Abstract
Microphysiological systems, including organoids, 3-D printed tissue constructs and organ-on-a-chips (organ chips), are physiologically relevant in vitro models and have experienced explosive growth in the past decades. Different from conventional, tissue culture plastic-based in vitro models or animal models, microphysiological systems recapitulate key microenvironmental characteristics of human organs and mimic their primary functions. The advent of microphysiological systems is attributed to evolving biomaterials, micro-/nanotechnologies and stem cell biology, which enable the precise control over the matrix properties and the interactions between cells, tissues and organs in physiological conditions. As such, microphysiological systems have been developed to model a broad spectrum of organs from microvasculature, eye, to lung and many others to understand human organ development and disease pathology and facilitate drug discovery. Multiorgans-on-a-chip systems have also been developed by integrating multiple associated organ chips in a single platform, which allows to study and employ the organ function in a systematic approach. Here we first discuss the design principles of microphysiological systems with a focus on the anatomy and physiology of organs, and then review the commonly used fabrication techniques and biomaterials for microphysiological systems. Subsequently, we discuss the recent development of microphysiological systems, and provide our perspectives on advancing microphysiological systems for preclinical investigation and drug discovery of human disease.
Keywords: microphysiological systems, organoids, 3-D printing, organ chips, anatomy, physiology, microenvironment
1. Introduction
The investigation of human disease and its treatment has mainly been relying on tissue culture plastic-based in vitro models and animal models. Although the conventional in vitro models, in which the cells are cultured on two-dimensional (2-D) plastic surfaces, have advanced our understanding of biology and pathology, the cell behaviors significantly deviate from their in vivo counterparts and the models do not recapitulate the in vivo cell-cell and cell-extracellular matrix (ECM) interactions, not to mention the intra- and inter-organ interactions. On the other side, animal models allow the investigation in a living system, yet they are costly and time-consuming. Moreover, the genome, anatomy and physiology of animals are not the same as human, and thus the pathophysiology and the responses of animals to the drug treatment can differ from those of human, which may result in false data of drug testing.1 Therefore, there is an urgent demand for in vitro models that have critical features and appropriate complexity of human organs and overcome the limitations of the conventional in vitro and in vivo models.
In the past decade, microphysiological systems, including organoids, three-dimensional (3-D) bioprinted tissue constructs and organs-on-a-chip systems (organ chips), have attracted increasing attention and been extensively explored because they can provide human organ-like in vitro models.2, 3 Human organs are complex networks and contain physical (matrix micro-/nanostructures and stiffness), mechanical (fluidic forces and mechanical stretch) and biochemical (such as growth factors and cytokines) characteristics.4, 5 These anatomical and physiological characteristics have shown profound influences on organ development and function.4, 6–9 Hence, microphysiological systems should include these key characteristics to establish the primary function of the human organ, and keep cell culture and analysis processes easy to perform compared to animal models. The microphysiological systems have many advantages, including but not limited to 1) 3-D structures and microenvironmental features resembling the human organ, 2) controlled cell-cell and cell-matrix interactions in the physiologically relevant condition, and 3) in situ monitoring of the disease initiation and progression as well as the organ responses to drugs.10, 11
Endowed with these advantages, microphysiological systems have been employed in various areas. One of their application areas is to investigate human developmental biology. For example, the early human embryogenesis and the neuroectoderm regionalization have been modeled by using microscale patterns or in a microfluidic device.12–14 Fetal lung branching development has also been established in a microfluidic platform by precisely controlling the internal mechanical force.15 The second area is to study the disease initiation and progression. For example, a small airway-on-a-chip has been built with the lung epithelial cells derived from patients with chronic obstructive pulmonary disease (COPD) to analyze organ-level lung pathophysiology in vitro.16 Another area is to evaluate the efficiency of therapeutic drugs. Thanks to the evolving biomaterials and micro-/nanotechnologies, microphysiological systems have been developed rapidly from simple 3-D organoids to sophisticated, biomimetic systems. There is no doubt that microphysiological systems will have great potentials to improve human healthcare in the future.
Different from the previous excellent reviews on microphysiological systems,5, 17–19 this review focuses on the design principles and fabrication of microphysiological systems and their implementation in a variety of applications as outlined in Figure 1. We first articulate and exemplify the design principles of microphysiological systems with the emphasis on anatomical structures and physiological conditions of organs. We next discuss fabrication techniques and biomaterials that have been widely employed to engineer microphysiological systems. We then review recent development of microphysiological systems by highlighting the design principles. In the end, we provide our perspectives regarding the challenges and future advancement of microphysiological systems.
Figure 1. Overview of the design, fabrication and applications of microphysiological systems.

The figure is adapted with permissions from refs 4 (Copyright 2010, The American Association for the Advancement of Science), 20 (Copyright 2016, Dove Medical Press), 21 (Copyright 2019, American Chemical Society), 22 (Copyright 2018, American Chemical Society), 44 (Copyright 2016, Elsevier), 92 (Copyright 2016, Springer Nature), 118 (Copyright 2016, American Chemical Society), 131 (Copyright 2015, PLOS), 180 (Copyright 2018, Royal Society of Chemistry), 216 (Copyright 2017, Springer Nature), 230 (Copyright 2018, Springer Nature), 235 (Copyright 2010, Royal Society of Chemistry).
2. Design principles of microphysiological systems
Microphysiological systems have been developing to establish primary function of human organs in vitro. It is thus paramount to recapitulate the anatomical and physiological characteristics of human organs. Moreover, the use of patient-specific cells in microphysiological systems at an integrative system level is critical for personalized medicine. In addition, microphysiological systems should be relatively straightforward and convenient for real time monitoring and analysis. In this section, the design principles including anatomy, physiology, cell sources of microphysiological systems are discussed and exemplified.
2.1. Anatomy
The anatomical structure of a tissue or organ is essential to its function, and the structural change could result in organ dysfunction and disease.23 For example, the villi and microvilli structures in the small intestine enlarge its surface area by more than 600 folds, which enables complete food digestion and nutrient absorption. Villous destruction (blunting or flattening of the villi) can result in abnormal digestion and absorption in the small intestine and symptoms such as diarrhea, weight loss, and iron-deficiency anemia.24 Another example is human lamina cribrosa, the main structural component of optic nerve head in human eyes. The lamina cribrosa is formed by stacks of collagenous cribriform plates, and have nerve bundle pores that are often irregular in shape with the cross-sectional area varying from 500 to 22500 μm2.25 The pore size decreases in glaucomatous eyes.26 Thus, the anatomical structure must be taken into account in designing microphysiological systems.
The cornea is an excellent example to manifest the importance of the anatomical structure in the design of microphysiological systems. Located in the front of eye, the cornea serves as a barrier for protecting the ocular contents, a surface for refracting light and a route for drug permeation.27 The cornea is highly ordered, being composed of epithelium, Bowman’s layer, stroma, Descemet’s membrane and endothelium (Figure 2A).28 The external epithelium consists of 3 to 7 layers of epithelial cells. The middle stroma contains keratocytes interspersed in highly organized collagen lamellae.29 The Descemet’s membrane is composed of mainly type IV collagen, laminin, and fibronectin for the support of the corneal endothelium. The innermost endothelium is a monolayer for maintaining stromal dehydration. In addition, corneal innervation is distributed throughout the epithelium and stroma for protecting eye from stimuli such as sunlight and touch.30
Figure 2. Exemplification of the design principles of microphysiological systems.

(A) Schematic and histological images of human cornea. Scale bar is 50 μm. Adapted with permission from ref 28. Copyright 2015, Mary Ann Liebert, Inc. (B) In vitro cornea model consisting of multiple epithelial layers, stroma and innervation. Adapted with permission from ref 44. Copyright 2016, Elsevier. (C) Illustration of the BBB. Adapted with permission from ref 62. Copyright 2016, Springer Nature. (D) A microfluidic chip recapitulating the physiological (stiffness, fluidic flows and cell-cell interactions) characteristics of the BBB. Adapted with permission from ref 198. Copyright 2015, AIP Publishing. (E) Illustration of the mechanical stretching of lung alveoli during breathing. Adapted with permission from ref 4. Copyright 2010, The American Association for the Advancement of Science. (F) A lung chip recreating the alveolar-capillary interface with fluidic flows and cyclic mechanical stretching. Adapted with permission from ref 2. Copyright 2010, The American Association for the Advancement of Science.
Functional corneal epithelium and endothelium have been constructed on various biomaterials such as collagens,31 silk films,32 synthetic hydrogels33 and amniotic membranes.34 For example, human amniotic membrane has been used to promote the proliferation yet suppress the differentiation of human limbal epithelial cells. After implanted to rabbit corneas with chemical injury, the corneal epithelium is reconstructed.34 To function normally, the endothelium should have a minimum cell density of 400 cells/mm2.35 Low cell density can cause cornea swelling and vision blurred, namely Fuchs’ corneal dystrophy.35 Palchesko et al. have prepared a membrane consisting of type IV collagen and laminin to mimic the composition and structure of Descemet’s membrane. On the membrane human corneal endothelial cells form a confluent monolayer with a density of 1700 cells/mm2 after 14 days of culture.36 Gelatin methacrylate with micro and nanopillars are also fabricated to generate human corneal endothelial layers. 1 μm pillars are shown to support the formation of functional endothelial monolayer with a cell density of nearly 2000 cells/mm2, accompanied with the upregulation of tight junction protein zonula occludens-1 (ZO-1).37
The structural and optical properties of the cornea are attributed to the mechanical properties and transparency of the stroma.38 It is thus critical to engineer transparent stroma substrates with mechanical properties comparable to the in vivo counterpart. Type I collagen has been used to form layers of fibrils with good transparency at a concentration of 90 mg/mL,29 and produce transparent stromal equivalent with similar mechanical properties to in vivo stroma.39, 40 When grown on silk substrates with groove structure, the human corneal stromal stem cell (hCSSC)-derived keratocytes can secret multilayered lamellae with highly ordered collagen fibrils, which is similar to human corneal stromal tissue in both component and structure.41 The human corneal keratocytes are also encapsulated into the bio-ink composed of type I collagen and sodium alginate to create the stroma equivalent via 3-D printing. This printed equivalent exhibits native human cornea-like structure with the cells remaining high viability for 7 days.42
While the single-layered tissue has been developed to mimic a certain part of the cornea, more complex substitutes are needed to resemble the multilayered cornea. Alaminos et al. have developed a multilayered cornea model in a transwell system containing a bottom corneal endothelial cell layer on the porous membrane, a middle stromal substitute of keratocytes in a gel of fibrin and agarose and top epithelial cell layers.43 The formed full-thickness rabbit cornea substitute has a lamina structure with elongated keratocytes similar to the native corneal stroma, a stratified epithelium, and a confluent endothelial cell monolayer. Moreover, innervation has been introduced into a corneal construct to establish the interactions between corneal tissue and corneal innervation (Figure 2B).44 Three grating-structured silk films are stacked to mimic the stroma and seeded with hCSSCs, while another flat silk film seeded with epithelial cells is put on the top of the film stacks. The top flat silk film is pre-stamped with nerve growth factor (NGF) to guide the chicken dorsal root ganglion (DRG) axons to grow from the stromal layer to the top epithelial layers. Because of the presence of the innervation, the maturity of the epithelium is enhanced and the engineered corneal construct remains transparency with dense innervation and multilayered epithelium for up to one month. This full thickness, innervated, corneal chip has been successfully applied to study the hyperglycemia of the cornea45 and nociceptive-related responses.46
The efforts have also been made to include anatomical structures in other microphysiological systems. For example, a gut chip has been engineered to have villi-like gut epithelium and exhibits about 1.5-fold higher efficiency of glucose absorption than the Caco-2 monolayer cultured in the transwell.47 More microphysiological systems resembling the anatomical structure of the targeted organ will be discussed in Section 4.
2.2. Physiology
The physiological conditions of an organ are vital to the development and maintenance of its normal functions. The physiological characteristics (physical, mechanical and biochemical factors) of human organ have profound influences on the functions of cells and organs.48–50 Any deviation in the physiological conditions could lead to dysfunction of the organ and even induce lesions. For example, the stiffness of human lamina cribrosa can be altered by the aging51 and disease state.52 Its stiffness in pseudoexfoliation syndrome (an age-related disease, characterized by the accumulation of microscale granular amyloid-like protein fibers in the eye) eyes is reported to be 10.1±1.4 kPa, softer than 17.2±2.7 kPa in normal human eyes52; while its stiffness in glaucoma is higher, ranging from 100 to 900 kPa.53
The importance of physiological characteristics in microphysiological systems has been underlined in the development of microvasculature chips. Being an essential part of organs, the microvascular system not only transports oxygen, nutrients and metabolic waste products but also supports intra-/inter-organ interactions.54 The formation and function of the microvasculature are tightly controlled by physiological factors such as fluidic forces, ECM stiffness and interactions with surrounding cells.55 The fluidic shear stress can influence the phenotype and function of vascular endothelial cells, for instance, morphogenesis and sprouting of human umbilical vein endothelial cells (HUVECs).56 The shear stress also affects the barrier function of microvascular endothelium. It has been demonstrated that the elevated shear stress induces the formation of a continuous pattern of VE-cadherin in the endothelial layer.57 Moreover, the ECM stiffness can profoundly affect the barrier function of microvascular endothelium. Cell-cell contacts and tight junction formation of endothelial layers are enhanced when the cells are cultured on the substrate of stiffness between 1 and 20 kPa,58, 59 while increasing the substrate stiffness over 30 kPa leads to the disruption of tight junctions.59, 60 In addition, the thrombin-induced endothelial barrier disruption recovers more efficiently when the cells are cultured on the substrate of 8.6 kPa in stiffness, compared to that of cells grown on the softer (0.55 kPa) or stiffer (42 kPa) substrates.61 Further, in the microvasculature of the central nervous system (CNS), the blood-brain-barrier (BBB) is regulated by not only the fluidic force and ECM stiffness (see Section 4.1), but also the interactions between endothelial cells and other cell types such as astrocytes and pericytes (Figure 2C). Co-culturing with the astrocytes promotes the formation of tight junctions and enhances the barrier function of the endothelial layer.62 Pericytes can also influence the endothelial sprouting, depending on the status of pericytes.63 Long endothelial sprouts with good barrier properties form when the endothelial cells are co-cultured with the pericytes expressing both α-smooth muscle actin (α-SMA) and desmin, while no sprout forms when the pericytes mainly express α-SMA.
Moreover, many organs such as blood vessels, heart, lung and gastrointestinal (GI) tract function under mechanical stimuli. For example, the lung alveoli experience cyclic mechanical stretch – breath-induced dilation and constriction (Figure 2E). A human breathing lung-on-a-chip has thus been designed by incorporating 1-D mechanical stretch in a microfluidic platform. As shown in Figure 2F, human lung epithelial cells and endothelial cells are co-cultured on the apical and basal sides of a microporous polydimethylsiloxane (PDMS) membrane, respectively. Two side chambers are attached to the vertically arranged co-culture microchannels via thin walls. The thin walls can be deformed by applying vacuum to stretch and relax the elastomeric PDMS membrane to mimic the lung breathing movement and thus provide cyclic mechanical strains to the epithelial and endothelial layers. The nanotoxicity study on the lung chip reveals that the mechanical stretch elevates the toxicity of nanoparticles by accentuating the endothelial and epithelial uptake of nanoparticles.4 The mechanical stretch has also been shown to enhance the IL2-induced vascular leakage, which highlights the crucial role of mechanical stretch in lung diseases such as pulmonary edema.64
While organs perform their function in their specific physiological conditions, these organs interact with each other through the circulatory system. To model organ function at the system level, multiple organ chips should be integrated into one single platform or a multiorgans-on-a-chip (multiorgan chip), which allows the component organ chips to function properly yet enables inter-organ communications and interactions.65–67 Inter-organ interactions can be implemented in two ways. One is to first mature all component organ chips individually to make them phenotypically stable, and then connect them in a circulatory microfluidic platform. Another way is to connect the component organ chips with the inter-organ barriers and mature all the components in an integrative platform. As such, the flow rates in the multiorgan chip system need to be precisely controlled to allow the system to function properly. In addition, multiorgan chips need to be designed by scaling the size of the component organs according to the actual size of the organs. The most common rule is to scale down the organ size based on the physiological liquid-to-cell ratio and determine flow rates based on in vivo residence times.68–71 A liver-tumor-marrow chip has been developed based on the scaling down rules and exhibited improved predictability of the toxicity and metabolism of anticancer drugs such as 5-fluorouracil (see Section 4.10).70, 71
Moreover, it is critical to develop common media/blood surrogate that is able to not only support the growth of cells derived from different organs but also maintain their functions. The common strategy of preparing common media is to combine different media at a defined ratio. For example, a multiorgan chip of liver and intestine modules is successfully maintained in mixed media and able to recapitulate the clinic pharmacokinetic and toxicity profile of terfenadine.72 However, the use of mixed media is not ideal especially when incorporating more organ components into a multiorgan chip. A more promising solution is to develop chemically defined serum-free media, which can avoid the media variation caused by serum. For examples, a 4-organ chip of cardiac, muscle, neuronal and liver modules is maintained in serum-free media for at least 14 days.73
2.3. Cell Sources
In addition to anatomical and physiological criteria, appropriate cell sources are critical to the success of microphysiological systems. The cell types are determined according to the organ of interest and its function. Generally speaking, commercially available cell lines are easier to culture than primary cells or pluripotent stem cells. However, cell lines can behave differently from primary cells. Hence, microphysiological systems using cells lines do not always faithfully resemble the organ physiology and function. For example, muscle constructs built on mouse myoblast cell line C2C12 are less mature than those using mouse muscle progenitor cells, which exhibit more abundant α-actinin cross-striations.74 In addition, the lack of patient-specific characteristics makes cell lines unsuitable for personalized medicine. Therefore, it is ideal to use human primary cells, in particular the cells isolated from the patient for personalized medicine because patient-specific disease models provide a promising way to understand disease pathology, identify novel disease biomarkers, and test patient-specific drug efficiency. For example, lung epithelial cells isolated from patients with chronic obstructive pulmonary disease have been used to construct lung small airway chips, which recapitulate lung pathophysiological conditions and demonstrate their potentials for drug discovery.16, 64, 75 Additionally, cancer exhibits a large variety of genetic mutations among patients and the genetic variations can impact the efficiency of drug treatment. As such, recreation of cancer-on-chips with the patient’s cancer cells will undoubtedly facilitate determination of the type and dose of anticancer drugs for a specific patient.
However, some obstacles exist regarding human primary cells, including limited cell sources and difficult maintenance of the cells in vitro without losing their functions. For example, human retinal ganglion cells are known to difficult to culture in vitro. Therefore, advanced cell culture technologies are highly desirable to efficiently maintain and expand primary cells in vitro without sacrificing their functionality. Stem cells represent a promising alternative for cell sources as they are able to differentiate to a variety of cell types. In particular, human pluripotent stem cells such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have been widely used to construct microphysiological systems. Various organ models, such as microvasculature76 and kidney,77 have been developed by using adult cells derived from human ESCs (hESCs). However, ethical issues with hESCs restrict their applications. In contrast, human iPSCs (hiPSCs) draw tremendous attention in the organ modeling. HiPSCs are genetically reprogrammed from the adult primary cells and endowed with self-renewal and multilineage differentiation potentials similar to hESCs. HiPSCs have been demonstrated to differentiate into multiple cell types including endothelial cells, vascular cells, neuronal cells and pericytes.78–81 Over the past few years, hiPSCs have been employed in the development of patient-specific organ chips.82, 83 For example, hiPSCs derived from Huntington’s disease patients are differentiated to brain microvascular endothelial-like cells, astrocytes and neurons to build patient-specific BBB chips. These BBB chips exhibit individual variation in dextran permeation, and thus can be potentially used for predicting patient-specific drug permeability.83 Despite their huge potentials, challenges exist for using hiPSCs. For example, cells derived from hiPSCs usually exhibit immature function characteristics and heterogenic subpopulation, which limits the development of consistent and reliable microphysiological systems. Additionally, since hiPSC differentiation protocols are not comprehensively developed, not all types of cells can be generated reliably from hiPSCs currently.
