Abstract
The human epidermal growth factor receptor 2 (HER2) is amplified in approximately 20% of breast cancers, and HER2 receptor targeting therapy is associated with a significant improvement in disease-free and overall survival. In several clinical trials, the pathologic complete response (pCR) rate was significantly increased with combined pertuzumab and trastuzumab treatment in HER2-amplified breast cancer. Although the efficacy and safety of anti-HER2 dual blockade therapy has been reported, the markers that predict the response are still unclear. This study aimed to investigate the relationship between the level of HER2 amplification and the pCR in trastuzumab and pertuzumab neoadjuvant therapy.
Twenty-two HER2-amplified early breast cancer patients who had received neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) therapy were included in this study. HER2/CEP17 ratio and average HER2 copy number were measured by fluorescence in situ hybridization analysis. The relationship between level of HER2 amplification and tumor pCR status was investigated.
The median age was 47.5 years (range, 36–62). 31.8% of the patients were hormone receptor (HR) positive and 68.2%% of the patients were HR negative. The pCR (ypN0/is ypN0) rate in the breast and axilla was 68.2%. The patients who experienced a pCR had a median HER2/CEP17 ratio of 7.08 (range, 3.16–10.40) and average HER2 copy number of 17.00 (range, 5.85–37.50). The patients who did not experience a pCR had a median ratio of 4.70 (range, 1.06–9.00) and median HER2 copy number of 12.00 (range, 5.85–20.95) (P = .030, P = .174), respectively.
pCR was highly correlated with HER2/CEP17 ratio in neoadjuvant anti-HER2 dual blockade. This suggests that the HER2/CEP17 ratio can be used as a predictive marker for pCR in neoadjuvant trastuzumab and pertuzumab therapy.
Keywords: breast cancer, fluorescence in situ hybridization, human epidermal growth factor receptor 2, neo adjuvant therapy, pathological response, pertuzumab, trastuzumab
1. Introduction
The human epidermal growth factor receptor 2 (HER2) gene is amplified in approximately 20% of all breast cancers.[1,2]HER2 gene over-expression is associated with poor clinical outcome.[3,4] Treatment with trastuzumab, a recombinant monoclonal antibody against HER2, in HER2-positive breast cancer patients significantly improved the effectiveness of treatment.[5–7] Accordingly, a trastuzumab-based chemotherapy regimen has become the standard treatment for HER2-amplified breast cancer in the adjuvant, neoadjuvant, and metastatic setting.[8–10]
More recently, the treatment of HER2-amplified breast cancer patients has been expanded with the introduction of dual HER2 block therapy using pertuzumab. In the APHINITY study, the addition of pertuzumab to trastuzumab in adjuvant chemotherapy significantly improved invasive-disease-free survival in operable breast cancer patients.[11] The NeoSphere and TRYPHAENA studies confirmed the improvement of pathologic complete response (pCR) when pertuzumab was added in neoadjuvant treatment.[12,13] First-line treatment of recurrent HER2 breast cancer patients also improved progression-free survival in the pertuzumab, trastuzumab, and docetaxel groups compared with the placebo, trastuzumab, and docetaxel groups.[14] Unfortunately, while treatment with pertuzumab has a higher pCR rate than previously, pertuzumab treatment is not effective in all HER2-amplified breast cancer patients. In luminal breast cancer patients, even if the hormone receptor is positive, the scores and responses are significant.[15] Similarly, even when the HER2 gene is amplified, there is a difference in the level of HER2 amplification. There have been few studies showing that the level of HER2 amplification is correlated with response in treatment with trastuzumab in patients with HER2-positive breast cancer.[16–18] There is still no study showing the relationship between HER2 amplification levels and response in dual HER2 block therapy with pertuzumab and trastuzumab. We therefore investigated the relationship between HER2 amplification and pCR in HER2 dual blockade using fluorescence in situ hybridization (FISH) analysis.
