Abstract
Objective
The coronavirus disease 2019 (COVID-19) has spread worldwide since December 2019. Neurologic symptoms have been reported as part of the clinical spectrum of the disease. We aimed to determine whether neurologic manifestations are common in hospitalized patients with COVID-19 and to describe their main characteristics.
Methods
We systematically reviewed all patients diagnosed with COVID-19 admitted to the hospital in a Spanish population during March 2020. Demographic characteristics, systemic and neurologic clinical manifestations, and complementary tests were analyzed.
Results
Of 841 patients hospitalized with COVID-19 (mean age 66.4 years, 56.2% men), 57.4% developed some form of neurologic symptom. Nonspecific symptoms such as myalgias (17.2%), headache (14.1%), and dizziness (6.1%) were present mostly in the early stages of infection. Anosmia (4.9%) and dysgeusia (6.2%) tended to occur early (60% as the first clinical manifestation) and were more frequent in less severe cases. Disorders of consciousness occurred commonly (19.6%), mostly in older patients and in severe and advanced COVID-19 stages. Myopathy (3.1%), dysautonomia (2.5%), cerebrovascular diseases (1.7%), seizures (0.7%), movement disorders (0.7%), encephalitis (n = 1), Guillain-Barré syndrome (n = 1), and optic neuritis (n = 1) were also reported, but less frequent. Neurologic complications were the main cause of death in 4.1% of all deceased study participants.
Conclusions
Neurologic manifestations are common in hospitalized patients with COVID-19. In our series, more than half of patients presented some form of neurologic symptom. Clinicians need to maintain close neurologic surveillance for prompt recognition of these complications. The mechanisms and consequences of severe acute respiratory syndrome coronavirus type 2 neurologic involvement require further studies.
Since December 2019, the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) has spread worldwide.1 COVID-19 was defined as a pandemic by WHO in March 2020.2 As of April 30, 2020, a total of 3,231,701 cases of COVID-19 and 229,447 deaths had been reported. Spain has the second greatest number of deaths adjusted by population worldwide.3
Neurotropism is one common feature of previously described pathogenic coronavirus types such as severe acute respiratory syndrome coronavirus (2002) and Middle East respiratory syndrome coronavirus (2012).4 It has been suggested that SARS-CoV-2 could reach the CNS via circulation or upper nasal transcribial routes. Endothelium, glial cells, and neurons have been reported to express angiotensin-converting enzyme receptor 2 (ACE2), which makes them a potential target of SARS-CoV-2, since the virus enters the cells through this receptor.5
Neurologic manifestations of COVID-19 have been described since the beginning of the pandemic.6 Nonspecific symptoms such as headache or dizziness can be associated with viral infection syndrome.7 Anosmia and dysgeusia are intriguing symptoms seen in early phases of COVID-19 infection.8 Neurologists have to deal with patients with neurologic complications from the disease, such as CNS dysfunction, global (altered level of consciousness) or focal (stroke, encephalitis, seizures) complications, or peripheral nervous system (PNS) and skeletal muscle complications like myopathy.6,9–12 As a consequence of the hyperactivation of the immune system,13 different autoimmune complications can also be expected.13–15 As such, we hypothesize that neurologic symptoms are common in COVID-19 infection and attempt to describe their main characteristics. Here, we report a systematic review of neurologic manifestations of COVID-19 among patients with SARS-CoV-2 infection admitted to our hospitals in Albacete in March 2020.
Methods
Study design and data collection
We conducted a retrospective, observational study in 2 centers (Complejo Hospitalario Universitario de Albacete and Hospital General de Almansa) in the province of Albacete (Castilla-La Mancha, Spain). We reviewed the medical records of all patients admitted to our hospitals from March 1 to April 1, 2020, diagnosed with COVID-19. All had a confirmed laboratory diagnosis of COVID-19, either with a positive result for immunoglobulin G (IgG)/immunoglobulin M (IgM) antibodies against SARS-CoV-2 in a blood test or through detection of SARS-CoV-2 RNA with real-time reverse transcription–polymerase chain reaction (rt-PCR) of throat swab samples.
We reviewed electronic medical records, laboratory parameters, radiologic examinations (head CT or brain MRI), and neurophysiologic tests, including EEG and EMG, if indicated. Demographic data such as age, sex, previous comorbidities (hypertension, diabetes, dyslipidemia, smoking habit, obesity, heart disease, chronic kidney disease [CKD], immunosuppression, cancer, neurologic diseases), and relevant previous treatments (antithrombotic therapy, angiotensin-converting enzyme inhibitors [ACEI], angiotensin II receptor blockers [ARB], statins) were recorded.
