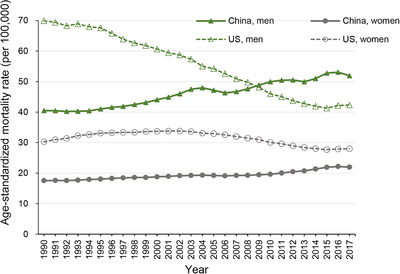
Trends in the age‐standardized mortality rates of lung cancer in China and the United States for both sexes at all ages, during 1990‐2017
3.2. Trends in mortality of lung cancer attributable to smoking
Trends in ASMR of lung cancer attributable to smoking from 1990 to 2017 in China and the US are shown in Figure 2. The highest rate in China was 41.94/100,000 in men and 5.14/100,000 in women in 2017. In the US, the highest rate was 59.61/100,000 in men in 1990 and 24.98/100,000 in women in 1995. Over the past decades, China showed a low smoking‐attributable ASMR in women, compared with the US. However, the smoking‐attributable ASMR in men was higher in China than in the US since 2008. Overall, the ASMR of lung cancer attributable to smoking was increasing in China and decreasing in the US.
FIGURE 2.
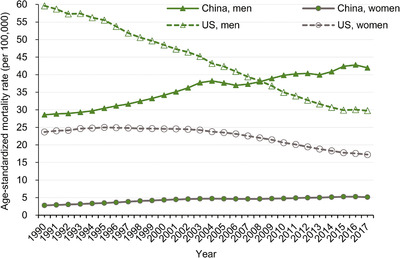
Trends in the age‐standardized rates of lung cancer mortality attributable to smoking in China and the United States during 1990‐2017, at all ages
The RR of a particular age, period, or birth cohort was calculated based on these coefficients of age, period, and cohort effects estimated by using the age‐period‐cohort model (Table 1). Similar trends of age and cohort effects were observed among adults aged 30 years and older in both China and the US. The period effect increased rapidly in China and kept stable in US men while it was still slightly increasing in US women. The goodness‐of‐fit statistics and the best‐fitting model are presented in Supplementary Table S1, and estimated coefficients for the age, period, and cohort effects are shown in Supplementary Table S2.
TABLE 1.
The relative risks of lung cancer mortality attributable to smoking due to age, period, and cohort effects in China and the US
| China | The US | |||||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| Factor | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Age | ||||||||
| 30‐34 | 0.04 | 0.02 to 0.09 | 0.01 | 0.00 to 7.59 | 0.01 | 0.00 to 0.07 | 0.02 | 0.00 to 0.11 |
| 35‐39 | 0.10 | 0.06 to 0.16 | 0.05 | 0.00 to 0.83 | 0.06 | 0.03 to 0.12 | 0.08 | 0.04 to 0.18 |
| 40‐44 | 0.25 | 0.18 to 0.34 | 0.17 | 0.03 to 0.98 | 0.19 | 0.12 to 0.29 | 0.23 | 0.14 to 0.39 |
