Abstract
Objective
To determine whether MRI-based cerebrovascular reactivity (CVR) can predict cognitive performance independently of Alzheimer pathologic markers, we studied the relationship between cognition, CVR, and CSF-derived β-amyloid42 (Aβ42) and tau in a group of elderly individuals with mixed Alzheimer and vascular cognitive impairment and dementia.
Methods
This was a cross-sectional study of 72 participants 69 ± 8 years of age consisting of individuals with normal cognition (n = 28) and cognitive impairment (n = 44) (including 36 with mild cognitive impairment [MCI] and 8 with mild dementia). CVR was measured with hypercapnia-MRI. Whole-brain CVR (percent blood oxygen level–dependent per 1 mm Hg Etco2) was used to estimate vasodilatory capacity. Montreal Cognitive Assessment (MoCA) scores, cognitive domains scores, and a global composite cognitive score were obtained. AD biomarkers included CSF assays of Aβ42 and tau.
Results
Whole-brain CVR was lower in the impaired (mean ± SE, 0.132 ± 0.006%/mm Hg) compared to the normal (0.151 ± 0.007%/mm Hg) group (β = −0.02%/mm Hg; 95% confidence interval [CI] −0.038 to −0.001). After adjustment for CSF Aβ42 and tau, higher whole-brain CVR was associated with better performance on the MoCA (β = 29.64, 95% CI 9.94–49.34) and with a global composite cognitive score (β = 4.32, 95% CI 0.05–8.58). When the CVR marker was compared with the Fazekas score based on white matter hyperintensities and vascular risk-score in a single regression model predicting the MoCA score, only CVR revealed a significant effect (β = 28.09, 95% CI 6.14–50.04), while the other 2 measures were not significant.
Conclusions
CVR was significantly associated with cognitive performance independently of AD pathology. Whole-brain CVR may be a useful biomarker for evaluating cognitive impairment related to vascular disease in older individuals.
Classification of evidence
This study provides Class II evidence that CVR was significantly associated with cognitive performance independent of AD pathology.

Most older individuals with dementia and mild cognitive impairment (MCI) have mixed brain pathologies, the most common being Alzheimer disease (AD) and vascular cognitive impairment and dementia (VCID).1,2 Biomarkers for VCID pathology are insufficiently established.3,4
VCID pathology is often assessed with white matter (WM) hyperintensities (WMH) or lacunar infarcts.5,6 However, these measures have limitations: quantification is not trivial and may require manual delineation,7 and correlations between WMH and cognitive performance are modest.8,9
Cerebrovascular reactivity (CVR), as determined by MRI, is a recently developed measure of the ability of brain vessels to contract or dilate in response to a vasoactive challenge such as carbon dioxide (CO2) inhalation.10,11 This measure of vessel perfusion reserve may be a useful biomarker in VCID because of its direct relationship to microcirculatory function in that prior evidence suggests that the hemodynamic response to CO2 is mediated primarily by the dilation of small resistance vessels (e.g., arterioles) via several parallel pathways involving pH, K+ channels, and nitric oxide synthase.10 Several reports in the literature have examined CVR among cognitively normal elderly, patients with MCI, and patients with dementia, with some showing a significant group differences12–14 and others reporting no difference among such groups.15–17 However, none of these studies assessed AD biomarkers and thus the interrelationships between CVR and AD biomarkers, and their combined influence on cognitive function has never been examined.
The aim of this study is to determine whether MRI-based CVR can predict cognitive performance independently of Alzheimer pathology. We hypothesize that CVR can predict diagnostic category and cognitive performance in a group of elderly individuals with MCI, mild dementia, and normal cognition. We further hypothesize that these associations are independent of CSF-derived β-amyloid42 (Aβ42) and tau.
Methods
Standard protocol approvals, registrations, and patient consents
This study was conducted under approval by the Institutional Review Board of the Johns Hopkins University School of Medicine. All participants provided informed written consent before participating in the study.
Participants
Seventy-two participants (mean age 69 years, 55.6% women) were recruited in a cross-sectional study design at the Johns Hopkins University School of Medicine between April 2017 and May 2018. Table 1 provides the participant characteristics.
Table 1.
Participant characteristics
Study participants were recruited from several sources: the Johns Hopkins Alzheimer's Disease Research Center (an AD research center), the Memory and Alzheimer's Treatment Center (an outpatient memory clinic), and the Johns Hopkins Comprehensive Diabetes Center (a clinical care and research center). The recruitment from the Diabetes Center was designed to enrich the study population for participants with individuals who had multiple vascular risk factors and thereby broaden the range of CVR values. Ten of the 72 participants enrolled in this study were recruited from the Diabetes Center, of whom 4 were cognitively normal and 6 were impaired.