3. Fabrication of microphysiological systems
The evolving micro-/nanotechnologies and biomaterials enable precise control over cell-cell and cell-matrix interactions and matrix properties in 3-D, and thus significantly advance the development of microphysiological systems. The selection of suitable fabrication techniques and biomaterials are critical to engineer microphysiological systems. The commonly used fabrication techniques, including organoid formation, 3-D bioprinting and microfluidic techniques are herein reviewed, followed by the discussion on biomaterials, both summarized in Table 1.
Table 1.
Summary of common fabrication techniques and biomaterials for microphysiological systems
| Techniques | Fabrication Methods | Biomaterials | Cost (US$) | Complexity | Example models |
|---|---|---|---|---|---|
| Organoid |
Hanging drops: Pros:
Pros:
Pros:
|
Natural Biomaterials: Matrigel, Collagen, Alginate, Silk, Hyaluronic acid, Fibrin… Synthetic Biomaterials: Polyethylene glycol, Amikagel, Polyglycolic acid, Poly-L-lactic acid… |
102–103 |
|
Brain84 Retina85 Stomach86 Liver87 Kidney88 Gut89 Tumor90 |
| 3-D Bioprinting |
Inkjet: Pros:
Pros:
Pros:
|
Natural biomaterials: Gelatin, Alginate, Collagen, Fibrin, Hyaluronic acid, decellularized ECM Silk, Chitosan, Cellulose… Synthetic biomaterials: Polycaprolactone, Polylactic acid, Poly(lactic-co-glycolic) acid, Polyvinylpyrrolidone, Poly(ethylene glycol)… |
10–103 |
|
Liver87 Vasculature91 Kidney92 Skin93 Muscle94 Tumor95 |
| Microfluidics |
Pros:
|
Polydimethylsiloxane, Polystyrene, Poly(methyl methacrylate), Polyurethane… |
10–103 |
|
Lung4, 96 Liver87, 97 Kidney92, 98 Gut99 Vasculature91, 100 Heart101 Bone102, 103 |
3.1. Organoid formation
Organoids are in vitro 3-D organ models composed of multicellular clusters and hydrogel matrix. The cells such as cancer cells, progenitor or stem cells such as hESCs and hiPSCs104 are encapsulated in the hydrogel matrix, exhibit similar composition, architecture and functionality to the organ of origin, and gain the capability of self-organization and self-renewal. The hydrogel matrix enables cell-cell and cell-matrix interactions in 3-D, mimicking the physiological conditions of organs more closely than 2-D models. To date, various types of organoids have been developed to model a variety of organs including brain,105 retina,106 stomach,107 liver,108 kidney,109 gut110 and tumor.90
Organoids are generally formed by encapsulating cells in a 3-D matrix by using different methods such as hanging drops, microwell array lading and microfluidic droplet formation (Figure 3A). In the hanging drop method, small drops of cell suspension or cell/hydrogel mixture are placed on a culture plate, which is then inverted. The cells can aggregate and grow to form spheroids due to the gravity effect.111, 112 In the microwell array lading, cells are trapped in microwells to form cell aggregates with the size controlled by the microwell dimensions. The microwell dimensions have shown to influence the differentiation of stem cells. For example, 450 μm microwells result in a larger size of embryoid bodies and favor cardiogenesis of mouse ESCs, while 150 μm microwells lead to a smaller size of embryoid bodies and bias endotheliogenesis.113 For the microfluidic droplet formation, cells/hydrogel mixture is injected through one microchannel and broken into droplets by the stream(s) of hydrophobic fluids such as non-toxic oil from other microchannel(s), usually via shear force or through flow focusing. The use of the microfluidic device can achieve high-throughput generation of cell-encapsulated microgels. The size of microgels can be controlled by the dimensions of the microchannels, the properties of the hydrophobic solution and/or surfactants, and the flow rates of hydrogel-cell mixture and the hydrophobic solution.114
Figure 3. Commonly used fabrication techniques to engineer microphysiological systems.
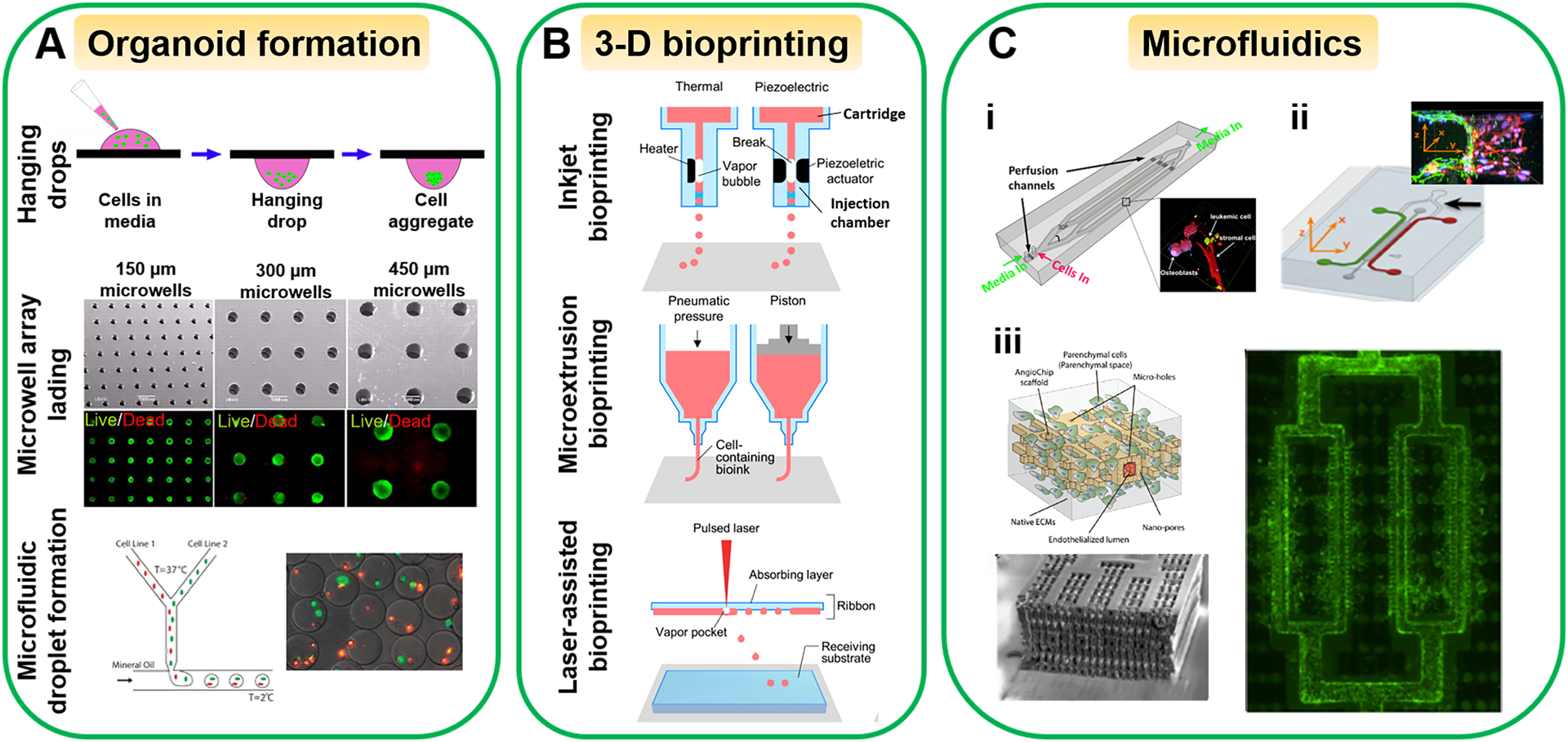
(A) Organoid formation, (B) 3-D bioprinting, and (C) Microfluidic techniques. (C-i) An engineered 3-D bone marrow with leukemic cells in microchannels as a tumor-on-a-chip. Adapted with permission from ref 131. Copyright 2015, PLOS. (C-ii) The tumor-vascular interface on a 3-microchannel chip for tumor cell intravasation study. Adapted with permission from ref 136. Copyright 2012, National Academy of Sciences. (C-iii) An AngioChip with perfusable, branched, 3-D microchannel network. Adapted with permission from ref 137. Copyright 2016, Springer Nature. The microwell array lading images in (A) were adapted with permission from ref 113. Copyright 2009, National Academy of Sciences. The microfluidic droplet formation images in (A) were adapted with permission from ref 114. Copyright 2011, Oxford University Press. (B) was adapted with permission from ref 118. Copyright 2016, American Chemical Society.
3.2. 3-D bioprinting
3-D bioprinting is an additive manufacturing approach to create tissue constructs by using 3-D printers and bio-inks (biomaterials and/or living cells). Applying 3-D bioprinting to build microphysiological systems is promising because this technique is capable of recreating the cellular architecture similar to human tissues/organs by precisely coordinating multiple types of cells in the construct.42, 115–118 Based on the working principle, 3-D bioprinting methods can be categorized into three types: inkjet bioprinting, microextrusion bioprinting and laser-assisted bioprinting (Figure 3B).
Inkjet bioprinting is derived from conventional inkjet printing.119 In this bioprinting, bio-ink is stored in a cartridge that is connected to an injection chamber. A heater or piezoelectric actuator is placed inside the injection chamber to generate microbubbles to eject the bio-ink out as droplets from the injection chamber.120 3-D layer-by-layer structures can be produced by depositing the droplets on a receiving layer. The inkjet bioprinting has been used to fabricate a variety of tissues and organs such as skin, liver, muscle and blood vessels.114, 117, 121 Lee et al. have printed perfusable vascular channels consisting of HUVECs in gelatin. The formed vascular channels exhibit normal vasculature function such as acting as a barrier for bovine serum albumin and dextran and supplying nutrient support to the tissue within 5 mm of distance.117 It is a favorable way to fabricate complex tissue structures because of its high-throughput capability, high reproducibility and low cost.122 Its drawback is that the size and placement of the generated droplets cannot be controlled precisely.
The microextrusion bioprinting is the most commonly used technique to prepare tissue constructs. It utilizes pneumatic pressure or mechanical force to dispense bio-ink through a nozzle continuously and deposit it on the substrate.116, 123, 124 For example, a liver-on-a-chip has been created by printing gelatin methacryloyl bio-ink containing hepatic spheroids in the middle chamber of the chip.87 The engineered chip displays hepatic function up to 30 days and the observed toxic response to the acetaminophen is similar to the animal study. The microextrusion bioprinting is able to build tissue structures with high integrity. However, its printing speed is lower than the other two methods. The shear stress in the nozzle could deform the cells and reduce cell viability.
Different from the aforementioned bioprinting methods, laser-assisted bioprinting is a nozzle-free approach, which contains three major components: a laser beam source, an absorption layer and a receiving substrate. The bio-ink is suspended on the bottom of the absorption layer, which is made of metals to convert laser energy to heat. When the laser is applied to the absorption layer, the heat is generated to vaporize the bio-ink to form a high-pressure bubble, which propels bio-ink droplets onto the substrate.125, 126 3-D structures are created by repeating the procedures. For example, a 3-D human skin tissue is created by printing human keratinocytes and fibroblasts in collagen with the cellular arrangement comparable to native skin tissue.127 Because of the high accuracy of laser targeting, this printing method can print 3-D structures in high resolution and precision. Yet, it suffers from low efficiency, low cell viability and high cost.128
3.3. Microfluidic techniques
Microfabrication techniques are essential to engineer microphysiological systems such as microwells and microfluidics for organoid formation. Because a significant portion of microphysiological systems are microfluidic-based we herein discuss microfluidics related microfabrication techniques and their applications. Microfluidics can work as a circulatory system to transport nutrients, gases and metabolites, provide a spatiotemporal control of signaling molecules, and create physiologically relevant mechanical stimulation. Therefore, microfluidics have been extensively applied in microphysiological systems.
The microfluidics can be designed, fabricated and assembled by applying photolithography, soft lithography, and various assembly techniques. In the photolithography process, the shape and lateral dimensions of microfluidics are designed by using computer-aided design software such as AutoCAD, printed on a photomask, and transferred to a photoresist layer supported on a silicon (Si) wafer with UV exposure. After development, a photoresist mold is generated, which can be further etched down to the underneath Si substrate or directly used as a master mold. The vertical dimensions of the microfluidics are determined by the depth etched to the Si substrate or the thickness of the photoresist layer. Working microfluidic components can be produced by applying soft lithography, which involves casting liquid polymer precursors such as PDMS prepolymer against the master mold followed by curing. Through the inexpensive process, the microfluidic components can be replicated in great quantities. The microfluidic systems can be assembled reversibly through van der Waals force and secured by applying mechanical clamping, or irreversibly by bonding chemically modified surfaces of the components. Moreover, the bonding strength of microfluidic systems can be tuned by applying PDMS adhesive-based assembly technique.129
Microfluidic technologies enable creation of dynamic, physiologically relevant systems. Culture media can be perfused through microchannels to the components of the microphysiological system. Microchannels are also used to connect the component organs in multiorgan chips and allow the inter-organ communications and interactions.70, 130 In addition, 3-D tissue microenvironment can be established in microfluidic platforms with controlled cell-cell and cell-matrix interactions. For example, primary human bone marrow stromal cells, osteoblasts and human leukemic cells are encapsulated in type I collagen matrix and embedded in microchannels as a tumor-on-a-chip (Figure 3C-i), where the matrix stiffness and fluidic shear stress are controlled in the physiological ranges. The leukemic cells exhibit decreased chemotherapeutic drug sensitivity in the tumor chip compared with the 2-D models, indicating the protective role of bone marrow microenvironment in tumor cell survival against drug treatment.131 Because of the microsized channels, the fluidic flows are usually laminar flows and can be precisely controlled to study their influences on cells.132 The laminar flows have also been utilized to generate chemical and physical gradients to study directional cell migration,133 cell-cell junction integrity100 and differentiation.134 Moreover, by using multichannel microfluidic platforms the cell-cell interaction models have been constructed to study the effect of tumor microenvironment.135, 136 For example, the tumor-vascular interface has been recreated in a 3-D microfluidic platform (Figure 3C-ii), which allows the real-time imaging of tumor cell intravasation and precise quantification of endothelial barrier function.136
Moreover, microfluidic technologies can implement other anatomical structures and physiological conditions of an organ in organ chips. Shown in Figure 3C-iii, an AngioChip is comprised of a perfusable, branched, 3-D microchannel network coated with endothelial cells. The AngioChip supports the assembly of parenchymal cells on the matrix surrounding the network and allows intercellular communications of monocytes and endothelial cells.137 Additionally, in the lung-on-a-chip (Figure 2F), the co-culture of epithelial cells and endothelial cells on the microporous PDMS membrane experiences cyclic mechanical loading caused by deformation of side walls.4 Other mechanical loadings such as tension and compression can also be modeled in the microfluidic platforms.138
Additionally, peripherals such as pumps, valves and microscopes are essential to operate and monitor organ chips. Advancement in microfabrication techniques allows the integration of the peripherals in the organ chip. For example, on-chip microvalves and pumps have been developed and integrated into the microfluidic platform to actuate different types of cell culture media in a controlled manner.139 A novel design of microfluidic device permits the on-chip measurement of interstitial fluid pressure using interferometric microscopy.140 More peripherals are expected to be miniaturized and employed in organ chips.
3.4. Biomaterials
Biomaterials are critical to cell-matrix interactions and fabrication of microphysiological systems, and are generally categorized as natural and synthetic biomaterials (Table 1). Natural biomaterials include protein-based biomaterials (such as collagen and fibrin), polysaccharide-based biomaterials (such as hyaluronic acid and cellulose) and decellularized ECM. They are biocompatible and able to support cell adhesion and growth, yet have low mechanical strength and batch-to-batch inconsistency in the composition. By comparison, synthetic biomaterials can have defined composition and tunable properties, which are advantageous for engineering microphysiological systems. Usually, synthetic biomaterials need to be functionalized with biological motifs because of their low bioactivity. The selection of biomaterials relies on the application, function, and fabrication of the microphysiological system.
The biomaterials used to form organoids are usually hydrogel-based. The widely used, murine sarcoma-derived Matrigel can support the growth of a broad spectrum of cell types, and has been utilized to form different types of organoids including stem cell-derived,141 cancer cell-derived142 and primary human adult cell-derived organoids such as prostate epithelial organoids.90 However, the composition of Matrigel is not defined, and hence it is challenging to control the organoid formation robustly and reliably. Alternatively, other natural biomaterials including alginate, fibrin, silk and hyaluronic acid are used to engineer intestine, kidney, brain and many other organ organoids.143–145 For example, alginate hydrogel has been used to form human intestinal organoids with hESCs or hiPSCs.143 The resulted human intestinal organoids successfully resemble human intestine and exhibit crypt-villus structures after in vivo transplantation. Synthetic hydrogels with defined chemical and/or physical properties have also been used to prepare organoids.146–148 For example, poly(ethylene glycol) (PEG) hydrogel conjugated with biodegradable acrylate groups, RGD (Arg-Gly-Asp) and laminin-111 has also been used to form intestinal organoids.147 RGD and laminin-111 provide anchoring sites to facilitate cell adhesion and growth, and the acrylate groups are used to tune the matrix stiffness through hydrolysis, thus achieving the physical and chemical manipulation of the organoid growth.
A wide range of natural and synthetic biomaterials have been used as bio-inks for 3-D bioprinting of microphysiological systems. The rheological (especially viscosity) and crosslinking properties of the bio-ink are critical to bioprinting techniques. Typically, inkjet bioprinting requires bio-inks of low viscosity (<10 mPa·s) to form microdroplets,149 as exemplified in the perfusable vascular channels built on gelatin (see Section 3.2).117 As an example of synthetic biomaterials, PEG mixed with human primary dermal fibroblasts is printed to form dermal equivalents, which are successfully maintained for 7 weeks.150 Different from inkjet bioprinting, microextrusion bioprinting requires bio-inks of high viscosity (> 30 mPa·s) and can encapsulate cells at a high density.149 Natural biomaterials including collagen, alginate, silk, cellulose and decellularized ECM, and synthetic biomaterials such as PEG, polyethylene oxide (PEO), polycaprolactone (PCL) and polyurethane have been used for microextrusion bioprinting. For example, collagens are used to prepare the microphyisological systems of cornea,42 skin151 and heart116 by using microextruson bioprinting. Decellularized porcine brain ECM is also used for engineering glioblastoma chips through microextrusion bioprinting.95 Laser-assisted bioprinting usually uses biomaterials of viscosity in 1–300 mPa·s range. Using high viscous bio-inks in laser-assisted bioprinting, the laser irritation induced cell death can be reduced. For example, compared to the 0.5% (w/v) alginate bio-ink, 1% alginate significantly increases the viability of Ea.hy926 endothelial cells after printing.152 Note that biomaterial composites are also widely used for 3-D bioprinting because they can provide improved performances to overcome the drawbacks of their ingredients. For example, adding alginate improves crosslinking properties of nanofibrillated cellulose without significantly altering its viscosity, and the composites successfully support human chondrocyte growth for cartilage tissue engineering.153
While organ chips are commonly built on PDMS because it is transparent, gas permeable and easy to process, other materials include poly(methyl methacrylate),154 polycarbonate,155 polystyrene156 and polyurethane157 are also used. To recapitulate the in vivo microenvironment, cells are embedded into biomaterial matrix, usually hydrogels, to create 3-D microenvironment inside organ chips. The biomaterials listed in Table 1 for organoid formation and 3-D bioprinting are also used for organ chips.
