Supplemental Digital Content is available in the text
Keywords: 8 edition tumor-node-metastasis staging systems, American joint committee on cancer, functional pancreatic neuroendocrine neoplasms, grading, non-functional
Abstract
Histologically, the World Health Organization has classified pancreatic neuroendocrine neoplasms (p-NENs) into well-differentiated pancreatic neuroendocrine tumors (G1/G2 p-NETs) and poorly-differentiated pancreatic neuroendocrine carcinoma (G3 p-NECs) based on tumor mitotic counts and Ki-67 index. Recently, the 8th edition of American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging manual has incorporated some major changes in 2017 that the TNM staging system for p-NENs should only be applied to well-differentiated G1/G2 p-NETs, while poorly-differentiated G3 p-NECs be classified according to the new system for pancreatic exocrine adenocarcinomas. However, this new manual for p-NENs has seldom been evaluated.
Data of patients with both G1/G2 and G3 non-functional p-NENs (NF-p-NENs) from our institution was retrospectively collected and analyzed using 2 new AJCC 8th staging systems. We also made survival comparisons between the 8th and 7th edition system separately for different subgroups.
For G1/G2 NF-p-NETs, there were 52 patients classified in AJCC 8th edition stage I, 40 in stage II, 41 in stage III and 19 in stage IV. As for G3 NF-p-NECs, 17, 19, 24, and 18 patients were respectively defined from AJCC 8th edition stage I to stage IV. In terms of the AJCC 7th staging system, the 230 patients with NF-p-NENs were totally distributed from stage I to stage IV (94, 63, 36, 37, respectively). For the survival analysis of both G1/G2 NF-p-NETs and G3 NF-p-NECs, the AJCC 7th edition system failed to discriminate the survival differences when compared stage III with stage II or stage IV (P > .05), while the 8th edition ones could perfectly allocate patients into 4 statistically different groups (P < .05). The HCIs of AJCC 8th stage for G1/G2 NF-p-NETs [HCI=0.658, 95% confidence interval (CI)=0.602–0.741] and stage for G3 NF-p-NECs (HCI=0.704, 95% CI=0.595–0.813) was both statistically larger than those of AJCC 7th stage for different grading NF-p-NENs [(HCI=0.578, 95% CI=0.557–0.649; P=.031), (HCI=0.546, 95% CI=0.531–0.636; P = .019); respectively], indicating a more accurate predictive ability for the survivals of NF-p-NENs.
Our data suggested the 2 new AJCC 8th staging systems were superior to its 7th edition for patients with both G1/G2 NF-p-NETs and G3 NF-p-NECs.
1. Introduction
Pancreatic neuroendocrine neoplasms (p-NENs) are an interesting, diverse, uncommon, and heterogenous group of tumors with a varied behavior, course, prognosis, as well as increasing prevalence.[1–3] P-NENs mainly consist of functional tumors with distinctive manifestations related to hormone overproduction (F-p-NENs) and non-functional ones (NF-p-NENs).[1–3] Accounting for nearly 60% to 90% of all p-NENs, the annual incidence rate of NF-p-NENs has been increasing from 1.4 to 3.0 new cases per million from 1973 to 2004.[4,5] NF-PNENs may not produce hormones or peptides, produce them at low levels and without hormone-related symptoms, or secrete peptides that cause no symptoms.[6,7] As the most common subgroup of p-NENs, NF-p-NENs mostly occurred in the 4th or 5th decade of life and generally diagnosed at more advanced stages on admission, because of their relatively indolent nature and slow growth causing a delay in onset of symptoms, such as abdominal pain, abdominal mass, weight loss, jaundice, and others.[8,9] There is an increasing number of incidental diagnoses of NF-p-NENs, with the widespread use of high-quality imaging techniques.[2,3,8,9] Although over 60% of NF-p-NENs are malignant when first diagnosed, NF-p-NENs often present much better survival than pancreatic exocrine adenocarcinomas (p-EACs).[2,5,8–10]
Due to the rarity and heterogeneity, the ability to define patients with p-NENs into prognostic groups has always been challenging. The classification and staging systems for p-NENs are mainly proposed by the European Neuroendocrine Tumor Society (ENETS) in 2006,[11] the World Health Organization (WHO) in 2010,[12] and the American Joint Committee on Cancer (AJCC) in 2010,[13] which have all been validated for important prognostic value for the survival of p-NENs, with their own scopes and features of applications.[14–18] ENETS is the first to propose a 4-stage tumor-nodes-metastasis (TNM) classification for gastrointestinal and pancreatic NETs which has been widely used in clinic, especially in European countries.[11,15] The WHO 2010 grading system is based on tumor mitotic counts and Ki-67 index which classified p-NENs into well-differentiated pancreatic neuroendocrine tumors (G1/G2 p-NETs) and poorly-differentiated pancreatic neuroendocrine carcinoma (G3 p-NECs).[12] The AJCC 2010 staging system for p-NENs is originally applied to p-EACs which discriminated between localized tumors (stage I), locally advanced but resectable tumors (stage II), locally advanced and unresectable tumors (stage III), and distantly metastasized tumors (stage IV).[13]
Recently in 2017, AJCC updates its 8th staging manual for p-EACs, as well as its first formal application for p-NENs.[19] However, some major changes are proposed in the new AJCC manual. For example, the specific TNM staging system for p-NENs should only be applied to well-differentiated p-NETs (G1/G2), which adopts the system by ENETS in 2006.[11] On the other hand, high-grade p-NECs (G3) should be classified according to the new system for p-EACs (Table 1). The clinical significance of AJCC 8th staging manual for p-NENs has seldom been validated.[20,21] Whether those changes could significantly improve the prognostic ability or accuracy for the survivals of p-NENs is still unclear.
Table 1.
