Abstract
Objective
To define the radiologic features and natural history of nonoptic pathway tumors (non-OPTs) in children with neurofibromatosis type 1 (NF1).
Methods
We performed a retrospective cross-sectional analysis of 64 children with NF1 harboring 100 probable non-OPTs. Age at diagnosis, sex, tumor location, number of tumors, symptomology, concurrent OPT, radiographic progression (defined as qualitative and quantitative increases in size), and treatment were assessed. Tumor volumes were measured from initial presentation until treatment or end of disease progression.
Results
Sixty-three percent of probable non-OPTs progressed over time, where radiographic progression was concomitantly associated with clinical progression. Fifty-two percent of patients had incidentally identified probable non-OPTs. Twenty-five percent of patients were symptomatic at initial diagnosis, all of whom harbored tumors that grew on subsequent scans and required tumor-directed therapy. There were no clinical differences between probable non-OPTs localized to the brainstem vs other locations with respect to age, sex, concurrent optic pathway glioma, symptomology, and treatment. The average time from diagnosis to stabilization or decrease in tumor size was 2.34 years (SD, 2.15 years). Nineteen biopsied lesions were all histopathologically confirmed as tumor. Six children (9%) had deep extensive tumors, who presented earlier (mean age at diagnosis, 3.88 years), required multiple treatments, and had a shorter mean progression-free survival (48 months).
Conclusions
Over half of children with NF1 in this study developed probable non-OPTs, the majority of which were clinically and radiographically progressive. While brainstem and nonbrainstem gliomas share similar clinical features and natural history, deep extensive tumors comprise a distinct aggressive group of tumors that warrant close attention.
Neurofibromatosis type 1 (NF1; OMIM 162200) is one of the most common cancer predisposition syndromes, in which affected individuals develop benign and malignant tumors affecting the nervous system.1 In addition to the hallmark peripheral nerve sheath tumors, there is an increased incidence of brain tumors, which are typically low-grade gliomas2 involving the optic pathway (optic pathway gliomas [OPGs]; 15% of children with NF1),3 brainstem,4 as well as other locations within the CNS.5–8
The development of uniform vision screening methods,9 coupled with the identification of potential risk factors for NF1-OPG clinical progression, including patient sex (female),10,11 tumor location (postchiasmal),12 and patient age (<2 years),12 have improved treatment decision-making.13 In comparison, far less is known about nonoptic pathway tumors (non-OPTs). Prior studies investigating these tumors arising outside of the optic pathway described relatively few patients (20–44 patients), with variations in the reported frequencies of these tumors in individuals with NF1.2,6–8,14 In addition, the lack of consistent radiographic criteria to distinguish gliomas from T2 hyperintensities has limited progress in this area.
For this reason, we leveraged the expertise of 4 NF1 tertiary referral centers to develop uniform radiologic criteria to classify lesions as probable tumor when they have MRI characteristics suspicious for tumor.4 Using this classification scheme, we previously found that 39 of 68 children (∼60%) who underwent brain MRI harbored lesions radiologically classified as probable tumors outside of the optic pathway.8 Moreover, many of these probable tumors underwent treatment, arguing that more detailed analyses are necessary to characterize these commonly encountered brain lesions in this at-risk population. To address this knowledge gap, we systematically characterized probable non-OPTs in the largest series of children with NF1 reported to date.
Methods
Study population
This study consisted of a retrospective analysis of the electronic medical records of 64 participants with NF1 and a brain lesion classified as a probable tumor outside of the optic pathway who were followed at St. Louis Children's Hospital (Washington University School of Medicine [WUSM]) over the course of a 19-year period (2000–2019). This study was approved by the institutional review board (IRB) at WUSM. All participants included in the study had a diagnosis of NF1 based on NIH Consensus Development criteria.15 Every patient in this study was continuously followed by either D.H.G. or S.M.M., and only 2/64 (3%) patients were lost to follow-up. The remaining 62 patients (97%) are still actively followed by either D.H.G. or S.M.M. In accordance with the clinical practice at most tertiary care NF1 subspecialty programs, individuals included in this study did not undergo baseline or routine surveillance MRIs. Rather, they received MRIs when there was concern for new clinical signs/symptoms, worsening of preexisting clinical signs/symptoms, or when there was a history of a known brain tumor. Thus, the frequency of the MRIs obtained varied from patient to patient based on his or her individual needs. Each patient included in this study had at least 5 brain MRIs of record and the MRI protocols used for tumor surveillance were similar over the duration of this study.