Severity of COVID-19 was defined according to the 2007 Infectious Diseases Society of America/American Thoracic Society criteria.16 Time of onset (days since first COVID-19 symptoms) and clinical phase of the disease were assessed for each neurologic symptom. Clinical phases of COVID-19 were divided into stage I (early infection), stage IIA (pulmonary involvement without respiratory insufficiency), stage IIB (respiratory insufficiency), and stage III (systemic hyperinflammation).17
Our research group categorized neurologic manifestations into nonspecific symptoms (headache, dizziness, myalgia), neuropsychiatric disorders (insomnia, depression, anxiety, psychosis), CNS disorders (direct viral infection, disorders of consciousness, seizures, stroke), PNS disorders (cranial neuropathies, anosmia/dysgeusia, peripheral neuropathy), myopathy, and demyelinating events.
Key laboratory results (creatine kinase [CK], lymphocyte count, C-reactive protein [CRP], ferritin, and D-dimer) and the extent of lung involvement on chest X-ray were documented. Antiviral drugs, antibiotics, immunomodulatory therapies (corticosteroids, β-interferon, IV immunoglobulins [IVIg], baricitinib, anakinra, tocilizumab, sarilumab), and low-molecular-weight heparin (LMWH) dose (prophylactic or anticoagulant) were recorded.
Standard protocol approvals, registrations, and patient consents
This is a retrospective and noninterventional study; we did not perform any experiments in human participants. This study was registered in our center (identifier: 2020/04/043) and it received approval from our institutional ethical standards committee. We obtained a waiver of written informed consent since all data were collected retrospectively and anonymously.
Statistical analysis
The statistical analysis was performed using SPSS software, version 25 (SPSS, Chicago, IL). The ratios were compared using the χ2 test, and the Fisher exact test when the sample size was too small. The comparison between quantitative variables was performed using the Student t test, considering a value p less than 0.05 as statistically significant. We also calculated the confidence interval (CI) and odds ratio (OR). A multivariate analysis was performed if indicated.
Data availability
After publication, the data will be made available to other researchers on reasonable request to the corresponding author.
Results
A total of 841 patients were admitted to the hospital with confirmed SARS-CoV-2 infection (85.7% by PCR, 12.1% by IgG/IgM rapid test, 2.2% both) between March 1 and April 1, 2020. A total of 329 (39.1%) had severe COVID-19, 77 (9.16%) were admitted to the intensive care unit (ICU), and 197 (23.4%) died during the course of their hospital admission. Neurologic complications were considered to be the fundamental cause of patient death in 8 cases (4.1% of total deaths).
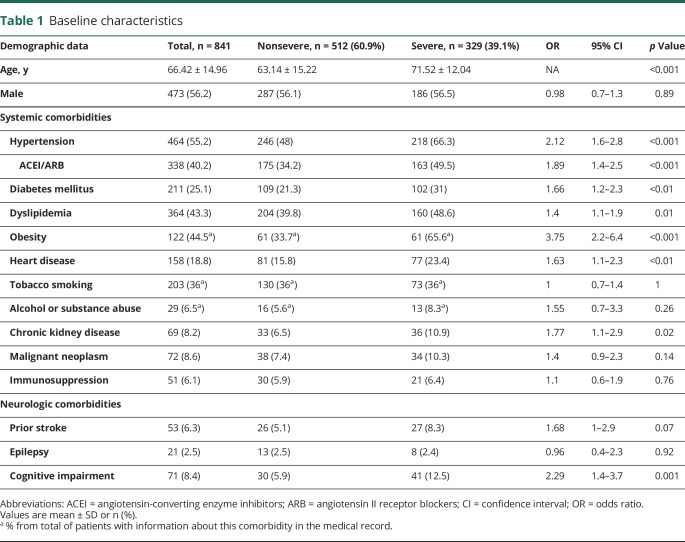
We show cohort baseline characteristics and comorbidities in table 1. The mean age was 66.4 years and 56.2% were men. The most common systemic comorbidities were hypertension (55.2%), obesity (44.5%), dyslipidemia (43.3%), tobacco smoking (36%), diabetes mellitus (25.1%), and heart disease (18.8%).
Table 1.
Baseline characteristics
Severe COVID-19 infection was associated with obesity (OR 3.75; p < 0.001), hypertension (OR 2.12; p < 0.001), use of ACEI/ARB therapies (OR 1.89; p < 0.001), CKD (OR 1.77; p = 0.02), diabetes (OR 1.66; p < 0.01), heart disease (OR 1.63; p < 0.01), and dyslipidemia (OR 1.4; p = 0.01). Patients in the severe disease group were older than those in the mild disease group (p < 0.001). Sex and immunosuppression were not risk factors for severe prognosis. In the multivariate analysis, the only independent predictor for severe disease was obesity (OR 3.06, 95% CI 1.41–6.67; p = 0.005).