2. Material and methods
2.1. Patients
We retrospectively analyzed HER2-positive breast cancer patients using trastuzumab and pertuzumab neoadjuvant chemotherapy among 22 patients diagnosed at Kosin University Gospel Hospital from January 2018 to December 2019. HER2 status was ascertained by immunohistochemistry (IHC) test. Patients with HER2 2+ were diagnosed as positive; a HER2/CEP17 ratio of 2.0 or higher or an average HER2 copy number of 6.0 or higher was considered amplified using FISH analysis.[19] All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. Patients previously treated with chemotherapy, radiation therapy, or targeted therapy were excluded. Patients whose left ventricular ejection fraction (LVEF) was 50% or less prior to chemotherapy as determined by echocardiography or multiple gated acquisition (MUGA) scan were excluded. Cases of metastatic disease, bilateral breast cancer, other malignancies, inadequate bone marrow function, and impaired liver function were also excluded. This study protocol was reviewed and approved by the institutional review board of Kosin University Gospel Hospital.
2.2. Procedure
All patients were diagnosed with invasive carcinoma through core needle biopsy (CNB) or excisional biopsy of breast lesions. Fine needle aspiration (FNA) cytology was performed when metastasis to an axillary lymph node was suspected. Breast ultrasonography (USG), breast magnetic resonance imaging (MRI), chest and abdominal computed tomography (CT), and bone scan were conducted for clinical staging.
All patients received 75 mg/m2 docetaxel, carboplatin area under the curve (AUC) of 6, an 8 mg/kg loading dose of trastuzumab, followed by a 6 mg/kg maintenance dose, and an 840 mg pertuzumab loading dose, followed by 420 mg maintenance dose every 3 weeks for 6 cycles. No patients required dose reduction due to tolerance difficulties. Tumor clinical response was assessed by physical breast examination at every cycle. After 3 cycles, breast USG and breast MRI were taken to confirm the tumor response. There were no cases of conversion to surgery without completing the scheduled chemotherapy. All patients were evaluated for cardiac function through performance of an echocardiographic or MUGA scan before starting chemotherapy.
The type of surgery was determined after consultation with the patient. However, total mastectomy was performed for multicentric malignant lesions, nipple invasion, and expectation of cosmetic problems as well as in patients who refused conserving surgery. All reconstructions were performed immediately, and the opposite side was also performed depending on the patient's condition.
2.3. HER2 scoring
IHC staining was performed on 4-μm sections of formalin-fixed paraffin-embedded tissue by BOND-MAX autostainer (Leica Biosystems, Wertzlar, Germany). Antigen retrieval procedure was accomplished using Bond Epitope Retrieval Solution 2 (Leica Biosystems) for 20 minutes at 100 °C. The sections were incubated with primary polyclonal antibody for HER2 (1: 700, DAKO, Glostrup, Denmark). A negative control stain without primary antibody was performed. HER2 immunoreactivity was assessed using the following scoring approach: 0, no immunoreactivity or weak incomplete membrane staining within <10% of tumor cells; 1+, weak incomplete membrane staining within >10% of tumor cells; 2+, weak to moderate incomplete membrane staining within >10% of tumor cells or strong complete membrane staining within <10% of tumor cells; 3+, strong complete membrane staining within >10% of tumor cells.
2.4. Fluorescence in situ hybridization analysis
HER2 gene copy number was evaluated using the PathVysion HER2 DNA Probe kit (Abbott Molecular, Des Plaines, IL/ Inter Medico, Markham, Canada). The deparaffinized 4-μm section was immersed in 0.2N HCl for 20 minutes. The Slide was placed in pretreatment solution at 98 °C for 30 minutes and then proteaseized at 37 °C for 5 minutes. Slides were hybridized with a PathVysion HER2 DNA probe mixture containing HER2 DNA probe (labeled with spectrum red) and CEP17 DNA probe (labeled with spectrum green) (Fig. 1). The CEP17 DNA probe allows correction of HER2 gene copy number relative to the copy number of chromosome 17. Slide glass cover slip was applied and sealed with rubber. The slides were then denatured at 74 °C for 2 minutes and hybridized overnight at 37 °C in a humidified hybridization chamber (ThermoBrite, Abbott Vysis, Downers Grove, IL). The nest day, the slides were washed in a post-hybridization buffer at 73.5 °C for 2 minutes and dried in the dark. The nuclei were then subsequently counterstained with 10 μL of 4′,6-diamidino-2-phenylin-dole (DAPI). Slides were stored in the dark at 4 °C until signal enumeration.