Standard protocol approvals, registration, and patient consents
The WUSM IRB does not require full board approval or patient consent for minimal risk retrospective studies.
Assessment of probable tumors
Probable tumors were identified using radiographic criteria developed by 4 tertiary NF referral centers to analyze brainstem gliomas in children with NF1.4,8 Lesions were identified as being probable non-OPTs if they met at least 1 of the following radiologic criteria: (1) a T2-hyperintense lesion with T1-hypointensity greater than cortical gray matter, (2) a T2-hyperintense lesion with mass effect, or (3) an enhancing lesion (figure 1). Conversely, a nonspecific T2 hyperintensity was defined as a lesion that had no associated T1 hypointensity, no mass effect, and no contrast enhancement. Tumor size was calculated using the ABC/2 method, which has been used and validated as a clinically applicable tool to measure intracerebral hemorrhage and volumes of ischemic infarctions in patients with stroke, as well as tumor volumes of meningiomas and vestibular schwannomas.16–18 An experienced neuroradiologist (M.S.G.) trained J.M. to measure probable tumor volumes. Probable tumor volumes were measured by a single investigator (J.M.) from the time of initial diagnosis on MRI to the time at which there was either no change or a decrease in tumor size on MRI. This included changes in size that occurred as a result of tumor treatment or in the absence of tumor treatment. The time to initial change in tumor size, as well as the time to change in tumor size on subsequent MRI scans, were measured. Radiographic progression was established when lesions showed both (1) an unbiased (i.e., blinded to clinical data or MRI report) qualitative increase in the size of the probable tumor on MRI as determined by J.M. and (2) an objective quantitative increase in the size of the probable tumor on at least 1 dimension as measured on the fluid-attenuated inversion recovery sequence; changes in contrast enhancement were not factored into the assessment of progression. Discrepancies or uncertainties about particular lesions were arbitrated by an experienced neuroradiologist (M.S.G.).
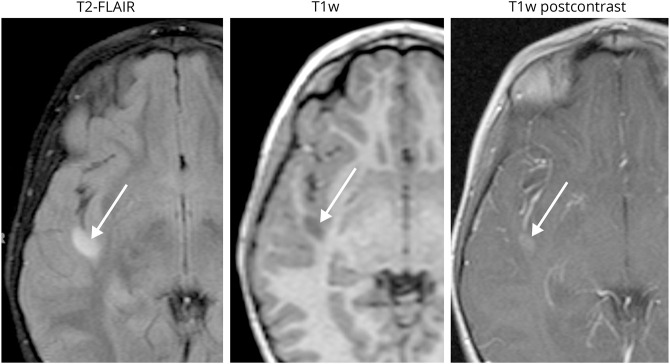
Figure 1. Radiographic appearance of a typical non-optic pathway tumor.
This right temporal lobe lesion exhibits slight surrounding mass effect, T1 signal hypointensity greater than that of gray matter, and mild enhancement. This mass enlarged radiologically and was subsequently resected. Following histopathology analysis, the lesion was diagnosed as a WHO grade II diffuse astrocytoma.
Record review and data collection
Data analyzed included the age at tumor diagnosis, patient sex (male/female), total number of probable non-OPTs (both brainstem and nonbrainstem), tumor location (basal ganglia, cerebellum, medulla, midbrain, entire brainstem, pons, temporal lobe, thalamus, frontal lobe, intraventricular, and deep extensive tumors), clinical signs/symptoms, presence of a concurrent optic pathway glioma (yes/no), family history of NF1 (yes/no), treatment (chemotherapy, radiation, or surgery), and average length of follow-up from the initial MRI scan at time of diagnosis to the last MRI scan of record.