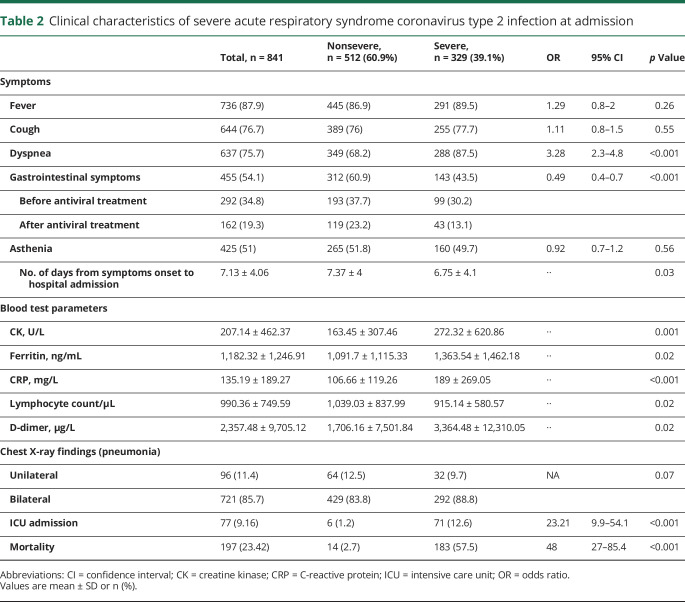
Clinical manifestations of COVID-19 at admission are detailed in table 2. The most common features were fever, cough, and dyspnea. Mean time from clinical onset to hospital admission was 7.13 days. At admission, elevated CK, ferritin, CRP, and D-dimer plus lower lymphocyte count were associated with severe disease. The most frequently used treatment protocol (standard protocol, 747 [89%]) included hydroxychloroquine, lopinavir/ritonavir, N-acetylcysteine, and azithromycin. In some patients, lopinavir/ritonavir was replaced by emtricitabine/tenofovir (50 [6%]) or ribavirin (21 [2.5%]). In most cases, antibiotics such as levofloxacin, doxycycline, ceftriaxone, or teicoplanin were prescribed to treat bacterial superinfection. Different doses of IV methylprednisolone pulses were added for 3–5 days: 125 mg (26.9%), 250 mg (15.54%), or >250 mg (5.2%). A total of 182 patients (21.6%) received immunomodulatory treatment, either as monotherapy (147 [17.5%]) or as a variety of polytherapy strategies (35 [4.2%]). Β-interferon was the most applied immunomodulatory drug (86 [10.2%]) followed by baricitinib (49 [5.8%]), IVIg (35 [4.2%]), and anakinra (31 [3.7%]). Among the patients who had neurologic manifestations, 102 (21.3%) received immunotherapy, 54 of them before clinical onset. LMWH was used at a prophylactic dose in 492 (64.7%) cases and at an anticoagulant dose in 268 (35.3%), if previously anticoagulated or D-dimer level was above 1,000 μg/L.
Table 2.
Clinical characteristics of severe acute respiratory syndrome coronavirus type 2 infection at admission
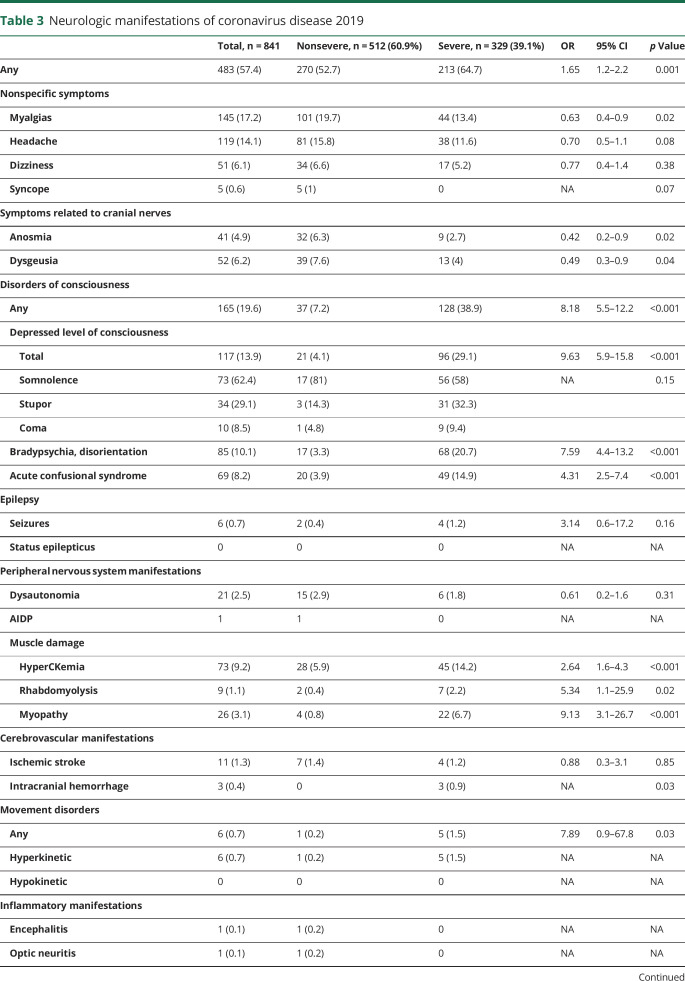
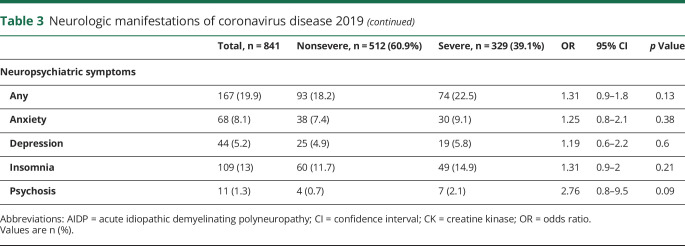
Neurologic manifestations of SARS-CoV-2 infection are shown in table 3. Up to 57.4% of patients developed at least one neurologic symptom. Within nonspecific symptoms, the most commonly reported were myalgias and headache. These symptoms appeared early in the evolution of the disease (mean time from onset 3.8 days) and up to 70.6% patients were at stage I of COVID-19.