It is highly desirable for the biomaterials to recapitulate the physical and biochemical properties of the native organ ECM such as architecture, stiffness and adhesive moieties. ECM displays hierarchically arranged architectures in vivo, which have proven to influence cell behavior.50 As such, biomaterials have been micro-/nanostructured to regulate cell behaviors to improve the function of the engineered microphysiological system. For example, as discussed in Section 2.1, 1 μm gelatin methacrylate pillars support the formation of functional human corneal endothelial monolayer.37 Notably, electrospinning technique is able to produce nanofibrous matrix using a variety of materials with similar composition and structure to native ECM. The fiber diameter, pore size and structures can be controlled mainly by polymer solution properties and process parameters such as needle diameter, flow rate, and power supply voltage.158 For example, aligned poly(lactic-co-glycolic acid) (PLGA) fibers generated by electrospinning have been used to induce to alignment of ganglion cells for corneal engineering.159 Electrospinning technique thus has great promise in engineering microphysiological systems.
4. Microphysiological systems
A variety of microphysiological systems have been developed as summarized in Table 2. Some representative systems are discussed in this section with the particular interest in the anatomical and physiological characteristics of the organs.
Table 2.
Representative microphysiological systems
| Organs | Design considerations | Fabrication/Biomaterials | Applications | Refs | ||
|---|---|---|---|---|---|---|
| Anatomy | Physiology | Cells | ||||
| Micro-vasculature | endothelial layer of BBB | fluidic shear stress | HUVECs, hCMEC/D3 | Microfluidics | drug testing | 160, 161 |
| co-culture of BBB | fluidic shear stress | microfluidics | ||||
| hBMVEC/pericytes/astrocytes | drug testing | 100 | ||||
| blood vessel | fluidic shear stress | HUVECs/mouse fibroblasts/mouse smooth muscle cells | microextrusion bioprinting, microfluidics | system development* | 91 | |
| Brain | multi-layered neural tissue | – | rat embryonic neurons and astrocytes, mouse neural stem cells, mouse primary cortical neurons |
microextrusion bioprinting | system development | 163–165 |
| brain | ||||||
| fluidic shear stress | hESCs | microfluidics, microwell array lading | drug testing | 169 | ||
| Eye | corneal epithelial layer | – | human limbal cells | human amniotic membrane | epithelium transplantation | 34 |
| corneal stroma | ||||||
| – | human keratocytes | microextrusion bioprinting | system development | 42 | ||
| corneal endothelial layer | mechanical properties | primary human corneal endothelial cells, human, sheep or bovine corneal endothelial cell lines | hydrogel substrates | system development | 36, 171–173 | |
| 3-D corneal tissue | cell alignment | human corneal epithelial cells/hCSSCs/ chicken DRG | nanogratings | system development | 44 | |
| full-thickness cornea | – | |||||
| hESC-derived limbal epithelial cell-like cells/corneal endothelial cell-like cells | decellularized porcine cornea | cornea transplantation | 174 | |||
| 3-D retina | – | retinal progenitor cells cell line/RPE | – | system development | 85 | |
| ocular surface | blink-induced mechanical forces | primary human corneal epithelial cells, immortalized human conjunctival epithelial cells and primary human keratocytes | microfluidics | drug testing | 8 | |
| Heart | aligned myocardium | |||||
| mechanical properties, fluidic shear stress | embryonic cardiomyoblast line | microfluidics | system development | 176 | ||
| laminar cardiac tissue | fluidic shear stress | hiPSC-derived cardiomyocytes | microfluidics | drug testing | 177 | |
| heart components | mechanical properties | hESC derived cardiomyocytes | microextrusion bioprinting | system development | 116 | |
| Lung | alveolar epithelial layer | mechanical stretch | human bronchial epithelial cells | – | drug testing | 178 |
| alveolar-capillary interface | mechanical stretch | human alveolar epithelial cells, airway epithelial cells/microvascular endothelial cells | microfluidics | nanotoxicity study, lung disease modeling | 4, 16, 64 | |
| alveolar respiratory membrane | – | non-small cell lung cancer cells/human fetal lung fibroblasts/HUVECs | microfluidics, electrospinning | drug testing | 96 | |
| Liver | hepatocyte aggregate | fluidic shear stress | rat hepatocytes | microfluidics | drug testing | 179 |
| human hepatocyte line | inkjet bioprinting, microwell array lading, microfluidics | drug testing | 87 | |||
| hepatic sinusoid | ||||||
| fluidic shear stress | mouse hepatocytes | microfluidics | drug testing | 97 | ||
| Kidney | nephron | – | hiPSCs, hESCs | microwell array lading | drug testing | 88 |
| proximal tubule | fluidic shear stress | |||||
| human proximal tubule epithelial cells | microfluidics | system development | 181 | |||
| Gut | intestinal villi | – | human epithelial colorectal adenocarcinoma cells, human epithelial cell line | alginate micropits | system development | 182 |
| intestinal epithelium | ||||||
| fluidic shear stress | human epithelial cells | microfluidics | system development | 99, 183, 184 | ||
| intestinal epithelial organoids | – | hiPSCs | microwell array lading | system development | 185 | |
| Tumor | tumor-vascular interface | fluidic shear stress | ||||
| HUVECs, human lung fibroblasts, monocytes, human breast cancer cell line, human melanoma cancer cell line | microfluidics | system development | 187 | |||
| tumor-stromal interaction | fluidic shear stress | bone marrow stromal cells/liver tumor cell line | microfluidics | system development | 188 | |
| compartmentalized structure of glioblastima | oxygen gradient | patient-derived glioblastoma cells and vascular endothelial cells | microextrusion bioprinting | drug testingdrug testing | 95 | |
Notes:
system development: no application reported
HUVEC: human umbilical vein endothelial cells; hCMEC/D3: human cerebral microvessel endothelial cells; hBMVEC: human brain-derived microvascular endothelial cells; hCSSCs: human corneal stromal stem cells; hCFs: human corneal fibroblasts; DRG: dorsal root ganglion; hESCs: human embryonic stem cells; RPE: retinal pigment epithelium; hPSCs: human pluripotent stem cells; hiPSCs: human induced pluripotent stem cells; MSCs: human mesenchymal stromal cells; HDMECs: human dermal microvascular cells
4.1. Microvasculature
The microvasculature is the fundamental system throughout human body that not only transports oxygen, nutrients and metabolic wastes but also functions as a barrier in some organs such as BBB. Dysfunction of microvascular systems is associated with diseases such as tumor and inflammation, sickle-cell disease and malaria.189, 190 The mechanistic understanding of endothelial barrier dysfunction is limited by the lack of physiologically relevant in vitro models.
Various microvasculature models have been developed to resemble specific microvasculature systems including BBB, kidney proximal tubule and liver sinusoid.191, 192 Microvascular structures have been built on hydrogel-based microfluidic chips, and the vascular diameter, wall shear stress and stiffness of post-capillary venules are mimicked (Figure 4A, B).193 Endothelial cells from different origins including HUVECs, human dermal microvascular endothelial cells and human lung microvascular endothelial cells have been tested in the chips (Figure 4C, D). On the chips, parasite plasmodium falciparum-infected red blood cells alone result in the dysfunction of the microvasculature by increasing endothelial permeability (Figure 4E), which highlights the potential of these chips.
Figure 4. A microvasculature-on-a-chip to study endothelial barrier dysfunction.
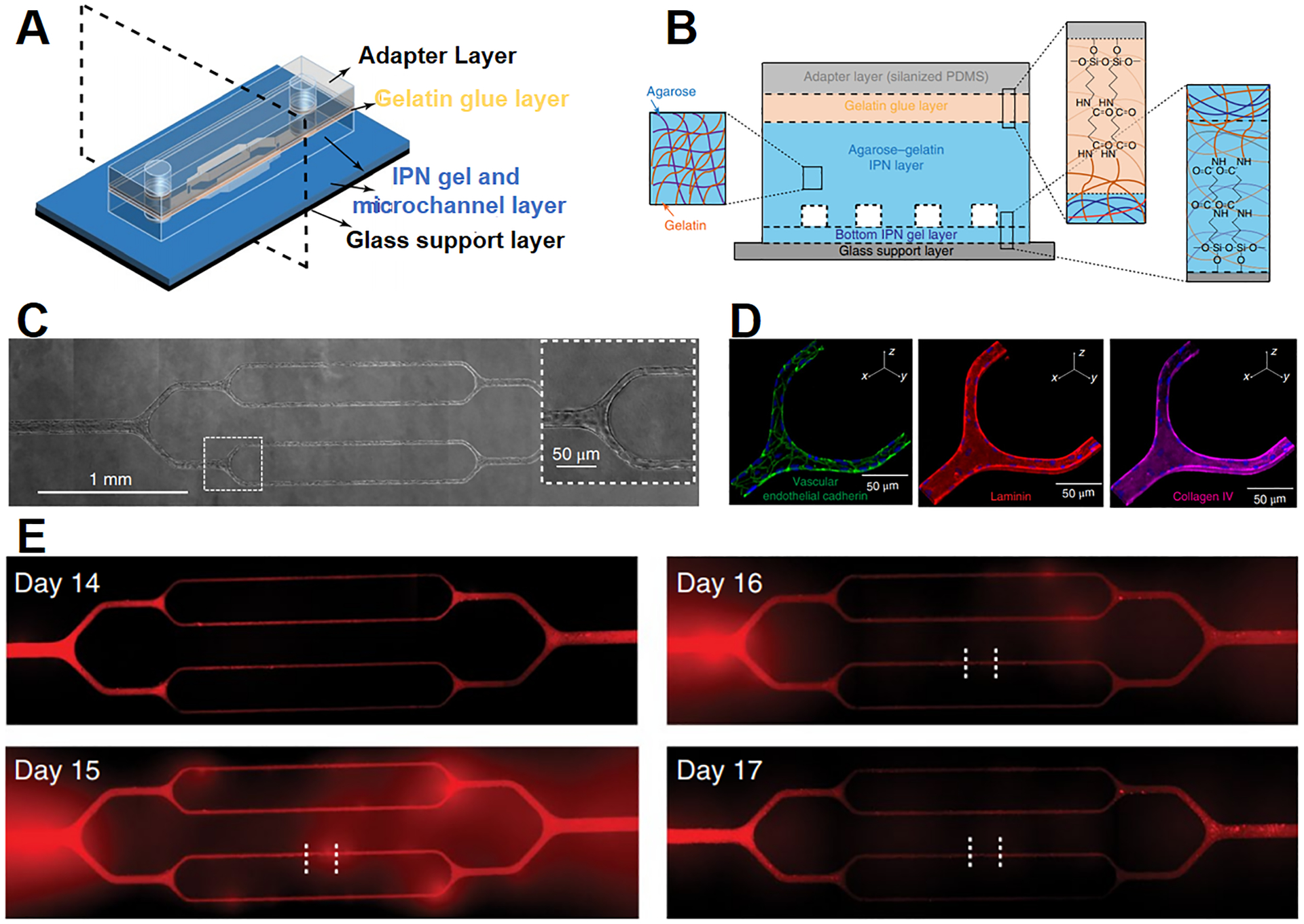
Schematic illustration of (A) the design and (B) the cross-sectional view of the microvasculature chip. (C) Brightfield images of the microvasculature chip with endothelial cells cultured for 14 days. The enlarged view of the boxed area shows the cells reach confluency in microchannels. (D) Confocal images of VE-cadherin (green), laminin (red) and collagen IV (purple) of HUVECs after 14 days of culture. The nuclei are in blue. (E) Fluorescent images demonstrate the change in endothelial permeability after the treatment of plasmodium falciparum-infected RBCs (iRBCs). Endothelial layer is treated with iRBCs for 4 h at day 14, fluorescent images show increased permeability of the cell layer at day 15 and 16, while the barrier function is recovered at day 17. Adapted with permission from ref 193. Copyright 2018, Springer Nature.
Of great interest is the BBB, which is a dynamic structure that regulates the transport of circulating compounds from the blood into the CNS. The BBB consists of microvascular endothelial cells, joined together by tight junction proteins and surrounded by the basement membrane, astrocytes and pericytes (Figure 2C).194 The key challenges in modeling the BBB include achieving a transendothelial electrical resistance (TEER) comparable to the BBB in vivo, having a low permeability to block polar or large compounds, and limiting paracellular permeability by the tight junctions.162, 195 Conventional, static, transwell-based in vitro models fail to reach the necessary TEER and permeability to closely mimic the BBB. It is known that fluid flow across endothelial cells in vivo affects tight junction formation.195 Co-culture of endothelial cells and astrocytes also increases TEER and decreases permeability.195 Recent BBB chips introduce the fluidic shear stress in the co-culture platform to improve the tight junction integrity196, 197 and increase the TEER value up to 300 Ω cm2 from 25 – 40 Ω cm2 of the transwell models.162 For example, Brown et al. have fabricated a BBB chip composed of two chambers separated by polycarbonate porous membrane.100 Human brain microvascular endothelial cells are cultured on the sidewalls of lower chamber to form vascular structure, and the astrocytes and pericytes are embedded in 3-D collagen gel and cultured in the upper chamber (Figure 2D). This BBB chip demonstrates effective blocking of fluorescence-labeled dextran diffusing from vascular chamber to brain chamber. Note that the incorporation of hiPSC-derived endothelial cells in a BBB chip, together with co-culture with neuronal progenitor cells and retinoic acid treatment, is reported to increase the TEER values up to 4000 Ω cm2.198 Nevertheless, most of the reported TEER values of current models are far below physiological values (up to 8,000 Ω cm2)199 and the models do not have satisfactory permeability. As a result, only 7% of CNS drugs reach the market compared to 15% for other therapeutic drugs.200 It is highly desirable to establish the biomimetic BBB chip that recapitulates the key characteristics of the BBB in vivo to advance our understanding of related diseases such as brain metastasis and facilitate the discovery of CNS drugs.
4.2. Brain
As the most sophisticated organ in human body, brain consists of a complex network of a variety of neurons, which are considered the basis for brain function.24 The axon and dendrites of the neuron are responsible for communicating with target cells and receiving information from adjacent neurons at the area of synapses, respectively; neuron damage is associated with various neurodegenerative diseases such as head trauma and multiple sclerosis.201 To study neuronal transport and regeneration, various models have been developed. For example, axonal and soma chambers, which are connected via microgrooves, are fabricated on a PDMS platform. These chambers have the same diameter but different heights (the soma chambers are deeper). When mouse cortical and hippocampal neurons are seeded in the soma chambers, the axons grow along the microgrooves towards the axonal chambers and thus can be isolated for analysis without the contamination of somata.202 In another study, rat hippocampal neurons are grown in both the axonal and soma chambers of the platform. Their dendrites extend along the microgrooves, which enables the instigation of the synapse-to-nucleus signaling.203
Moreover, 3-D multi-layered neural networks have been constructed by using 3-D bioprinting.163–165 For example, a 3-D multilayered structure of neurons is formed by 3-D printing gellan gum mixed with mouse primary neurons.165 Compared with 3-D bioprinting, organoids provide a sample way for stem cells to differentiate and self-organize into a brain-like structure.105, 166–168 It has been demonstrated that organoids are formed from hiPSCs that are cultured in nonadherent substrates and differentiated by using brain-derived neurotrophic factor (BDNF) and neurotrophic factor 3 (NT3). The organoids contain astrocytes and neurons found in both deep and superficial cortical layers. After 137-day culture, the two types of neurons self-assemble to form multi-layered human cortical spheroids (hCSs). The transcriptional profiles of the organoids are similar to those of the mid-fetal prenatal brain.167 Similarly, 3-D ventral forebrain organoids are generated by culturing hiPSCs in the presence of growth factors FGF2 and EGF. A ventral forebrain organoid and a hCS are further cultured in a microcentrifudge tube, and fused after 3 days. The process of saltatory migration of interneurons and generation of neural circuits are observed. The organoids model Timothy syndrome (TS, a severe neurodevelopmental disorder) by using hiPSCs from the patient of TS.168 Additionally, uniformed human brain organoids are formed by culturing and differentiating hESCs in PDMS chambers with 2 mm diameter, and their viability is enhanced by culturing at air-liquid interface to reduce hypoxia. After cannabis treatment, the neuron maturation and outgrowth of the organoids are reduced, which are consistent with previous animal studies.169 Although efforts have been made to mimic parts of brain structure, advanced models are needed to reflect the complex structure and signaling network of the brain.
4.3. Eye
It is estimated that there will be about 2.01 million people with blindness and about 6.95 million people with visual impairment by 2050 in the US alone,204 which poses an urgent need to develop eye substitutes for transplantation and biomimetic eye models for drug development. Human eye consists of several sophisticated components such as cornea and retina. The development of cornea models has been discussed in the Design Principles section (Section 2.1), and this section will focus on the model development for other parts of eye.
The retina, lining the back of the eye, consists of retinal pigment epithelium (RPE) and five major types of neurons including photoreceptors, bipolar cells, horizontal cells, amacrine cells and ganglion cells, which are supported by two types of glial cells, Müller cells and astrocytes.205 The disruption of the RPE monolayer by abnormal choroidal vessels penetrating is a common reason for numerous ophthalmic diseases such as age-related macular degeneration (AMD, a common ophthalmic disease caused by degeneration of photoreceptors). Compared to direct injection of retinal pigment epithelial cells, RPE transplantation offers a more effective way of treating AMD.206 Natural and synthetic biomaterials have been used as substrates to produce RPE monolayer.159, 207, 208 For example, micropatterned PLGA sheets are used to form stable RPE monolayer by promoting the proliferation and tight junction formation of RPE-J cells. The formed RPE monolayer firmly attaches to the macular after injected into the subretinal space of a swine eye.159 Moreover, the RPE-choroid complex acts as an outer blood-retinal barrier to protect the retina from outer vascular. An in vitro model has been constructed by using a fibrin gel to separate and support the RPE layer and blood vessel network in a microfluidic platform. By applying exogenous vascular endothelial growth factor and antiangiogenic compound, pathological angiogenesis and treatment process have been observed in this model, similar to the in vivo pathogenesis and treatment of abnormal choroidal vessel increment.209 To mimic the complex retina microenvironment, a 3-D retinal model has been developed by combining hiPSC-derived retinal organoids (ROs) and hiPSC-derived RPE into a microfluidic platform, which provides vasculature-like perfusion. The ROs contain all five major types of retina neurons and show inner and outer segment formation similar to in vivo retina. The toxicity studies of anti-malaria drug chloroquine (CQ) and the antibiotic gentamicin (GM) in the models demonstrate the pathological effects on the cells and the barrier function of the RPE, being consistent with previous reports.210
The axons of retinal ganglion cells form the optic nerve and transmit visual signals to the brain. Guiding the axons from transplanted cells to the optic nerve is a great challenge in the retinal regeneration. Thus, aligned electrospun nanofibers have been utilized to guide and improve the growth of ganglion cells, mimicking axon orientation in vivo.211, 212 On the aligned PLGA nanofibers containing polypyrrole functionalized graphene the ganglion cells grow along the fiber direction up to 160 μm in the cell length with electrical stimulation, compared to randomly oriented cells with 100 μm in length on random nanofibers.212 Nevertheless, it is still challenging to develop retina models that closely mimic the cellular organization and function of human retina.