Statistical analysis
Continuously distributed data adhering to conventional normality assumptions were reported as mean and SD, and compared using analysis of variance methods. Nonparametric data were analyzed using the Mann-Whitney U test. Categorical variables were reported as frequencies and proportions, and were compared using odds ratios (ORs).
Data availability
All de-identified data are available on request.
Results
A total of 64 patients were included in this study, comprising 34 female and 30 male patients (table 1). The median age at initial tumor diagnosis of all patients was 6.0 years, with an age range of 0.8–17.0 years. These patients harbored a total of 106 lesions, which were classified as probable tumors outside of the optic pathway (probable non-OPT). Thirty-three of the 64 patients (52%) had probable non-OPTs that were discovered as incidental findings, while 31 patients (48%) harbored probable non-OPTs on MRI following clinical indications of a suspected brain tumor. There were 6 hypothalamic tumors, of which 5 changed in size over time. All of these tumors appeared distinct on MRI, but only occurred in patients with an OPG. Since hypothalamic gliomas may represent an extension of concurrent OPGs, they were excluded from further analysis. Of the remaining 100 probable non-OPTs included in analysis, a greater proportion increased in size over time (radiographically progressive) than those that remained unchanged (stable) (63% vs 37%). Among the symptomatic progressive non-OPTs, there was a correlation between radiographic progression and clinical progression. Of the 16 patients with clinical signs/symptoms at presentation, changes on MRI were associated with worsening clinical signs/symptoms in all but 1 patient. This single patient initially presented with only developmental delay. While there was no association between patient sex or a positive family history of NF1 and radiographic progression, patients tended to have a progressive non-OPT if they had a concurrent OPG (42% vs 20%; OR, 1.1 [95% confidence interval (CI), 0.6–2.1]). Patients with progressive tumors that increased in size over time were slightly more likely to have distinct probable tumors in multiple locations (68% vs 57%; OR, 1.3 [95% CI 0.5–2.9]).
Table 1.
Study demographics
Clinical signs/symptoms and treatment
Progressive non-OPTs were more likely to present with clinical signs or symptoms, and none of the patients with stable non-OPTs had clinical signs or symptoms (25% vs 0%; OR, 26.1 [95% CI 1.5–448.5]). Presenting clinical signs and symptoms included headache, nausea/vomiting, seizures, cranial nerve abnormalities, ataxia, hemiparesis, and gait abnormalities. Headache was the most common clinical symptom (56%), followed by ataxia and gait disturbances (22%). There was no association between the location of a tumor and any specific clinical signs/symptoms. In this regard, probable non-OPTs in different locations frequently presented with the same clinical signs/symptoms.
Progressive non-OPTs were also more likely to be treated than stable probable tumors, since no patients with only stable probable tumors received treatment (66% vs 0%). Patients with progressive non-OPTs were treated due to the presence of clinical signs/symptoms or rapid growth. In total, 27 of 63 patients (43%) underwent treatment with chemotherapy, radiation, or surgical resection, and the type of treatment varied by tumor location. These 27 patients do not include those who only underwent ventriculoperitoneal shunt placement or endoscopic third ventriculostomy specifically for hydrocephalus (n = 4, including 3 with midbrain non-OPTs and 1 cerebellar non-OPT). Only 4 of 17 (24%) patients with basal ganglia probable tumors underwent treatment: 3 of whom were receiving chemotherapy for a concurrent OPG, and 1 of whom underwent surgery and chemotherapy due to concerns for potential hemiparesis. Comparatively, 6 of 12 (50%) individuals with probable non-OPTs in the cerebellum underwent surgical resection, both with (n = 4) and without (n = 2) concurrent chemotherapy.