Clinical and cognitive evaluation included a medical, neurologic, and psychiatric history; a neurologic examination; a psychiatric rating scale for depression; the Clinical Dementia Rating scale; and a comprehensive neuropsychological battery. All participants with existing neurologic conditions, including history of major strokes, brain trauma, multiple sclerosis, or respiratory problems such as chronic obstructive pulmonary disease or asthma, were excluded. All enrolled participants were required to be >50 years of age and to have a study partner who could act as an informant.
Consensus diagnosis
Each individual case was summarized and reviewed for a consensus diagnosis by a clinical team at Johns Hopkins consisting of a psychiatrist, a geriatrician, and a neuropsychologist. The clinical team reviewed a clinical summary prepared for each case that included demographic information; occupation history; family history of dementia; the medical, neurologic, and psychiatric history; and current medication use. The clinical summary also included any evidence of cognitive decline (where relevant) based on the Clinical Dementia Rating interview, administered to each participant and a close family member or friend. The clinical team also reviewed the neuropsychological test results, with z scores for each participant, and an indication of which scores were 0.5 SD or lower than the age and education normative values for each test. From these data, the team determined whether a participant met syndromic criteria for having normal cognition, MCI, or dementia. These clinical diagnostic procedures for generating syndromic diagnoses follow the recommendations of the National Institute on Aging/Alzheimer's Association work groups.18 The clinical team did not generate etiologic diagnoses for the participants.
The participants were divided into 2 groups based on the consensus diagnosis procedures: normal controls (n = 28) and impaired participants (n = 44), which included those with MCI (n = 36) and mild dementia (n = 8). The clinical diagnoses were made without knowledge of the MRI and CSF data.
MRI procedures
MRI scans were performed on a 3T scanner (Achieva, Philips Medical Systems, Best, the Netherlands). A high-resolution (1 × 1 × 1 mm3) T1-weighted sequence was performed for anatomic reference. The T1 sequence used a magnetization-prepared rapid acquisition gradient echo with repetition time (TR) of 8.1 milliseconds, echo time (TE) of 3.7 milliseconds, shot interval of 2,100 milliseconds, inversion time (TI) of 1,100 milliseconds, and duration of 3 minutes 57 seconds.
A fluid-attenuated inversion recovery (FLAIR) image was collected to quantify WMH. FLAIR MRI used TR/TI/TE of 4,800/1,650/301 milliseconds, field of view of 240 × 240 × 165 mm3, and resolution of 1.1 × 1.1 × 1.1 mm2.
CVR, which provides an index of the capacity of brain small vessels to react to vasoactive challenge, was assessed with a procedure based on the inhalation of a gas enriched in CO2, while continuously acquiring blood oxygen level–dependent (BOLD) MRIs.19 Specifically, participants were fitted with a mouthpiece and a nose clip, and CO2-enriched air (5% CO2, 21% oxygen, and 74% nitrogen) was administered with an airbag, with a valve to switch between room air and CO2-rich air. Subjects breathed 70 seconds of room air and 50 seconds of CO2-rich air in an interleaved manner for a total of 430 seconds. Breathing rate and Etco2 were recorded with capnography. MRIs were continuously acquired during the entire 430-second period. The scan parameters were as follows: gradient echo planar imaging, voxel size of 3.4 × 3.4 × 3.8 mm3, matrix size of 64 × 64 × 36, sensitivity encoding (SENSE) factor of 2, TR of 1,500 milliseconds, TE of 21 milliseconds, flip angle of 90°, and number of dynamics 281. These parameters were chosen on the basis of our previous technical development studies.20
Each participant rated the comfort level of the scanning procedure (based on a scale from 0–10 with 10 indicating maximum comfort) during the standard MRI scan and the CVR portion of the scan.
Image processing
CVR data postprocessing was conducted using in-house MATLAB (MathWorks, Natick, MA) scripts reported previously.21 Briefly, the data processing was based on a general linear model (SPM, University College London), in which the BOLD image intensity was the dependent variable and Etco2 time course was the regressor. The hemodynamic delay was included the analysis by performing the regression for 30 times, each with a delay increment of 1,500 milliseconds (i.e., 1 TR). The regression that yields the lowest sum-of-square residual is considered the best fit, and the corresponding fitting coefficients are used to compute CVR. The hemodynamic delay was allowed to vary across individuals and across brain regions. The hemodynamic response function (e.g., gamma variate function) that is often used in task-based fMRI analysis was not included in the model because our previous research suggested that inclusion of the hemodynamic response function yielded less reliable estimates,22 presumably because the change of arterial CO2 during the CVR experiment is substantially slower than the change of neural activity in an fMRI experiment. Both whole-brain and lobar CVR values were obtained. For image normalization, the individual CVR data were transformed into Montreal Neurological Institute template space via T1 magnetization-prepared rapid acquisition gradient echo using the T1-multiatlas toolbox of MRICloud (mricloud.org). For lobar CVR values, the MRICloud tool segmented the brain and defined the following lobar gray matter regions: frontal, parietal, temporal, and occipital lobes. The region-of-interest mask was applied to the BOLD data to obtain region-of-interest time courses, from which CVR was computed. The final CVR is written in the units of percent BOLD signal change per 1 mm Hg of Etco2.