Definitions of American Joint Committee on Cancer 7th edition and 8th edition tumor-node-metastasis staging systems for pancreatic neuroendocrine neoplasms and analysis for non-functional-pancreatic neuroendocrine neoplasms in the present study.
| AJCC 7th edition staging system for p-EACsA(N = 152 +78) C | AJCC 8th edition staging system for G1/G2 p-NETsB(N = 152) | AJCC 8th edition staging system for G3 p-NECsB(N = 78) | |
| T/N/M staging definitions | |||
| T1 | Tumor limited to the pancreas, < 2 cm in greatest diameter; | Tumors limited to pancreas, 2 cm or less in greatest dimension; | T tumor 2 cm or less in greatest dimension; |
| T2 | Tumor limited to the pancreas, > 2 cm in greatest diameter; | Tumors limited to pancreas more than 2 cm but less than 4 cm in greatest dimension; | Tumor more than 2 cm but no more than 4 cm in greatest dimension; |
| T3 | Tumor extends beyond the pancreas, but not involving the celiac axis or superior mesenteric artery; | Tumors limited to pancreas, more than 4 cm in greatest dimension or tumors invading duodenum or bile duct; | Tumor more than 4 cm in greatest dimension; |
| T4 | Tumor involves the celiac axis or superior mesenteric artery (unresectable tumor). | Tumors perforates visceral peritoneum (serosa) or invades other organs or adjacent structures. | Tumor involves coeliac axis, superior mesenteric artery and/or common hepatic artery. |
| N0 | No regional lymph node metastasis; | No regional lymph node metastasis; | No regional lymph node metastasis; |
| N1 | Regional lymph node metastasis; | Regional lymph node metastasis. | Metastases in 1 to 3 regional lymph nodes; |
| N2 | NA. | NA. | Metastases in 4 or more regional lymph nodes. |
| M0 | No distant metastasis; | No distant metastasis; | No distant metastasis; |
| M1 | Distant metastasis. | Distant metastasis. | Distant metastasis. |
| Clinical staging definitions--- (Cases) | |||
| Stage I | T1 N0 M0 (A) --- (52) D / (10) E;T2 N0 M0 (B) --- (19) / (13); | T1 N0 M0--- (52) D; | T1 N0 M0 (A) --- (10) E;T2 N0 M0(B) --- (7); |
| Stage II | T3 N0 M0 (A) --- (25) / (9);T1-3 N1 M0(B) --- (17) / (12); | T2 N0 M0(A) --- (12);T3 N0 M0(B) --- (28); | T3 N0 M0(A) --- (11);Any T N1 M0(B)--- (8); |
| Stage III | T4 Any N M0--- (20) / (16); | T4 N0 M0(A) --- (15);Any T N1 M0(B) --- (26); | Any T N2 M0--- (8);T4 Any N M0--- (16); |
| Stage IV | Any T Any N M1--- (19) / (18). | Any T Any N M1 --- (19). | Any T Any N M1--- (18). |
| Cross-tabulation of 3 AJCC TNM staging systems--- (Cases) | |||||
| For NF-p-NENs by AJCC 7th system | |||||
| Stage I | Stage II | Stage III | Stage IV | Total | |
| For G1/G2 NF-p-NETs by AJCC 8th system | |||||
| Stage I | 52 | 0 | 0 | 0 | 52 |
| Stage II | 19 | 21 | 0 | 0 | 40 |
| Stage III | 0 | 21 | 20 | 0 | 41 |
| Stage IV | 0 | 0 | 0 | 19 | 19 |
| Total | 71 | 42 | 20 | 19 | 152 |
| For G3 NF-p-NECs by AJCC 8th system | |||||
| Stage I | 17 | 0 | 0 | 0 | 17 |
| Stage II | 6 | 13 | 0 | 0 | 19 |
| Stage III | 0 | 8 | 16 | 0 | 24 |
| Stage IV | 0 | 0 | 0 | 18 | 18 |
| Total | 23 | 21 | 16 | 18 | 78 |
Our previous work has preliminarily evaluated the applications of AJCC 8th edition TNM staging systems respectively for high-grade p-NECs (G3)[20] and well-differentiated p-NETs (G1/G2).[21] However, due to the obvious heterogeneity of p-NENs consisting of 2 main functional and non-functional subgroups, those studying results needed to be further validated. In the present study, undertaken at a large single specialist center in China, we described the clinical features of NF-p-NENs with different grading subgroups. Based on our previous efforts,[20,21] we emphasized to comprehensively analyze the distribution characteristics and survival differences between each AJCC new stage for the outcome of patients with both G1/G2 NF-p-NETs and G3 NF-p-NECs. Additionally, we compared the 7th and 8th edition of the AJCC TNM staging systems in overall prognostic accuracy for the survivals of patients with NF-p-NENs who underwent an operation. We limited our study to NF-p-NENs to reduce the confounding effects caused by the heterogeneity of p-NENs, as they accounted for the largest portion of p-NENs and best represent the biological behaviors of this disease.
2. Materials and methods
2.1. Patient enrollments
Patients who were histopathologically diagnosed as p-NENs after an operation from January 2002 to December 2017 were retrospectively identified from West China Hospital, Sichuan University, after which we excluded those with F-p-NENs, such as insulinomas and gastrinomas. Patients with only clinical suspicion but without pathological confirmation of NF-p-NENs were not enrolled as well. Patients with hereditary syndromes, including multiple endocrine neoplasia type I, von Hippel-Lindau syndrome, were also excluded. For enrolled patients, demographic, clinical, operative, and pathological data were systematically compiled from their electronic or paper-based medical records. Our research was approved by the Institutional Review Board of West China Hospital, Sichuan University. Written informed consent was obtained on admission from all included patients, in accordance with the general principles of the Helsinki Declaration.[22]
2.2. Tumor features
Tumors that were histopathologically diagnosed as G1/G2/G3 p-NENs without recognizable and typical syndromes related to hormone overproduction were clinically defined as NF-p-NENs. As WHO defined in 2010,[12] G1 p-NETs have a mitotic count of less than 2 per 10 high-power fields (HPFs) and a Ki-67 index less than or equal to 2%; G2 p-NETs have mitotic counts of 2 to 20 per 10 HPFs and a Ki-67 of 3% to 20%; G3 p-NECs is characterized by mitotic counts greater than 20 per 10 HPFs and a Ki-67 greater than 20%. Local lymph node, adjacent, and distant organ were all routinely explored at surgery to exclude the potential local invasion or distant metastasis. Radical resection for both the local and metastatic lesion meant negative surgical margins, both grossly and microscopically. Tumors were retrospectively recorded and grouped according to the prescribed AJCC 7th and 8th edition classifications of their TNM staging systems (Table 1), based on pathologic tumor size, number of positive lymph nodes, distant lesion diagnosis.[13,19] Tumors with undefined TNM stages (because of missing values with respect to tumor clinical-pathological features or patients follow-up data) were also excluded from the final analysis. Finally, we designed our present study as the consort diagram showed [Supplemental Digital Content (Fig. S1)].