Figure 1.
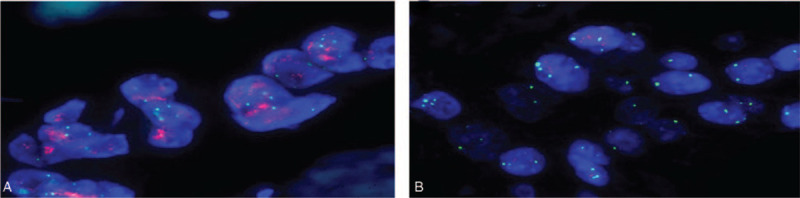
Fluorescence in situ hybridization (FISH) is shown for HER2 gene amplification on Thin-Prep specimens and on corresponding histologic sections (HER2: red signal, CEP17: green signal). (A) A case with positive HER2 amplification status (DAPI counterstain; ×1000). (B) A case with negative HER2 amplification status (DAPI counterstain; ×1000). DAPI = 4′,6-diamidino-2-phenylin-dole.
2.5. Statistics
General characteristics of study subjects are presented as number (%). The HER2/CEP17 ratio and average number of HER2 copies were tested for normality through the Kolmogorov–Smirnov test and Shapiro-Wilk test, and we verified that these 2 variables were normally distributed. According to pCR status, we compared HER2 amplification using Fisher exact test, and data are presented as mean value with 95% confidence interval using box plot. For all analyses, a P value of <.05 was considered statistically significant. All statistical analysis was done with SPSS version 25.0 (IBM Co., Armonk, NY).
3. Results
3.1. Patient characteristics
The baseline characteristics of all 22 patients are summarized in Table 1. Median age was 47.5 years (range, 36–62). The number of patients in tumor stage T1 was 2 (2.9%), in T2 was 13 (59.1%), and in T3–4 was 7 (31.8%). The number of patients in nodal stage N1 was 15 (68.2%), in N2 was 4 (18.2%), and in N3 was 3 (13.6%). Seven patients (31.8%) were hormone receptor positive; 15 were hormone receptor negative (68.2%). In tissue samples obtained from core needle biopsy, the histologic grade was G2 in 19 patients (86.4%) and G3 in 3 patients (13.6%). The Ki-67 value was over 20% in most patients (n = 16, 72.7%). There were 12 premenopausal patients (54.5%) and 10 postmenopausal patients (45.5%). Ten patients (45.5%) underwent total mastectomy, and 9 of these underwent reconstruction immediately after nipple areolar complex-preserving mastectomy. Twelve patients (54.5%) underwent partial mastectomy. Sixteen patients (72.7%) had sentinel lymph node biopsy (SLNB) and 4 (18.2%) had axillary lymph node dissection (ALND). When postoperative pathologic examination was confirmed, 15 patients (68.2%) were ypT0 ypN0 (absence of invasive cancer and in situ cancer in breast and axillary lymph nodes), 19 (86.4%) were ypT0/is ypN0 (absence of invasive cancer in breast and in axillary lymph nodes, irrespective of remaining ductal carcinoma in situ (DCIS) in the primary tumor) and 20 (90.9%) were ypT0/is (absence of invasive cancer in the breast irrespective of the presence of DCIS or nodal involvement).
Table 1.
