Abstract
The United States radiation medical countermeasures (MCM) programme for radiological and nuclear incidents has been focusing on developing mitigators for the acute radiation syndrome (ARS) and delayed effects of acute radiation exposure (DEARE), and biodosimetry technologies to provide radiation dose assessments for guiding treatment. Because a nuclear accident or terrorist incident could potentially expose a large number of people to low to moderate doses of ionising radiation, and thus increase their excess lifetime cancer risk, there is an interest in developing mitigators for this purpose. This article discusses the current status, issues, and challenges regarding development of mitigators against radiation-induced cancers. The challenges of developing mitigators for ARS include: the long latency between exposure and cancer manifestation, limitations of animal models, potential side effects of the mitigator itself, potential need for long-term use, the complexity of human trials to demonstrate effectiveness, and statistical power constraints for measuring health risks (and reduction of health risks after mitigation) following relatively low radiation doses (<0.75 Gy). Nevertheless, progress in the understanding of the molecular mechanisms resulting in radiation injury, along with parallel progress in dose assessment technologies, make this an opportune, if not critical, time to invest in research strategies that result in the development of agents to lower the risk of radiation-induced cancers for populations that survive a significant radiation exposure incident.
Keywords: radiation-induced cancer, radiation mitigator, radioprotector, radiation risk
1. Introduction
The possibility of exposure of a large number of people to radiation following a nuclear incident has raised the public’s concerns about the increases in the chances of developing cancers (Knebel et al 2011). Further, the earthquake/tsunami off the coast of Japan in March 2011 renewed attention to the debate on radiation-induced health risks from nuclear power industry mishaps and other accidental releases of radioactivity into the environment (Boice 2012, González et al 2013, Normile 2011). The primary defences against such mishaps are prevention, sheltering in place with delayed evacuation, and restricting the consumption of contaminated foodstuffs. Following a release of radioactivity from a nuclear power plant accident or due to an explosion of a radiological dispersal device (RDD), there is the potential need for agents that block radionuclide uptake and/or enhance excretion of ingested and inhaled radioactive elements. Since preventive strategies can never be 100% effective, it is important for public health officials, radiation researchers and professionals to consider developing a second tier medical intervention strategy to mitigate the health risks, i.e., cancer, from accidental radiation exposure. This article focuses on the potential for assessing and reducing the future cancer risk to large populations following a major nuclear incident.
In considering a strategy of risk reduction, it is critical for the general public and public health officials to understand that lifetime risk for developing cancer for an individual is not exclusively related to accidental exposure to radiation but is dependent on several factors including age at the time of radiation exposure and other variables including genetics, dietary habits, voluntary and involuntary exposures to carcinogens, such as cigarette smoke, and so forth. For example, in the US, the lifetime risk of developing cancer in the absence of radiation exposure is already quite high, i.e. 42% of the population is likely to develop cancer during their lifetimes from factors other than radiation exposure (ACS 2013, NA/NRC 2006). This high baseline rate of developing cancer in the absence of radiation exposure poses a challenge in the determination of probability of causation of cancer in an individual as well as for the development of other late effects. In general, the guidelines for determining the probability of causation under the DHHS (Department of Health and Human Services) Energy Employees Occupational Illness Compensation Program Act of 2000 (DHHS 2002), states that a determination is required that an individual’s cancer in question is due to an accidental or occupational exposure to radiation with a probability of a 50% or greater.