2.3. Statistical analysis
Data were presented as median for quantitative variables, or as numbers and their frequencies as proportions for categorical variables, which were compared by Student t tests (or analysis of variance) and Chi-squared test (or Fisher exact test) according to variable distribution wherever possible. Follow-up was mainly conducted by telephone, e-mail, mail, or outpatient clinic review between January and June of 2018. The primary outcome was overall survival (OS), which was calculated either as the time in months between the date of surgery and the date of death or last follow-up, and presented as either median survival time (MST) or 5-year survival rate with a hazard ratio (HR) and 95% confidence interval (CI). Kaplan–Meier curves depicting OS were computed and compared with the log-rank test being used to verify significance in the survival differences of AJCC 7th and 8th staging systems. Multivariate analysis was performed by Cox proportional hazards model to adjust for pathological variables, which were known to be associated with prognosis. Weighted Cohen's κ coefficient was computed to evaluate the inter-rater agreements of AJCC 7th or 8th staging systems for different grading subgroups of NF-p-NENs. To validate and compare the prognostic accuracy for OS of NF-p-NENs, a Harrell's C-index (HCI) was calculated and compared (R software version 3.2.4) for each staging system of AJCC 7th and 8th manual. A larger HCI value indicated a better model for predicting outcome.[23,24] Statistical analyses were carried out using IBM SPSS 22.0 statistical software. A P value of less than .05 was considered statistically significant and all tests were 2-sided.
3. Results
3.1. Patients demographics and tumor features
According to the inclusion criteria above, we finally enrolled 230 consecutive patients with NF-p-NENs, including 152 patients with G1/G2 NF-p-NETs, and 78 ones with G3 NF-p-NECs (Table 2). Our study consists of 118 males and 112 females, with a median age of 53.4 years (Ranging from 14.0–86.7). Abdominal pain was the most common clinical manifestation of NF-p-NENs (55.2%), while 28.7% patients was diagnosed incidentally on admission. Tumors were located almost equally in pancreas, with median size of 3.5 cm (Ranging from 0.5–7.5). Seventy-six patients were pathologically confirmed to have local lymph invasion, while 37 ones were detected to present distant metastasis. Ultrasound guided fine needle aspiration was performed only for 34 patients preoperatively, who were all diagnosed again as NF-p-NENs through an operation. One hundred ninety patients obtained radical resection, with both grossly and microscopically negative surgical margins, and distal pancreatectomy was the most common surgical procedure (37.0%). There were 68 patients who received regularly postoperatively medical therapy (chemotherapy, radiotherapy, and molecular targeted therapy, alone, or with their combinations). One hundred eighty-three patients were kept in touch with at last follow-up, in which 87 ones were dead. For different grading subgroups of NF-p-NENs, patients with G1/G2 NF-p-NETs was notably younger than those with G3 NF-p-NECs (P = .018), whose tumor size was also statistically smaller (P = .041). Other comparisons between G1/G2 NF-p-NETs and G3 NF-p-NECs, such as patients’ gender and symptoms, tumor location, local or distant metastasis, surgical procedures, and medical therapy were not significant (P > .05).
Table 2.
The baseline demographics and tumor features of non-functional pancreatic neuroendocrine neoplasms in the present study.
| Patients, No. (%) | ||||
| Factor | G1/G2 NF-p-NETs(N = 152) | G3 NF-p-NECs(N = 78) | NF-p-NENs(N = 230) | P valueA |
| Patients gender | .436 | |||
| Male | 70 (46.1) | 48 (61.5) | 118 (51.3) | |
| Female | 82 (53.9) | 30 (38.5) | 112 (48.7) | |
| Age at diagnosis, yr | .018 | |||
| Median | 49.0 | 61.2 | 53.4 | |
| Range | 14.0–78.3 | 17.4–86.7 | 14.0–86.7 | |
| Clinical symptoms | .685 | |||
| Abdominal pain | 70 (46.1) | 50 (64.1) | 120 (52.2) | |
| Abdominal mass | 61 (40.1) | 42 (53.8) | 103 (44.8) | |
| Jaundice | 26 (17.1) | 38 (48.7) | 64 (27.8) | |
| Bleeding | 8 (5.3) | 14 (17.9) | 22 (9.6) | |
| Incidental diagnosis | 38 (24.0) | 28 (35.9) | 66 (28.7) | .112 |
| Tumor location | .097 | |||
| Head/uncinate | 76 (50.0) | 50 (64.1) | 126 (54.8) | |
| Body/tail | 76 (50.0) | 28 (35.9) | 104 (45.2) | |
| Tumor size | .041 | |||
| Median, cm | 2.5 | 4.0 | 3.5 | |
| Range, cm | 0.5–4.2 | 1.5–7.5 | 0.5–7.5 | |
| <2cm | 62 (40.8) | 20 (25.6) | 82 (35.6) | |
| 2cm≤ and <4cm | 38 (25.0) | 27 (34.6) | 65 (28.3) | |
| ≥4cm | 52 (34.2) | 31 (39.8) | 83 (36.1) | |
| Local lymph metastases | .136 | |||
| No | 114 (75.0) | 40 (51.3) | 154 (66.9) | |
| Yes, No.≤3 | 18 (11.8) | 12 (15.4) | 30 (13.1) | |
| Yes, No.>3 | 20 (13.2) | 26 (33.3) | 46 (20.0) | |
| Distant metastasis | 19 (12.5) | 18 (23.1) | 37 (16.1) | .323 |
| US-guided-FNA | 20 (13.2) | 14 (17.9) | 34 (14.8) | .962 |
| Surgical procedure | .158 | |||
| LRP | 38 (25.0) | 15 (19.2) | 53 (23.0) | |
| DP | 62 (40.8) | 23 (29.5) | 85 (37.0) | |
| PD | 42 (27.6) | 24 (30.8) | 66 (28.7) | |
| BPB | 10 (6.7) | 16 (20.5) | 26 (11.3) | |
| Radical resectionC | 136 (89.5) | 54 (69.2) | 190 (82.6) | .085 |
| Medical therapyD | 48 (31.6) | 20 (25.6) | 68 (29.6) | .159 |
| Patient out of contact | 39 (25.7) | 8 (10.3) | 47 (20.4) | .284 |
| Dead at follow-up | 50 (32.9) | 37 (47.4) | 87 (37.8) | .089 |
3.2. AJCC stage distributions for G1/G2 NF-p-NETs
As Table 1 described, on the basis of the definitions of different TNM staging systems, 52 patients with G1/G2 NF-p-NETs were grouped in AJCC 8th edition stage I, 40 in stage II, 41 in stage III and 19 in stage IV. As for the AJCC 7th staging system for the same patients, there were respectively 71, 42, 20, and 19 ones classified from stage I to stage IV. According to the cross-tabulation of both staging systems, patients with G1/G2 NF-p-NETs defined as AJCC 8th edition stage II (n = 40) were respectively distributed in stage I (n = 19) and stage II (n = 21) by AJCC 7th staging system, while patients in AJCC 8th edition stage III (n = 41) were grouped in AJCC 7th edition stage II (n = 21) and stage III (n = 20), respectively. Patients in AJCC 8th edition stage I (n = 52) or stage IV (n = 19) were similarly classified by AJCC 7th staging system. The Weighted Cohen's κ coefficient of AJCC 7th and 8th staging systems for G1/G2 NF-p-NETs was 0.713 (95% CI = 0.561–0.824, P = .027), indicating a roughly agreement and moderate discrepancy.