Baseline characteristics of patients.
| Total, N = 22 | |
| Age (yrs), mean (range) | 47.5 (36–62) |
| Clinical stage | |
| Tumor stage, no. (%) | |
| T1 | 2 (9.1) |
| T2 | 13 (59.1) |
| T3–T4 | 7 (31.8) |
| Nodal stage, no. (%) | |
| N1 | 15 (68.2) |
| N2 | 4 (18.2) |
| N3 | 3 (13.6) |
| Hormone receptor status, no. (%) | |
| Positive | 7 (31.8) |
| Negative | 15 (68.2) |
| Histologic grade, no. (%) | |
| G1 | 0 (0) |
| G2 | 19 (86.4) |
| G3 | 3 (13.6) |
| Ki-67, no. (%) | |
| <10% | 3 (13.6) |
| 10–20% | 3 (13.6) |
| ≥20% | 16 (72.7) |
| Menopause status, no. (%) | |
| Pre-menopause | 12 (54.5) |
| Post-menopause | 10 (45.5) |
3.2. HER2/CEP17 ratio and pathologic response
Patients who experienced a pCR as defined by ypT0 ypN0 had a median HER2/CEP17 ratio of 7.08 (range, 3.16–10.40). Those who did not have a pCR had a median HER2/CEP17 ratio of 4.70 (range, 1.06–9.00; P = .030, Fig. 2A). Patients who experienced pCR with ypT0/is ypN0 had a median ratio of 6.87 (range, 2.54–10.40). Those who did not have a pCR had a median HER2/CEP17 ratio of 3.15 (range, 1.06–5.90; P = .029, Fig. 2B). The tumors which responded with ypT0/is had a median HER2/CEP17 ratio of 6.51 (range, 2.54–10.40). The median ratio in those that did not have a pCR were 2.11 (range, 1.06–3.15; P = .017, Fig. 2C) (Table 2).
Figure 2.
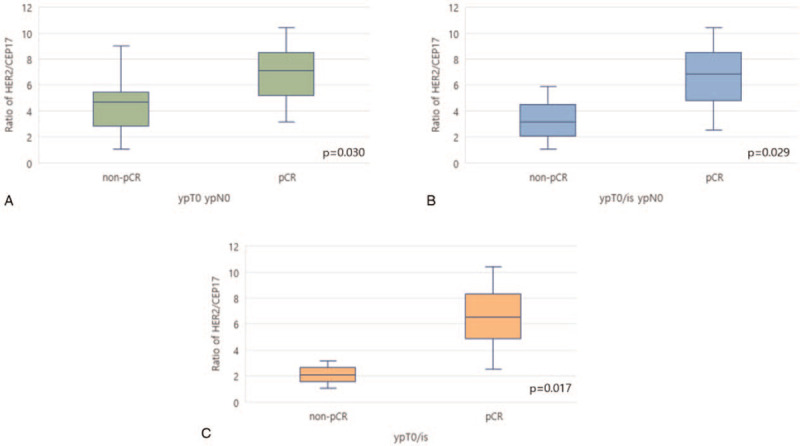
HER2/CEP17 ratios in tumors from patient who did not (left box blot) or did (right box blot) experience pCR as defined by ypT0 ypN0 (A), ypT0/is ypN0 (B), ypT0/is (C). pCR = pathologic complete response.
Table 2.
Median (range) level of HER2 amplifications and pCR as defined by ypT0 N0, ypT0/is N0, ypT0/is.