The shape of the dose–response curve for radiation-induced cancer remains a subject of much debate. While there are exceptions, for the purposes of radiation protection it is assumed that there is a proportional relationship between exposure and future cancer risk. A linear, no-threshold model is used to assess risk at doses below 100 mSv for these purposes, recognising that epidemiological information in the low-dose domain, i.e. below 100 mSv, cannot unambiguously support or refute the model assumptions (ICRP 2007). For example, recent data from the Radiation Effects Research Foundation (RERF, Japan) seems to be more in line with a linear relationship between radiation dose and cancer risk (Ozasa et al 2012). How the increased lifetime risk may be considered for using a mitigator is discussed later in this paper. Development of radiation medical countermeasures (MCM) for specific organ system damage, i.e. acute radiation syndromes (ARS), in the immediate aftermath of a radiation event is discussed in detail elsewhere (DiCarlo et al 2011, Grace et al 2011). The challenges in developing agents for health risks that may not be apparent until years after exposure are in table 1. Given the substantial public concerns about radiation exposure on their health and radiation-related cancer risks, this paper brings together opinions of the experts on issues of radiation risk and the experimental difficulties in the development of mitigators. This paper is intended to benefit scientists in this field of research and development, the public at large, and the public health policy makers to better understand ways to address the late occurring health risks.
Table 1.
Challenges for developing mitigators for radiation-induced cancer.
| ● Biomarkers for cancer |
| ○ Radiation—biomarker for dose |
| ○ Radiation risk—biomarker for radiation damage |
| ○ Cancer biomarker—is there one to assess person’s risk of cancer in general or of a specific cancer and efficacy of an intervention? |
| ● Individuals at risk |
| ○ Based on underlying genetic susceptibility |
| ○ Family history (no known genetic marker) |
| ○ Individual exposure history to genotoxic injury including lifestyle choices (e.g., smoking) |
| ○ Children? Pregnant women? |
| ○ Threshold dose at which to consider mitigation |
| ● Operative molecular and biological mechanisms during latency |
| ○ Are any subject to ‘chemo-modification’? |
| ○ What mechanisms can be affected by an individual’s effort and what are beyond intervention? |
| ● Drug development |
| ○ Develop a drug for this purpose? |
| ○ Utilise an existing drug—repurposing? |
| ○ Assess safety |
| ○ Assess efficacy of common dietary foods or nutraceuticals |
| ● Cost versus benefit |
| ○ At what excess lifetime risk is intervention appropriate? |
| ○ Toxicity, expense, time and excess stress from an intervention |
2. Radiation concerns and public perception
The general public is known to have a perceived ‘fear of radiation’ (Dauer et al 2011) that is felt to be well beyond the actual risk (Ropeik 2013). This fear was enhanced as a consequence of the Fukushima disaster (Brumfiel 2013). Public concerns have raised more interest for agents to mitigate the cancer risks associated with radiation, including exposures from diagnostic imaging (Mettler et al 2011). While there are, at present no such radiomitigants, the problem of finding treatments for reducing cancer risks a rising from accidental radiation exposures is different from that arising from medical radiation exposures. Clinically directed radiation exposures are relatively well characterised with respect to several factors (e.g. dose of radiation, age, gender, organ sites, etc) that will not be well defined in a radiation accident. Regarding diagnostic imaging, Kuefner et al (2012) have reported that a mixture of antioxidants and glutathione-elevating compounds in human peripheral blood lymphocyte (HPBL) cultures when exposed in vitro to a radiological dose of 10 mGy, reduces the number of γ-H2AX foci, a surrogate marker for DNA damage. The observed reduction in γ-H2AX foci suggests that antioxidant pretreatment may provide protection from radiation-induced DNA damage in a diagnostic setting. Since DNA damage is thought to be a precursor to radiation-induced cancer, these and related compounds may provide protection against cancer induction and thus reduce the risk of cancer. These findings need to be validated (Brink and Boice 2012) but even if confirmed it is unlikely that any strategy that relies on treatment of people prior to their radiation exposure would be useful in an accidental exposure situation, except perhaps for first responders. Some first responders may benefit from pre-exposure prophylaxis if they anticipate exposure up to 0.25 Gy (or a very few possibly up to 0.5–0.75 Gy for ‘lifesaving or protection of large populations’) (see table 2.2 in the PAG (protective action guides) Manual, US EPA 2013). Recently, Mettler et al (2011) reviewed pharmacological strategies for minimising the cancer risk from diagnostic radiation doses and concluded that there are no proven chemical compounds that could be administered after diagnostic radiation to reduce cancer risk. Since, at present, there are no drugs currently available from clinical practice that can be used for the post-exposure treatment, specific research and development efforts that are needed to address the issues provided in table 1. A first consideration is what radiation dose may be a reasonable threshold to consider as a trigger for monitoring and commencing potential mitigation activities (e.g. >0.75 Gy) and in what special populations this threshold may need a reassignment, e.g. children, pregnant women, and special populations with genetic susceptibilities, to afford ARS, DEARE and cancer risk–benefit.