| 45‐49 | 0.44 | 0.35 to 0.56 | 0.29 | 0.07 to 1.17 | 0.47 | 0.34 to 0.65 | 0.51 | 0.35 to 0.76 |
| 50‐54 | 0.75 | 0.62 to 0.91 | 0.63 | 0.21 to 1.91 | 0.95 | 0.73 to 1.23 | 0.96 | 0.70 to 1.31 |
| 55‐59 | 1.13 | 0.96 to 1.32 | 1.00 | 0.39 to 2.52 | 1.65 | 1.34 to 2.03 | 1.55 | 1.21 to 2.00 |
| 60‐64 | 1.83 | 1.61 to 2.07 | 1.92 | 0.91 to 4.07 | 2.50 | 2.11 to 2.97 | 2.29 | 1.86 to 2.81 |
| 65‐69 | 2.53 | 2.28 to 2.80 | 3.63 | 1.98 to 6.65 | 3.34 | 2.90 to 3.85 | 3.09 | 2.60 to 3.67 |
| 70‐74 | 3.32 | 3.04 to 3.63 | 4.86 | 2.94 to 8.01 | 4.01 | 3.52 to 4.56 | 3.73 | 3.18 to 4.37 |
| 75‐79 | 3.72 | 3.41 to 4.05 | 6.41 | 4.08 to 10.06 | 4.42 | 3.85 to 5.07 | 4.04 | 3.40 to 4.78 |
| 80‐84 | 3.98 | 3.62 to 4.38 | 7.14 | 4.44 to 11.48 | 4.50 | 3.82 to 5.28 | 3.96 | 3.23 to 4.84 |
| 85‐89 | 3.81 | 3.39 to 4.27 | 5.82 | 3.31 to 10.22 | 4.03 | 3.31 to 4.91 | 3.39 | 2.65 to 4.33 |
| 90‐94 | 3.35 | 2.91 to 3.85 | 6.05 | 3.04 to 12.05 | 2.96 | 2.33 to 3.75 | 2.49 | 1.85 to 3.35 |
| Period | ||||||||
| 1992 | 0.63 | 0.57 to 0.68 | 0.51 | 0.32 to 0.82 | 1.01 | 0.89 to 1.15 | 0.86 | 0.73 to 1.01 |
| 1997 | 0.75 | 0.70 to 0.79 | 0.72 | 0.53 to 0.96 | 1.01 | 0.93 to 1.10 | 0.94 | 0.85 to 1.04 |
| 2002 | 0.96 | 0.92 to 1.00 | 0.94 | 0.82 to 1.08 | 1.01 | 0.97 to 1.06 | 1.02 | 0.96 to 1.08 |
| 2007 | 1.12 | 1.08 to 1.17 | 1.12 | 0.98 to 1.28 | 0.99 | 0.95 to 1.04 | 1.06 | 1.00 to 1.13 |
| 2012 | 1.33 | 1.25to 1.41 | 1.46 | 1.09 to 1.95 | 0.97 | 0.89 to 1.05 | 1.06 | 0.95 to 1.18 |
| 2017 | 1.50 | 1.37 to 1.63 | 1.77 | 1.12 to 2.81 | 1.01 | 0.88 to 1.15 | 1.08 | 0.92 to 1.26 |
| Cohort | ||||||||
| 1902‐1906 | 2.49 | 1.99 to 3.13 | 3.67 | 1.21 to 11.15 | 2.52 | 1.72 to 3.67 | 1.84 | 1.13 to 3.02 |
| 1907‐1911 | 2.32 | 1.91 to 2.81 | 3.11 | 1.17 to 8.27 | 2.56 | 1.81 to 3.62 | 1.99 | 1.27 to 3.10 |
| 1912‐1916 | 2.15 | 1.81 to 2.56 | 2.70 | 1.13 to 6.50 | 2.56 | 1.85 to 3.55 | 2.18 | 1.44 to 3.32 |
| 1917‐1921 | 2.04 | 1.74 to 2.40 | 2.57 | 1.14 to 5.78 | 2.52 | 1.84 to 3.46 | 2.36 | 1.58 to 3.55 |
| 1922‐1926 | 1.99 | 1.70 to 2.32 | 2.56 | 1.17 to 5.59 | 2.40 | 1.76 to 3.29 | 2.39 | 1.60 to 3.58 |
| 1927‐1931 | 1.88 | 1.61 to 2.20 | 2.26 | 1.02 to 5.03 | 2.26 | 1.64 to 3.11 | 2.30 | 1.53 to 3.47 |
| 1932‐1936 | 1.70 | 1.44 to 2.01 | 2.15 | 0.91 to 5.06 | 2.03 | 1.46 to 2.84 | 2.21 | 1.45 to 3.39 |