4.4. Heart
Heart dysfunction is one of the major causes of death worldwide. Engineered biomimetic heart can provide a novel way to study heart regeneration and test new drugs.213 As the major part of human heart, the myocardium spirals around the ventricular chambers to facilitate the anisotropic electric propagation and pumps blood from the heart to the systemic circulation via its contraction. The myocardium contains parallel-arranged cardiomyocytes, whose alignment and elongation are critical to the myocardial function,214 and its contraction is stimulated by mechanical, chemical, and electrical signals.24
Different approaches have been taken to promote the alignment and elongation of cardiomyocytes.215, 216 For example, multilayer cantilevers with the microgrooves are fabricated by applying 3-D microextrusion bioprinting to guide the growth of hiPSCs-derived cardiomyocytes (Figure 5A). The cardiomyocytes are aligned on the microgrooves of 60 μm in filament spacing and form the anisotropic laminar tissue with the electric propagation similar to native ventricle (Figure 5B). Thicker cantilevers containing micropins are also fabricated to support four layers of cardiomyocytes and construct a laminar tissue with the thickness matching that of the heart (Figure 5C, D).216 Additionally, it is desirable to build a myocardium model with the capability to characterize the performance of the electrical property and mechanical contractions. A cylindrical heart chip has thus been engineered by culturing neonatal rat cardiomyocytes on a fibrinogen matrix to form 3-D cardiac construct after cell maturation. A flexible probe is incorporated into the chip to provide the strain loading to the construct and its contractility is calculated by measuring the lateral displacement with an optical microscope. With the applied strain, frequency and adrenergic stimulation, the contractility changes of the engineered cardiac construct are comparable to those of cardiac tissues in vivo.217 Moreover, components and the whole organ of human heart have been 3-D printed with collagen. Notably, by using gelatin microparticles to support the collagen assembly, the 3-D bioprinting achieves a high fidelity and a resolution of 20 μm. The 3-D printed heart recapitulates the anatomic structure of human heart, and the printed components exhibit their abilities for improving cell infiltration and microvascularization, mimicking ventricle contraction and potential propagation and maintaining integrity under physiologic heart valve pressures.116
Figure 5. A heart-on-a-chip capable of monitoring cardiac contractility.
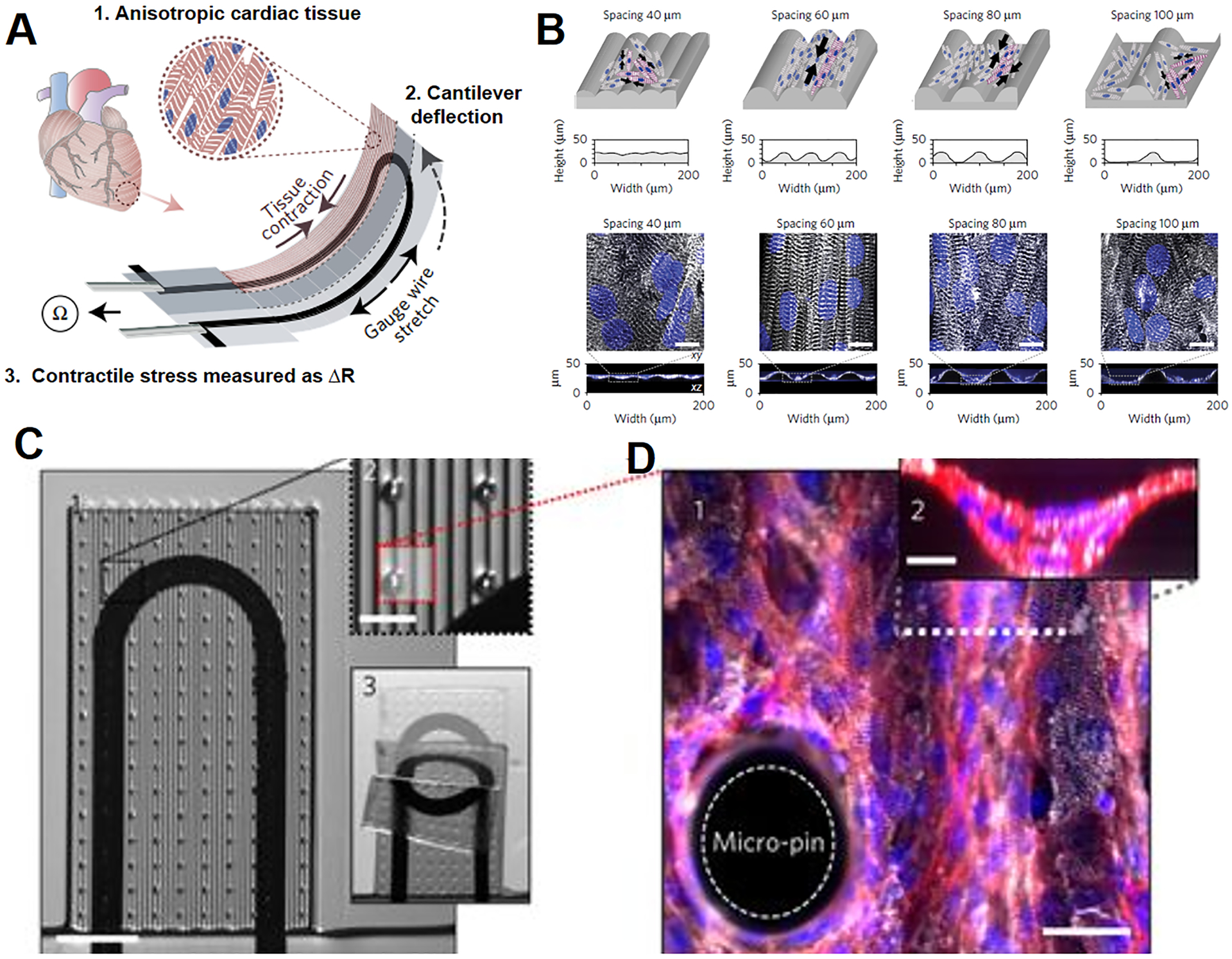
(A) Illustration of the heart chip design. Cardiomyocytes form anisotropic myocardium on the engineered cantilever, which is deflected by myocardial contraction and stretches the soft strain gauge. The resistance change is used to calculate the contractile stress of myocardium. (B) The microgrooves are engineered on the cantilever to guide the elongation of cardiomyocytes. White: α-actinin; Blue: DAPI. Scale bars are 10 μm. (C) 1. Modified cantilever containing micropins is used to support approximately four cardiomyocyte layers. 2. Close-up of the micropins. 3. A cantilever under deflecting. (D) 1. Fluorescent image of the myocardial tissue on the modified cantilever. Scale bar is 30 μm. 2. A cross-sectional image of the myocardial tissue. Scale bar is 10 μm. White: α-actinin; Red: actin; Blue: DAPI. Adapted with permission from ref 216. Copyright 2017, Springer Nature.
4.5. Lung
The minimal functional units of lung, alveoli, experience breath induced mechanical stretch and enable the exchange of oxygen and carbon dioxide between alveolar space and the capillaries. Alveolar dysfunction is the major cause of some lung diseases such as chronic obstructive pulmonary disease and pulmonary edema.218, 219 Hence, physiologically relevant alveolar models hold great potentials for studying the pathology of lung diseases and evaluating drugs and nanotoxins.
The aforementioned lung chip recapitulates the anatomical and physiological characteristics of alveoli (Section 2.2, Figure 2F).4 The co-culture of human alveolar epithelial cells and human pulmonary microvascular endothelial cells via a flexible PDMS microporous membrane mimics the interface of airway and capillary. The air-liquid interface of the airway is recreated in the upper microchannel, and the capillary blood flow is resembled in the lower microchannel. In addition, the vacuum modulated deformation of the two side walls generates 1-D, cyclic mechanical stretch. The study on the lung chip demonstrates that the cyclic stretch influences the cellular responses to nanoparticles and pulmonary infection. This lung chip design has been adapted to model several lung diseases.16, 64 For example, the drug-induced pulmonary edema with the interleukin-2 (IL-2) treatment is modeled, and the mechanical stretch is found to play a critical role in the IL-2 induced vascular leakage, independent of the circulating immune cells.64 In other lung chip designs, asthma and chronic obstructive pulmonary disease are modeled by treating normal lung epithelial cell layer with IL-13 and using the epithelial cells from the patient with chronic obstructive pulmonary disease, respectively.16
Different from the flat, elastomeric PDMS membrane, nanofibers have been utilized to construct the basement membrane to closely mimic the lung anatomy. Recently, electrospun PLGA nanofibrous membrane is used to separate the two parallel channels in a lung cancer chip.96 Human fetal lung fibroblasts (HFL1) and HUVECs are cultured on one side of the nanofibrous membrane, whereas human non-small cell lung cancer cells (A549) are cultured on another side of the membrane. HFL1 cells are found to attenuate the antitumor efficiency of gefitinib, an inhibitor of epidermal growth factor receptor, by secreting IGF-1 to activate the PI3K/Akt signal pathway of A549 cells. However, there is no mechanical stretch employed in this lung cancer chip. In another design, a microimpedance tomography (MITO) system is integrated into a lung chip and demonstrates a high sensitivity on detecting a 7% drop of the impedance resulted from chemically impaired epithelial permeability and cell density-induced mechanical changes at the alveolar interface.178 While many anatomical and physiological characteristics have been considered in the lung models, it is highly desirable to incorporate these characteristics into a single platform to faithfully resemble the lung microenvironment.
4.6. Liver
Liver plays an important role in diverse metabolic processes and is susceptible to various diseases. Liver toxicity is one of the most common reasons for the failure of drug testing in clinical phases,220 which makes it appealing to develop in vitro models recapitulating the functional units of liver. The functional units of liver are lobules which consist of multiple hepatic sinusoids, hepatocytes and several non-parenchymal cells such as liver sinusoidal endothelial cells and hepatic stellate cells.221
Hepatocyte function is affected by the hepatocytes’ interactions with endothelial cells, fibroblasts and other cells, thus efforts have been made to recreate these interactions. For example, when the hepatocyte aggregates are formed from rat primary hepatocytes and co-cultured with J2–3T3 fibroblasts, they produce albumin 2-fold higher than that hepatocyte-only controls and extend the albumin production period from 20 days to more than 7 weeks. Additionally, compared with the hepatocytes on 2-D, the 3-D hepatic organoids that are formed by mixing the hepatocyte aggregates with the fibroblasts in PEG hydrogel display higher Cytochrome P450 enzyme activity, which is important for drug metabolism and hepatotoxicity.222 To closely mimic the hepatic sinusoid structure, a 3-D microwell-based, multilayered chip has been developed (Figure 6A, B). The lower microwell layer is used for hepatic spheroids culture, and the middle microporous membrane permits the nutrients and waste exchange yet serves as a barrier to protect the hepatic spheroids from the high fluid shear stress in the upper layer. Compared to the 2-D static model, the hepatic spheroids generated in the 3-D chip exhibit enhanced cell polarity, which is required for most hepatic function (Figure 6C).180
Figure 6. A liver-on-a-chip with perfusion culture of 3D hepatic spheroids.

(A) Schematic illustration of the hepatic sinusoid structure in vivo and the corresponding chip design. (B) A cross-sectional view of the multilayered liver chip. The lower microwell layer is used for hepatic spheroids culture, and the middle microporous membrane permits the nutrients and waste exchange yet serves as a barrier to protect the hepatic spheroids from the high fluid shear stress in the upper layer. (C) Fluorescent images of MRP-2 and ZO-1 in 2-D hepatic monolayer and 3-D hepatic spheroids. Adapted with permission from ref 180. Copyright 2018, Royal Society of Chemistry.
Because of the complicacy of liver structure and function, not all the essential anatomical and physiological characteristics have been recreated in the liver models. For example, more relevant cell types and more metabolic activities should be included in the liver models. In addition, the flow in the liver sinusoid results in a gradient of biochemical factors such as hormones and oxygen, leading to the gradual changes in cell morphology and various metabolic activities across the sinusoid. A human liver chip is thus developed by co-culturing primary human hepatocytes, human endothelial cells (EA.hy926), immune cells (U937) and stellate cells (LX-2) in a single chamber microfluidic chip.223 This liver chip exhibits a similar level of in vivo albumin production and is able to reproduce the acute toxic responses of liver to troglitazone, a type 2 diabetes drug (previously approved but withdrawn in the US because of its acute liver toxicity). The oxygen tension variance in hepatic sinusoid is also modeled by controlling media flow rate in this liver chip, which successfully recapitulates zonation dependent protein secretions of hepatic sinusoid.224 The liver chip with high oxygen tension shows oxidative phosphorylation bias in function and albumin secretion, while low oxygen tension liver chip biases functions of glycolysis and α1AT secretion. In addition, to mimic the gradual change in metabolic activities, a microfluidic gradient generator has been engineered and linked to a cell culture chamber. By infusing different inducers through the generator, this chip mimics some in vivo metabolic activities of the liver sinusoid.179
4.7. Kidney
Kidney is responsible for filtration of blood, reabsorption of water and solutes, and removal of waste. Its minimal structural and functional unit is nephron, which is composed of a corpuscle and a renal tubule. As the major part of the corpuscle, glomerulus is a tuft of capillaries and responsible for filtration. The renal tubule is formed by squamous epithelium and contains proximal tubule as the major segment.24
The structure and function of glomerulus are modeled in a chip similar to the lung chip (Figure 2F) by growing hiPSCs-derived glomerular podocytes and human glomerular endothelial cells on each side of a PDMS microporous membrane. The cyclic mechanical stretch has been shown to promote the differentiation of mesoderm cells to podocytes and the formation of a glomerular capillary wall.225 A glomerulus-on-a-chip has also been developed to mimic hypertensive glomerulopathy by controlling hydrodynamic factors in two microfluidic channels lined by closely opposed layers of glomerular endothelial cells and podocytes. When the flow rate increases, cytoskeleton is rearranged and cell-cell connection becomes loose, resulting in glomerular filtration barrier damage and glomerular leakage, in accordance with in vivo studies.226
To build a proximal tubule model, human proximal tubule epithelial cells are grown inside polyethersulfone hollow fibers. The formed epithelial monolayer demonstrated proximal tubule functions such as uremic toxin clearance and albumin reabsorption.227 Moreover, a convoluted proximal tubule construct is engineered by using 3-D microextrusion bioprinting (Figure 7). The fluidic shear stress through the lumen facilitates the formation of confluent proximal tubule epithelial cell monolayer and its maturation. Notably, the response of the engineered proximal tubule to nephrotoxin Cyclosporine A is similar to the in vivo observation, exhibiting dose-dependent epithelial barrier disruption.92 Nevertheless, human kidney contains more than 10 cell types and the components of nephron have their unique structures and coordinate to execute their functions; advanced kidney models are thus needed to reflect the complexity and realize the functions of the kidney.
Figure 7. A renal proximal tubule-on-a-chip.
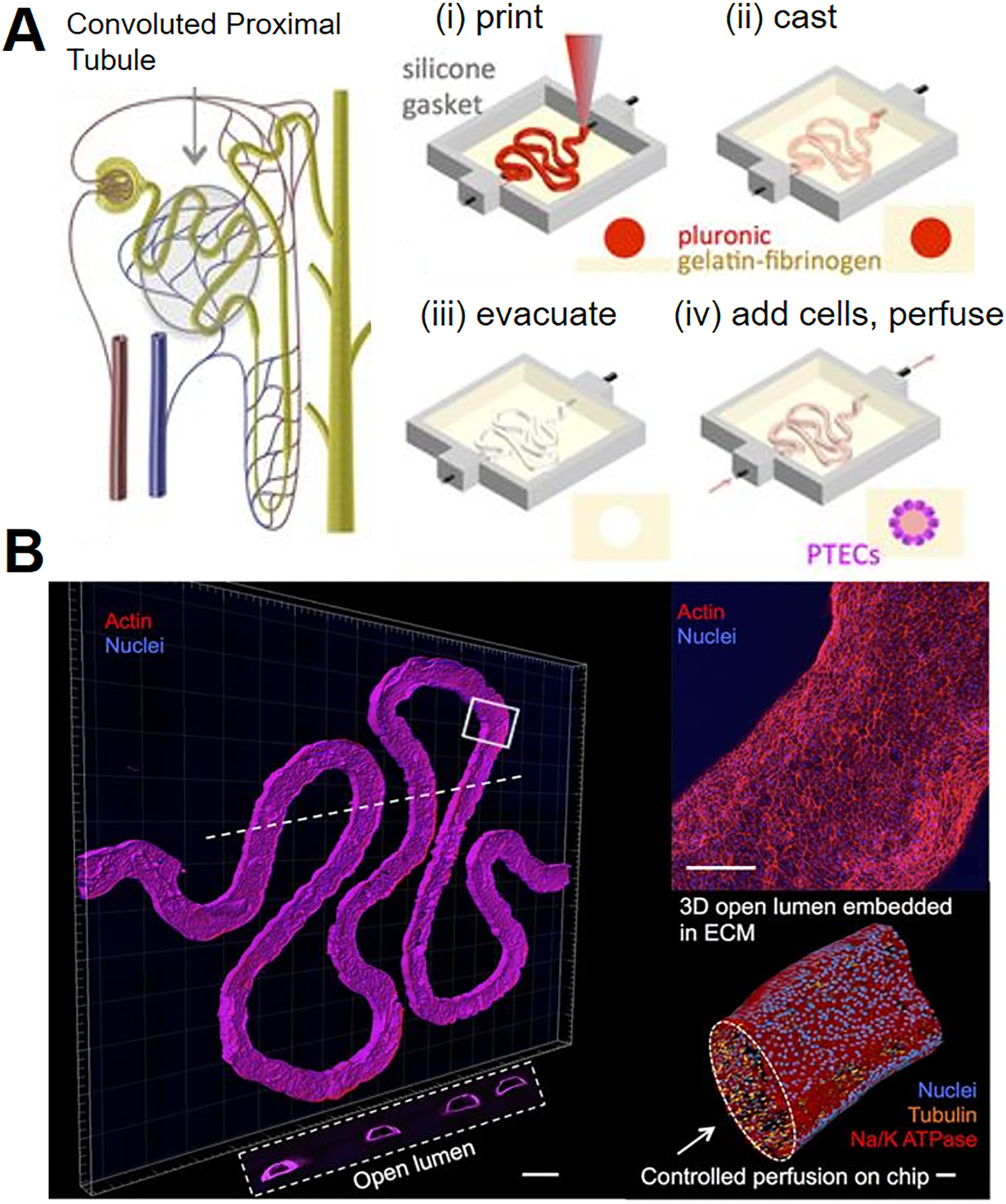
(A) Schematic illustration of a nephron containing proximal tubule and the fabrication process of the proximal tubule chip. (B) 3-D confocal images of the formed proximal tubule. Scale bar is 500 μm. Upper right image: the enlarged view of the proximal tubule. Scale bar is 200 μm. Lower right image: a 3-D rendering of proximal tubule to show the open lumen. Scale bar is 50 μm. The white dash line shows the location of the cross-sectional view in the white box. Adapted with permission from ref 92. Copyright 2016, Springer Nature.