WM CVR was also calculated. We used a procedure developed previously.23 that was similar to the whole-brain CVR processing method described above. The WM mask used for signal averaging was obtained by brain segmentation using MRICloud. WM is known to contain fewer blood vessels; thus, BOLD signal in the WM is less sensitive to physiologic challenges compared to the gray matter. Therefore, the analysis of WM CVR is considered exploratory in this study.
A subspecialty board-certified neuroradiologist reviewed the T2 FLAIR images and generated Fazekas scores (range 0–3) to quantify WMH in deep WM and periventricular WM, respectively.24 The total Fazekas score represented the average of the periventricular and deep WM scores.
Cognitive assessments
The detailed neuropsychological battery mentioned above included a test for global cognition and tests that focused on several cognitive domains. The Montreal Cognitive Assessment (MoCA) was used to evaluate global cognitive status. The remaining tests were divided into 4 cognitive domains, and scores were generated for each cognitive domain by creating a z score for each test score and averaging the z scores within each domain. The domains included (1) verbal episodic memory (Logical Memory delayed-recall; Hopkins Verbal Learning Test recall over trials 1–5), (2) executive function (Digit Span backwards, Trial Making Test Part B, Digit Symbol Test, Stroop Color-Word score), (3) processing speed (Trial Making Test Part A, Stroop Color and Word scores), and (4) language (Multilingual Naming Test, Letter [words that begin with F & L] and Category [animal, vegetables] fluency tasks). A composite overall cognitive score was computed by averaging the 4 domain scores.
CSF collection and assays
CSF was collected via lumbar puncture, and the samples were available in 47 participants. CSF Aβ42 (picograms per milliliter) was estimated with the Lumipulse G1200 assay (Fujirebio, Malvern, PA), for which the intra-assay coefficient of variation was 3.1%.25 CSF tau (picograms per milliliter) was analyzed with the Neurology 3-plex Simoa immunoassay (Quanterix, Lexington, MA) on the SR-X platform. All samples were analyzed over 2 plates with the same lot number, and an average value for the 2 runs was recorded. A CSF standard was run across both plates to measure plate-to-plate variability. The interassay coefficient of variation for CSF tau was 7.1%.26
Vascular risk score
Vascular risk factors consist of multiple domains, some of which are strongly correlated with each other. In this study, the quantification of the vascular risk score (VRS) was guided by recent findings in the Atherosclerosis Risk in Communities (ARIC) Study, a large, longitudinal, community-based study that is currently focused on examining the impact of vascular risks across the life span on late-life cognitive decline (e.g., Gottesman et al.27). Each vascular risk was coded as a binary variable, and the composite score was calculated as the sum of 5 measures: (1) hypertension (1 if current, 0 if remote/absent), (2) hypercholesterolemia (1 if current, 0 if remote/absent), (3) diabetes mellitus (1 if current, 0 if remote/absent), (4) smoking (1 if smoked >100 cigarettes in his/her life, 0 if not), and (5) obesity (body mass index [BMI]; 1 if BMI >30 kg/m2, 0 if not). BMI was calculated as weight in kilograms divided by height in meters squared. This VRS therefore ranges from 0 to 5.27
Statistical analysis
Group differences in continuous variables were assessed by t tests. We used χ2 tests for dichotomous variables. Cross-sectional analyses using linear regression models were performed within the entire group of participants to test associations between CVR and measures of cognition and its domains. These models included age, sex, and education as covariates. SPSS 24.0 was used for the analyses (IBM, Armonk, NY). For cognition, we examined global cognitive status using MoCA score, cognitive domains using domain-specific cognitive scores, and overall cognition using a composite cognitive score by combining domain scores.
Next, the models were adjusted for CSF-derived Aβ42 and tau to determine whether the relationship between CVR and cognition was independent of the level of AD pathology (as measured by CSF biomarkers). The models also explored whether Aβ42 and tau are predictive of cognition. Because clinical trials are typically focused on impaired individuals, the above-described linear regression models were also conducted among only those participants with cognitive impairments (MCI + dementia).