3.3. AJCC stage distributions for G3 NF-p-NECs
Also in Table 1, in view of the criteria of AJCC 8th TNM staging system for G3 NF-p-NECs, there were 17 patients defined in stage I, 19 in stage II, 24 in stage III and 18 in stage IV. With regard to the AJCC 7th TNM system for the same objectives, a total of 23, 21, 16, and 18 patients were respectively defined from stage I to stage IV. Referring to the cross-tabulation of both staging systems, patients with G3 NF-p-NECs defined as AJCC 8th edition stage II (n = 19) were respectively distributed in stage I (n = 6) and stage II (n = 13) by AJCC 7th staging system, while patients in AJCC 8th edition stage III (n = 24) were grouped in AJCC 7th edition stage II (n = 8) and stage III (n = 16), respectively. Patients in AJCC 8th in stage I (n = 17) or stage IV (n = 18) were similarly classified by AJCC 7th staging system. The Weighted Cohen's κ coefficient of AJCC 7th and 8th staging systems for G3 NF-p-NECs was 0.751 (95% CI = 0.497–0.859, P = .018), also indicating a roughly agreement and moderate discrepancy.
3.4. Survival analysis of G1/G2 NF-p-NETs by AJCC stages
The median follow-up time of our study was 62.6 months, ranging from 6.1 to 187.2 months. When the follow-up ended in June 2018, there were 47 patients out of contact (20.4%), including 39 patients with G1/G2 NF-p-NETs (25.7%) and 8 ones with G3 NF-p-NECs (10.3%), which were all censored in the final survival analysis model. There were 87 deaths (37.8%), including 50 patients with G1/G2 NF-p-NETs (32.9%) and 37 ones with G3 NF-p-NECs (47.4%). For the whole group patients with NF-p-NENs, the calculated 5-year accumulated OS was 53.4%, with a MST of 68.4 months [95%CI = 54.3–82.5 months; Supplemental Digital Content (Fig. S2)]. For NF-p-NENs with G1, G2 and G3 subgroups, the calculated OS at 5 years was statistically different 66.3%, 55.3%, and 31.9%, with a MST of NA (not applicable), 68.4 (95%CI = 57.1–79.7 months) and 42.2 months (95%CI = 23.9–60.4 months), respectively [P < .05; Supplemental Digital Content (Fig. S3)]. For G3 NF-p-NECs with morphologically well- and poorly-differentiated subgroups, the calculated 5-year OS was 58.0% and 27.9%, with a MST of NA and 32.1 months (95%CI = 21.8–42.4 months), respectively [P = .018; Fig. Supplemental Digital Content (Fig. S4)].
For patients with G1/G2 NF-p-NETs by AJCC 7th TNM staging system, there were 17, 15, 10, and 8 deaths from stage I to stage IV, with a calculated 5-year OS of 79.7% (MST = 93.2 months, 95%CI = 68.6–117.7 months), 61.6% (MST = 72.9 months, 95%CI = 59.7–82.1 months), 39.3% (MST = 43.1 months, 95%CI = 38.2–47.9 months) and 19.2% (MST = 36.4 months, 95%CI = 20.1–52.7 months), respectively. Specifically, survivals of patients in AJCC 7th stage I were notably better than those in stage II (P = .016), or stage III (P < .001), or stage IV (P < .001), as well as those in stage II compared with stage IV (P = .001), while comparisons between stage III and stage II or stage IV were not significant (P = .111, P = .133, respectively; Fig. 1A). According to the AJCC 8th staging system for G1/G2 NF-p-NETs, there were respectively 8 dead patients for stage I, 15 for stage II, 19 for stage III and 8 for stage IV, with a calculated 5-year OS of 81.9% (MST = 104.8 months, 95%CI = 89.5–120.1 months), 76.9% (MST = 72.9 months, 95%CI = 72.4–81.3 months), 34.9% (MST = 55.5 months, 95%CI = 34.5–76.4 months) and 19.2% (MST = 36.4 months, 95%CI = 20.1–52.7 months). Survival comparisons between AJCC 8th stage I and stage II (P = .017), or stage III (P < .001), or stage IV (P < .001), between stage II and stage III (P = .008), or stage IV (P < .001), between stage III and stage IV (P = .044) were all statistically significant (Fig. 1B).
Figure 1.
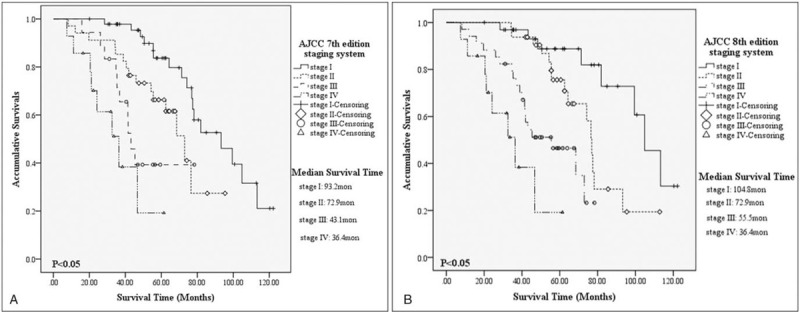
Kaplan–Meier estimates for survival outcomes of G1/G2 non-functional pancreatic neuroendocrine tumors, according to the American Joint Committee on Cancer 7th edition staging system (A) and the 8th edition one (B).
3.5. Survival analysis of G3 NF-p-NECs by AJCC stages
For patients with G3 NF-p-NECs by AJCC 7th staging classification, there were respectively 7, 8, 10, and 12 deaths from stage I to stage IV, with a calculated 5-year OS of 57.2% (MST=NA; 95%CI=NA), 44.2% (MST=41.7 months, 95%CI=18.1–65.2 months), NA (MST=26.9 months, 95%CI= 17.3–36.4 months) and NA (MST=17.1 months, 95%CI=7.6–26.5 months). Patients in AJCC 7th stage I present a notably longer survival than those in stage II (P=.021), or stage III (P < .001), or stage IV (P < .001), as well as those in stage II compared with stage IV (P=.001), while survival comparisons between stage III and stage II or stage IV were not significant (P=.079, P = .126, respectively; Fig. 2A). On the other hand, there were respectively 4 dead patients for AJCC 8th stage I, 8 for stage II, 13 for stage III and 12 for stage IV, with a calculated 5-year OS of 66.6% (MST = NA, 95%CI = NA), 34.6% (MST = 57.3 months, 95%CI = 29.5–85.0 months), NA (MST = 30.8 months, 95%CI = 34.4–37.2 months) and NA (MST = 17.1 months, 95%CI = 7.6–26.5 months). Survival comparisons between AJCC 8th stage I and stage II (P = .035), or stage III (P < .001), or stage IV (P < .001), between stage II and stage III (P = .044), or stage IV (P < .001), between stage III and stage IV (P = .027) were all statistically significant as well (Fig. 2B).