4.8. Gut
The small intestine is the main site for digestion and absorption of nutrients and development of mucosal immunity. Therefore, physiologically relevant intestinal models will benefit the studies of the nutrient metabolism, oral drug pharmacokinetics and intestinal disease development.228 One of the unique features in the intestine is the villi, which are covered by intestinal epithelium and significantly increase the surface area of small intestine for digestion and absorption.24
The lung chip design shown in Figure 2F has been adapted to explore the roles of the fluidic flow and cyclic peristaltic mechanical strain in the development of intestinal structure. Human intestinal epithelial (Caco-2) cells are grown on the porous PDMS membrane to form villi-like gut epithelium, which exhibits about 1.5-fold higher efficiency of glucose absorption than the Caco-2 monolayer cultured in the transwell.47 This chip design is also modified to mimic the interactions between the obligate anaerobic bacteria, the major bacteria in the gut that regulate the nutrient absorption and immune response, and the host cells. The anaerobic bacteria and intestinal epithelial cells are co-cultured in the chip, and the anoxic and oxic culture media are infused to the upper and lower channels respectively to control the oxygen gradient. This chip successfully mimics the oxygen gradient microenvironment in human gut and shows that the presence of the anaerobic bacteria increases the TEER of the epithelium.229
Furthermore, to model the interactions between microvasculature and intestine, which are critical to the intestinal function, the intestinal organoid fragments and human intestinal microvascular endothelial cells are grown on the apical and basal channels of the aforementioned chip to form villi-like intestinal structure and the endothelial barrier, respectively. The amount of mucus secreted by the epithelial cells in this chip is 10-fold higher than that of Caco-2 cells alone. Compared to the intestinal organoids alone, the expression levels of genes associated with digestive function and host defense response observed in the gut chip are closer to the in vivo results.230
4.9. Tumor
Cancer metastasis is responsible for about 90% of cancer deaths.231 Cancer metastasis is a complex and multistep process involving tumor cells disseminating from a primary tumor, entering into the blood vessel, and extravasating across the endothelial layer to colonize in a secondary site.232 In the process, interactions between tumor cells and other cells including stromal cells, endothelial cells and the cells in the secondary site play critical roles. Modeling these cell interactions will advance the understanding of the cancer metastasis and the evaluation of anticancer drugs.233, 234
A microfluidic chip with three parallel channels has been designed to study the interactions between liver cancer cells (HuH7) and bone marrow stromal cells (HS5).188 The middle channel is separated by rectangular micropillars from two side channels, where the HS5 and HuH7 cells are cultured, respectively. The HS5 and HuH7 cells migrate to the middle channel and interact with each other, which significantly accelerates the proliferation of HuH7 cells yet causes the death of HS5 cells. Note that both cells are cultured on the 2-D PDMS surface and this chip does not resemble the 3-D cell-cell interactions in vivo. In another study, carcinoma-associated fibroblasts (CAFs) and the gland adenoid cystic carcinoma (ACC) cells are embedded in 3-D matrices and injected into two chambers of a microfluidic chip, respectively (Figure 8A-i, ii).235 The chambers are centered horizontally to facilitate the evaluation of the interaction between CAFs and AAC cells. The study helps identify GM6001 (an MMP inhibitor) as a promising candidate for inhibiting the CAFs-promoted ACC invasion (Figure 8A-iii). The tumor-vascular interface is also recreated in a microfluidic chip by growing the tumor cells and the endothelial cells in two side channels that are separated by 3-D hydrogel.136 In this chip, the presence of macrophages or the simulation of TNF-α impairs endothelial barrier, leading to elevated tumor cell intravasation rates. Moreover, the interactions between the circulating breast cancer cell MDA-MB-231 and human dermal microvascular endothelium are modeled in a microfluidic chip, which consists of two vertical arranged chambers, separated by a porous polyester membrane.186 The endothelial cells are cultured on the apical side of the membrane and MDA-MB-231 cells are perfused in the top chamber. When C-X-C motif chemokine ligand 12 (CXCL12) is added into the bottom chamber, CXCL12 interacts with the C-X-C motif chemokine receptor 4 (CXCR4) on the endothelium to enhance the adhesion of MDA-MB-231 cells, independent of the CXCR4 on tumor cells.
Figure 8. Tumor-on-a-chip systems (A) to study tumor-CAF interactions and (B) to identify patient-specific responses to chemoradiotherapy.
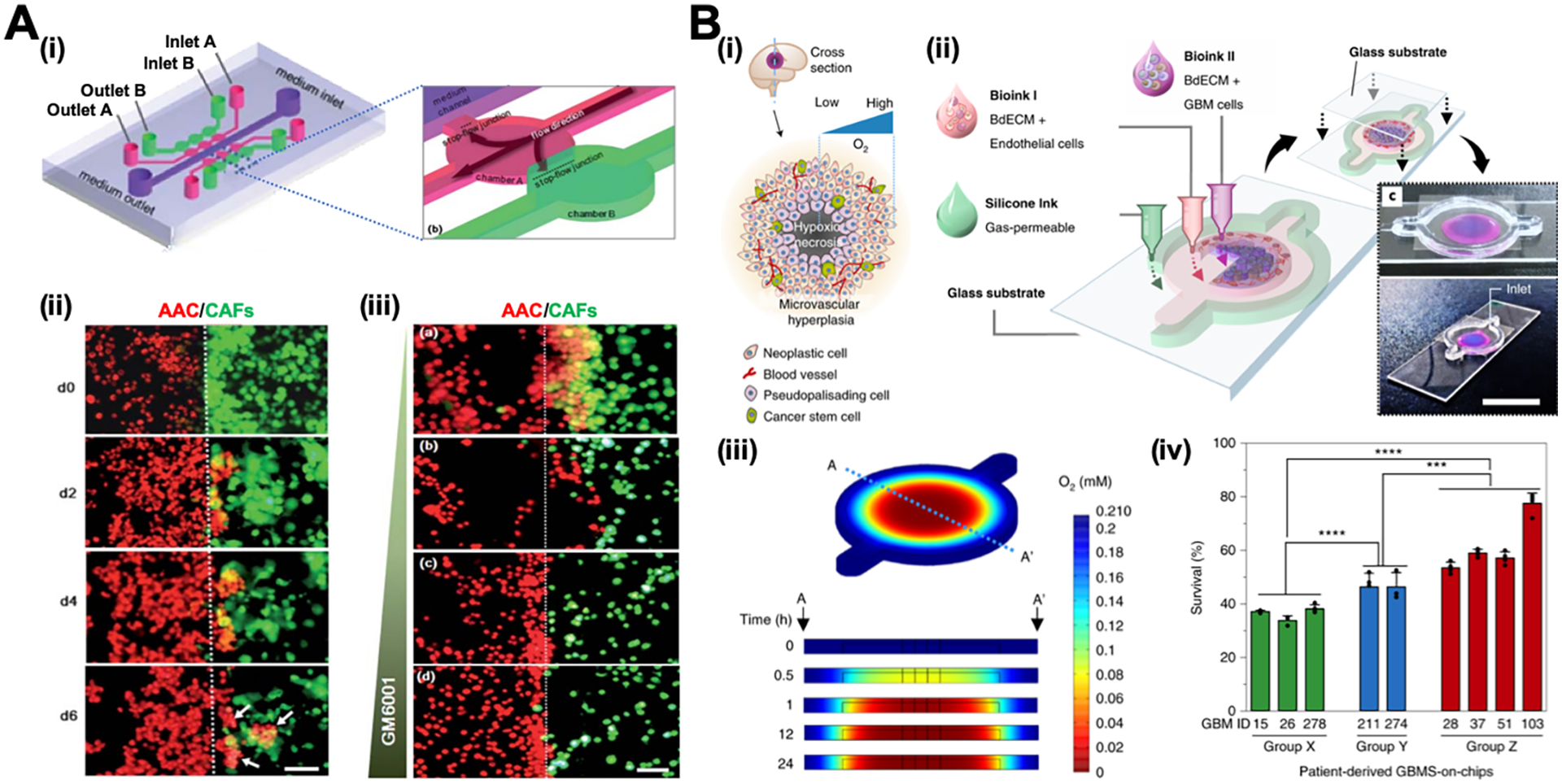
(A) i: Schematic illustration of the chip design. ii: Invasion of AAC cells co-cultured with CAFs over time. White arrows show that AAC cells form spheroids in CAF-containing matrix. iii: Invasion of AAC cells co-cultured with CAFs under the treatment of GM6001 at the concentration of (a) 0 mg/mL; (b) 10 mg/mL; (c) 50 mg/mL; and (d) 100 mg/mL. Scale bars are 100 μm. Adapted with permission from ref 235. Copyright 2010, Royal Society of Chemistry. (B) i: Schematic illustration of the heterogeneous structure of the glioblastoma tissue. ii: Schematic illustration and photos of the glioblastoma chip. iii: Heatmap images of oxygen distribution in the glioblastoma chip along the crosssection A–A’. iv: The percentage survival after chemoradiation of the glioblastoma chip prepared by using patient-specific glioblastoma cells. The patients exhibited low-to-moderate resistance in Group X and high resistance in Group Y to chemoradiation treatment, while experienced extremely aggressive tumor progression in Group Z after the treatment. Adapted with permission from ref 95. Copyright 2019, Springer Nature.
The selective organ-homing effect of tumor cells has also been studied in a three-channel chip, in which the middle channel mimics 3-D bone or muscle microenvironments and two side channels are perfused with tumor cells.236 Breast cancer cells exhibit a significantly higher extravasation rate in bone-mimicking microenvironment than the cells in the muscle-mimicking microenvironment, indicating the organ-specific extravasation of cancer cells.
Recapitulating the structural, physical and biochemical properties of native tumors in a tumor chip is critical to determine appropriate cancer treatment. Recently, a human-glioblastoma-on-a-chip is engineered by constructing a compartmentalized cancer-stroma concentric-ring structure via 3-D bioprinting technique.95 The patient-derived glioblastoma cells and vascular endothelial cells are mixed with decellularized ECM as bio-ink and printed on a glass slide with the tumor cells in the center and the endothelial cells in the outer region surrounding the tumor cells, which mimics the structure of glioblastoma in vivo (Figure 8B-i, ii). A glass coverslip is placed on top of the chip so that the oxygen can only penetrate from the outmost region to the center, thus creating a radial oxygen gradient similar to the oxygen distribution inside the glioblastoma (Figure 8B-iii). This patient-specific glioblastoma chip is shown to successfully reproduce the observed resistance differences of glioblastoma patients after concurrent chemoradiation using temozolomide (Figure 8B-iv).
4.10. Multiorgans-on-a-chip systems
The development of multiorgans-on-a-chip (multiorgan chip) systems enables the organ-organ interactions in an integrative and dynamic system, thus providing more robust and realistic platforms for pathophysiological studies and treatment of human disease.237 Multiorgan chips represent promising platforms to study pharmacokinetics/pharmacodynamics (PK/PD) and absorption, distribution, metabolism, and excretion (ADME) of drugs.
For example, a multiorgan chip consisted of liver, tumor and marrow chambers has been developed to evaluate the drug metabolism.71 To mimic the physiological condition, the organ-specific cells are cultured in different 3-D hydrogels with the stiffnesses comparable to that of the corresponding organ. The component organ chambers are connected via microchannels, and the fluidic flow rates and residence times are determined based on the blood flows in human body. The metabolism of a prodrug (Tegafur) to its functional unit (5-fluorouracil) in liver and the consequent drug-induced cell death are successfully demonstrated in the multiorgan chip. Many other multiorgan chips have been developed for toxicity assessment. For example, liver-kidney chips are built to predict the chemical toxicity in a high-throughput manner and the studies highlight the metabolic cooperation between liver and kidney in response to small molecules such as ammonia, dimethylsulfoxide, N-acetyl-para-aminophenol and aristolochic acid I.238, 239 Another study with a liver-gut chip tests the toxicity of 50 nm carboxylated polystyrene nanoparticles and indicates that the intestinal cell layer can act as the barrier to prevent the penetration of more than 90% of nanoparticles.240 This chip further shows that a small portion of penetrated nanoparticles can cause liver injury by inducing aspartate aminotransferase release.
In order to model and control the fluidic flow, pumps are usually utilized in most of the multiorgan chips. However, one of the major issues is that the pump may generate bubbles in the chip, which could cause cell death and fail the drug test.71 Hence, a pumpless approach is developed to control fluidic flow as demonstrated in a GI tract-liver chip.241 The fluidic flow that connects the two organs is driven by gravity and the flow rate is controlled by the hydraulic resistances of the microchannels. By taking the pumpless gravity-driven approach, a 13 organ modules including the barrier and non-barrier types of tissues have been integrated into a microfluidic chip, which exhibits the high viability rate of five cell lines for at least 7 days.242
5. Perspectives
Driven by the pressing need for physiologically relevant in vitro models, microphysiological systems have experienced explosive growth in the past decade and shown a huge promise in pathophysiological study, diagnosis and treatment of human diseases, and drug development. Microphysiological systems can be further improved by faithfully mimicking the in vivo organ microenvironment, in particular the key physical (matrix stiffness and micro-/nanostructures) and mechanical (shear stress and mechanical stretch) characteristics, and accurately coordinating intra- and inter-organ interactions in a 3-D integrative system.
The ECM which the cells interact with often displays various degrees of stiffness and hierarchically arranged micro-/nanostructures.243–245 The stiffness is not only tissue specific but also can be altered by the disease state. For example, the lung tissue is softer in emphysema,246 but stiffer in fibrotic tissues than the normal lung.247, 248 The stiffness of the mammary tissue in breast cancer patients is also higher than its normal condition.249 Although the physical characteristics such as stiffness and nanostructures have been taken into account during the development of microphysiological systems to some extent, current systems do not recapitulate these key characteristics satisfactorily. Notably, as a ubiquitous component of ECM, the basement membrane, which separates tissues such as epithelia, endothelia, and the nervous systems from connective tissue compartment,250 is soft and manifests significant nanoscale structures.251, 252 However, conventional cell culture platforms and even some organ chips such as the BBB chips mainly use flat, porous membranes made of stiff materials such as PC, polyethylene terephthalate (PET) or polytetrafluoroethylene (PTFE) or PDMS for co-culture. The flat membranes do not resemble the nanostructured membrane. In addition, the supraphysiologically stiff membrane (Young’s modulus: 0.4–2.7 GPa,253 compared to 0.1–1 kPa,254 the stiffness of brain) favors cell adhesion yet compromises cell-cell interactions. Electrospinning technology provides a promising tool to engineer nanofibrous substrates to resemble the basement membrane.96, 255 For example, aligned poly(ε-caprolactone) nanofibrous (100–300 nm) substrates are shown to promote the expression of adherens junction protein VE-cadherin of HUVEC.255 Yet, the electrospun nanofibrous membrane is much stiffer than the in vivo counterpart..
While the significance of fluidic shear stress is well known, the mechanical stimulation has not been implemented in microphysiological systems until the development of the lung chips. The mechanical stimulation is critical to the development and normal function of human organs; alterations in the mechanical stimulation can result in dysfunction and even disease of the organ.8 For examples, cardiac growth and morphogenesis have been shown to require mechanical loading.256, 257 Functional dysphagia is associated with abnormally low esophageal traction forces.258 Note that the recently developed lung chips manifest the significance of mechanical stimulation in evaluating nanomaterial toxicity, disease development and drug efficiency.4, 16, 64 Nevertheless, the mechanical stimulation has not been satisfactorily incorporated into the developed microphysiological systems. Even the uniaxial stretch in the lung chips does not faithfully resemble 3-D radial stretch in lung breathing. It is believed that the dimensionality of mechanical stimulation, such as in vivo 2-D circumferential stretch of blood vessels and 3-D radial stretch observed during heart beating and lung breathing movement, and their magnitude affect cell behaviors and organ function.259 In this regard, the mechanical stimulation in the microphysiological system should faithfully resemble the in vivo physiological conditions.
Of equal importance is to integrate and coordinate the key characteristics in 3-D microenvironment. In addition to using electrospun nanofibrous membranes for co-culture as described above, PCL nanofibers are deposited into microfluidic channels to culture macrophages, which exhibit enhanced interleukin-6 section under circulating lipopolysaccharide stimulation.260 However, these key microenvironmental characteristics have not been fully integrated into microphysiological systems. For example, biomimetic BBB chips that incorporates the nanofibrous membrane for co-culture of endothelial cells with astrocytes and pericytes and blood pressure-induced circumferential stretch is still absent. It is also vital to control the organization of cells and matrix in 3-D. As discussed in the Design Principles section, the fibril organization in the corneal stroma is critical to the transparency of cornea.29 Moreover, a recent study shows that hMSCs preferentially undergo osteogenesis when grown on top of hydrogel surfaces, but predominantly differentiate to chondrocytes when encapsulated within the same gels.261
Further, the applications of microphysiological systems usually require a long-term cell culture. During the culture period, the alterations in physicochemical properties of the system could induce the unexpected variation of the outcomes such as inaccurate prediction of drug efficiency. For example, lung fibroblasts overproduce ECM proteins in fibrogenesis and induce ECM stiffening, which can further promote fibrosis via a positive feedback loop.247 It is therefore critical to control the physiological conditions and perform real-time monitoring in the microphysiological system, such as pH value, oxygen tension, glucose and lactate levels, and matrix properties among many others. Microsensor systems are promising because the miniaturization facilitates the integration of sensors in microphysiological systems, which enable continuous monitoring and real-time data recording. One example is an electro-optics-based multi-analyte microsensor that has been developed to acquire and record both pH and oxygen levels in a microfluidic system for three days.262 Another example is a microbead-based electrochemical immunosensor that has been designed to measure the biomarkers secreted by the cells in a microfluidic chip.263 However, the sensory systems have not been satisfactorily integrated into the current organ chips. One of the challenges is to scale down the sensor to be compatible with the organ chip.264 Another challenge is that the microsensors based on electrochemical and biological reactions suffer from the sensitivity drift and need to be calibrated frequently.265 Advanced microsensor technology is demanded to implement the real-time monitoring of microphysiological systems.
Evolving stem cell biology, biomaterials and micro-/nanotechnologies have contributed to the advent of microphysiological systems, and the innovations in these technologies will continue to advance the engineering, operation and monitoring of the systems. Biomimetic microphysiological systems will inevitably facilitate preclinical investigation and expedite the drug development of human diseases, while they reduce, if not eliminate the use of animals.
Acknowledgment
The authors would like to acknowledge funding support for Yong Yang from the National Institute of Health (NIH) (R15GM122953).
Footnotes
Disclosure
The authors declare no conflict of interest.