A linear regression model was used to examine the relationship between CVR and VRS, with age and sex as covariates. The relationship of CVR, VRS, and WMH to cognition was also examined together in a single model after adjustment for age, education, sex, and CSF Aβ42 and tau.
For all tests, a value of p ≤ 0.05 was considered significant. Bonferroni correction was performed for tests involving domain-specific cognitive scores because we had 4 cognitive domains.
The primary research question was to evaluate whether CVR was significantly associated with cognitive performance independently of AD pathology. The Classification of Evidence is Class II.
Data availability
The data, methods, and materials used to conduct the research in the article have been documented in the Methods section, and summary measures are provided in table 1. Data not provided in the article because of space limitations will be shared at the request of other investigators for the purposes of replicating procedures and results.
Results
Participants
A total of 72 participants were enrolled in the study (normal n = 28, impaired n = 44). For the purpose of this study, the mild dementia and MCI groups were combined due to the small number of participants in the mild dementia group. Table 1 summarizes participant characteristics. CVR data from 3 participants were excluded in the final analyses due to technical issues. Cognition, CVR, Fazekas score, and VRS were obtained from all 69 participants, while CSF Aβ42 and tau were obtained from 47 participants who had CSF collected. The mean self-reported comfort level (0 = least comfortable, 10 = maximal comfort) for the standard MRI scan was 8.7 ± 1.4 (mean ± SD). The mean comfort level for the CVR scan was 6.5 ± 2.6. The comfort level of the CVR scan was significantly lower than that of the standard MRI (p < 0.001), but all participants were able to complete the CVR scans. Figure 1A shows averaged CVR maps in normal and impaired participants. Figure 1B shows their whole-brain CVR values. CVR values were significantly lower in the impaired relative to the normal group (β = −0.02%/mm Hg; 95% confidence interval [CI] −0.038 to −0.001; p = 0.038) after accounting for age and sex in a regression model.
Figure 1. Comparison of CVR between normal (n = 26) and impaired participants (n = 43).
(A) Averaged cerebrovascular reactivity (CVR) maps for normal and impaired (mild cognitive impairment [MCI] + dementia) participants. (B) Bar plots (mean ± SE) of whole-brain CVR in normal and impaired participants. BOLD = blood oxygen level–dependent.
Associations between CVR and cognition
We first examined the associations between CVR and global cognition. Figure 2 shows a scatterplot between CVR and MoCA score across participants. Table 2 summarizes the linear regression results. The whole-brain CVR showed a positive association with MoCA score after adjustment for age, sex, and education (p = 0.003; table 2 gives β values and 95% CIs). Higher whole-brain CVR was also associated with a better composite cognitive score (p = 0.020, table 2). Next, we examined associations between CVR and individual cognitive domains. Higher whole-brain CVR was significantly associated with better performance in the language domain score (p = 0.016, uncorrected p = 0.004, table 2) but not with executive function (p = 0.22, uncorrected p = 0.059, table 2), episodic memory (p = 0.48, uncorrected p = 0.15, table 2), and processing speed (p = 0.45, uncorrected p = 0.14, table 2).
Figure 2. Scatterplot between CVR and MoCA score across participants (n = 69).

BOLD = blood oxygen level–dependent; CVR = cerebrovascular reactivity; MoCA = Montreal Cognitive Assessment.
Table 2.
Linear regression models for the association of whole-brain CVR with MoCA score, overall cognition, and cognitive domains
We then tested whether the associations between CVR and cognition were independent of AD pathology, as measured by CSF Aβ42 and tau. The results are summarized in table 2. It was found that higher CVR (p = 0.004, table 2) and higher CSF Aβ42 (p = 0.013, table 2), but not CSF tau (p = 0.052, table 2), were independently associated with better MoCA scores after adjustment for age, education, and sex. Similarly, the composite cognitive score was significantly related to CVR (p = 0.048, table 2) and CSF Aβ42 (p = 0.001, table 2), but not CSF tau (p = 0.11, table 2), after adjustment for age, education, and sex. These findings suggest that AD pathology is not a mediating variable (or common cause) explaining the association of CVR and cognition. For domain-specific cognitive scores, after adjustment for CSF Aβ42 and tau, none of the domain scores were associated with whole-brain CVR (processing speed: p = 0.13, uncorrected p = 0.035; language scores: p = 0.10, uncorrected p = 0.027; executive function: p = 0.40, uncorrected p = 0.12; episodic memory: p = 0.86, uncorrected p = 0.39) (table 2). We also conducted additional analyses to examine the relationship between CVR and AD pathology and observed that CVR itself was not associated with CSF Aβ42 (p = 0.70) or CSF tau (p = 0.81) after controlling for age and sex.