Figure 2.
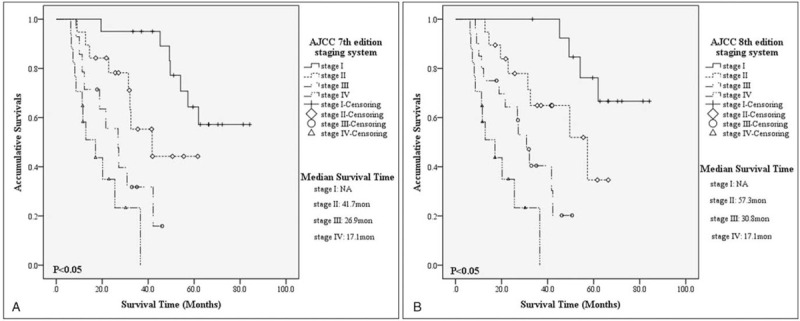
Kaplan–Meier estimates for survival outcomes of G3 non-functional-pancreatic neuroendocrine carcinomas, according to the American Joint Committee on Cancer 7th edition staging system (A) and the 8th edition one (B).
3.6. Prognostic value of AJCC stages for NF-p-NENs
With variables such as patients gender, age, clinical symptom, tumor location, grading, stage, and surgical procedure in multivariate analysis separately performed by Cox proportional hazards model, AJCC 7th stage for NF-p-NENs (P < .001; with stage I as the reference: stage II HR of death=1.53, 95% CI = 1.29–3.07, P = .116; stage III HR of death=2.92, 95% CI = 2.75–7.46, P = .352; stage IV HR of death=5.14, 95% CI = 4.13–10.13, P = .024), AJCC 8th stage for G1/G2 NF-p-NETs (P < .001; with stage I as the reference: stage II HR of death=1.46, 95% CI=1.12–2.64, P=.045; stage III HR of death=2.97, 95% CI=2.19–6.46, P=.023; stage IV HR of death=5.03, 95% CI=3.53–9.02, P < .001), AJCC 8th stage for G3 NF-p-NECs (P < .001; with stage I as the reference: stage II HR of death=1.53, 95% CI=1.29–3.07, P = .116; stage III HR of death=2.92, 95% CI = 2.75–7.46, P = .352; stage IV HR of death=5.14, 95% CI = 4.13–10.13, P = .024) were all demonstrated to be independent predictors of survival (Table 3). Finally, we separately performed analysis of concordance index for different models, the value of AJCC 8th stage for G1/G2 NF-p-NETs (HCI=0.658, 95% CI=0.602–0.741) and stage for G3 NF-p-NECs (HCI=0.704, 95% CI=0.595–0.813) was both statistically larger than those of AJCC 7th stage for NF-p-NENs [(HCI=0.578, 95% CI=0.557–0.649; P = .031), (HCI=0.546, 95% CI=0.531–0.636; P = .019); respectively], meant they were more informative about prognostic accuracy for patients with different grading NF-p-NENs.
Table 3.
Multivariate analysis of prognostic factors for non-functional pancreatic neuroendocrine neoplasms.
| AJCC 7th stage for all NF-p-NENsA | AJCC 8th stage for G1/G2 NF-p-NETsB | AJCC 8th stage for G3 NF-p-NECsC | ||||
| Variable | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Patients gender | ||||||
| Female | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| Male | 1.29 (1.18–1.92) | .313 | 1.08 (0.98–1.52) | .147 | 1.12 (0.89–1.22) | .198 |
| Age at diagnosis | ||||||
| <Median | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| ≥Median | 1.75 (1.13–2.12) | .289 | 1.54 (1.09–1.95) | .147 | 1.42 (0.99–1.82) | .183 |
| Diagnosis on admission | ||||||
| Incidental | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| Symptomatic | 1.32 (0.78–2.02) | .267 | 1.65 (1.13–1.89) | .235 | 1.44 (0.99–1.97) | .331 |
| Tumor location | ||||||
| Head/uncinate | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| Body/tail | 1.82 (1.48–3.24) | .158 | 1.79 (1.25–2.48) | .246 | 2.04 (0.57–2.97) | .098 |
| Surgical procedure | .146 | .353 | .278 | |||
| Local resection | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | |||
| Distal pancreatectomy | 5.97 (3.29–12.04) | .041 | 5.54 (3.25–11.78) | .032 | 6.09 (3.47–13.65) | .015 |
| Pancreaticoduodenectomy | 7.83 (4.18–13.64) | .015 | 7.25 (3.11–10.86) | .025 | 8.12 (5.51–15.26) | .007 |
| Biopsy | 16.24 (6.36–33.07) | <.001 | 18.83 (7.25–38.36) | <.001 | 18.15 (7.07–34.25) | <.001 |
| WHO 2010 grading classification | <.001 | .039 | ||||
| G1 | 1.0 (referent) | 1.0 (referent) | NA | |||
| G2 | 3.97 (3.02–7.64) | .048 | 4.14 (3.22–8.15) | .039 | ||
| G3 | 18.52 (13.74–41.35) | <.001 | NA | |||
| AJCC 7th stage for all NF-p-NENs | <.001 | |||||
| I | 1.0 (referent) | NA | NA | |||
| II | 1.53 (1.29–3.07) | .116 | ||||
| III | 2.92 (2.75–7.46) | .352 | ||||
| IV | 5.14 (4.13–1.13) | .024 | ||||
| AJCC 8th stage for G1/G2 NF-p-NETs | <.001 | |||||
| I | NA | 1.0 (referent) | NA | |||
| II | 1.46 (1.12–2.64) | .045 | ||||
| III | 2.97 (2.19–6.46) | .023 | ||||
| IV | 5.03 (3.53–9.02) | <.001 | ||||
| AJCC 8th stage for G3 NF-p-NECs | <.001 | |||||
| I | NA | NA | 1.0 (referent) | |||
| II | 1.57 (1.01–2.52) | .038 | ||||
| III | 2.63 (2.05–6.48) | .017 | ||||
| IV | 5.12 (3.36–8.45) | <.001 | ||||
4. Discussion
Although accounting for the majority of all p-NENs and with an increasing prevalence, NF-p-NENs are still very uncommon.[4,5] Unlike F-p-NENs with a wide range of typical clinical presentations depending on which hormone is hypersecreted, NF-p-NENs manifest nonspecific symptoms (such as abdominal pain, weight loss, jaundice, etc), which are often caused by tumor invasion or encroachment or displacement of contiguous structures, leading to an advanced stage and elderly onset of illness on admission.[4–9,25] Nowadays, more and more asymptomatic NF-p-NENs are detected by cross-sectional imaging or endoscopic studies as incidental findings.[9,23] Patients with NF-p-NENs could benefit from radical resections, depending on tumor size and location within the pancreas.[7–9,25,26] But recent studies have focused on the controversy for small NF-p-NENs, for many favor 2 cm as the cutoff size to surgery or observation with the considerations of clinical symptoms on admission and tumor grade mainly acquired by ultrasound guided fine needle aspiration.[7,9,25]