References
- 1.Greek R; Menache A, Systematic reviews of animal models: methodology versus epistemology. Int. J. Med. Sci 2013, 10 (3), 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Low L; Tagle D, Tissue chips–innovative tools for drug development and disease modeling. Lab Chip 2017, 17 (18), 3026–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SE; Georgescu A; Huh D, Organoids-on-a-chip. Science 2019, 364 (6444), 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh D; Matthews BD; Mammoto A; Montoya-Zavala M; Yuan Hsin H; Ingber DE, Reconstituting organ-level lung functions on a chip. Science 2010, 328 (5986), 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronaldson-Bouchard K; Vunjak-Novakovic G, Organs-on-a-chip: a fast track for engineered human tissues in drug development. Cell Stem Cell 2018, 22 (3), 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter CM; Lundberg MH; Harrington LS; Warboys CM; Warner TD; Berson RE; Moshkov AV; Gorelik J; Weinberg PD; Mitchell JA, Role of shear stress in endothelial cell morphology and expression of cyclooxygenase isoforms. Arterioscler. Thromb. Vasc. Biol 2011, 31 (2), 384–391. [DOI] [PubMed] [Google Scholar]
- 7.Anlaş AA; Nelson CM, Tissue mechanics regulates form, function, and dysfunction. Curr. Opin. Cell. Biol 2018, 54, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo J; Byun WY; Alisafaei F; Georgescu A; Yi Y-S; Massaro-Giordano M; Shenoy VB; Lee V; Bunya VY; Huh D, Multiscale reverse engineering of the human ocular surface. Nat. Med 2019, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K; Shi L; Linthicum W; Man K; He X; Wen Q; Rojanasakul LW; Rojanasakul Y; Yang Y, Substrate stiffness-dependent carbon nanotube-induced lung fibrogenesis. Nano Lett. 2019, 19 (8), 5443–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplin JD; Granados NG; James MR; Montazami R; Hashemi N, Microfluidic organ-on-a-chip technology for advancement of drug development and toxicology. Adv. Healthc. Mater 2015, 4 (10), 1426–1450. [DOI] [PubMed] [Google Scholar]
- 11.Wagner I; Materne E-M; Brincker S; Süßbier U; Frädrich C; Busek M; Sonntag F; Sakharov DA; Trushkin EV; Tonevitsky AG; Lauster R; Marx U, A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013, 13 (18), 3538–3538. [DOI] [PubMed] [Google Scholar]
- 12.Shao Y; Taniguchi K; Gurdziel K; Townshend Ryan F.; Xue X; Yong Koh Meng A.; Sang J; Spence Jason R.; Gumucio Deborah L.; Fu J, Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater 2016, 16, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue X; Sun Y; Resto-Irizarry AM; Yuan Y; Yong KMA; Zheng Y; Weng S; Shao Y; Chai Y; Studer L; Fu J, Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater 2018, 17 (7), 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y; Xue X; Shao Y; Wang S; Esfahani SN; Li Z; Muncie JM; Lakins JN; Weaver VM; Gumucio DL, Controlled modelling of human epiblast and amnion development using stem cells. Nature 2019, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson CM; Gleghorn JP; Pang M-F; Jaslove J; Goodwin K; Varner VD; Miller E; Radisky DC; Stone HA, Microfluidic chest cavities reveal that transmural pressure controls the rate of lung development. Development 2017, 4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benam KH; Villenave R; Lucchesi C; Varone A; Hubeau C; Lee H-H; Alves SE; Salmon M; Ferrante TC; Weaver JC; Bahinski A; Hamilton GA; Ingber DE, Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13 (2), 151–157. [DOI] [PubMed] [Google Scholar]
- 17.Ahadian S; Civitarese R; Bannerman D; Mohammadi MH; Lu R; Wang E; Davenport-Huyer L; Lai B; Zhang B; Zhao Y, Organ-on-a-chip platforms: a convergence of advanced materials, cells, and microscale technologies. Adv. Healthc. Mater 2018, 7 (2), 1700506. [DOI] [PubMed] [Google Scholar]
- 18.Zheng F; Fu F; Cheng Y; Wang C; Zhao Y; Gu Z, Organ-on-a-chip systems: microengineering to biomimic living systems. Small 2016, 12 (17), 2253–2282. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B; Korolj A; Lai BFL; Radisic M, Advances in organ-on-a-chip engineering. Nat. Rev. Mater 2018, 3 (8), 257. [Google Scholar]
- 20.Kuecker CM; Vivian EM, Patient considerations in type 2 diabetes–role of combination dapagliflozin–metformin XR. Diabetes Metab. Syndr. Obes 2016, 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paek J; Park SE; Lu Q; Park K-T; Cho M; Oh JM; Kwon KW; Yi Y.-s.; Song JW; Edelstein HI, Microphysiological engineering of self-assembled and Perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS nano 2019, 13 (7), 7627–7643. [DOI] [PubMed] [Google Scholar]
- 22.Yin F; Zhu Y; Wang Y; Qin J, Engineering brain organoids to probe impaired neurogenesis induced by cadmium. ACS Biomater. Sci. Eng 2018, 4 (5), 1908–1915. [DOI] [PubMed] [Google Scholar]
- 23.Kaushik G; Leijten J; Khademhosseini A, Concise review: organ engineering: design, technology, and integration. Stem Cells 2017, 35 (1), 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.College, O., Anatomy and physiology. Rice University: 2013. [Google Scholar]
- 25.Ogden TE; Duggan J; Danley K; Wilcox M; Minckler DS, Morphometry of nerve fiber bundle pores in the optic nerve head of the human. Exp. Eye Res 1988, 46 (4), 559–568. [DOI] [PubMed] [Google Scholar]
- 26.Tezel G; Trinkaus K; Wax MB, Alterations in the morphology of lamina cribrosa pores in glaucomatous eyes. Br. J. Ophthalmol 2004, 88 (2), 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rönkkö S; Vellonen K-S; Järvinen K; Toropainen E; Urtti A, Human corneal cell culture models for drug toxicity studies. Drug Deliv. Transl. Res 2016, 6 (6), 660–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghezzi CE; Rnjak-Kovacina J; Kaplan DL, Corneal tissue engineering: recent advances and future perspectives. Tissue Eng. Part B Rev 2015, 21 (3), 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tidu A; Ghoubay-Benallaoua D; Lynch B; Haye B; Illoul C; Allain JM; Borderie VM; Mosser G, Development of human corneal epithelium on organized fibrillated transparent collagen matrices synthesized at high concentration. Acta Biomater. 2015, 22, 50–58. [DOI] [PubMed] [Google Scholar]
- 30.Shaheen BS; Bakir M; Jain S, Corneal nerves in health and disease. Surv. Ophthalmol 2014, 59 (3), 263–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buznyk O; Pasyechnikova N; Islam MM; Iakymenko S; Fagerholm P; Griffith M, Bioengineered corneas grafted as alternatives to human donor corneas in three high-risk patients. Clin. Transl. Sci 2015, 8 (5), 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gil ES; Park S-H; Marchant J; Omenetto F; Kaplan DL, Response of human corneal fibroblasts on silk film surface patterns. Macromol. Biosci 2010, 10 (6), 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T; Kan K; Nishida K; Yamato M; Okano T, Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials 2013, 34 (36), 9010–9017. [DOI] [PubMed] [Google Scholar]
- 34.Du Y; Chen J; Funderburgh JL; Zhu X; Li L, Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol. Vis 2003, 9, 635–643. [PMC free article] [PubMed] [Google Scholar]
- 35.Rao SK; Leung A; Young AL; Fan D; Lam D, Is there a minimum endothelial cell count for a clear cornea after penetrating keratoplasty? Indian J. Ophthalmol 2000, 48 (1), 71. [PubMed] [Google Scholar]
- 36.Palchesko RN; Funderburgh JL; Feinberg AW, Engineered basement membranes for regenerating the corneal endothelium. Adv. Healthc. Mater 2016, 5 (22), 2942–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizwan M; Peh GSL; Ang HP; Lwin NC; Adnan K; Mehta JS; Tan WS; Yim EKF, Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [DOI] [PubMed] [Google Scholar]
- 38.Skalicky SE, Ocular and Visual Physiology. Springer: 2016. [Google Scholar]
- 39.Ahearne M; Wilson SL; Liu KK; Rauz S; El Haj AJ; Yang Y, Influence of cell and collagen concentration on the cell-matrix mechanical relationship in a corneal stroma wound healing model. Exp. Eye Res 2010, 91 (5), 584–591. [DOI] [PubMed] [Google Scholar]
- 40.Liu W; Deng C; McLaughlin CR; Fagerholm P; Lagali NS; Heyne B; Scaiano JC; Watsky MA; Kato Y; Munger R; Shinozaki N; Li F; Griffith M, Collagen–phosphorylcholine interpenetrating network hydrogels as corneal substitutes. Biomaterials 2009, 30 (8), 1551–1559. [DOI] [PubMed] [Google Scholar]
- 41.Wu J; Rnjak-Kovacina J; Du Y; Funderburgh ML; Kaplan DL; Funderburgh JL, Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 2014, 35 (12), 3744–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaacson A; Swioklo S; Connon CJ, 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res 2018, 173, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alaminos M; Sánchez-Quevedo M. a. D. C.; Munõz-Ávila JI; Serrano D; Medialdea S; Carreras I; Campos A, Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest. Ophthalmol. Vis. Sci 2006, 47 (8), 3311–3317. [DOI] [PubMed] [Google Scholar]
- 44.Wang S; Ghezzi CE; Gomes R; Pollard RE; Funderburgh JL; Kaplan DL, In vitro 3D corneal tissue model with epithelium, stroma, and innervation. Biomaterials 2017, 112, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deardorff PM; McKay TB; Wang S; Ghezzi CE; Cairns DM; Abbott RD; Funderburgh JL; Kenyon KR; Kaplan DL, Modeling diabetic corneal neuropathy in a 3D in vitro cornea system. Sci. Rep 2018, 8 (1), 17294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siran W; Ghezzi CE; Cairns DM; Pollard RE; Chen Y; Gomes R; McKay TB; Pouli D; Jamali A; Georgakoudi I; Funderburgh JL; Kenyon K; Hamrah P; Kaplan DL, Human corneal tissue model for nociceptive assessments. Adv. Healthc. Mater 2018, 7 (19), 1800488. [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ; Ingber DE, Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol 2013, 5 (9), 1130–1140. [DOI] [PubMed] [Google Scholar]
- 48.Mammoto T; Ingber DE, Mechanical control of tissue and organ development. Development 2010, 137 (9), 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K; Bruce A; Mezan R; Kadiyala A; Wang L; Dawson J; Rojanasakul Y; Yang Y, Nanotopographical modulation of cell function through nuclear deformation. ACS Appl. Mater. Interfaces 2016, 8 (8), 5082–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y; Wang K; Gu X; Leong KW, Biophysical regulation of cell behavior—cross talk between substrate stiffness and nanotopography. Engineering 2017, 3 (1), 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu B; McNally S; Kilpatrick JI; Jarvis SP; O’Brien CJ, Aging and ocular tissue stiffness in glaucoma. Surv. Ophthalmol 2018, 63 (1), 56–74. [DOI] [PubMed] [Google Scholar]
- 52.Braunsmann C; Hammer CM; Rheinlaender J; Kruse FE; Schaffer TE; Schlotzer-Schrehardt U, Evaluation of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Invest. Ophthalmol. Vis. Sci 2012, 53 (6), 2960–2967. [DOI] [PubMed] [Google Scholar]
- 53.Sigal IA; Flanagan JG; Ethier CR, Factors influencing optic nerve head biomechanics. Invest. Ophthalmol. Vis. Sci 2005, 46 (11), 4189–4199. [DOI] [PubMed] [Google Scholar]
- 54.Kim S; Kim W; Lim S; Jeon JS, Vasculature-on-a-chip for in vitro disease models. Bioengineering 2017, 4 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Resnick N; Yahav H; Shay-Salit A; Shushy M; Schubert S; Zilberman LC; Wofovitz E, Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol 2003, 81 (3), 177–199. [DOI] [PubMed] [Google Scholar]
- 56.Song JW; Munn LL, Fluid forces control endothelial sprouting. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (37), 15342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price GM; Wong KH; Truslow JG; Leung AD; Acharya C; Tien J, Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010, 31 (24), 6182–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mammoto A; Mammoto T; Kanapathipillai M; Wing Yung C; Jiang E; Jiang A; Lofgren K; Gee EP; Ingber DE, Control of lung vascular permeability and endotoxin-induced pulmonary oedema by changes in extracellular matrix mechanics. Nat. Commun 2013, 4, 1759. [DOI] [PubMed] [Google Scholar]
- 59.You H; Padmashali RM; Ranganathan A; Lei P; Girnius N; Davis RJ; Andreadis ST, JNK regulates compliance-induced adherens junctions formation in epithelial cells and tissues. J. Cell Sci 2013, 126 (Pt 12), 2718–2729. [DOI] [PubMed] [Google Scholar]
- 60.Bordeleau F; Mason BN; Lollis EM; Mazzola M; Zanotelli MR; Somasegar S; Califano JP; Montague C; LaValley DJ; Huynh J, Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (3), 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birukova AA; Tian X; Cokic I; Beckham Y; Gardel ML; Birukov KG, Endothelial barrier disruption and recovery is controlled by substrate stiffness. Microvasc. Res 2013, 87, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbott NJ; Rönnbäck L; Hansson E, Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci 2006, 7 (1), 41. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y; Chen J; Craven M; Choi NW; Totorica S; Diaz-Santana A; Kermani P; Hempstead B; Fischbach-Teschl C; Lopez JA; Stroock AD, In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (24), 9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huh D; Leslie DC; Matthews BD; Fraser JP; Jurek S; Hamilton GA; Thorneloe KS; McAlexander MA; Ingber DE, A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med 2012, 4 (159), 159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esch MB; Sung JH; Yang J; Yu C; Yu J; March JC; Shuler ML, On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’devices. Biomed. Microdevices 2012, 14 (5), 895–906. [DOI] [PubMed] [Google Scholar]
- 66.Wang YI; Carmona C; Hickman JJ; Shuler ML, Multiorgan microphysiological systems for drug development: strategies, advances, and challenges. Adv. Healthc. Mater 2018, 7 (2), 1701000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esch MB; Ueno H; Applegate DR; Shuler ML, Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 2016, 16 (14), 2719–2729. [DOI] [PubMed] [Google Scholar]
- 68.Esch MB; King TL; Shuler ML, The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng 2011, 13, 55–72. [DOI] [PubMed] [Google Scholar]
- 69.Moraes C; Labuz JM; Leung BM; Inoue M; Chun T-H; Takayama S, On being the right size: scaling effects in designing a human-on-a-chip. Integr. Biol 2013, 5 (9), 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sung JH; Kam C; Shuler ML, A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 2010, 10 (4), 446–455. [DOI] [PubMed] [Google Scholar]
- 71.Sung JH; Shuler ML, A micro cell culture analog (microCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 2009, 9 (10), 1385–1394. [DOI] [PubMed] [Google Scholar]
- 72.Vernetti L; Gough A; Baetz N; Blutt S; Broughman JR; Brown JA; Foulke-Abel J; Hasan N; In J; Kelly E, Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci. Rep 2017, 7, 42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oleaga C; Bernabini C; Smith AS; Srinivasan B; Jackson M; McLamb W; Platt V; Bridges R; Cai Y; Santhanam N; Berry B; Najjar S; Akanda N; Guo X; Martin C; Ekman G; Esch MB; Langer J; Ouedraogo G; Cotovio J; Breton L; Shuler ML; Hickman JJ, Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep 2016, 6, 20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langelaan ML; Boonen KJ; Rosaria-Chak KY; van der Schaft DW; Post MJ; Baaijens FP, Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med 2011, 5 (7), 529–539. [DOI] [PubMed] [Google Scholar]
- 75.Benam KH; Novak R; Nawroth J; Hirano-Kobayashi M; Ferrante TC; Choe Y; Prantil-Baun R; Weaver JC; Bahinski A; Parker KK; Ingber DE, Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip. Cell Syst. 2016, 3 (5), 456–466. [DOI] [PubMed] [Google Scholar]
- 76.van der Meer AD; Orlova VV; ten Dijke P; van den Berg A; Mummery CL, Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip 2013, 13 (18), 3562–3568. [DOI] [PubMed] [Google Scholar]
- 77.Morizane R; Lam AQ; Freedman BS; Kishi S; Valerius MT; Bonventre JV, Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol 2015, 33 (11), 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song L; Wang K; Li Y; Yang Y, Nanotopography promoted neuronal differentiation of human induced pluripotent stem cells. Colloids Surf. B Biointerfaces 2016, 148, 49–58. [DOI] [PubMed] [Google Scholar]
- 79.Ikuno T; Masumoto H; Yamamizu K; Yoshioka M; Minakata K; Ikeda T; Sakata R; Yamashita JK, Efficient and robust differentiation of endothelial cells from human induced pluripotent stem cells via lineage control with VEGF and cyclic AMP. PLoS One 2017, 12 (3), e0173271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L; Geng Z; Nickel T; Johnson C; Gao L; Dutton J; Hou C; Zhang J, Differentiation of human induced-pluripotent stem cells into smooth-muscle cells: two novel protocols. PLoS One 2016, 11 (1), e0147155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lippmann ES; Azarin SM; Kay JE; Nessler RA; Wilson HK; Al-Ahmad A; Palecek SP; Shusta EV, Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol 2012, 30 (8), 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sances S; Ho R; Vatine G; West D; Laperle A; Meyer A; Godoy M; Kay PS; Mandefro B; Hatata S, Human iPSC-derived endothelial cells and microengineered organ-chip enhance neuronal development. Stem Cell Rep. 2018, 10 (4), 1222–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vatine GD; Barrile R; Workman MJ; Sances S; Barriga BK; Rahnama M; Barthakur S; Kasendra M; Lucchesi C; Kerns J, Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24 (6), 995–1005. [DOI] [PubMed] [Google Scholar]
- 84.Eiraku M; Watanabe K; Matsuo-Takasaki M; Kawada M; Yonemura S; Matsumura M; Wataya T; Nishiyama A; Muguruma K; Sasai Y, Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3 (5), 519–32. [DOI] [PubMed] [Google Scholar]
- 85.Dutt K; Cao Y, Engineering retina from human retinal progenitors (Cell Lines). Tissue Eng. Part A 2009, 15 (6), 1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noguchi TK; Kurisaki A, Formation of stomach tissue by organoid culture using mouse embryonic stem cells. Methods Mol Biol 2017, 1597, 217–228. [DOI] [PubMed] [Google Scholar]
- 87.Bhise NS; Manoharan V; Massa S; Tamayol A; Ghaderi M; Miscuglio M; Lang Q; Zhang YS; Shin SR; Calzone G, A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8 (1), 014101. [DOI] [PubMed] [Google Scholar]