In total, 19 of the 100 lesions classified as probable tumor were assessed with histopathology. In symptomatic patients, 11 were WHO grade I tumors and 2 were WHO grade II tumors (1 patient who initially had a WHO grade II tumor later developed a primitive neuroectodermal tumor [PNET]). In comparison, 4 asymptomatic probable tumors were classified as WHO grade I tumors and 2 were WHO grade II tumors. Importantly, all biopsied probable tumors were pathologically confirmed to be neoplasm. There was no association between tumor grade and progression-free survival (PFS) across all tumors included in this study (the average PFS of WHO grade I tumors was 60 months and the average PFS of WHO grade II tumors was 87 months).
Brainstem gliomas versus nonbrainstem gliomas
The majority of lesions classified as probable non-OPTs were located outside of the brainstem (68/100 [68%]; table 2). The median age at tumor diagnosis was 6 years (range, 1–17 years) for probable non-OPTs located both within and outside of the brainstem. There were no statistically significant differences between brainstem and nonbrainstem probable tumors with respect to the presence of a concurrent OPG (63% vs 66%), multiple probable tumors (63% vs 77%), or clinical signs/symptoms (13% vs 16%). A similar percentage of brainstem and nonbrainstem probable tumors were progressive (56% vs 66%), with comparable average times to initial change on MRI (22 months vs 17 months). In addition, a similar percentage of brainstem and nonbrainstem probable tumors underwent treatment (22% vs 21%). Thus, there were no clinically significant differences observed between brainstem and nonbrainstem probable tumors.
Table 2.
Brainstem gliomas compared to nonbrainstem tumors

Probable tumors that increased in size over time
Of the probable non-OPTs that progressed over time (n = 63), the majority were located in the basal ganglia (n = 17 [27%]), cerebellum (n = 12 [19%]), medulla (n = 9 [14%]), midbrain (n = 7 [11%]), or diffusely across the bilateral temporal lobes, thalami, and basal ganglia (classified as deep extensive tumors, n = 6 [9%]). The average time to initial change in size on MRI was 16 months across all locations, with a range of 1–125 months. Medullary, midbrain, thalamic, and cerebellar probable tumors exhibited the slowest growth rates, with an average time to change on MRI of 23 months. In striking contrast, deep extensive tumors had the shortest average time to change on MRI (6 months). Probable tumors in the cerebellum had the largest mean percent volume change (251%; SD, 633) from the initial MRI to the last MRI or the MRI prior to treatment. There was no statistically significant difference in the average time to initial change in size on MRI, the percentage of symptomatic non-OPTs, mean percent tumor volume change from the initial MRI to the last MRI or the MRI prior to tumor treatment, or the percentage of progressive non-OPTs that required treatment among the different locations of the progressive non-OPTs.
Deep extensive tumors
In this study, we propose that “deep extensive tumors” involving the bilateral temporal lobes, thalami, and basal ganglia represent a distinct clinical and radiographic entity (n = 6 [9%]; table 3 and figure 2). These lesions were evenly distributed between the sexes, but occurred in slightly younger patients, with an average age at diagnosis of 3.88 years (compared to an average of 6.78 years across all locations). Each of these patients had a concurrent bilateral OPG. However, even though these deep extensive tumors were contiguous with the bilateral OPGs, they involved many structures outside of the typically defined optic pathway (e.g., basal ganglia and hippocampi) and were radiographically more progressive than typical OPGs (as well as other probable non-OPTs). Four patients were symptomatic at the time of diagnosis, with 1 patient presenting with developmental regression and epilepsy, 1 with headache and epilepsy, 1 with headache, and 1 only with developmental regression (table 3). All deep extensive tumors were clinically progressive, and there was a trend towards deep extensive tumors being more likely to be clinically progressive when compared to those arising in any other location (OR, 8.48 [95% CI, 0.5–155.0]). Patients with these tumors had the shortest average time to change in size on MRI (6 months, range 1–19 months), and underwent multiple rounds of treatment with a combination of different chemotherapeutic agents alone or chemotherapy plus surgery. Three of these tumors were WHO grade I tumors (pilocytic astrocytomas), 1 tumor was a WHO grade II glioma (diffuse astrocytoma), and 1 tumor was a WHO grade IV PNET. One patient did not undergo biopsy. The PFS for patients with these tumors following the last treatment was 48 months. There was no significant difference in the average PFS between those who had a WHO grade I (60 months) tumor and those who had a WHO grade II tumor (87 months). The patient with the WHO grade IV PNET was a boy who presented at the age of 7 years with headaches, nausea, and vomiting as well as epilepsy. He underwent multiple surgical resections, multiple rounds of chemotherapy, and radiation. He died from complications of his tumor 9 years after his initial presentation, at 16 years of age.