We then repeated these analyses in the subset of participants with cognitive impairments (MCI + mild dementia). These results are summarized in table 3. The association between CVR and MoCA score was significant after adjustment for age, sex, and education (p = 0.017, table 3). Similarly, the association between CVR and composite cognitive score was significant (p = 0.033, table 3). For domain cognitive scores, the association between CVR and the language domain was significant (p = 0.024, uncorrected p = 0.006, table 3) but not for the other domains (table 3).
Table 3.
Associations of whole-brain CVR with MoCA score, overall cognition, and cognitive domains within the impaired group (MCI + dementia) (n = 44)

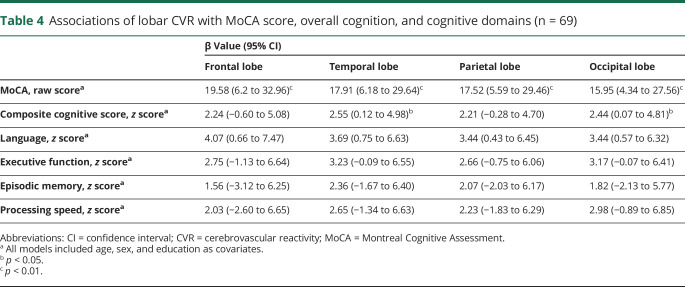
We also analyzed the associations between lobar CVR and the cognitive scores. Overall, the lobar results were similar to that of whole-brain CVR in that CVR was associated with MoCA score and with several neuropsychological scores (table 4). Analyses of gray matter CVR were similar to the lobar findings (data not shown).
Table 4.
Associations of lobar CVR with MoCA score, overall cognition, and cognitive domains (n = 69)
Although CVR in the WM is less frequently reported due to fewer blood vessels in this tissue type and thereby lower BOLD signal change, as an exploratory analysis, we also examined WM CVR and its relationship to diagnostic category and cognition (table 5). WM CVR was significantly lower in the impaired relative to the normal group (0.086 ± 0.003, mean ± SE, for the impaired group and 0.105 ± 0.005 for the normal group; p = 0.001) after accounting for age and sex. WM CVR was also significantly associated with MoCA score (p = 0.002, table 5) and composite cognitive score (p = 0.19, table 5) after adjustment for age, sex, and education. The association of WM CVR with MoCA score remained significant (p = 0.02, table 5) after adjustment for CSF Aβ42 and tau but not with composite cognitive score (p = 0.097, table 5). WM CVR was not associated with any of the domain cognitive scores.
Table 5.
Linear regression models for the association of WM CVR with MoCA score, overall cognition, and cognitive domains, and after adjustment for Aβ42 and tau
Relationship among CVR, WMH, and cognition
Next, we examined whether CVR accounts for variations in cognition beyond those explained by a WMH Fazekas score. It was found that whole-brain CVR and MoCA score remained significant (β = 29.69, 95% CI 9.81–49.56, p = 0.004) after adjustment for Fazekas score, age, sex, education, and CSF Aβ42 and tau. Similarly, whole-brain CVR was significantly associated with composite cognitive scores (β = 4.39, 95% CI 0.331–8.45; p = 0.035) after adjustment for Fazekas score, age, education, sex, and CSF Aβ42 and tau. Fazekas scores were not associated with CVR (p = 0.92).
In an examination of the association between WMH and cognitive function after adjustment for age, sex, and education, Fazekas scores were not associated with the MoCA score (p = 0.494), composite cognitive score (p = 0.092), or any of the domain cognitive scores.
Associations among CVR, VRS, WMH, and MoCA score
CVR was not associated with the composite VRS (p = 0.703). When all 3 candidates of the VCID marker, i.e., CVR, VRS, and Fazekas score, were included in a single regression model predicting the MoCA score (after adjustment for age, education, sex, and CSF Aβ42 and tau), only CVR revealed a significant effect (β = 28.09, 95% CI 6.14–50.04; p = 0.014), while the other 2 measures were not significant (VRS: p = 0.714; WMH: p = 0.507).
When the association between VRS and cognitive function was examined separately, the VRS scores were not associated with the MoCA score (p = 0.349), composite cognitive score (p = 0.437), or any of the domain cognitive scores.