- 88.Morizane R; Lam AQ; Freedman BS; Kishi S; Valerius MT; Bonventre JV, Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat. Biotechnol 2015, 33 (11), 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Workman MJ; Gleeson JP; Troisi EJ; Estrada HQ; Kerns SJ; Hinojosa CD; Hamilton GA; Targan SR; Svendsen CN; Barrett RJ, Enhanced utilization of induced pluripotent stem cell-derived human intestinal organoids using microengineered chips. Cell. Mol. Gastroenterol. Hepatol 2018, 5 (4), 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drost J; Karthaus WR; Gao D; Driehuis E; Sawyers CL; Chen Y; Clevers H, Organoid culture systems for prostate epithelial and cancer tissue. Nat. Protoc 2016, 11 (2), 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Q; Liu Z; Lin Z; Qiu J; Liu Y; Liu A; Wang Y; Xiang M; Chen B; Fu J, 3D bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng 2017, 3 (3), 399–408. [DOI] [PubMed] [Google Scholar]
- 92.Homan KA; Kolesky DB; Skylar-Scott MA; Herrmann J; Obuobi H; Moisan A; Lewis JA, Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep 2016, 6, 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pourchet LJ; Thepot A; Albouy M; Courtial EJ; Boher A; Blum LJ; Marquette CA, Human Skin 3D Bioprinting Using Scaffold-Free Approach. Adv. Healthc. Mater 2017, 6 (4). [DOI] [PubMed] [Google Scholar]
- 94.Choi YJ; Kim TG; Jeong J; Yi HG; Park JW; Hwang W; Cho DW, 3D cell printing of functional skeletal muscle constructs using skeletal muscle-derived bioink. Adv. Healthc. Mater 2016, 5 (20), 2636–2645. [DOI] [PubMed] [Google Scholar]
- 95.Yi H-G; Jeong YH; Kim Y; Choi Y-J; Moon HE; Park SH; Kang KS; Bae M; Jang J; Youn H; Paek SH; Cho D-W, A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat. Biomed. Eng 2019, 3 (7), 509–519. [DOI] [PubMed] [Google Scholar]
- 96.Yang X; Li K; Zhang X; Liu C; Guo B; Wen W; Gao X, Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab Chip 2018, 18 (3), 486–495. [DOI] [PubMed] [Google Scholar]
- 97.Delalat B; Cozzi C; Rasi Ghaemi S; Polito G; Kriel FH; Michl TD; Harding FJ; Priest C; Barillaro G; Voelcker NH, Microengineered bioartificial liver chip for drug toxicity screening. Adv. Funct. Mater 2018, 28 (28). [Google Scholar]
- 98.Jang K-J; Mehr AP; Hamilton GA; McPartlin LA; Chung S; Suh K-Y; Ingber DE, Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol 2013, 5 (9), 1119–1129. [DOI] [PubMed] [Google Scholar]
- 99.Shah P; Fritz JV; Glaab E; Desai MS; Greenhalgh K; Frachet A; Niegowska M; Estes M; Jager C; Seguin-Devaux C; Zenhausern F; Wilmes P, A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun 2016, 7, 11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brown JA; Pensabene V; Markov DA; Allwardt V; Neely MD; Shi M; Britt CM; Hoilett OS; Yang Q; Brewer BM; Samson PC; McCawley LJ; May JM; Webb DJ; Li D; Bowman AB; Reiserer RS; Wikswo JP, Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015, 9 (5), 054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Agarwal A; Goss JA; Cho A; McCain ML; Parker KK, Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13 (18), 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Torisawa YS; Spina CS; Mammoto T; Mammoto A; Weaver JC; Tat T; Collins JJ; Ingber DE, Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 2014, 11 (6), 663–669. [DOI] [PubMed] [Google Scholar]
- 103.Torisawa YS; Mammoto T; Jiang E; Jiang A; Mammoto A; Watters AL; Bahinski A; Ingber DE, Modeling hematopoiesis and responses to radiation countermeasures in a bone marrow-on-a-chip. Tissue Eng. Part C Methods 2016, 22 (5), 509–515. [DOI] [PubMed] [Google Scholar]
- 104.Dutta D; Heo I; Clevers H, Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med 2017, 23 (5), 393–410. [DOI] [PubMed] [Google Scholar]
- 105.Lancaster MA; Renner M; Martin C-A; Wenzel D; Bicknell LS; Hurles ME; Homfray T; Penninger JM; Jackson AP; Knoblich JA, Cerebral organoids model human brain development and microcephaly. Nature 2013, 501 (7467), 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eiraku M; Takata N; Ishibashi H; Kawada M; Sakakura E; Okuda S; Sekiguchi K; Adachi T; Sasai Y, Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 2011, 472 (7341), 51. [DOI] [PubMed] [Google Scholar]
- 107.Stange DE; Koo B-K; Huch M; Sibbel G; Basak O; Lyubimova A; Kujala P; Bartfeld S; Koster J; Geahlen JH, Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 2013, 155 (2), 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huch M; Gehart H; Van Boxtel R; Hamer K; Blokzijl F; Verstegen MM; Ellis E; Van Wenum M; Fuchs SA; de Ligt J, Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160 (1), 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koehler KR; Mikosz AM; Molosh AI; Patel D; Hashino E, Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 2013, 500 (7461), 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Finkbeiner SR; Zeng X-L; Utama B; Atmar RL; Shroyer NF; Estes MK, Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio 2012, 3 (4), e00159–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gaebler M; Silvestri A; Haybaeck J; Reichardt P; Lowery CD; Stancato LF; Zybarth G; Regenbrecht CR, Three-dimensional patient-derived in vitro sarcoma models: promising tools for improving clinical tumor management. Front. Oncol 2017, 7, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lv D; Hu Z; Lu L; Lu H; Xu X, Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett 2017, 14 (6), 6999–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hwang YS; Chung BG; Ortmann D; Hattori N; Moeller HC; Khademhosseini A, Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl. Acad. Sci. U. S. A 2009, 106 (40), 16978–16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tumarkin E; Tzadu L; Csaszar E; Seo M; Zhang H; Lee A; Peerani R; Purpura K; Zandstra PW; Kumacheva E, High-throughput combinatorial cell co-culture using microfluidics. Integr. Biol 2011, 3 (6), 653–662. [DOI] [PubMed] [Google Scholar]
- 115.Derby B, Printing and prototyping of tissues and scaffolds. Science 2012, 338 (6109), 921–926. [DOI] [PubMed] [Google Scholar]
- 116.Lee A; Hudson A; Shiwarski D; Tashman J; Hinton T; Yerneni S; Bliley J; Campbell P; Feinberg A, 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365 (6452), 482–487. [DOI] [PubMed] [Google Scholar]
- 117.Lee VK; Kim DY; Ngo H; Lee Y; Seo L; Yoo SS; Vincent PA; Dai G, Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35 (28), 8092–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jang J; Yi H-G; Cho D-W, 3D printed tissue models: present and future. ACS Biomater. Sci. Eng 2016, 2 (10), 1722–1731. [DOI] [PubMed] [Google Scholar]
- 119.Boland T; Xu T; Damon B; Cui X, Application of inkjet printing to tissue engineering. Biotechnol. J 2006, 1 (9), 910–917. [DOI] [PubMed] [Google Scholar]
- 120.Ringeisen BR; Othon CM; Barron JA; Young D; Spargo BJ, Jet-based methods to print living cells. Biotechnol. J 2006, 1 (9), 930–948. [DOI] [PubMed] [Google Scholar]
- 121.Lee VK; Lanzi AM; Haygan N; Yoo SS; Vincent PA; Dai G, Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell. Mol. Bioeng 2014, 7 (3), 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roth EA; Xu T; Das M; Gregory C; Hickman JJ; Boland T, Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25 (17), 3707–3715. [DOI] [PubMed] [Google Scholar]
- 123.Melchels FP; Domingos MA; Klein TJ; Malda J; Bartolo PJ; Hutmacher DW, Additive manufacturing of tissues and organs. Prog. Polym. Sci 2012, 37 (8), 1079–1104. [Google Scholar]
- 124.Yi H-G; Lee H; Cho D-W, 3D printing of organs-on-chips. Bioengineering 2017, 4 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Odde DJ; Renn MJ, Laser-guided direct writing of living cells. Biotechnol. Bioeng 2000, 67 (3), 312–318. [DOI] [PubMed] [Google Scholar]
- 126.Zadpoor AA; Malda J, Additive manufacturing of biomaterials, tissues, and organs. Ann. Biomed. Eng 2017, 45 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- 127.Koch L; Deiwick A; Schlie S; Michael S; Gruene M; Coger V; Zychlinski D; Schambach A; Reimers K; Vogt PM; Chichkov B, Skin tissue generation by laser cell printing. Biotechnol. Bioeng 2012, 109 (7), 1855–1863. [DOI] [PubMed] [Google Scholar]
- 128.Bishop ES; Mostafa S; Pakvasa M; Luu HH; Lee MJ; Wolf JM; Ameer GA; He TC; Reid RR, 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4 (4), 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y; Leong KW, Microfluidic platforms with nanoscale features In Microfluidic Cell Culture Systems (Second Edition), Borenstein JT; Tandon V; Tao SL; Charest JL, Eds. Elsevier: 2019; pp 65–90. [Google Scholar]
- 130.Chen HJ; Miller P; Shuler ML, A pumpless body-on-a-chip model using a primary culture of human intestinal cells and a 3D culture of liver cells. Lab Chip 2018, 18 (14), 2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bruce A; Evans R; Mezan R; Shi L; Moses BS; Martin KH; Gibson LF; Yang Y, Three-dimensional microfluidic tri-culture model of the bone marrow microenvironment for study of acute lymphoblastic leukemia. PLoS One 2015, 10 (10), e0140506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang Y; Kulangara K; Sia J; Wang L; Leong KW, Engineering of a microfluidic cell culture platform embedded with nanoscale features. Lab Chip 2011, 11 (9), 1638–1646. [DOI] [PubMed] [Google Scholar]
- 133.Berthier E; Beebe D, Gradient generation platforms: new directions for an established microfluidic technology. Lab Chip 2014, 14 (17), 3241–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cimetta E; Cannizzaro C; James R; Biechele T; Moon RT; Elvassore N; Vunjak-Novakovic G, Microfluidic device generating stable concentration gradients for long term cell culture: application to Wnt3a regulation of beta-catenin signaling. Lab Chip 2010, 10 (23), 3277–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boussommier-Calleja A; Li R; Chen MB; Wong SC; Kamm RD, Microfluidics: a new tool for modeling cancer–immune interactions. Trends Cancer 2016, 2 (1), 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zervantonakis IK; Hughes-Alford SK; Charest JL; Condeelis JS; Gertler FB; Kamm RD, Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (34), 13515–13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang B; Montgomery M; Chamberlain MD; Ogawa S; Korolj A; Pahnke A; Wells LA; Massé S; Kim J; Reis L, Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater 2016, 15 (6), 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aziz AUR; Geng C; Fu M; Yu X; Qin K; Liu B, The role of microfluidics for organ on chip simulations. Bioengineering 2017, 4 (2), 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Im SB; Uddin MJ; Jin GJ; Shim JS, A disposable on-chip microvalve and pump for programmable microfluidics. Lab Chip 2018, 18 (9), 1310–1319. [DOI] [PubMed] [Google Scholar]
- 140.Tien J; Li L; Ozsun O; Ekinci KL, Dynamics of interstitial fluid pressure in extracellular matrix hydrogels in microfluidic devices. J. Biomech. Eng 2015, 137 (9), 091009. [DOI] [PubMed] [Google Scholar]
- 141.Yin X; Mead Benjamin E.; Safaee H; Langer R; Karp Jeffrey M.; Levy O, Engineering stem cell organoids. Cell Stem Cell 2016, 18 (1), 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Drost J; Clevers H, Organoids in cancer research. Nat. Rev. Cancer 2018, 18 (7), 407–418. [DOI] [PubMed] [Google Scholar]
- 143.Capeling MM; Czerwinski M; Huang S; Tsai Y-H; Wu A; Nagy MS; Juliar B; Sundaram N; Song Y; Han WM, Nonadhesive alginate hydrogels support growth of pluripotent stem cell-derived intestinal organoids. Stem Cell Rep. 2019, 12 (2), 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Homan KA; Gupta N; Kroll KT; Kolesky DB; Skylar-Scott M; Miyoshi T; Mau D; Valerius MT; Ferrante T; Bonventre JV, Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 2019, 16 (3), 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yan Y; Bejoy J; Marzano M; Li Y, The use of pluripotent stem cell-derived organoids to study extracellular matrix development during neural degeneration. Cells 2019, 8 (3), 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cruz-Acuña R; Quirós M; Farkas AE; Dedhia PH; Huang S; Siuda D; García-Hernández V; Miller AJ; Spence JR; Nusrat A; García AJ, Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol 2017, 19 (11), 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gjorevski N; Lutolf MP, Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat. Protoc 2017, 12 (11), 2263–2274. [DOI] [PubMed] [Google Scholar]
- 148.Yamada M; Utoh R; Ohashi K; Tatsumi K; Yamato M; Okano T; Seki M, Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions. Biomaterials 2012, 33 (33), 8304–8315. [DOI] [PubMed] [Google Scholar]
- 149.Hölzl K; Lin S; Tytgat L; Van Vlierberghe S; Gu L; Ovsianikov A, Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8 (3), 032002. [DOI] [PubMed] [Google Scholar]
- 150.Rimann M; Bono E; Annaheim H; Bleisch M; Graf-Hausner U, Standardized 3D bioprinting of soft tissue models with human primary cells. J. Lab. Autom 2016, 21 (4), 496–509. [DOI] [PubMed] [Google Scholar]
- 151.Lee V; Singh G; Trasatti JP; Bjornsson C; Xu X; Tran TN; Yoo S-S; Dai G; Karande P, Design and fabrication of human skin by three-dimensional bioprinting. Tissue Eng. Part C Methods 2014, 20 (6), 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Catros S; Guillotin B; Bačáková M; Fricain J-C; Guillemot F, Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by laser-assisted bioprinting. Appl. Surf. Sci 2011, 257 (12), 5142–5147. [Google Scholar]
- 153.Markstedt K; Mantas A; Tournier I; MartÍnez Ávila H. c.; Hagg D; Gatenholm P, 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16 (5), 1489–1496. [DOI] [PubMed] [Google Scholar]
- 154.Miller PG; Chen CY; Wang YI; Gao E; Shuler ML, Multiorgan microfluidic platform with breathable lung chamber for inhalation or intravenous drug screening and development. Biotechnology and bioengineering 2020, 117 (2), 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gale BK; Jafek AR; Lambert CJ; Goenner BL; Moghimifam H; Nze UC; Kamarapu SK, A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3 (3), 60. [Google Scholar]
- 156.Chan CY; Goral VN; DeRosa ME; Huang TJ; Yuen PK, A polystyrene-based microfluidic device with three-dimensional interconnected microporous walls for perfusion cell culture. Biomicrofluidics 2014, 8 (4), 046505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu W-I; Sask KN; Brash JL; Selvaganapathy PR, Polyurethane-based microfluidic devices for blood contacting applications. Lab Chip 2012, 12 (5), 960–970. [DOI] [PubMed] [Google Scholar]
- 158.Castiaux AD; Spence DM; Martin RS, Review of 3D cell culture with analysis in microfluidic systems. Anal. Methods 2019, 11 (33), 4220–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fujie T; Mori Y; Ito S; Nishizawa M; Bae H; Nagai N; Onami H; Abe T; Khademhosseini A; Kaji H, Micropatterned polymeric nanosheets for local delivery of an engineered epithelial monolayer. Adv. Mater 2014, 26 (11), 1699–1705. [DOI] [PubMed] [Google Scholar]
- 160.Yeon JH; Na D; Choi K; Ryu SW; Choi C; Park JK, Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices 2012, 14 (6), 1141–8. [DOI] [PubMed] [Google Scholar]
- 161.Shao X; Gao D; Chen Y; Jin F; Hu G; Jiang Y; Liu H, Development of a blood-brain barrier model in a membrane-based microchip for characterization of drug permeability and cytotoxicity for drug screening. Anal. Chim. Acta 2016, 934, 186–93. [DOI] [PubMed] [Google Scholar]
- 162.Booth R; Kim H, Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB). Lab Chip 2012, 12 (10), 1784–1792. [DOI] [PubMed] [Google Scholar]
- 163.Lee W; Pinckney J; Lee V; Lee J-H; Fischer K; Polio S; Park J-K; Yoo S-S, Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 2009, 20 (8), 798–803. [DOI] [PubMed] [Google Scholar]
- 164.Lee Y-B; Polio S; Lee W; Dai G; Menon L; Carroll RS; Yoo S-S, Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Experimental neurology 2010, 223 (2), 645–652 [DOI] [PubMed] [Google Scholar]
- 165.Lozano R; Stevens L; Thompson BC; Gilmore KJ; Gorkin Iii R; Stewart EM; in het Panhuis M; Romero-Ortega M; Wallace GG, 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [DOI] [PubMed] [Google Scholar]
- 166.Eiraku M; Watanabe K; Matsuo-Takasaki M; Kawada M; Yonemura S; Matsumura M; Wataya T; Nishiyama A; Muguruma K; Sasai Y, Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3 (5), 519–532. [DOI] [PubMed] [Google Scholar]
- 167.Paşca AM; Sloan SA; Clarke LE; Tian Y; Makinson CD; Huber N; Kim CH; Park J-Y; O’Rourke NA; Nguyen KD, Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12 (7), 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Birey F; Andersen J; Makinson CD; Islam S; Wei W; Huber N; Fan HC; Metzler KRC; Panagiotakos G; Thom N, Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545 (7652), 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ao Z; Cai H; Havert DJ; Wu Z; Gong Z; Beggs JM; Mackie K; Guo F, One-stop Microfluidic Assembly of Human Brain Organoids to Model Prenatal Cannabis Exposure. Anal. Chem 2020. [DOI] [PubMed] [Google Scholar]
- 170.Lawrence BD; Marchant JK; Pindrus MA; Omenetto FG; Kaplan DL, Silk film biomaterials for cornea tissue engineering. Biomaterials 2009, 30 (7), 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Palchesko RN; Lathrop KL; Funderburgh JL; Feinberg AW, In vitro expansion of corneal endothelial cells on biomimetic substrates. Sci. Rep 2015, 5 (1), 7955–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Levis HJ; Peh GSL; Toh K-P; Poh R; Shortt AJ; Drake RAL; Mehta JS; Daniels JT, Plastic compressed collagen as a novel carrier for expanded human corneal endothelial cells for transplantation. PLoS One 2012, 7 (11), e50993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ozcelik B; Brown KD; Blencowe A; Daniell M; Stevens GW; Qiao GG, Ultrathin chitosan–poly(ethylene glycol) hydrogel films for corneal tissue engineering. Acta Biomater. 2013, 9 (5), 6594–6605. [DOI] [PubMed] [Google Scholar]
- 174.Zhang C; Du L; Sun P; Shen L; Zhu J; Pang K; Wu X, Construction of tissue-engineered full-thickness cornea substitute using limbal epithelial cell-like and corneal endothelial cell-like cells derived from human embryonic stem cells. Biomaterials 2017, 124, 180–194. [DOI] [PubMed] [Google Scholar]
- 175.Nunes SS; Miklas JW; Liu J; Aschar-Sobbi R; Xiao Y; Zhang B; Jiang J; Masse S; Gagliardi M; Hsieh A; Thavandiran N; Laflamme MA; Nanthakumar K; Gross GJ; Backx PH; Keller G; Radisic M, Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 2013, 10 (8), 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Giridharan GA; Nguyen MD; Estrada R; Parichehreh V; Hamid T; Ismahil MA; Prabhu SD; Sethu P, Microfluidic cardiac cell culture model (muCCCM). Anal. Chem 2010, 82 (18), 7581–7587. [DOI] [PubMed] [Google Scholar]
- 177.Mathur A; Loskill P; Shao K; Huebsch N; Hong S; Marcus SG; Marks N; Mandegar M; Conklin BR; Lee LP; Healy KE, Human iPSC-based cardiac microphysiological system for drug screening applications. Sci. Rep 2015, 5, 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mermoud Y; Felder M; Stucki J; Stucki A; Guenat OT, Microimpedance tomography system to monitor cell activity and membrane movements in a breathing lung-on-chip. Sens. Actuators B Chem 2018, 255, 3647–3653. [Google Scholar]