| 1937‐1941 | 1.53 | 1.27 to 1.84 | 1.72 | 0.66 to 4.43 | 1.77 | 1.24 to 2.52 | 2.02 | 1.28 to 3.17 |
| 1942‐1946 | 1.29 | 1.05 to 1.58 | 1.41 | 0.49to 4.09 | 1.52 | 1.04 to 2.23 | 1.77 | 1.09 to 2.87 |
| 1947‐1951 | 1.19 | 0.95 to 1.49 | 1.05 | 0.32 to 3.48 | 1.27 | 0.84 to 1.92 | 1.45 | 0.86 to 2.44 |
| 1952‐1956 | 1.21 | 0.94 to 1.55 | 0.93 | 0.24 to 3.60 | 1.03 | 0.66 to 1.62 | 1.11 | 0.63 to 1.95 |
| 1957‐1961 | 1.01 | 0.76 to 1.33 | 0.79 | 0.17 to 3.58 | 0.88 | 0.54 to 1.44 | 0.94 | 0.51 to 1.75 |
| 1962‐1966 | 0.71 | 0.52 to 0.98 | 0.50 | 0.09 to 2.81 | 0.71 | 0.42 to 1.22 | 0.84 | 0.43 to 1.64 |
| 1967‐1971 | 0.69 | 0.48 to 0.99 | 0.49 | 0.07 to 3.41 | 0.49 | 0.27 to 0.91 | 0.59 | 0.28 to 1.25 |
| 1972‐1976 | 0.46 | 0.29 to 0.73 | 0.29 | 0.02 to 3.54 | 0.30 | 0.14 to 0.67 | 0.34 | 0.13 to 0.89 |
| 1977‐1981 | 0.28 | 0.14 to 0.55 | 0.19 | 0.01 to 6.84 | 0.22 | 0.07 to 0.72 | 0.22 | 0.05 to 0.91 |
| 1982‐1986 | 0.21 | 0.07 to 0.68 | 0.20 | 0.00 to 88.24 | 0.19 | 0.02 to 1.57 | 0.16 | 0.01 to 2.24 |
| 1987‐1991 | 0.16 | 0.01 to 2.62 | 0.20 | 0.00 to 721617.14 | 0.16 | 0.00 to 52.93 | 0.14 | 0.00 to 212.43 |
Notes : RR: relative risk [RR = exp(coefficient)]; CI: confidence interval.
3.3. Age effect
Age effect demonstrated an increasing trend in younger age groups and a decreasing tendency in older age groups in both Chinese and the US adults (Figure 3a, Table 1), suggesting that the RR of smoking‐attributable mortality varied between younger and older age groups. In China, the RR was higher in women than in men as age increases, suggesting that aging could intensify lung cancer mortality in women. From the 30‐34 to 90‐94 age groups, the RR of lung cancer mortality attributable to smoking increased by 89.16 times in men and 493.21 times in women in China; in the US, it increased by 203.54 times in men and 131.51 times in women (Supplementary Table S2). These coefficients were calculated to the exponential value (exp(coef.) = ecoef.). For example, for age effect of China men, the risk of lung cancer due to smoking in 90‐94 age group was 89.16 (exp (β 90–94 − β mean) − (β 30‐34 − β mean) = exp (β 90‐94 − β 30‐34) ≈ 89.16) times the risk in 30‐34 age group. This finding indicated that older age generally increased the risk of lung cancer death.
FIGURE 3.