Table 3.
Deep extensive tumors in children with neurofibromatosis type 1 (NF1)

Figure 2. Fat-suppressed fluid-attenuated inversion recovery images of a deep extensive tumor in a child with neurofibromatosis type 1 involving the bilateral temporal lobes, thalami, and basal ganglia.
Discussion
Extra-optic pathway tumors (non-OPTs) represent an underrecognized clinical entity in children with the NF1 cancer predisposition syndrome. Despite the fact that these tumors account for approximately one-third of all CNS neoplasms in the pediatric NF1 population,7 few studies have systemically characterized these tumors with respect to frequency, location, natural history, and treatment. In this regard, several groups have reported that non-OPTs occur in 33%–57% of children with NF1,7,8 undergoing treatment in 23%–28% of cases.4,8 Given the frequency of these brain tumors in this population, we applied uniform radiographic criteria to evaluate the clinical characteristics and natural history of 100 probable non-OPTs from 64 children with NF1. This study raises several important points relevant to the management of children with NF1 and probable non-OPTs.
First, consistent with the designation of NF1 as a brain tumor predisposition syndrome, more than 60% of children with non-OPTs had a concurrent OPG, and 64% harbored more than 1 probable brain tumor. In this regard, children with OPGs are at increased risk for development of multiple tumors outside of the optic pathway, and the majority of these tumors will increase in size over time. In addition, in contrast to a prior study reporting that 14% of children and adults with NF1 and a non-OPT harbored more than 1 CNS tumor, our study found that 64% of children with NF1 and a non-OPT had more than 1 lesion classified as a probable tumor.14 This is likely in part due to the use of more sensitive, uniform radiologic criteria. While these radiographic criteria will require independent validation by other centers beyond the 4 centers in the original study,4 it should be appreciated that all 19 non-OPT lesions biopsied in this study were in fact pathologically confirmed to be tumor, the majority of tumors exhibited radiologic progression over time, and of those that progressed, the majority were treated. It is therefore likely that the majority of the lesions classified as probable tumor on MRI were indeed tumors. While more accurate estimates would require sampling of all T2-hyperintense lesions in this population, brain biopsy of asymptomatic patients is not currently part of routine medical care of patients with NF1 and would therefore not be an ethically permissible study.
While the overall mortality from these tumors in children with NF1 is low (1.6% in this study), the high incidence of intracranial neoplasms in children with NF1 is of particular importance, given that 42% of the children in our cohort eventually underwent tumor-directed treatment due to clinical symptoms or rapid radiographic progression. Interestingly, of the 27 individuals in this study with multiple probable non-OPTs that progressed over time, only 1 patient had multiple tumors develop and grow simultaneously. The remainder of patients had probable non-OPTs that developed and changed size at different times, with each individual probable tumor exhibiting a unique growth trajectory. This finding illustrates the importance of closely monitoring each individual probable tumor independently, as within a single patient, individual probable non-OPTs can vary in size, location, progression, and need for treatment.