- 179.Kang YBA; Eo J; Mert S; Yarmush ML; Usta OB, Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci. Rep 2018, 8 (1), 8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ma LD; Wang YT; Wang JR; Wu JL; Meng XS; Hu P; Mu X; Liang QL; Luo GA, Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip 2018, 2547–2562. [DOI] [PubMed] [Google Scholar]
- 181.Weber EJ; Chapron A; Chapron BD; Voellinger JL; Lidberg KA; Yeung CK; Wang Z; Yamaura Y; Hailey DW; Neumann T, Development of a microphysiological model of human kidney proximal tubule function. Kidney international 2016, 90 (3), 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sung JH; Yu J; Luo D; Shuler ML; March JC, Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 2011, 11 (3), 389–392. [DOI] [PubMed] [Google Scholar]
- 183.Kim HJ; Li H; Collins JJ; Ingber DE, Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (1), E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim HJ; Huh D; Hamilton G; Ingber DE, Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12 (12), 2165–2174. [DOI] [PubMed] [Google Scholar]
- 185.Takahashi Y; Sato S; Kurashima Y; Yamamoto T; Kurokawa S; Yuki Y; Takemura N; Uematsu S; Lai CY; Otsu M; Matsuno H; Osawa H; Mizushima T; Nishimura J; Hayashi M; Yamaguchi T; Kiyono H, A refined culture system for human induced pluripotent stem cell-derived intestinal epithelial organoids. Stem Cell Rep. 2018, 10 (1), 314–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Song JW; Cavnar SP; Walker AC; Luker KE; Gupta M; Tung YC; Luker GD; Takayama S, Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One 2009, 4 (6), e5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Boussommier-Calleja A; Atiyas Y; Haase K; Headley M; Lewis C; Kamm RD, The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 2018, 180–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Menon NV; Chuah YJ; Cao B; Lim M; Kang Y, A microfluidic co-culture system to monitor tumor-stromal interactions on a chip. Biomicrofluidics 2014, 8 (6), 064118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Munzel T; Sinning C; Post F; Warnholtz A; Schulz E, Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann. Med 2008, 40 (3), 180–196. [DOI] [PubMed] [Google Scholar]
- 190.Roudsari LC; West JL, Studying the influence of angiogenesis in in vitro cancer model systems. Adv. Drug Deliv. Rev 2016, 97, 250–9. [DOI] [PubMed] [Google Scholar]
- 191.Du Y; Li N; Yang H; Luo C; Gong Y; Tong C; Gao Y; Lu S; Long M, Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip 2017, 17 (5), 782–794. [DOI] [PubMed] [Google Scholar]
- 192.Wang YI; Abaci HE; Shuler ML, Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng 2017, 114 (1), 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Qiu Y; Ahn B; Sakurai Y; Hansen CE; Tran R; Mimche PN; Mannino RG; Ciciliano JC; Lamb TJ; Joiner CH; Ofori-Acquah SF; Lam WA, Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat. Biomed. Eng 2018, 2 (6), 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zheng W; Jiang B; Wang D; Zhang W; Wang Z; Jiang X, A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip 2012, 12 (18), 3441–3450. [DOI] [PubMed] [Google Scholar]
- 195.Weksler BB; Subileau EA; Perriere N; Charneau P; Holloway K; Leveque M; Tricoire-Leignel H; Nicotra A; Bourdoulous S; Turowski P; Male DK; Roux F; Greenwood J; Romero IA; Couraud PO, Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19 (13), 1872–4. [DOI] [PubMed] [Google Scholar]
- 196.Virumbrales-Munoz M; Ayuso JM; Lacueva A; Randelovic T; Livingston MK; Beebe DJ; Olivan S; Pereboom D; Doblare M; Fernandez L; Ochoa I, Enabling cell recovery from 3D cell culture microfluidic devices for tumour microenvironment biomarker profiling. Sci. Rep 2019, 9 (1), 6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shang M; Soon RH; Lim CT; Khoo BL; Han J, Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 2019, 19 (3), 369–386. [DOI] [PubMed] [Google Scholar]
- 198.Wang YI; Abaci HE; Shuler ML, Microfluidic bloodloodofluidic modelling of the tumor m-like barrier properties for drug permeability screening. Biotechnol. Bioeng 2017, 114 (1), 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Reichel A; Begley DJ; Abbott NJ, An overview of in vitro techniques for blood-brain barrier studies In The Blood-Brain Barrier, Springer: 2003; pp 307–324. [DOI] [PubMed] [Google Scholar]
- 200.Pangalos MN; Schechter LE; Hurko O, Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat. Rev. Drug Discov 2007, 6 (7), 521–532. [DOI] [PubMed] [Google Scholar]
- 201.Medana IM; Esiri MM, Axonal damage: a key predictor of outcome in human CNS diseases. Brain 2003, 126 (3), 515–530. [DOI] [PubMed] [Google Scholar]
- 202.Taylor AM; Blurton-Jones M; Rhee SW; Cribbs DH; Cotman CW; Jeon NL, A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature methods 2005, 2 (8), 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Taylor AM; Dieterich DC; Ito HT; Kim SA; Schuman EM, Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron 2010, 66 (1), 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Varma R; Vajaranant TS; Burkemper B; Wu S; Torres M; Hsu C; Choudhury F; McKean-Cowdin R, Visual impairment and blindness in adults in the united states: demographic and geographic variations from 2015 to 2050. JAMA Ophthalmol. 2016, 134 (7), 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bach M, Vision—how it works and what can go wrong. Perception 2017, 46 (7), 882–884. [Google Scholar]
- 206.Maruotti J; Sripathi SR; Bharti K; Fuller J; Wahlin KJ; Ranganathan V; Sluch VM; Berlinicke CA; Davis J; Kim C; Zhao L; Wan J; Qian J; Corneo B; Temple S; Dubey R; Olenyuk BZ; Bhutto I; Lutty GA; Zack DJ, Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (35), 10950–10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stanzel BV; Liu Z; Somboonthanakij S; Wongsawad W; Brinken R; Eter N; Corneo B; Holz FG; Temple S; Stern JH; Blenkinsop TA, Human RPE stem cells grown into polarized RPE monolayers on a polyester matrix are maintained after grafting into rabbit subretinal space. Stem Cell Rep. 2014, 2 (1), 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Thumann G; Viethen A; Gaebler A; Walter P; Kaempf S; Johnen S; Salz AK, The in vitro and in vivo behaviour of retinal pigment epithelial cells cultured on ultrathin collagen membranes. Biomaterials 2009, 30 (3), 287–294. [DOI] [PubMed] [Google Scholar]
- 209.Chung M; Lee S; Lee BJ; Son K; Jeon NL; Kim JH, Wet-AMD on a chip: modeling outer blood-retinal barrier in vitro. Adv. Healthc. Mater 2018, 7 (2), 1700028. [DOI] [PubMed] [Google Scholar]
- 210.Achberger K; Probst C; Haderspeck J; Bolz S; Rogal J; Chuchuy J; Nikolova M; Cora V; Antkowiak L; Haq W, Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kador KE; Montero RB; Venugopalan P; Hertz J; Zindell AN; Valenzuela DA; Uddin MS; Lavik EB; Muller KJ; Andreopoulos FM; Goldberg JL, Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials 2013, 34 (17), 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yan L; Zhao B; Liu X; Li X; Zeng C; Shi H; Xu X; Lin T; Dai L; Liu Y, Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl. Mater. Interfaces 2016, 8 (11), 6834–6840. [DOI] [PubMed] [Google Scholar]
- 213.Mendis S; Davis S; Norrving B, Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015, 46 (5), e121–e122. [DOI] [PubMed] [Google Scholar]
- 214.Sheehy SP; Grosberg A; Qin P; Behm DJ; Ferrier JP; Eagleson MA; Nesmith AP; Krull D; Falls JG; Campbell PH; McCain ML; Willette RN; Hu E; Parker KK, Toward improved myocardial maturity in an organ-on-chip platform with immature cardiac myocytes. Exp. Biol. Med 2017, 242 (17), 1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kobuszewska A; Tomecka E; Zukowski K; Jastrzebska E; Chudy M; Dybko A; Renaud P; Brzozka Z, Heart-on-a-chip: an investigation of the influence of static and perfusion conditions on cardiac (H9C2) cell proliferation, morphology, and alignment. SLAS Technol. 2017, 22 (5), 536–546. [DOI] [PubMed] [Google Scholar]
- 216.Lind JU; Busbee TA; Valentine AD; Pasqualini FS; Yuan H; Yadid M; Park SJ; Kotikian A; Nesmith AP; Campbell PH; Vlassak JJ; Lewis JA; Parker KK, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater 2017, 16 (3), 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sidorov VY; Samson PC; Sidorova TN; Davidson JM; Lim CC; Wikswo JP, I-wire heart-on-a-chip I: three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater. 2017, 48, 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Barnes PJ; Burney PGJ; Silverman EK; Celli BR; Vestbo J; Wedzicha JA; Wouters EFM, Chronic obstructive pulmonary disease. Nat. Rev. Dis. Primers 2015, 1, 15076. [DOI] [PubMed] [Google Scholar]
- 219.Clements JA, Pulmonary edema and permeability of alveolar membranes. Arch. Environ. Occup. Health 1961, 2 (3), 104–107. [PubMed] [Google Scholar]
- 220.Baker M, Tissue models: a living system on a chip. Nature 2011, 471 (7340), 661–665. [DOI] [PubMed] [Google Scholar]
- 221.Beckwitt CH; Clark AM; Wheeler S; Taylor DL; Stolz DB; Griffith L; Wells A, Liver ‘organ on a chip’. Exp. Cell Res 2018, 363 (1), 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li CY; Stevens KR; Schwartz RE; Alejandro BS; Huang JH; Bhatia SN, Micropatterned cell-cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues. Tissue Eng. Part A 2014, 20 (15–16), 2200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vernetti LA; Senutovitch N; Boltz R; DeBiasio R; Ying Shun T; Gough A; Taylor DL, A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med 2016, 241 (1), 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee-Montiel FT; George SM; Gough AH; Sharma AD; Wu J; DeBiasio R; Vernetti LA; Taylor DL, Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems. Exp. Biol. Med 2017, 242 (16), 1617–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Musah S; Dimitrakak N; Camacho DM; Church GM; Ingber DE, Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat. Protoc 2018, 13 (7), 1662–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou M; Zhang X; Wen X; Wu T; Wang W; Yang M; Wang J; Fang M; Lin B; Lin H, Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci. Rep 2016, 6, 31771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jansen J; Fedecostante M; Wilmer M; Peters J; Kreuser U; van den Broek P; Mensink R; Boltje T; Stamatialis D; Wetzels J, Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep 2016, 6, 26715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.International Transporter C; Giacomini KM; Huang SM; Tweedie DJ; Benet LZ; Brouwer KL; Chu X; Dahlin A; Evers R; Fischer V; Hillgren KM; Hoffmaster KA; Ishikawa T; Keppler D; Kim RB; Lee CA; Niemi M; Polli JW; Sugiyama Y; Swaan PW; Ware JA; Wright SH; Yee SW; Zamek-Gliszczynski MJ; Zhang L, Membrane transporters in drug development. Nat. Rev. Drug Discov 2010, 9 (3), 215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Shin W; Wu A; Massidda MW; Foster C; Thomas N; Lee DW; Koh H; Ju Y; Kim J; Kim HJ, A robust longitudinal co-culture of obligate anaerobic gut microbiome with human intestinal epithelium in an anoxic-oxic interface-on-a-chip. Front. Bioeng. Biotechnol 2019, 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kasendra M; Tovaglieri A; Sontheimer-Phelps A; Jalili-Firoozinezhad S; Bein A; Chalkiadaki A; Scholl W; Zhang C; Rickner H; Richmond CA; Li H; Breault DT; Ingber DE, Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep 2018, 8 (1), 2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Seyfried TN; Huysentruyt LC, On the origin of cancer metastasis. Crit. Rev. Oncog 2013, 18 (1–2), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Friedl P; Alexander S, Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011, 147 (5), 992–1009. [DOI] [PubMed] [Google Scholar]
- 233.Phan DT; Wang X; Craver BM; Sobrino A; Zhao D; Chen JC; Lee LY; George SC; Lee AP; Hughes CC, A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 2017, 17 (3), 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sobrino A; Phan DT; Datta R; Wang X; Hachey SJ; Romero-López M; Gratton E; Lee AP; George SC; Hughes CC, 3D microtumors in vitro supported by perfused vascular networks. Sci. Rep 2016, 6 (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu T; Lin B; Qin J, Carcinoma-associated fibroblasts promoted tumor spheroid invasion on a microfluidic 3D co-culture device. Lab Chip 2010, 10 (13), 1671–1677. [DOI] [PubMed] [Google Scholar]
- 236.Kim C; Kasuya J; Jeon J; Chung S; Kamm RD, A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 2015, 15 (1), 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Luni C; Serena E; Elvassore N, Human-on-chip for therapy development and fundamental science. Curr. Opin. Biotechnol 2014, 25, 45–50. [DOI] [PubMed] [Google Scholar]
- 238.Shintu L; Baudoin R. g.; Navratil V; Prot J-M; Pontoizeau C. m.; Defernez M; Blaise BJ; Domange C. l.; Péry AR; Toulhoat P, Metabolomics-on-a-chip and predictive systems toxicology in microfluidic bioartificial organs. Anal. Chem 2012, 84 (4), 1840–1848. [DOI] [PubMed] [Google Scholar]
- 239.Chang S-Y; Weber EJ; Sidorenko VS; Chapron A; Yeung CK; Gao C; Mao Q; Shen D; Wang J; Rosenquist TA, Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI insight 2017, 2 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Esch MB; Mahler GJ; Stokol T; Shuler ML, Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip 2014, 14 (16), 3081–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Esch MB; Ueno H; Applegate DR; Shuler ML, Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab Chip 2016, 16 (14), 2719–29. [DOI] [PubMed] [Google Scholar]
- 242.Miller PG; Shuler ML, Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol. Bioeng 2016, 113 (10), 2213–2227. [DOI] [PubMed] [Google Scholar]
- 243.Ma Z; Ramakrishna S, Nanostructured extracellular matrix. Encyclopedia of Nanoscience and Nanotechnology 2004, 7, 641–655. [Google Scholar]
- 244.Nemir S; West JL, Synthetic materials in the study of cell response to substrate rigidity. Ann. Biomed. Eng 2009, 2–20. [DOI] [PubMed] [Google Scholar]
- 245.Discher DE; Mooney DJ; Zandstra PW, Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324 (5935), 1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Parameswaran H; Majumdar A; Suki B, Linking microscopic spatial patterns of tissue destruction in emphysema to macroscopic decline in stiffness using a 3D computational model. PLoS Comput. Biol 2011, 7 (4), e1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu F; Mih JD; Shea BS; Kho AT; Sharif AS; Tager AM; Tschumperlin DJ, Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol 2010, 190 (4), 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Booth AJ; Hadley R; Cornett AM; Dreffs AA; Matthes SA; Tsui JL; Weiss K; Horowitz JC; Fiore VF; Barker TH; Moore BB; Martinez FJ; Niklason LE; White ES, Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care. Med 2012, 186 (9), 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Paszek MJ; Zahir N; Johnson KR; Lakins JN; Rozenberg GI; Gefen A; Reinhart-King CA; Margulies SS; Dembo M; Boettiger D; Hammer DA; Weaver VM, Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8 (3), 241–254. [DOI] [PubMed] [Google Scholar]
- 250.Yim EKF; Leong KW, Significance of synthetic nanostructures in dictating cellular response. Nanomedicine 2005, 1 (1), 10–21. [DOI] [PubMed] [Google Scholar]
- 251.Brody S; Anilkumar T; Liliensiek S; Last JA; Murphy CJ; Pandit A, Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue. Eng 2006, 12 (2), 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stratman AN; Malotte KM; Mahan RD; Davis MJ; Davis GE, Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114 (24), 5091–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yang Y; Kulangara K; Lam RTS; Dharmawan R; Leong KW, Effects of topographical and mechanical property alterations induced by oxygen plasma modification on stem cell behavior. ACS Nano 2012, 6 (10), 8591–8598. [DOI] [PubMed] [Google Scholar]
- 254.Engler AJ; Sen S; Sweeney HL; Discher DE, Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677–689. [DOI] [PubMed] [Google Scholar]
- 255.Whited BM; Rylander MN, The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng 2014, 111 (1), 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Voronov DA; Alford PW; Xu G; Taber LA, The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev. Biol 2004, 272 (2), 339–350. [DOI] [PubMed] [Google Scholar]
- 257.Kira Y; Nakaoka T; Hashimoto E; Okabe F; Asano S; Sekine I, Effect of long-term cyclic mechanical load on protein synthesis and morphological changes in cultured Myocardial cells from neonatal rat. Cardiovasc. Drugs Ther 1994, 8 (2), 251–262. [DOI] [PubMed] [Google Scholar]
- 258.Williams D; Thompson DG; Marples M; Heggie L; O’Hanrahan T; Bancewicz J, Diminished oesophageal traction forces with swallowing in gastro-oesophageal reflux disease and in functional dysphagia. Gut 1994, 35 (2), 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rogers RS; Dharsee M; Ackloo S; Sivak JM; Flanagan JG, Proteomics analyses of human optic nerve head astrocytes following biomechanical strain. Mol. Cell. Proteomics 2012, 11 (2), M111. 012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen C; Mehl BT; Sell SA; Martin RS, Use of electrospinning and dynamic air focusing to create three-dimensional cell culture scaffolds in microfluidic devices. Analyst 2016, 141 (18), 5311–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hogrebe NJ; Gooch KJ, Direct influence of culture dimensionality on human mesenchymal stem cell differentiation at various matrix stiffnesses using a fibrous self-assembling peptide hydrogel. J. Biomed. Mater. Res. A 2016, 104 (9), 2356–2368. [DOI] [PubMed] [Google Scholar]
- 262.Shaegh SAM; De Ferrari F; Zhang YS; Nabavinia M; Binth Mohammad N; Ryan J; Pourmand A; Laukaitis E; Banan Sadeghian R; Nadhman A; Shin SR; Nezhad AS; Khademhosseini A; Dokmeci MR, A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10 (4), 044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Riahi R; Shaegh SAM; Ghaderi M; Zhang YS; Shin SR; Aleman J; Massa S; Kim D; Dokmeci MR; Khademhosseini A, Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep 2016, 6, 24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kieninger J; Weltin A; Flamm H; Urban GA, Microsensor systems for cell metabolism - from 2D culture to organ-on-chip. Lab Chip 2018, 18 (9), 1274–1291. [DOI] [PubMed] [Google Scholar]
- 265.Alhadrami HA, Biosensors: classifications, medical applications, and future prospective. Biotechnol. Appl. Biochem 2018, 65 (3), 497–508. [DOI] [PubMed] [Google Scholar]