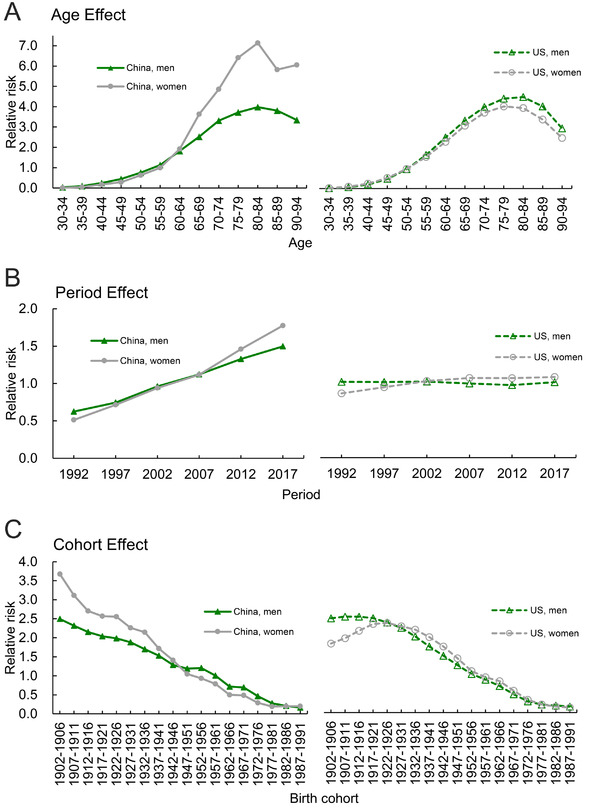
Relative risks estimated using the age‐period‐cohort analysis on lung cancer mortality attributable to smoking. A. age effect; B. period effect; C. birth cohort effect
3.4. Period effect
We observed that the risk of lung cancer attributable to smoking was increased with advancing time in China, but not in the US. The period effect rapidly increased in Chinese adults, while a stable trend was observed in the US adults during 1992‐2017 (Figure 3b, Table 1), suggesting that the RR of lung cancer mortality attributable to smoking increased with advancing time and Chinese population was being exposed to an increased risk of lung cancer deaths. The risk of lung cancer deaths was similarly controlled in the US adults of both sexes. In particular, from 1992 to 2017, the RR of mortality increased by 2.39 times in men and 3.46 times in women in China; in the US, it decreased by 0.41% in men and increased by 1.25 times in women (Supplementary Table S2).
3.5. Cohort effect
The cohort effect continuously decreased in China and US with the exception of individual birth cohorts (from 1902‐1906 to 1917‐1921 birth cohorts) (Figure 3c, Table 1). From 1902‐1906 to 1987‐1991 birth cohorts, th mortality of lung cancer attributable to smoking generally showed a decreasing trend in both China and US.
4. DISCUSSION
In the present study, we performed an age‐period‐cohort analysis to estimate the age, period, and birth cohort effects of the mortality of lung cancer attributable to smoking among adults aged 30 years and older in China and the US. We observed similar trends of age and cohort effects in both Chinese and the US adults. The period effect increased rapidly in Chinese adults and kept relatively stable in the US adults while it was still slightly increasing in the US women.
In the present study, we also found that from 1990 to 2017, the mortality of lung cancer was increasing for both sexes in China but not in the US, which was consistent with a previous study reported that lung cancer mortality was decreasing in the US but rising in China [4]. The mortality of lung cancer attributable to smoking also has generally increased in China, which was possibly contributed to the high prevalence of smoking [8] and a large burden of tobacco assumption in China [9]. The mortality of lung cancer attributable to smoking has decreased in the US, which may be due to the decreasing smoking prevalence in the US. As reported, an aggressive and successful smoking cessation campaign has been conducted in the US [1]. The varied smoking rates have driven an increasing trend of lung cancer mortality in China and a decreasing trend in the US. The continuing rise in lung cancer mortality attributable to smoking in China reinforces the need for strengthening implementation of the preventive actions, such as intensifying the smoking cessation campaign and tobacco control.
In the present study, similar trends of the age effect on lung cancer mortality attributable to smoking were observed between Chinese and the US adults aged 30 years and over. The age effect increased from the youngest age group to the 80‐84 age group and subsequently decreased to the 90‐94 age group in both countries, suggesting that aging has driven the trend of the increasing mortality of lung cancer [18, 19]. Different trends of the age effect were observed between sexes in China. The age‐period‐cohort analysis demonstrated that the RR of age effect increased faster in women than in men, suggesting that the increasing age effect may be especially intensified in women. This finding was possibly related to the perceived acceptability of female smoking [20]. Thus, women smoking prevalence needs to be closely monitored to discontinue an increase in smoking‐related deaths in Chinese women.