For the most part, non-OPTs arising outside of the brainstem exhibited many similarities with their brainstem glioma counterparts, and, as a group, shared clinical characteristics that can guide their surveillance and clinical management. Analogous to brainstem gliomas, non-brainstem probable tumors occurred in slightly older children than those with OPGs (mean age, 7 years), and they had no significant differences with respect to sex, location, family history, symptomology, time to progression, and need for treatment. The most common locations for non-brainstem gliomas were the basal ganglia and cerebellum, and were just as likely to be associated with a concurrent OPG and multiple brain tumors. In total, over 60% of probable tumors increased in size over time, with an average time to progression of 17 months (SD, 23 months), and only patients with probable non-OPTs that were radiographically progressive exhibited clinical signs/symptoms.
The type of treatment the patients received varied by location. Whereas the majority of basal ganglia tumors did not require tumor-specific therapy (94%), 50% of cerebellar non-OPTs required surgical resection. Furthermore, the majority of progressive non-OPTs stopped growing either on their own or secondary to treatment in an average of 2.34 years (SD, 2.15 years). The minority of probable tumors that remained stable were monitored with surveillance MRIs for a median of 4.7 years (range, 2–13 years), which surpassed the average time to progression observed for all non-OPTs included in this study. As these probable tumors were not biopsied, it is unclear which of these lesions represented tumors that remained dormant over time vs non-tumoral lesions. However, these findings suggest that if a lesion suspicious for probable tumor does not grow within 2 years, it is unlikely to grow afterwards, arguing that there may be a finite amount of time that neuroimaging surveillance is necessary.
We identified a small subset of children (n = 6 [9%]) with a particularly aggressive form of intracranial neoplasm, termed a deep extensive tumor. These individuals tended to be younger (mean age, 3.88 years), without a sex predominance, and harbored multiple other brain tumors, including concurrent OPGs. Each of these tumors had a distinct radiographic appearance when compared to both bilateral OPGs and other non-OPTs, which was characterized by an extensive, diffuse, and contiguous tumor that spanned the temporal lobes bilaterally, thalami, and basal ganglia, often with extension into the cerebral hemispheres. All of these tumors required aggressive treatment with multiple rounds of tumor-directed therapy, and despite the fact that most of these tumors were pathologically classified as low-grade gliomas, the mean PFS was only 48 months, implying that the radiographic appearance might be more critical than the pathologic appearance for determining prognosis and treatment. There were no statistically significant differences in PFS, degree of neurologic impairment, and need for treatment with chemotherapy/surgery between children with WHO grade I tumors and WHO grade II tumors. Although these tumors were all associated with bilateral OPGs, suggesting that they might share a similar pathogenic origin with OPGs, they encompassed regions outside of the optic pathway and demonstrated a distinct natural history. Future studies will be required to determine whether these deep extensive tumors evolve distinctly from OPGs and whether they harbor additional genetic alterations beyond NF1 inactivation19,20 that might contribute to their particular aggressive clinical behavior.
Being a retrospective review that primarily relied on medical records, there are inherent limitations to this study. Selection bias in particular may limit generalization of these findings to other cohorts of patients with NF1. However, every patient was followed by the same team of experienced physicians longitudinally at a single institution, and uniform radiographic criteria were used. Taken together, this report provides a focused analysis of non-OPTs radiologically classified as probable tumors in children with NF1, identifies a uniquely aggressive brain tumor entity, and establishes the foundation for additional studies that aim to improve the management of children with these probable CNS neoplasms.
Acknowledgment
The authors thank Robert C. McKinstry, MD, PhD, Department of Radiology at Washington University, for input during the establishment of the radiologic classification criteria used in this study; and Feng Gao, MD, PhD, for biostatistical consultation and review.
Glossary
- CI
confidence interval
- IRB
institutional review board
- NF1
neurofibromatosis type 1
- OPG
optic pathway glioma
- OPT
optic pathway tumor
- OR
odds ratio
- PFS
progression-free survival
- PNET
primitive neuroectodermal tumor
- WUSM
Washington University School of Medicine
Appendix. Authors


Study funding
This study was partially supported by unrestricted gifts from Schnuck Markets, Inc. and the Mrs. Edward J. Schnuck NF Fund (Dr. Gutmann).