| ypT0 ypN0 | ypT0/is ypN0 | ypT0/is | |||||||
| pCR | Non-pCR | P | pCR | Non-pCR | P | pCR | Non-pCR | P | |
| HER2/CEP17 ratio, mean (range) | 7.08 (3.16–10.40) | 4.70 (1.06–9.00) | .030 | 6.87 (2.54–10.40) | 3.15 (1.06–5.90) | .029 | 6.51 (2.54–10.40) | 2.11 (1.06–3.15) | .017 |
| Average HER2 copy number, mean (range) | 17.00 (5.85–37.50) | 12.00 (5.85–20.95) | .174 | 17.00 (5.85–37.50) | 11.30 (2.50–12.00) | .070 | 16.40 (5.85–37.50) | 7.25 (2.50–12.00) | .093 |
3.3. Average HER2 copy number and pathologic response
Patients who did have a pCR with ypT0 ypN0 had average HER2 copy number of 17.00 (range, 5.85–37.50). Those who did not have a pCR had average HER2 copy number of 12.00 (range, 5.85–20.95; P = .174, Fig. 3A). Patients who experienced pCR with ypT0/is ypN0 had average HER2 copy number of 17.00 (range, 5.85–37.50). Those who did not have a pCR had average HER2 copy number 11.30 (range, 2.50–12.00; P = .070, Fig. 3B). The tumors which responded with ypT0/is had average HER2 copy number of 16.40 (range, 5.85–37.50). The average copy number in those that did not have a pCR were 7.25 (range, 2.50–12.00; P = .093, Fig. 3C) (Table 2).
Figure 3.
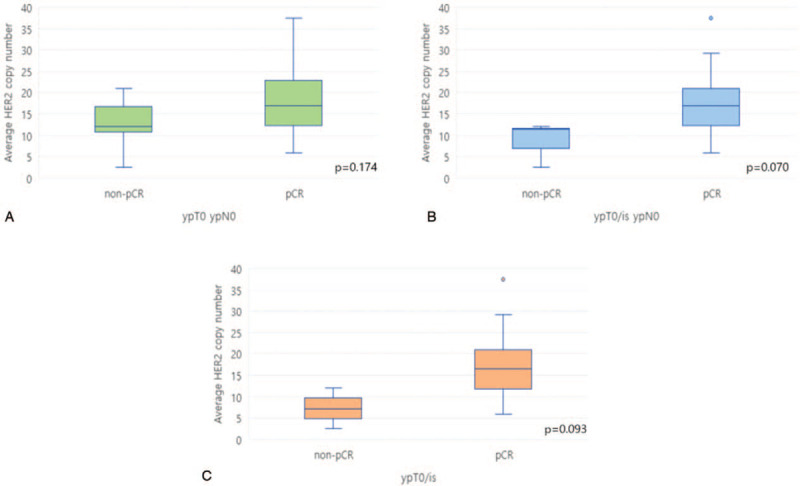
Average HER2 copy number in tumors from patient who did not (left box blot) or did (right box blot) experience pCR as defined by ypT0 ypN0 (A), ypT0/is ypN0 (B), ypT0/is (C). pCR = pathologic complete response.
4. Discussion
When large-scale trials of trastuzumab were first reported, many studies reported that the level of HER2 amplification was not related to the benefit from the adjuvant. In the HERA trial, HER2/CEP17 ratio, HER2 copy number, and CEP17 copy number were not associated with disease-free survival.[20] The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial also did not find a relationship between HER2 copy number and therapeutic benefits.[21] However, some studies in neoadjuvant therapy have focused on the level of HER2 amplification and its relationship to pCR. Arnould et al[16] first reported a relationship between level of HER2 amplification and rate of pCR in trastuzumab-based neoadjuvant treatment. Signal per nuclei was classified into no amplification, low amplification, and high amplification groups; pCR rate was high in the high amplification group. In a study by Singer et al,[17]HER2/CEP17 ratio was measured in patients undergoing neoadjuvant therapy including trastuzumab, and a higher pCR rate was reported when the cut-off value was higher than 6. In our study, only a correlation between HER2/CEP17 ratio and pCR rate could be confirmed because the number of patients was not large enough to divide by grade of amplification or to set a cut-off value. In addition, Singer et al[17] reported that pCR is more common in hormone receptor negative tumors. However, our study had too few patients to understand the relationship between hormone receptor staus and pCR.