Discussion
CVR measures the vasodilatory capacity of cerebral vasculature. Therefore, this index may provide a sensitive marker for VCID. Measurement of CVR has previously been restricted to relatively small cohorts of patients due to technical challenges such as the need to inject a pharmacologic agent, acetazolamide, and the use of radiotracers in PET28 or SPECT.17,29–34 Recent advances in MRI technologies made by our group and others have allowed larger-scale, longitudinal follow-up studies of CVR.21,35 To the best of our knowledge, the present report is the most comprehensive study of the relationship of CVR to cognitive impairment, in that CVR was compared not only between diagnostic categories but also to global cognition and domain-specific cognitive scores. We demonstrated that CVR is associated with 2 separate measures of global cognitive performance, the MoCA score and a composite cognitive score. These results were seen within the group as a whole, as well as in the participants with cognitive impairments, and are consistent with earlier reports of positive associations between CVR and global cognition measures such as the Mini-Mental State Examination.36 In addition, CVR was significantly associated with a language domain score. After adjustment for Aβ42 and tau, CVR was further associated with a processing speed score with an uncorrected p = 0.035. Poor performances in language, processing speed, and executive function are common manifestations in vascular pathologic conditions,28,37–41 whereas impairments in episodic memory are characteristic of individuals with Alzheimer dementia. Thus, CVR may have value in dissecting out the vascular contribution to cognitive impairment in mixed vascular/AD pathology.
Another aspect of this study is that the influence of CVR on cognition was examined in the context of concurrent AD pathology as measured by CSF Aβ42 and tau, which has not been done in previous studies.13,16,22,29,34 Given that most patients with cognitive impairment are likely to have a mixed pathology including AD and VCID, simultaneous investigation of these 2 types of biomarkers is important to better elucidate the underlying neurobiology in these patients. Our results showed that vascular and AD pathologic biomarkers can independently predict portions of variance in cognitive function. Among the AD markers, CSF Aβ42 seemed to be the most significantly associated with cognitive status. It was positively associated with all cognitive scores assessed in this study (table 2). CSF-derived tau was not significantly associated with any of the cognitive scores in either the entire sample or the impaired participants, although the coefficients in the linear regression models were always negative in sign, as might be expected.
We also showed that the association between CVR and cognition remained significant within the impaired group. This is relevant for future clinical trials of vascular cognitive impairment in which investigators may be tasked to select appropriate patients with cognitive impairment for the intervention. CVR may be useful in identifying patients whose impairment is attributed primarily to VCID as opposed to other pathologies such as AD or Lewy body disease. In addition, the impaired group may be expected to have a narrower dynamic range in terms of cognition and CVR compared to the whole-sample data. Thus, the ability of CVR to predict cognition within the impaired group suggests a high sensitivity of this measure to cognitive performance.
We did not observe a significant association between the VRS and CVR or cognition. This could be due to at least 3 reasons. The first is small effect size of VRS. Most prior studies that reported a significant impact of vascular risks on cognition have used a relatively large sample size (ranging from 223–15,744 participants).27,42 Thus, with a sample size of 69, we may be underpowered to detect a relationship between VRS and CVR. The second reason is delayed effect of vascular risks. Studies have reported significant associations between increased vascular risks in midlife and poorer cognition later in life,27 whereas their relationships at older ages are attenuated.43 Because VRS was not assessed in our participants early in their life, e.g., at middle age, this may have further reduced our ability to observe a significant relationship. The last reason is the effects of vascular risk–related medications. Most of the participants were undergoing medical treatments for their vascular conditions, e.g., antihypertensive drugs, and the treatment effects were not considered due to the diversity of their medications. This may have added confounding factors weakening the relationship of VRS to CVR and cognition. Nonetheless, from the results of studying the relationship of cognitive function to all 3 variables, i.e., CVR, WMH, and VRS, in a single model and comparing the associations between cognition and each of the variables, CVR showed the strongest association with cognition.
As a technical note, we point out that the measurement of CVR in this study required CO2 inhalation inside an MRI scanner, which involved additional equipment and personnel. There are ongoing efforts in the MRI field to develop CVR methods that do not require gas delivery (e.g., breath-hold– or resting-state–based methods),44,45 which may improve the tolerability and applicability of the CVR biomarker in future multisite studies or clinical trials of SVD. In addition, the present study used a BOLD-based MRI acquisition in the CVR experiment due to its high sensitivity. However, BOLD signal is known to reflect a complex interplaying between several physiologic parameters, and its relationship to Etco2 has been suggested to be nonlinear.46 Ongoing work in our laboratory aims to use CBF-based CVR to study cognitive function in patients with small vessel disease.