As we all know, TNM staging system is a simple and effective instrument for reliable prediction of survival estimates and patient stratification, which helps clinicians in guiding treatment decisions, provides researchers with a tool to adjust for cancer stage in evaluating treatment outcomes, and is informative to patients.[15,27,28] Since 1977, as the most authoritative international organization, the AJCC has established well-defined staging guidelines for solid tumors based on local tumor extent (T stage), dissemination to the regional lymph nodes (N stage), and metastatic spread to distant sites (M stage), which attempts to use anatomical and reproducible parameters to discriminate groups with different survival outcomes.[13,19] It was not until 2010 that AJCC staging guideline (i.e., 7th edition) started to introduce a classification for p-NENs, which derived from the staging algorithm for p-EACs.[13] However, use of a common staging system for both p-EACs and p-NENs might be oversimplified.[15,18,29,30] Meanwhile, the AJCC 7th staging manual for p-NENs recommended that tumor grade be recorded but did not include specific guidelines for grade assignment.[13,14] Afterwards, accumulated studies have demonstrated well-differentiated p-NETs (i.e., G1/G2) present notably different clinical features, histological behaviors and survival outcomes compared with high-grade p-NECs (i.e., G3), which should be re-recognized, re-staged and treated differently.[16,31–33]
Recently in 2017, AJCC incorporated some major changes in its 8th TNM staging manual for both p-EACs and p-NENs.[19] In the new AJCC manual, the WHO 2010 grading scheme has been uniformly proposed for all p-NENs. Most importantly, patients with different grading p-NENs should be staged by different system. The new AJCC system for p-NENs should only be applied to G1/G2 p-NETs, which adopted the criteria of ENETS 2006 staging system for p-NENs, while G3 p-NECs be classified according to the new AJCC staging system for p-EACs, which also made several changes (Table 1). Compared with the AJCC 7th staging manual for p-EACs, instead of being representative of “limited to” or “extends beyond” pancreas, the AJCC 8th T2 and T3 tumors were now defined as those with a maximum tumor diameter of >2, ≤4, and >4 cm. Moreover, N stage has been divided from a binary to a tripartite classification in the light of the number of positive regional lymph nodes. Thirdly, besides tumors with T4, any N, and M0, those with any T, N2, and M0 are also defined as Stage III.[19,34] These updates represented an important step toward adopting uniform and exclusive p-NENs staging systems which might be clinically applied with widespread acceptance.
Several studies have previously validated the 8th edition AJCC staging system for p-EACs.[35–37] However, for p-NENs, whether those changes in AJCC 8th manual could provide more reliable predictions of survival assessment and patient stratification, and better guide our clinical practice is still unclear. Our previous work has preliminarily evaluated the applications of AJCC 8th staging manual respectively for high-grade p-NECs (G3)[20] and well-differentiated p-NETs (G1/G2).[21] However, both studies enrolled a large portion of functional p-NENs (40.4% and 54.7%, respectively), especially insulinoma (28.8% and 47.6%, respectively), which inevitably increase the heterogeneity of p-NENs and influence the accuracy of related analysis. Moreover, we have demonstrated in our previous research that patients with functional and non-functional p-NENs should be staged according to different TNM staging system because of their varied biological behavior, clinical course and long-term outcome.[30] Therefore, in this study, to reduce the confounding effects caused by the heterogeneity of p-NENs, we restricted our eligible studying objects to NF-p-NENs as they accounted for the largest portion of p-NENs and best represent the biological behaviors of this disease. Based on our previous effort,[20,21] we emphasized to analyze and compare the distribution characteristics and survival differences between AJCC 7th and 8th staging systems for outcomes of both G1/G2 NF-p-NETs and G3 NF-p-NECs. According to our analysis, for patients with G1/G2 NF-p-NETs and those with G3 NF-p-NECs, the AJCC 7th edition staging system failed to discriminate the survival differences when compared its stage III with stage II or stage IV [(P = .111, P = .133; Fig. 1B), (P = .079, P = .126; Fig. 2B); respectively], while the 8th edition ones could perfectly allocate patients into 4 statistically significantly different groups (P < .05; Fig. 1A; Fig. 2A). The statistically larger HCIs of AJCC 8th staging systems for G1/G2 NF-p-NETs and G3 NF-p-NECs than those of 7th edition system for NF-p-NENs have also indicated that the novel AJCC systems were more informative about prognostic accuracy for the survival outcomes of patients with different grading NF-p-NENs. As we mentioned above, patients with G3 p-NECs should be treated differently from those with G1/G2 p-NETs.[16,31–33] The inclusion of p-NENs in the novel AJCC 8th staging manual represented an important step toward a uniform p-NENs nomenclature with the purpose of potentially widespread acceptance, suggesting that each grade of tumor should be grouped differently. Our present results also demonstrated the major update in AJCC 8th staging manual for NF-p-NENs were of great value for the survival assessments and patients’ stratifications which would better guide our clinical practices.