There are various studies that have proven the concept that dual HER2 blockade will be more effective than single anti-HER2 block therapy. The NeoALTTO trial showed that combined therapy using an anti-HER2 agent, the tyrosine kinase inhibitor lapatinib, and trastuzumab had a higher pCR rate than therapy with trastuzumab or lapatinib alone.[22] In the NeoSphere study, the pCR rate was about twice as high with dual HER2 blockade with anti-HER2 antibodies trastuzumab and pertuzumab than with a single block.[12] In the TRYPHAENA study, the combination of pertuzumab with trastuzumab and standard chemotherapy resulted in a high response rate.[13] In this study, when pertuzumab was added to docetaxel, cisplatin and trastuzumab treatment, pCR (ypT0/is ypN0) occurred in 19 of 22 patients (86%). This is much higher than the responses seen in the NeoALTTO trial (51.3%) and NeoSphere trial (45.8%). This discrepancy may be due to the small number of participants in our study or to differences in treatment regimen.
In the NOAH study, pCR rate increased when trastuzumab was added to conventional chemotherapy in HER2-positive, locally advanced or inflammatory breast cancer, which improved event-free survival.[23,24] In addition, in the TECHNO study, pCR rate was higher in the group in which trastuzumab was added, which improved disease-free survival and overall survival.[25] The NeoSphere trial also showed a benefit in long term efficacy in the group in which pertuzumab was added to trastuzumab and docetaxel in a 5-year analysis study.[26] By securing a larger number of samples and having a sufficient follow-up period, this study not only confirmed survival in patients with pCR after dual HER2 blockade but also confirmed the frequency and characteristics of recurrence according to the HER2/CEP17 ratio.
Being a retrospective single center study with a relatively small sample size, our study has some limitations. Therefore, our results must be interpreted with caution. Studies conducted on larger sample sizes are required. Nevertheless, this study provided evidence of the clinical impact of the HER2/CEP17 ratio in patients with HER2-positive breast cancer that were treated with double HER2 blockade. Our study provides direction for future preliminary clinical trials.
Recently, an antibody–drug conjugate, trastuzumab emtansine, with reduced side effects compared with conventional anticancer agents, has been introduced. Treatment with this antibody–drug conjugate has been evaluated in various studies. In patients with HER2-positive metastatic breast cancer, overall survival was improved in patients previously treated with trastuzumab and taxane.[27] A study comparing neoadjuvant TCPH and trastuzumab emtansine-added pertuzumab showed that the pCR rate was lower than the dual HER2 blockade group. However, serious adverse events were significantly reduced.[28] Current practice standards consider not only the efficacy of treatment, but also the effect of treatment on the cancer patient's quality of life. Therefore, developing a therapy with high efficacy and low toxicity is a research priority. In the future, we plan to study the relationship between HER2 targeted chemotherapy response and HER2/CEP17 ratio.
In conclusion, the HER2/CEP17 ratio was associated with pCR in dual HER2 blockade neoadjuvant treatment in HER2-positive breast cancer patients. Determining the degree of HER2 amplification by FISH analysis will help predict the effectiveness of treatment and will help in the selection of therapeutic agents.
Author contributions
All authors conceived and designed the study. Jin Hyuk Choi conducted the experiments, analyzed the data and wrote the paper. All authors contributed to manuscript revisions. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Footnotes
Abbreviations: ALND = axillary lymph node dissection, AUC = area under the curve, CEP17 = centromere enumerator probe 17, CNB = core needle biopsy, CT = computed tomography, DAPI = 4′,6-diamidino-2-phenylin-dole, DCIS = ductal carcinoma in situ, ECOG = Eastern Cooperative Oncology Group, FISH = fluorescence in situ hybridization, FNA = fine needle aspiration, HER2 = human epidermal growth factor receptor 2, IHC = immunohistochemistry, LVEF = left ventricular ejection fraction, MRI = magnetic resonance imaging, MUGA = multiple gated acquisition, pCR = pathologic complete response, SLNB = sentinel lymph node biopsy, USG = ultrasonography.