Table 3.
Neurologic manifestations of coronavirus disease 2019
Symptoms associated with cranial nerves were statistically more common in nonsevere pneumonia compared with severe cases: anosmia (32 [6.3%] vs 9 [2.7%]; p = 0.02) and dysgeusia (39 [7.6%] vs 13 [4%]; p = 0.04). The mean time of evolution of symptoms was 3.5 days (more than 60% from the first day). A total of 84.6% patients were at stage I.
Disorders of consciousness were the most repeatedly observed neurologic symptoms (19.6%), especially in the severe COVID-19 group compared to nonsevere (38.9% vs 7.2%, OR 8.18; p < 0.001). The mean time of onset was 9 days. These manifestations were statistically associated with older age (77.6 ± 12.1 vs 65.22 ± 14.87; p < 0.001), higher CK levels (386.85 ± 777.244 vs 181.72 ± 268.77; p ≤ 0.001), lower lymphocyte count (731 ± 12.1 vs 1,008.6 ± 296.6; p = 0.02), and advanced stages of COVID-19 (60 in stage IIB [38.7%] and 62 stage III [40%]). Of note was that bradypsychia/disorientation occurred in the context of marked hypoxemia (PaFi < 300) in 48.23% of cases.
Two patients presented with depressed level of consciousness and pyramidal signs during stage III of COVID-19. In both cases, the brain MRI was normal and the EEG showed moderate encephalopathy. The first had a normal CSF analysis, including a negative rt-PCR for SARS-CoV-2 RNA, and improved without treatment after some days. The second case was associated with mild acute kidney injury (creatinine 2.85 mg/dL, urea 160 mg/dL) and improved after dialysis. No lumbar puncture was performed in this patient so we do not have CSF data in this case.
Seizures occurred in 6 patients (0.7%), only one having been diagnosed with epilepsy previously. None of them were complicated by status epilepticus. Four cases occurred in severe stage disease, 2 of which were after intracranial hemorrhages. In 3 patients, seizures had a focal onset. It is noteworthy that seizures in the context of COVID-19 were associated with previous history of cognitive impairment (OR 11.28; p < 0.001), older age (80.67 ± 6 vs 66.31 ± 15; p = 0.02), higher CK levels (1,001.5 ± 1,816 vs 201.1 ± 435.3; p < 0.001), and higher CRP (391.9 ± 617 vs 133.1 ± 181.8; p < 0.001) at admission. The type of seizure was not related to the severity of the infection or other clinical parameters.
With respect to neuromuscular disorders, muscle damage was the most prominent feature. Nonspecific muscle manifestations such as myalgia, asthenia, and muscle fatigue were striking symptoms seen in early stages of the disease in more than half of the cohort. None was associated with disease severity or subsequent development of myopathy. HyperCKemia was detected at admission in 73 (9.2%) patients and rhabdomyolysis in 9 (1.1%). Clinical and examination data suggestive of myopathy were found in 26 cases (3.1%), 3 of which had hyperCKemia. Three patients with myopathy had compatible neurophysiology test results, but it was not possible to perform more studies, including muscle biopsy, due to the ongoing pandemic context. Myopathy tended to develop later in the disease, around the 12th day from onset. Longer stay in the ICU was the only independent predictor in multivariate analysis (OR 1.3, 95% CI 1.02–1.71; p = 0.03) that included other relevant variables such us previous treatment with statins, corticosteroids, hydroxychloroquine, immunomodulatory therapies, and severe disease. Another PNS alteration noted was dysautonomia, recorded in 21 (2.5%) patients, 15 of whom had nonsevere disease. One patient was diagnosed with acute inflammatory demyelinating polyneuropathy (AIDP) during the recovery phase of the disease.