Our research had several limitations. Firstly, it was a retrospective nature and single-center study, which might imply some degree of variation in collecting relevant data, such as the surgical techniques and lymph node samplings by surgeons, the interpretations of Ki-67 staining of cancer cells and morphological analysis of p-NENs by pathologists, the variabilities in postoperative treatments and the limited survival data. Moreover, we restrict our objects to NF-p-NENs to reduce the heterogeneity of p-NENs. But as a subgroup of NF-p-NENs, small number of patients with G3 NF-p-NECs (only 78 cases) were staged by the new AJCC system, which would influence the statistical analysis. Any prospectively designed and multi-center study with large volumes was still needed to confirm our demonstrations. Thirdly, according to the WHO grading criteria,[12] p-NENs might also be mixed adeno and neuroendocrine carcinoma, which was rare but possible to involve the pancreas and tumors with neuroendocrine components, as recently reported in the literatures.[38,39] However, the 8th AJCC manual emphasized that their new TNM staging systems should only be applied to patients with either G1/G2 p-NETs or G3 p-NECs, not to the rare entirety with mixed adeno and neuroendocrine carcinoma. So, we just enrolled those patients with G1/G2/G3 p-NENs in the inclusive criteria. Finally, some studies have reported G3 p-NECs consist of well-differentiated tumors and poorly-differentiated carcinomas, with different Ki-67 index, histological features, and survival outcomes.[31,40–42] Our results detected the intersection of survival curves for patients with G2 NF-p-NETs and those with G3 NF-p-NECs, although their survival difference was statistically significant [55.3% vs 31.9%, P < .05; Supplemental Digital Content (Fig. S3)]. Meanwhile, the subgroup analysis of patients with morphologically differently-differentiated G3 NF-p-NECs also demonstrated a notably different survivals [58.0% vs 27.9%, P = .018; Supplemental Digital Content (Fig. S4)]. However, the AJCC in 2017 considered all G3 p-NECs as a poorly-differentiated entirety, which ignored the heterogeneity of G3 p-NECs with morphologically differently-differentiated subgroups. This might be the potential defect of AJCC 8th staging manual which should be further studied or even revised in its future 9th manual. Despite these limitations, it was reasonable to use our data to validate the new AJCC TNM staging systems for p-NENs.
5. Conclusions
Together with our previous effort,[20,21] our present study validated again that the AJCC 8th edition TNM staging manual were more practical for p-NENs using a single-center database with NF-p-NENs. Our results showed that the 2 AJCC staging systems demonstrated good survival discriminations between their different new stages for the population of patients with G1/G2 NF-p-NETs and those with G3 NF-p-NECs. Meanwhile, we found increased prognostic accuracy for the 8th edition of the AJCC staging manual compared with the 7th one. Although with some limitations, our analysis still suggested the novel 8th edition of AJCC staging systems as superior and might support their wide use in clinical practice for patients with NF-p-NENs [Supplemental Digital Content (Fig. S1)].
Author contributions
In this paper, M. Yang contributed to this work as first authors; Y. Zhang and B. Xiang contributed equally as senior authors. Y. Zhang and B. Xiang designed the research, corrected and approved the final manuscript; M. Yang, Y. Zhang and L. Zeng Tan extracted the data, carried out the statistical analysis of studies, made the tables and figures and wrote the manuscript. B. L. Tian and X. B. Liu had important intelligent contributions and critically revised the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence interval, ENETS = European Neuroendocrine Tumor Society, F-p-NENs = functional pancreatic neuroendocrine neoplasms, G1 p-NETs = G1 pancreatic neuroendocrine tumors, G2 p-NETs = G2 pancreatic neuroendocrine tumors, G3 p-NECs = G3 pancreatic neuroendocrine carcinomas, HCI = Harrell's C-index, HPFs = high-power fields, HR = hazard ratio, MST = median survival time, NF-p-NENs = non-functional pancreatic neuroendocrine neoplasms, OS = overall survival, p-EACs = pancreatic exocrine adenocarcinomas, p-NENs = pancreatic neuroendocrine neoplasms, TNM = tumor-node-metastasis, WHO = World Health Organization.
How to cite this article: Yang M, Zeng L, Yao W-Q, Ke N-w, Tan C-l, Tian B-l, Liu X-b, Xiang B, Zhang Y. A comprehensive validation of the novel 8th edition of American Joint Committee on Cancer staging manual for the long-term survivals of patients with non-functional pancreatic neuroendocrine neoplasms. Medicine. 2020;99:46(e22291).
BX and YZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
A: The old AJCC 7th staging system was primarily applied for p-EACs, which was also suggested in 2010 for p-NENs.
B: The new AJCC 8th manual proposed for p-NENs in 2017 that well-differentiated p-NETs (G1/G2), and high-grade p-NECs (G3) should be grouped by 2 different new staging systems.
C: The 2 subgroups of NF-p-NENs (G1/G2 and G3) was classified separately by the old AJCC 7th staging system.
D: The present analysis of G1/G2 NF-p-NETs by AJCC 7th and 8th staging system (N = 152).
E: The present analysis of G3 NF-p-NECs by AJCC 7th and 8th staging system (N = 78).
AJCC = American Joint Committee on Cancer, G = grading, M = distant metastasis, N = regional lymph node, NA = not applicable, NF-p-NENs = non-functional pancreatic neuroendocrine neoplasms, p-EACs = pancreatic exocrine adenocarcinomas, p-NECs = pancreatic neuroendocrine carcinomas, p-NENs = pancreatic neuroendocrine neoplasms, p-NETs = pancreatic neuroendocrine tumors, T = primary tumor, TNM = tumor-node-metastasis.
A: Comparison of G1/G2 NF-p-NETs and G3 NF-p-NECs wherever possible.
B: Palliative and exploratory operations included.
C: Resections with negative surgical margins, both grossly and microscopically.
D: Chemotherapy, radiotherapy, and molecular targeted therapy, alone or with their combinations.
BP = biopsy, DP = distal pancreatectomy, G = grading, LRP = local resection of pancreatic tumor (enucleation included), NA = not applicable, NF-p-NECs = non-functional pancreatic neuroendocrine carcinomas, NF-p-NENs = non-functional pancreatic neuroendocrine neoplasms, NF-p-NETs = non-functional pancreatic neuroendocrine tumors, PD = pancreaticoduodenectomy, US-guided-FNA = ultrasound guided fine needle aspiration.
A, B, C: The potential prognostic value of AJCC 7th stage for all NF-p-NENs(A), AJCC 8th stage for G1/G2 NF-p-NETs(B) and G3 NF-p-NECs(C) was evaluated separately with those parameters in different multivariate Cox hazard models.
AJCC = American Joint Committee On Cancer, CI = confidence interval, G = grading, HR = hazard ratio, NA = not applicable, NF-p-NECs = non-functional pancreatic neuroendocrine carcinomas, NF-p-NENs = non-functional pancreatic neuroendocrine neoplasms, NF-p-NETs = non-functional pancreatic neuroendocrine tumors, WHO = World Health Organization.