How to cite this article: Choi JH, Jeon CW, Kim YO, Jung S. Pathological complete response to neoadjuvant trastuzumab and pertuzumab therapy is related to human epidermal growth factor receptor 2 (HER2) amplification level in HER2-amplified breast cancer. Medicine. 2020;99:46(e23053).
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2019M3E5D1A02070865). This study was also supported by a grant from Kosin University College of Medicine.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
CEP17 = centromere enumerator probe 17, HER2 = human epidermal growth factor receptor 2, non-pCR = absence of pathological complete response, pCR = pathological complete response.
References
- [1].Slamon D, Clark G, Wong S, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82. [DOI] [PubMed] [Google Scholar]
- [2].Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- [3].Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol 1997;15:2894–904. [DOI] [PubMed] [Google Scholar]
- [4].Gown AM, Goldstein LC, Barry TS, et al. High concordance between immunohistochemistry and fluorescence in situ hybridization testing for HER2 status in breast cancer requires a normalized IHC scoring system. Mod Pathol 2008;21:1271–7. [DOI] [PubMed] [Google Scholar]
- [5].Pritchard KI, Shepherd LE, O’Malley FP, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med 2006;354:2103–11. [DOI] [PubMed] [Google Scholar]
- [6].Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–72. [DOI] [PubMed] [Google Scholar]
- [7].Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-Positive breast cancer. N Engl J Med 2005;353:1673–84. [DOI] [PubMed] [Google Scholar]
- [8].Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265–74. [DOI] [PubMed] [Google Scholar]
- [9].Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809–20. [DOI] [PubMed] [Google Scholar]
- [10].Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2–positive operable breast cancer. J Clin Oncol 2005;23:3676–85. [DOI] [PubMed] [Google Scholar]
- [11].von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 2017;377:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gianni L, Pienkowski T, Im Y-H, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25–32. [DOI] [PubMed] [Google Scholar]
- [13].Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278–84. [DOI] [PubMed] [Google Scholar]
- [14].Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harvey JM, Clark GM, Osborne CK, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999;17:1474–81. [DOI] [PubMed] [Google Scholar]
- [16].Arnould L, Arveux P, Couturier J, et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res 2007;13:6404–9. [DOI] [PubMed] [Google Scholar]
- [17].Singer CF, Tan YY, Fitzal F, et al. Pathological complete response to neoadjuvant trastuzumab is dependent on HER2/CEP17 ratio in HER2-amplified early breast cancer. Clin Cancer Res 2017;23:3676–83. [DOI] [PubMed] [Google Scholar]
- [18].Kim J-W, Kim JH, Im S-A, et al. HER2/CEP17 ratio and HER2 immunohistochemistry predict clinical outcome after first-line trastuzumab plus taxane chemotherapy in patients with HER2 fluorescence in situ hybridization-positive metastatic breast cancer. Cancer Chemother Pharmacol 2013;72:109–15. [DOI] [PubMed] [Google Scholar]
- [19].Wolff A, Hammond M, Hicks D, et al. American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- [20].Dowsett M, Procter M, McCaskill-Stevens W, et al. Disease-free survival according to degree of HER2 amplification for patients treated with adjuvant chemotherapy with or without 1 year of Trastuzumab: The HERA Trial. J Clin Oncol 2009;27:2962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 2008;358:1409–11. [DOI] [PubMed] [Google Scholar]
- [22].Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER. Lancet 2010;375:377–84. [DOI] [PubMed] [Google Scholar]
- [24].Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014;15:640–7. [DOI] [PubMed] [Google Scholar]
- [25].Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2–overexpressing breast cancer: results from the TECHNO Trial of the AGO and GBG Study Groups. J Clin Oncol 2011;29:3351–7. [DOI] [PubMed] [Google Scholar]
- [26].Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791–800. [DOI] [PubMed] [Google Scholar]
- [27].Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2018;19:115–26. [DOI] [PubMed] [Google Scholar]