As for cerebrovascular diseases, 11 (1.3%) patients had ischemic stroke and 3 (0.4%) intracranial hemorrhage (ICH). Mean time of occurrence was 10 days after onset of COVID-19 symptoms. Cerebrovascular diseases were associated with higher D-dimer levels at admission (9,929 ± 28,286 vs 2,250 ± 293; p = 0.01). Two patients of the ischemic stroke group were on anticoagulant treatment (dabigatran and acenocoumarol) at the time of stroke onset. In the hemorrhage group, one patient was on LMWH (prophylactic dose) and he also had thrombopenia, while the other patient was receiving LMWH at an anticoagulant dose. ICH was only noticed in patients with severe disease (p = 0.03). Interestingly, one patient who had multiple brain hemorrhages also showed a brain MRI pattern resembling posterior reversible encephalopathy syndrome (figure 1). Unlike ICH, ischemic stroke occurred independently of COVID-19 severity, even in the absence of systemic manifestations. In fact, 4 patients had this serious complication in the recovery phase of the disease. The posterior arterial territory was involved in 4 cases (36.4%). The etiology was either undetermined or other determined etiology (modified TOAST classification) in a high proportion of patients. Highlights of this series were 2 cases of multiterritorial infarctions, 2 more with arterial dissections (1 extracranial internal carotid and 1 extracranial vertebral), and 1 case of CNS vasculitis.
Figure 1. Hemorrhages and posterior reversible encephalopathy syndrome–like features.
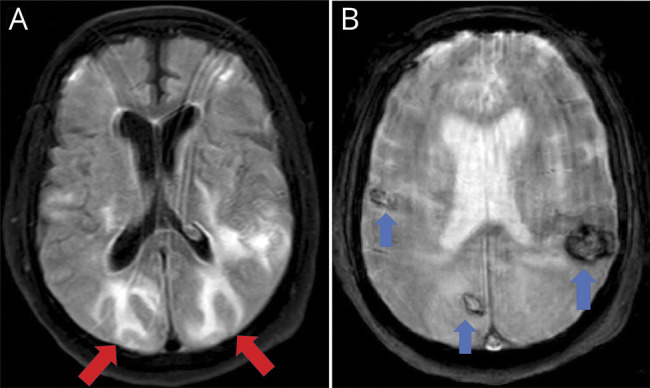
Images are from a 64-year-old man admitted to the intensive care unit due to severe bilateral pneumonia (real-time reverse transcription–polymerase chain reaction positive for severe acute respiratory syndrome coronavirus type 2) requiring mechanical ventilation. When endotracheal intubation was removed, the patient did not recover consciousness. Neuroimaging (MRI) showed bilateral subcortical hyperintense lesions with vasogenic edema in occipito-parietal lobes (A, axial fluid-attenuated inversion recovery sequence, red arrows) resembling posterior reversible encephalopathy syndrome. Gradient-echo sequences also revealed bilateral hypointense lesions compatible with several hemorrhages (B, axial T2 gradient echo sequence, blue arrows). MRI excluded other possibilities such as cerebral venous sinus thrombosis.
Six patients (0.7%) developed hyperkinetic movements (mostly myoclonic tremor) at a mean time of 8.3 days from the clinical onset of COVID-19. Three patients with prior neuropsychiatric history developed oromandibular dyskinesias, upper limb tremor, and rigidity within a rigid-akinetic syndrome exacerbated by severe pneumonia and neuroleptic use. The rest of the cases presented with myoclonic tremor involving the upper body, unilateral or bilateral, plus alteration in the level of consciousness. An appropriate neurologic workup was not performed in these cases.
When considering inflammatory manifestations, one patient had encephalitis, which presented as a stroke mimic due to the appearance of sudden language dysfunction (14th day from onset, stage IIA) with bilateral temporal hyperintensity in fluid-attenuated inversion recovery sequences of brain MRI (figure 2). CSF analysis was normal, including a negative rt-PCR for SARS-CoV-2 RNA. Another woman consulted for vision loss due to optic neuritis during the recovery phase (11th day from onset, PCR negative, IgM positive, IgG positive) without any sign of pneumonia. Previously, both had mild respiratory symptoms and dysgeusia. Neither of these patients received treatment before admission.
Figure 2. Bitemporal lobe involvement compatible with encephalitis.