References
- [1].Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- [2].Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589–97. [DOI] [PubMed] [Google Scholar]
- [4].Fitzgerald T, Hickner Z, Schmitz M, et al. Increasing incidence of nonfunctional neuroendocrine tumors of the pancreas. In: ASCO Gastrointestinal Cancers Symposium Orlando, Florida, 2007. [Google Scholar]
- [5].Franko J, Feng W, Yip L, et al. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg 2010;14:541–8. [DOI] [PubMed] [Google Scholar]
- [6].Oberg K, Modlin IM, De Herder W, et al. Consensus on biomarkers for neuroendocrine tumor disease. Lancet Oncol 2015;16:e435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bar-Moshe Y, Mazeh H, Grozinsky-Glasberg S. Non-functioning pancreatic neuroendocrine tumors: surgery or observation? World J Gastrointest Endosc 2017;9:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gullo L, Migliori M, Falconi M, et al. Nonfunctioning pancreatic endocrine tumors: a multicenter clinical study. Am J Gastroenterol 2003;98:2435–9. [DOI] [PubMed] [Google Scholar]
- [9].Kuo JH, Lee JA, Chabot JA. Nonfunctional pancreatic neuroendocrine tumors. Surg Clin North Am 2014;94:689–708. [DOI] [PubMed] [Google Scholar]
- [10].Fendrich V, Waldmann J, Bartsch DK, et al. Surgical management of pancreatic endocrine tumor. Nat Rev Clin Oncol 2009;67:419–28. [DOI] [PubMed] [Google Scholar]
- [11].Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman T, Carneiro F, Hruban R, Theise N, eds. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer (IARC); 2010:13–14. [Google Scholar]
- [13].Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed.New York, NY: Springer-Verlag; 2009. [Google Scholar]
- [14].Strosberg JR, Cheema A, Weber J, et al. Prognostic validity of a novel American Joint Committee on Cancer staging classification for pancreatic neuroendocrine tumors. J Clin Oncol 2011;29:3044–9. [DOI] [PubMed] [Google Scholar]
- [15].Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 2012;104:764–77. [DOI] [PubMed] [Google Scholar]
- [16].Yang M, Tian BL, Zhang Y, et al. Evaluation of the World Health Organization 2010 grading system in surgical outcome and prognosis of pancreatic neuroendocrine tumors. Pancreas 2014;43:1003–8. [DOI] [PubMed] [Google Scholar]
- [17].Ellison TA, Wolfgang CL, Shi C, et al. A single institution's 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg 2014;259:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang M, Zeng L, Zhang Y, et al. TNM staging of pancreatic neuroendocrine tumors: an observational analysis and comparison by both AJCC and ENETS systems from 1 single institution. Medicine (Baltimore) 2015;94:e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kakar S, Pawlik TM, Allen PJ. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer-Verlag; 2017. [Google Scholar]
- [20].Yang M, Zhang Y, Zeng L, et al. Survivals of patients with surgically treated and high-grade pancreatic neuroendocrine carcinomas: a comparative study between two American Joint Committee on Cancer 8th tumor-node-metastasis staging systems. Eur J Surg Oncol 2019;45:1054–60. [DOI] [PubMed] [Google Scholar]
- [21].Yang M, Zhang Y, Zeng L, et al. Prognostic validity of the American Joint Committee on Cancer 8th tumor-node-metastasis staging system for surgically treated and well-differentiated pancreatic neuroendocrine tumors: a comprehensive analysis of 254 consecutive patients from a large Chinese institution. Pancreas 2019;48:613–21. [DOI] [PubMed] [Google Scholar]
- [22].Crawley FP. The Limits of the Declaration of Helsinki. In: Address to Scientific Session. World Medical Association General Assembly: Helsinki, 2012. [Google Scholar]
- [23].Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- [24].Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011;30:1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gorelik M, Ahmad M, Grossman D, et al. Nonfunctioning incidental pancreatic neuroendocrine tumors: who, when, and how to treat? Surg Clin North Am 2018;98:157–67. [DOI] [PubMed] [Google Scholar]
- [26].Yang M, Zeng L, Zhang Y, et al. Surgical treatment and clinical outcome of nonfunctional pancreatic neuroendocrine tumors: a 14-year experience from one single center. Medicine (Baltimore) 2014;93:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Andersen BL, Shapiro CL, Farrar WB, et al. Psychological responses to cancer recurrence. Cancer 2005;104:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cartwright LA, Dumenci L, Siminoff LA, et al. Cancer patients’ understanding of prognostic information. J Cancer Educ 2014;29:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Strosberg JR, Cheema A, Weber JM, et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg 2012;256:321–5. [DOI] [PubMed] [Google Scholar]
- [30].Yang M, Ke NW, Zhang Y, et al. Functional and non-functional pancreatic neuroendocrine tumors: ENETS or AJCC TNM staging system? Oncotarget 2017;8:82784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 2016;103:186–94. [DOI] [PubMed] [Google Scholar]
- [32].Mizuno Y, Kudo A, Akashi T, et al. Sunitinib shrinks NET-G3 pancreatic neuroendocrine neoplasms. J Cancer Res Clin Oncol 2018;144:1155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Benetatos N, Hodson J, Marudanayagam R, et al. Prognostic factors and survival after surgical resection of pancreatic neuroendocrine tumor with validation of established and modified staging systems. Hepatobiliary Pancreat Dis Int 2018;17:169–75. [DOI] [PubMed] [Google Scholar]
- [34].Li X, Gou S, Liu Z, et al. Assessment of the American Joint Commission on cancer 8th edition staging system for patients with pancreatic neuroendocrine tumors: a surveillance, epidemiology, and end results analysis. Cancer Med 2018;7:626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].van Roessel S, Kasumova GG, Verheij J, et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg 2018;153:e183617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the American Joint Commission on cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol 2017;24:2023–30. [DOI] [PubMed] [Google Scholar]
- [38].Gurzu S, Bara T, Molnar C, et al. The epithelial-mesenchymal transition induces aggressivity of mucinous cystic neoplasm of the pancreas with neuroendocrine component: an immunohistochemistry study. Pathol Res Pract 2019;215:82–9. [DOI] [PubMed] [Google Scholar]
- [39].Gurzu S, Fetyko A, Bara T, et al. Gastrointestinal mixed adenoneuroendocrine carcinoma (MANEC): an immunohistochemistry study of 13 microsatellite stable cases. Pathol Res Pract 2019;215:152697. [DOI] [PubMed] [Google Scholar]
- [40].Sorbye H, Strosberg J, Baudin E, et al. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014;120:2814–23. [DOI] [PubMed] [Google Scholar]
- [41].Basturk O, Yang Z, Tang LH, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015;39:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol 2017;29:11–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