It should be noted that, although the present study has focused on small vessel disease and individuals with a history of major stroke were excluded from the study, large vessel pathology and small vessel pathology often coexist in patients, and poststroke dementia is a significant cause of vascular cognitive impairment.47 Indeed, prior work has shown that CVR is also a highly sensitive marker in large vessel diseases such as atherosclerosis and moyamoya disease.48,49 The mechanism of the CVR sensitivity in large vessel disease is thought to be related to the autoregulation of the brain in the presence of large vessel stenosis, resulting in compensatory dilation of small vessels at baseline and thereby reducing vascular dilatory capacity on CO2 stimulation.50
One of the limitations in the current study pertains to the modest sample size and relatively limited range of vascular pathology represented in the participants. We excluded participants with stents and prior cardiovascular or neurologic events such as major strokes that could have affected cognitive outcomes, thus precluding participants with the highest vascular burden. The majority of the participants were under medical care with controlled diabetes mellitus, hypertension, or hypercholesterolemia, thus limiting relationships between vascular risks and the measures of brain physiology. In addition, although the VRS used in our study has been used in studies by other authors, residual vascular risk may still be possible. Finally, while this study has controlled for AD pathologic markers such as CSF Aβ42 and tau, we have not ruled out the possibility that CVR actually reflects other pathologic mechanisms of dementias such as Lewy body pathology,1 which need to be tested in future investigations.
CVR, as a measure of the dilatory function of the brain vessel, can predict cognitive function independently of AD pathology. CVR was more sensitive in predicting cognition compared to WMH or vascular risk factors. CVR is a promising candidate biomarker for cognitive impairment due to vascular diseases.
Glossary
- Aβ42
β-amyloid42
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- BOLD
blood oxygen level–dependent
- CI
confidence interval
- CVR
cerebrovascular reactivity
- FLAIR
fluid-attenuated inversion recovery
- MCI
mild cognitive impairment
- MoCA
Montreal Cognitive Assessment
- TE
echo time
- TI
inversion time
- TR
repetition time
- VCID
vascular cognitive impairment and dementia
- VRS
vascular risk score
- WMH
white matter hyperintensities
Appendix. Authors


Footnotes
Class of Evidence: NPub.org/coe
Study funding
Partly supported by NIH UH2 NS100588.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 2.Beeri MS, Lin HM, Sano M, et al. Association of the haptoglobin gene polymorphism with cognitive function and decline in elderly African American adults with type 2 diabetes: findings from the Action to Control cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Study. JAMA Netw Open 2018;1:e184458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer's disease. Acta Neuropathol 2016;131:687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baykara E, Gesierich B, Adam R, et al. A novel imaging marker for small vessel disease based on skeletonization of white matter tracts and diffusion histograms. Ann Neurol 2016;80:581–592. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan A, Godin O, Jouvent E, et al. Impact of MRI markers in subcortical vascular dementia: a multi-modal analysis in CADASIL. Neurobiol Aging 2010;31:1629–1636. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prins ND, van Straaten EC, van Dijk EJ, et al. Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology 2004;62:1533–1539. [DOI] [PubMed] [Google Scholar]
- 8.Knopman DS, Griswold ME, Lirette ST, et al. Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke 2015;46:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 2002;52:335–341. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. Neuroimage 2019;187:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juttukonda MR, Donahue MJ. Neuroimaging of vascular reserve in patients with cerebrovascular diseases. Neuroimage 2019;187:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yezhuvath US, Uh J, Cheng Y, et al. Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimer's disease. Neurobiol Aging 2012;33:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glodzik L, Rusinek H, Brys M, et al. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab 2011;31:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvestrini M, Pasqualetti P, Baruffaldi R, et al. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 2006;37:1010–1015. [DOI] [PubMed] [Google Scholar]
- 15.Thomas BP, Sheng M, Tseng BY, et al. Reduced global brain metabolism but maintained vascular function in amnestic mild cognitive impairment. J Cereb Blood Flow Metab 2017;37:1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richiardi J, Monsch AU, Haas T, et al. Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 2015;36:33–41. [DOI] [PubMed] [Google Scholar]
- 17.Jagust WJ, Eberling JL, Reed BR, Mathis CA, Budinger TF. Clinical studies of cerebral blood flow in Alzheimer's disease. Ann NY Acad Sci 1997;826:254–262. [DOI] [PubMed] [Google Scholar]