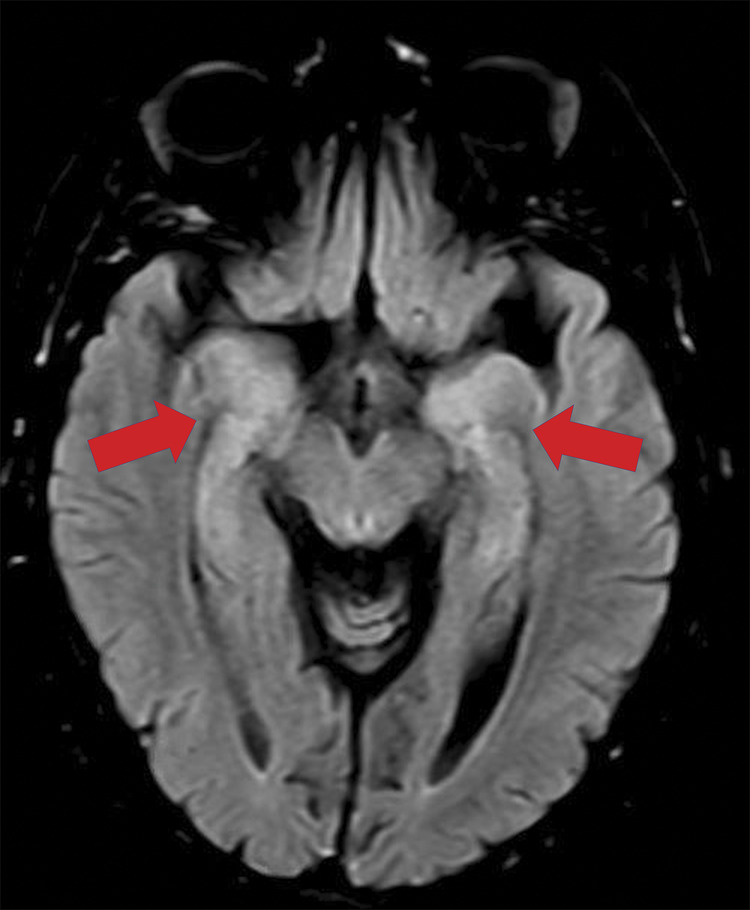
Images are from a 57-year-old woman referred to the hospital in the setting of stroke code due to acute aphasia. Rapid test (immunoglobulin G/immunoglobulin M against severe acute respiratory syndrome coronavirus type 2 [SARS-CoV-2]) was positive but no coronavirus disease 2019–related symptoms were found. MRI axial fluid-attenuated inversion recovery sequence showed bilateral hyperintensity within both temporo-mesial lobes (red arrows), compatible with encephalitis. CSF was normal, including real-time reverse transcription–polymerase chain reaction for RNA of SARS-CoV-2.
We detected neuropsychiatric symptoms in 167 (19.9%) patients, insomnia being the most frequent symptom, followed by anxiety, depression, and psychosis. None of these symptoms was associated with the severity of the disease.
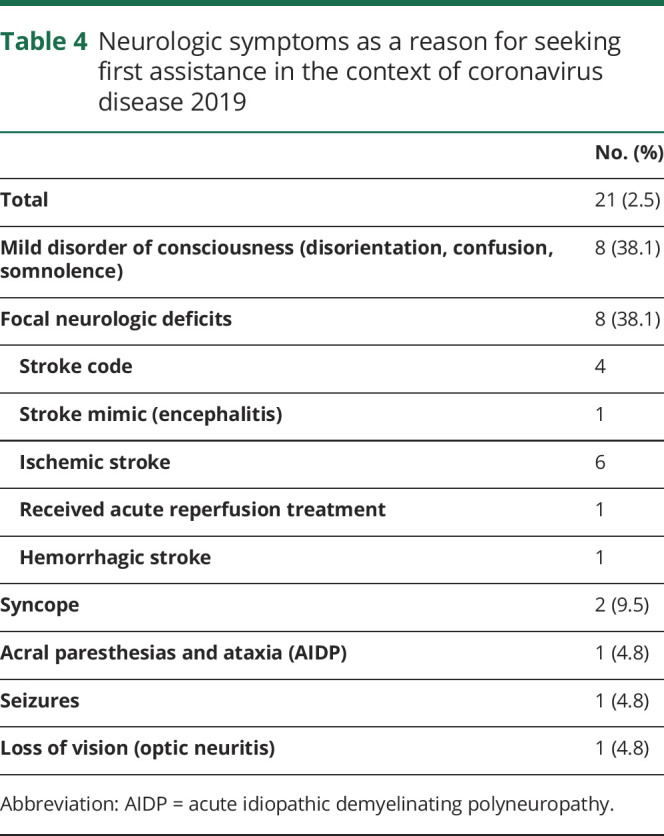
In 21 patients (2.5%), a neurologic manifestation was the main reason to visit the emergency department (table 4); almost a third of these patients consulted within the first 24 hours from onset. The most common symptoms were mild disorders of consciousness (n = 8) and focal neurologic deficits (n = 8).
Table 4.