We also found that the period effect markedly increased in Chinese adults aged from 30 years and older, which may be mainly explained by the increasing smoking rate in China. A previous study showed a rising prevalence of smoking rates in Chinese people [8]. According to the eight China Health and Nutrition Surveys (CHNS), an international collaborative project between the Carolina Population Center of the University of North Carolina and the National Institute of Nutrition and Food Safety of the Chinese Center for Disease Control and Prevention since 1989, the smoking prevalence decreased slightly in the Chinese population during 1991‐2011 [21]. Generally, there is a time lag of several decades between smoking initiation and the occurrence of smoking‐related cancers or chronic respiratory diseases [22]. Moreover, smoking is still a major cause of death in China [21]. Smoking‐attributable mortality of lung cancer will possibly continue increasing in the Chinese population. Interestingly, the period effect increased more rapidly in Chinese than in the US adults. In the present study, period effect tended to be stable for smoking‐attributable mortality of lung cancer in the US adults during the whole observation period, which is probably due to the successful smoking cessation campaign and decreased smoking prevalence in the US [4]. However, a recent national survey in the US was conducted to examine vaping and smoking prevalence among adolescents and demonstrated no changes of smoking prevalence but an increased use of JUUL (a nicotine salt‐based electronic cigarette with high nicotine concentration) [23]. Thus, the evolving vaping prevalence should be closely monitored in the US. Additionally, we found that the period effect increased rapidly in women than in men in both China and the US. Another study reported that the incidence of lung cancer tended to stable or decreased in men worldwide, while in women it was usually increasing, indicating the need for strengthening the implementation of tobacco control among women [2]. Our findings may also indicate inadequate measures or policies on tobacco control in Chinese adults, while such measures were effective in the US [24, 25]. Most importantly, tobacco control in China has remained particularly difficult due to interference by the tobacco industry [5, 6]. However, the public strongly supports the WHO Framework Convention on Tobacco Control measures, in addition to increases in cigarette price, which may reduce tobacco consumption among Chinese urban people [26].
Apart from smoking (defined as current and former use of any smoked tobacco product in the present study), electronic cigarettes (e‐cigarettes) also need attentions in both Chinese and the US population. E‐cigarettes are a prevalent smoking cessation aid worldwide. However, a consensus of their efficacy and safety has yet to be reached [27]. As reported, the rate of adverse events related to e‐cigarettes ranged from 49.1% to 51.6% according to 11 studies involving 6406 persons [28]. The common adverse events were mouth or throat irritation, anxiety, depressed mood, nausea, and insomnia [28, 29]. Smoking cigarettes and e‐cigarettes are widely popular among Chinese students, and 7.4% of cigarette users and 3.6% of e‐cigarette consumers were observed among 3194 participants [30]. E‐cigarette is increasingly popular among adults of 19‐29 years old in China [31], and the awareness and use of e‐cigarettes in China are lower than that in the US [32]. In the US, the prevalence of e‐cigarette smoking rapidly increased among the US adults since 2010 [33, 34], and it was more popular among the US younger population. Previous studies have indicated that the government should emphasize the potentially harmful and addictive properties of e‐cigarettes [32, 35]. Therefore, the governments of China and the US also could establish and implement regulations for e‐cigarette smoking. Overall, the smoking prevalence in China is mostly unchanged over the past decade [21, 36], and the Chinese population seems to face a very heavy burden of lung cancer. Moreover, among people aged 30‐59, the age when adults often consider quitting smoking, only 10% of male smokers have tried quitting in the past year in China [36]. It is necessary to educate the nation with the correct knowledge and adopt policies on tobacco control and smoking cessation to reduce lung cancer deaths in Chinese adults.
In the present study, the cohort effect almost peaked at the 1902‐1906 birth cohort and declined to the most recent birth cohort, except for individual birth cohorts, indicating a decreasing risk of lung cancer death in younger generations. This declining effect was possibly attributed to receiving good education and high awareness of knowledge about health in the younger generations [37]. As for older people, they tend to have poor appreciations regarding the risks of cancer or the damage of smoking to health. Therefore, older patients are at a higher risk of lung cancer deaths.
There are some limitations in the present study. First, despite the mortality data estimated by the 2017 GBD study which incorporated methods to adjust for incomplete or missing VR and VA data, general heterogeneity in data completeness and quality, and the redistribution of so‐called garbage codes (insufficiently specific or implausible cause of death codes), inaccurate data could not be completely excluded. Second, the quality of data collection in the US and China may be different, due to different data sources. Therefore, our data from the present study on the epidemiology of lung cancer mortality should be treated carefully.