Disclosure
J. Mahdi reports no disclosures relevant to the manuscript. M. Goyal has US government and foundation research funding, has received trip reimbursement from Capital Medical University, Tancheng Talent Office, and Shandong Madic Technologies Co. Ltd. in China, and has stock equity in IBM. J. Griffith and S. Morris report no disclosures relevant to the manuscript. D. Gutmann serves on the Editorial Board of Glia and Neuro-Oncology and receives license fee payments for the use of his GFAP-Cre mouse strain from the Tuberous Sclerosis Alliance. Go to Neurology.org/N for full disclosures.
References
- 1.Rosser T, Packer RJ. Intracranial neoplasms in children with neurofibromatosis 1. J Child Neurol 2002;17:630–637. [DOI] [PubMed] [Google Scholar]
- 2.Peltonen S, Kallionpää RA, Rantanen M, et al. Pediatric malignancies in neurofibromatosis type 1: a population-based cohort study. Int J Cancer 2019;145:2926–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Listernick R, Charrow J, Greenwald MJ, et al. Optic gliomas in children with neurofibromatosis type 1. J Pediatr 1989;114:788–792. [DOI] [PubMed] [Google Scholar]
- 4.Mahdi J, Shah AC, Sato A, et al. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology 2017;18:1584–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doganis D, Pourtsidis A, Tsakiris K, et al. Optic pathway gliomas in children: 10 years of experience in a single institution. Pediatr Hematol Oncol 2016;33:102–108. [DOI] [PubMed] [Google Scholar]
- 6.Sellmer L, Farschtsci S, Marangoni M, et al. Non-optic gliomas in adults and children with neurofibromatosis 1. Orphanet J Rare Dis 2017;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillamo JS, Creange A, Grill J, et al. Prognostic factors of CNS tumors in neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain 2003;126:152–160. [DOI] [PubMed] [Google Scholar]
- 8.Griffith JL, Morris SM, Mahdi J, et al. Increased prevalence of brain tumors classified as T2 hyperintensities in neurofibromatosis 1. Neurol Clin Pract 2018;8:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Listernick R, Ferner RE, Liu GT, et al. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 2007;61:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher MJ, Loguidice M, Gutmann DH, et al. Gender as a disease modifier in neurofibromatosis type 1 optic pathway gliomas. Ann Neurol 2014;75:799–800. [DOI] [PubMed] [Google Scholar]
- 11.Diggs-Andrews KA, Brwon JA, Gianino SM, et al. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol 2014;75:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway gliomas following chemotherapy: a multicenter retrospective analysis. Neuro Oncol 2012;14:790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.deBlank PMK, Fisher MJ, Liu GT, et al. Optic pathway gliomas in neurofibromatosis type 1: an update: surveillance, treatment indications, and biomarkers of vision. J Neuroophthalmol 2017;37:S23–S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne S, Connor S, Lascelles K, et al. Clinical presentation and prognostic indicators in 100 adults and children with neurofibromatosis 1 associated non-optic pathway gliomas. J Neurooncol 2017;133:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neurofibromatosis NIH. Consensus statement. Arch Neurol 1987;6:1–19. [Google Scholar]
- 16.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–1305. [DOI] [PubMed] [Google Scholar]
- 17.Opalak C, Parry M, Rock AK, et al. Comparison of ABC/2 estimation and volumetric computerized method for measurement of meningiomas using magnetic resonance imaging. J Neurooncol 2019;144:275–282. [DOI] [PubMed] [Google Scholar]
- 18.Yu YL, Lee MS, Juan CJ, et al. Calculating the tumor volume of acoustic neuromas: comparison of ABC/2 formula with planimetry method. Clin Neurol Neurosurg 2013;115:1371–1374. [DOI] [PubMed] [Google Scholar]
- 19.D'Angelo F, Ceccarelli M, Tala. The molecular landscape of gliomas in patients with neurofibromatosis I. Nat Med 2019;25:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DT, Hutter B, Jager N. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet 2013l;45:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All de-identified data are available on request.