Neurologic symptoms as a reason for seeking first assistance in the context of coronavirus disease 2019
Discussion
To our knowledge, this work comprises the largest hospital-based cohort of patients with COVID-19 to date in which neurologic symptoms were systematically analyzed. More than half of patients with COVID-19 (57.4%) developed at least one neurologic symptom, a proportion significantly higher than the 36.4% reported in previous studies.6
Our knowledge of COVID-19 remains limited since the pathogen was first described only a few months ago. Recently, the neurotropic capacity of SARS-CoV-2 has been explored in 2 articles. Moriguchi et al.11 described the first case where RNA of SARS-CoV-2 was detected in CSF by rt-PCR in a patient with encephalitis in the context of COVID-19. Paniz-Mondolfi et al.18 reported the presence of SARS-CoV-2 viral particles in the cytoplasm of frontal lobe neurons, as well as in brain endothelial cells in the postmortem examination of a patient with COVID-19 who presented with confusion and encephalopathy during the course of the infection. We have been unable to demonstrate direct CNS invasion by SARS-CoV-2 in our COVID-19 population who developed neurologic manifestations, since all CNS analyses performed were negative for the viral RNA. In addition to direct CNS infection, the intense systemic inflammatory response elicited by SARS-CoV-2 infection can lead to blood–brain barrier (BBB) breakdown. The increased BBB permeability may allow peripheral cytokines to pass into the CNS and thus an indirect neuroinflammatory reaction could be responsible for neurologic manifestations in COVID-19.19
In our sample, basal comorbidities were similar to those described in previous reports.20 However, we found higher rates of hypertension, diabetes, smoking, heart disease, and CKD than those communicated from China.6,21,22 Furthermore, although in our series these comorbidities were associated with severe SARS-CoV-2 infection, multivariate analysis allowed us to discover that, unlike other descriptions, in our series only obesity was a comorbidity capable of modifying risk of severe disease.22 Obesity and metabolic syndrome have been implicated in chronic inflammation, promoting a proinflammatory phenotype in macrophages that may be a key factor leading to the hyperinflammatory response seen in the later stage of infection by SARS-CoV-2.23 Accordingly, some authors support the idea that selective immunosuppressive therapy could be protective since the critical phase of COVID-19 is thought to be associated with an uncontrolled immune response.24 We have not found a relationship between severe disease and male sex, immunosuppression, smoking, or ACEI/ARB.
In our cohort, early (stage I) neurologic symptoms were mainly nonspecific. Anosmia and dysgeusia are particularly important as they may be independent markers of early infection. Previous studies report a high variability of the prevalence of these symptoms (from 5% to 70%),6,8,25 which may be underestimated in our cohort due to selection bias. In critical respiratory conditions, early mild nonspecific symptoms of the infection might be ignored, especially in a pandemic setting and in those cases presenting with an altered level of consciousness or confusion. Elucidating whether these early symptoms are due to local damage of nasal epithelium and invasion of the CNS via the olfactory bulb is a challenge for researchers that remains unresolved.8,26
Late neurologic complications (at stages II and III) include encephalopathy, myopathy, and autoimmune diseases. In our experience, most cases of altered consciousness were secondary to severe hypoxemia (PaFi < 300) and closely related to the severity of the disease. Previous reports suggest that this global brain dysfunction occurs in the context of multiorgan failure due to a conjunction of hypoxemia, BBB dysfunction, cerebrovascular disease, toxic metabolites (uremia, ammonium, electrolytes dysregulation), and cytokine release syndrome as observed in chimeric antigen receptor T-cell therapy–associated neurotoxicity.15,26,27 However, in several of our patients the study was incomplete, and the etiology remains unclear. Recently, Helms et al.28 described patients with severe COVID-19 who presented with encephalopathy and pyramidalism, similar to 2 cases highlighted in our series. Again, the neuropathologic features of these cases remain unclear.
The prevalence of symptoms and laboratory abnormalities associated with muscle damage like myalgias, asthenia, hyperCKemia, and rhabdomyolysis is slightly lower than reported in other case series of COVID-19 and infections by other coronavirus.29 The myopathy described in our cohort was linked to critical forms of the disease and longer admissions in the ICU. This may be better explained by multiorgan failure and critical illness myopathy, although other pathogenic mechanisms cannot be excluded. Regarding peripheral neuropathies, dysautonomia is suggestive of COVID-19 affecting small unmyelinated fibers; nevertheless, a central origin of dysautonomia cannot be excluded. Unlike that seen in the CNS, there is no evidence that SARS-CoV-2 is capable of directly damaging the peripheral nerves and it seems more likely that this damage is exerted indirectly by a cytokine storm or due to a dysimmune mechanism.6 Intriguingly, in our study we happen to describe 3 cases of classical dysimmune diseases (AIDP, encephalitis, and optic neuritis). These complications, barely reported as being associated with COVID-19 in the current literature, appeared in the recovery phase, thus a dysimmune or parainfectious response elicited by SARS-CoV-2 infection appears to be plausible in this disease.
With respect to acute cerebrovascular diseases, unusual features were observed in our series, including a high proportion of vertebrobasilar stroke and uncommon causes such as arterial dissection, CNS vasculitis, and infarction of different arterial territories without an identified cardioembolic source. Our research group has prepared a separate document in which we address each of these cases. We hypothesize that cerebrovascular manifestations of COVID-19 may arise as a result of a combination of hypercoagulability and endothelial damage. The latter could be triggered by cytokine release as well as direct viral injury by SARS-CoV-2, given that the endothelium also expresses ACE2 receptors.5,30
Most of the patients with vascular and inflammatory neurologic diseases had mild or no respiratory symptoms. Thus, in all patients presenting with a neurologic acute process during the COVID-19 outbreak, we recommend evaluation of the possibility of a subjacent SARS-CoV-2 infection by rt-PCR, serology, and, if possible, chest CT scan (especially during stroke codes, associated with the multimodal CT protocols).