5. CONCLUSIONS
The lung cancer mortality attributable to smoking is rapidly increasing in China while decreasing in the US from 1990 to 2017. The lung cancer burden is still heavy in the Chinese population when compared with the US population, as the period effect increased rapidly in Chinese adults and kept stable in the US adults. The increasing period effect may indicate a continuous effect of risk factors on the mortality of lung cancer. Smoking prevalence and aging also may continuously drive the increase in lung cancer mortality in China. Continuous efforts should be concentrated on educating the general public to increase the prevention and early detection of lung cancer in China. Behavioral intervention, such as smoking cessation, reducing smoking rates and environmental risk factors, could effectively reduce lung cancer mortality.
DECLARATIONS: ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and/or analyzed in the current study are available in the GBD repository, http://ghdx.healthdata.org/gbd-results-tool.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was funded by the National Key Research and Development Program of China [grant numbers 2018YFC1315302, 2017YFC1200502] and the National Natural Science Foundation of China [grant number 81773552].
AUTHORS’ CONTRIBUTIONS
XL drafted this manuscript. XL, YY, MW, and SM collected the data. XL and FW analyzed the data. XL, YY, MW, SM, FW, YW, RM, and CY revised this manuscript. CY conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We appreciate the works by the 2017 Global Burden of Disease study collaborators.
Liu X, Yu Y, Wang M, et al. The mortality of lung cancer attributable to smoking among adults in China and the United States during 1990–2017. Cancer Commun. 2020;40:611–619. 10.1002/cac2.12099
REFERENCES
- 1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Annals of Global Health. 2019;85(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miranda‐Filho A, Pineros M, Bray F. The descriptive epidemiology of lung cancer and tobacco control: a global overview 2018. Salud Publica Mex. 2019;61(3):219–29. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 4. Yang D, Liu Y, Bai C, Wang X, Powell CA. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 2020;468:82–7. [DOI] [PubMed] [Google Scholar]
- 5. Yang G, Wang Y, Wu Y, Yang J, Wan X. The road to effective tobacco control in China. Lancet. 2015;385(9972):1019–28. [DOI] [PubMed] [Google Scholar]
- 6. Lv J, Su M, Hong Z, Zhang T, Huang X, Wang B, et al. Implementation of the WHO Framework Convention on Tobacco Control in mainland China. Tob Control. 2011;20(4):309–14. [DOI] [PubMed] [Google Scholar]
- 7. Siemiatycki J, Karp I, Sylvestre MP, Pintos J. Estimating the proportion of cases of lung cancer legally attributable to smoking: a novel approach for class actions against the tobacco industry. Am J Public Health. 2014;104(8):e60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3(8):819–31. [DOI] [PubMed] [Google Scholar]
- 9. Shi JF, Liu CC, Ren JS, Parascandola M, Zheng R, Tang W, et al. Economic burden of lung cancer attributable to smoking in China in 2015. Tob Control. 2020;29(2):191–9. [DOI] [PubMed] [Google Scholar]
- 10. Wang F, Mubarik S, Zhang Y, Wang L, Wang Y, Yu C, et al. Long‐Term Trends of Liver Cancer Incidence and Mortality in China 1990–2017: A Joinpoint and Age‐Period‐Cohort Analysis. International Journal of Environmental Research and Public Health. 2019;16(16):2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Y, Schulhofer‐Wohl S, Fu WJJ, Land KC. The intrinsic estimator for age‐period‐cohort analysis: What it is and how to use it. Am J Sociol. 2008;113(6):1697–736. [Google Scholar]
- 12. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murray CJ, Lopez AD, Jamison DT. The global burden of disease in 1990: summary results, sensitivity analysis and future directions. Bull World Health Organ. 1994;72(3):495–509. [PMC free article] [PubMed] [Google Scholar]
- 15. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause‐specific mortality for 240 causes in China during 1990‐2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–72. [DOI] [PubMed] [Google Scholar]