- 18.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Demen 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Vis Exp 2014:52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi H, Thomas BP, Peng SL, Liu H, Lu H. On the optimization of imaging protocol for the mapping of cerebrovascular reactivity. J Magn Reson Imaging 2016;43:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011;21:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yezhuvath US, Lewis-Amezcua K, Varghese R, Xiao G, Lu H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed 2009;22:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas BP, Liu P, Park DC, van Osch MJ, Lu H. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J Cereb Blood Flow Metab 2014;34:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 25.Pereson S, Vandersteen A, Dekeyser F, et al. From INNOTEST to the fully automated chemiluminescent b-amyloid(1-42) and total tau assays on the LUMIPULSE® G instrument series: taking quantification of Alzheimer's disease CSF biomarkers to the next level. Alzheimers Demen 2015;11:P868. [Google Scholar]
- 26.Lambert J, Chang L, Song L, et al. Comparison of two platforms quantitating Fg/ml neurological biomarkers using single molecule arrays and digital ELISA: the Benchtop Reader Sr-X™ and the fully automated analyzer Hd-1™. Alzheimers Demen 2018;14:P706. [Google Scholar]
- 27.Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol 2003;2:89–98. [DOI] [PubMed] [Google Scholar]
- 29.Oishi M, Mochizuki Y, Takasu T. Regional differences in cerebrovascular reactivity to acetazolamide in Alzheimer's disease. J Clin Neurosci 1999;6:380–381. [DOI] [PubMed] [Google Scholar]
- 30.Kuwabara Y, Ichiya Y, Otsuka M, Masuda K, Ichimiya A, Fujishima M. Cerebrovascular responsiveness to hypercapnia in Alzheimer's dementia and vascular dementia of the Binswanger type. Stroke 1992;23:594–598. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi F, Meyer JS, Yamamoto M, Sakai F, Shaw T. Noninvasive regional cerebral blood flow measurements in dementia. Arch Neurol 1980;37:410–418. [DOI] [PubMed] [Google Scholar]
- 32.Stoppe G, Schutze R, Kogler A, et al. Cerebrovascular reactivity to acetazolamide in (senile) dementia of Alzheimer's type: relationship to disease severity. Dementia 1995;6:73–82. [DOI] [PubMed] [Google Scholar]
- 33.Bonte FJ, Devous MD Sr, Reisch JS, et al. The effect of acetazolamide on regional cerebral blood flow in patients with Alzheimer's disease or stroke as measured by single-photon emission computed tomography. Invest Radiol 1989;24:99–103. [DOI] [PubMed] [Google Scholar]
- 34.Knapp WH, von Kummer R, Kubler W. Imaging of cerebral blood flow-to-volume distribution using SPECT. J Nucl Med 1986;27:465–470. [PubMed] [Google Scholar]
- 35.Peng SL, Chen X, Li Y, Rodrigue KM, Park DC, Lu H. Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. Neuroimage 2018;174:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantin S, Villien M, Moreaud O, et al. Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. Neuroimage 2011;58:579–587. [DOI] [PubMed] [Google Scholar]
- 37.Garrett KD, Browndyke JN, Whelihan W, et al. The neuropsychological profile of vascular cognitive impairment—no dementia: comparisons to patients at risk for cerebrovascular disease and vascular dementia. Arch Clin Neuropsychol 2004;19:745–757. [DOI] [PubMed] [Google Scholar]
- 38.Tierney MC, Black SE, Szalai JP, et al. Recognition memory and verbal fluency differentiate probable Alzheimer disease from subcortical ischemic vascular dementia. Arch Neurol 2001;58:1654–1659. [DOI] [PubMed] [Google Scholar]
- 39.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 2004;62:912–919. [DOI] [PubMed] [Google Scholar]
- 40.Roman GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci 2004;226:81–87. [DOI] [PubMed] [Google Scholar]
- 41.Jokinen H, Kalska H, Mantyla R, et al. Cognitive profile of subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry 2006;77:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabin JS, Schultz AP, Hedden T, et al. Interactive associations of vascular risk and beta-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard Aging Brain Study. JAMA Neurol 2018;75:1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 2018;91:e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golestani AM, Wei LL, Chen JJ. Quantitative mapping of cerebrovascular reactivity using resting-state BOLD fMRI: validation in healthy adults. Neuroimage 2016;138:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bright MG, Murphy K. Reliable quantification of BOLD fMRI cerebrovascular reactivity despite poor breath-hold performance. Neuroimage 2013;83:559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhogal AA, Siero JC, Fisher JA, et al. Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. Neuroimage 2014;98:296–305. [DOI] [PubMed] [Google Scholar]
- 47.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donahue MJ, Ayad M, Moore R, et al. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J Magn Reson Imaging 2013;38:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandell DM, Han JS, Poublanc J, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 2008;39:2021–2028. [DOI] [PubMed] [Google Scholar]
- 50.Donahue MJ, Achten E, Cogswell PM, et al. Consensus statement on current and emerging methods for the diagnosis and evaluation of cerebrovascular disease. J Cereb Blood Flow Metab 2018;38:1391–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data, methods, and materials used to conduct the research in the article have been documented in the Methods section, and summary measures are provided in table 1. Data not provided in the article because of space limitations will be shared at the request of other investigators for the purposes of replicating procedures and results.