The main limitation to this work is the ever present pandemic context, which prevented us from performing full neurologic evaluation of every patient and a complete diagnostic workup. The data were obtained retrospectively, so selection bias may arise and some important information could be missing. Moreover, our work is a descriptive and retrospective series, and as such we could not determine without any doubt whether the neurologic problems of the patients were caused by the SARS-CoV-2 infection or by other factors such as cross-immunity, inflammatory reaction, or side effects of the treatments. Finally, this study is hospital-based, so it does not necessarily reflect the incidence of neurologic complications of patients with COVID-19 in the community and any findings must be considered with that in mind.
Neurologic manifestations are common in hospitalized patients with COVID-19. A wide variety of neurologic symptoms can appear during COVID-19 infection and may be related either to direct damage of neurologic tissues or indirectly due to cytokine release, respiratory insufficiency, critical illness, and side effects of pharmacologic treatment. Potentially severe conditions such as stroke or inflammatory diseases can appear in later stages, even during recovery. Since the global emergency is expected to persist for some time, we encourage first-line doctors to be aware of potential neurologic symptoms. We recommend that patients with altered levels of consciousness or confusion should be evaluated by a neurologist, especially in the absence of hypoxemia or marked metabolic alterations. We also encourage neurologists to be included in COVID-19 response teams as a matter of routine for adequate early recognition and management of neurologic manifestations in order to improve neurologic outcomes. With this in mind, the mechanisms of neurologic injury and their emerging long-term medical consequences require further investigation.
Acknowledgment
In memory of all the people in our region who have suffered the consequences of COVID-19 and to all of the workers in our hospitals who have given their all in the fight against this terrible disease.
Glossary
- ACE2
angiotensin-converting enzyme receptor 2
- ACEI
angiotensin-converting enzyme inhibitors
- AIDP
acute inflammatory demyelinating polyneuropathy
- ARB
angiotensin II receptor blockers
- BBB
blood–brain barrier
- CI
confidence interval
- CK
creatine kinase
- CKD
chronic kidney disease
- COVID-19
coronavirus disease 2019
- CRP
C-reactive protein
- ICH
intracranial hemorrhage
- ICU
intensive care unit
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- LMWH
low-molecular-weight heparin
- OR
odds ratio
- PNS
peripheral nervous system
- rt-PCR
real-time reverse transcription–polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus type 2
Appendix. Authors
Footnotes
COVID-19 Resources: NPub.org/COVID19
CME Course: NPub.org/cmelist
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med 2020;20:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19. Geneva: World Health Organization; 2020. [Google Scholar]
- 3.Johns Hopkins University and Medicine. COVID-19 Map. Johns Hopkins Coronavirus Resource Centre. Available at: coronavirus.jhu.edu/map.html. Accessed April 30, 2020. [Google Scholar]
- 4.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol Epub 2020 Feb 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995–998. [DOI] [PubMed] [Google Scholar]
- 6.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol Epub 2020 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med 2020;14:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicenter PCR-based case-control study. Eur J Neurol Epub 2020 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 2020;12:e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. SSRN Electronic J Epub 2020 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L, Xiong W, Liu D, et al. New-onset acute symptomatic seizure and risk factors in corona virus disease 2019: a retrospective multicenter study. Epilepsia Epub 2020 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol 2020;92:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020;19:383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezpeleta D, García-Azorín D. Manual COVID para el neurólogo general [Internet]. 1st ed. Madrid: SEN (Sociedad Española de Neurología); 2020. Available at: sen.es/attachments/article/2677/Manual_neuroCOVID-19_SEN.pdf. Accessed May 4, 2020. [Google Scholar]
- 16.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44(suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transpl 2020;39:405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paniz-Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol Epub 2020 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt MP, Bolding KA, Wayne CR, et al. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of postinfectious autoimmune encephalitis. Proc Natl Acad Sci 2020;117:6708–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA Epub 2020 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J Epub 2020 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frydrych LM, Bian G, O'Lone DE, Ward PA, Delano MJ. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J Leukoc Biol 2018;104:525–534. [DOI] [PubMed] [Google Scholar]
- 24.Novi G, Mikulska M, Briano F, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord 2020;42:102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med Epub 2020 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther Epub 2020 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou CK, Turtle CJ. Insight into mechanisms associated with cytokine release syndrome and neurotoxicity after CD19 CAR-T cell immunotherapy. Bone Marrow Transpl 2019;54(suppl 2):780–784. [DOI] [PubMed] [Google Scholar]
- 28.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020;382:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung TW, Wong KS, Hui AC, et al. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol 2005;62:1113–1117. [DOI] [PubMed] [Google Scholar]
- 30.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 2020;8:E46–E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
After publication, the data will be made available to other researchers on reasonable request to the corresponding author.