- 17. GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanasi E, Ayilavarapu S, Jones J. The aging population: demographics and the biology of aging. Periodontol 2000. 2016;72(1):13–8. [DOI] [PubMed] [Google Scholar]
- 19. Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–9. [DOI] [PubMed] [Google Scholar]
- 20. Sansone N, Yong HH, Li L, Jiang Y, Fong GT. Perceived acceptability of female smoking in China. Tob Control. 2015;24(Suppl 4):iv48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li S, Meng L, Chiolero A, Ma C, Xi B. Trends in smoking prevalence and attributable mortality in China, 1991‐2011. Prev Med. 2016;93:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill C. [Tobacco epidemiology]. Rev Prat. 2012;62(3):325, 7‐9. [PubMed] [Google Scholar]
- 23. Hammond D, Reid JL, Rynard VL, Fong GT, Cummings KM, McNeill A, et al. Prevalence of vaping and smoking among adolescents in Canada, England, and the United States: repeat national cross sectional surveys. BMJ. 2019;365:l2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lortet‐Tieulent J, Goding Sauer A, Siegel RL, Miller KD, Islami F, Fedewa SA, et al. State‐Level Cancer Mortality Attributable to Cigarette Smoking in the United States. JAMA Intern. Med.. 2016;176(12):1792–8. [DOI] [PubMed] [Google Scholar]
- 25. Mader EM, Lapin B, Cameron BJ, Carr TA, Morley CP. Update on Performance in Tobacco Control: A Longitudinal Analysis of the Impact of Tobacco Control Policy and the US Adult Smoking Rate, 2011‐2013. Journal of Public Health Management and Practice: JPHMP. 2016;22(5):E29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang T, Wu Y, Abdullah AS, Dai D, Li F, Wu J, et al. Attitudes and behavioral response toward key tobacco control measures from the FCTC among Chinese urban residents. BMC public health. 2007;7:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farsalinos K. Electronic cigarettes: an aid in smoking cessation, or a new health hazard? Therapeutic Advances in Respiratory Disease. 2018;12:1753465817744960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, Lu W, Liao S, Deng Z, Zhang Z, Liu Y, et al. Efficiency and adverse events of electronic cigarettes: A systematic review and meta‐analysis (PRISMA‐compliant article). Medicine (Baltimore). 2018;97(19):e0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. E‐cigarettes and smoking cessation . Similar efficacy to other nicotine delivery devices, but many uncertainties. Prescrire Int. 2015;24(165):271–6. [PubMed] [Google Scholar]
- 30. Zhu J, Shi F, Xu G, Li N, Li J, He Y, et al. Conventional Cigarette and E‐Cigarette Smoking among School Personnel in Shanghai. China: Prevalence and Determinants. International Journal of Environmental Research and Public Health. 2019;16(17):3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang X, Zhang X, Xu X, Gao Y. Perceptions and use of electronic cigarettes among young adults in China. Tobacco Induced Diseases. 2019;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang W, He Z, Feng N, Cai Y. Electronic cigarette use in China: Awareness, prevalence and regulation. Tobacco Induced Diseases. 2019;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco. 2015;17(10):1195–202. [DOI] [PubMed] [Google Scholar]
- 34. Bao W, Xu G, Lu J, Snetselaar LG, Wallace RB. Changes in Electronic Cigarette Use Among Adults in the United States, 2014‐2016. JAMA. 2018;319(19):2039–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang N, Cleland CM, Wang MP, Kwong A, Lai V, Lam TH. Perceptions and use of e‐cigarettes among young adults in Hong Kong. BMC Public Health. 2019;19(1):1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jha P. Smoking cessation and e‐cigarettes in China and India. BMJ. 2019;367:l6016. [DOI] [PubMed] [Google Scholar]
- 37. Cohen AK, Syme SL. Education: a missed opportunity for public health intervention. Am J Public Health. 2013;103(6):997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets generated and/or analyzed in the current study are available in the GBD repository, http://ghdx.healthdata.org/gbd-results-tool.