Radiation-inducible cancer is a stochastic event in which probability of occurrence increases with dose with no threshold (assuming the linear no-threshold model) and severity of the cancer is independent of radiation dose. The risk of excess cancers and potential heritable effects varies with age, sex, dose rate, radiation type, smoking status, underlying genetic susceptibility, and nutritional and other factors. A reasonable summary risk for an exposed general population used in this paper is 6% per Sv that is based on an increased excess lifetime cancer risk (ICRP 2007), which is likely to be used by the decision makers as is provided in the PAG manual (US EPA 2013).
The number of people at risk in an incident will depend on many factors (e.g. type of device, height of burst, natural or structural shielding, effectiveness of sheltering-in-place, and weather conditions). Looking at a range of scenarios, the number of people exposed to >0.75 Gy, where the increased lifetime risk is at least an additional 4%, could be in the tens to hundreds of thousands (Knebel et al 2011, Murrain-Hill et al 2011). There will be hundreds of thousands of concerned citizens who have a lower dose, and potentially a million unexposed people who are still concerned, and people who have some degree of post-traumatic stress, which is known to be a key consequence of any major disaster (Bromet 2012, 2014, Brumfiel 2013). Thus, we feel that the potential benefit from an efficacious ‘non-toxic chemopreventive agent’—should one exist—would be of benefit to overall individual and society recovery and resilience in the long-term.
3. Challenges to measuring and developing matrices for medical countermeasure (MCM) efficacy at low doses (<0.75 Gy)
Radiation epidemiological studies from diverse populations (atomic-bomb survivors, populations from therapeutic and diagnostic exposures, communities exposed to environmental and accidentally released sources of radiation) have indeed shown an increase in cancers occurring many years post-exposure. However, risk–benefit analysis and risk communication are keys to how the public will respond. Even for people who survive ARS-causing radiation doses, their long-term risk of fatal cancer is still predominately due to their baseline risk of dying from a fatal cancer because cancer is a common cause of death. With the average lifetime risk of dying from cancer in the United States being approximately 21% (ACS 2013, Siegel et al 2012) a whole body radiation dose sufficient to cause symptoms (approximately 0.75 Gy) would add only another 4% to the lifetime risk of cancer death bringing the risk to 25%. Furthermore, for the suggested maximum dose for first-responders of 0.25 Gy, the additional 1.5% brings their lifetime risk to approximately 22.5% (also see PAG Manual, US EPA 2013). This is not meant to minimise the concern but to point out that for the vast majority of concerned citizens and individuals exposed to doses below those requiring treatment for ARS symptoms (~0.75 Gy) or even ARS haematology syndrome (~2 Gy), the added lifetime risk must be balanced against any toxicity related to the use of a post-exposure mitigant (Jacob et al 1999, 2009, Thompson et al 1994). Although lowering of exposure is expected to decrease risk, greater is the difficulty in detecting any increase in the number of cancers possibly attributable specifically to the individual’s radiation exposure from the incident (Boice 2012), and in turn, demonstrating any mitigation of this risk.
Conducting various chemo-preventative and nutritional intervention studies is possible for various cancer risks, but it is not possible to directly measure radiation-inducible cancer resulting from whole body low-dose exposures, even in a controlled epidemiological study or a clinical trial to assess the efficacy of a mitigation strategy, as an exposed population of the size needed to accurately address these issues, does not exist. Therefore, MCM development requires assumptions and extrapolation from knowledge from experimental systems and animal models of radiation injury.
4. Mechanistic approaches for developing MCM drugs
It is generally agreed that carcinogenesis is a multistep process involving initiation, promotion, and progression, although it is not proven for all forms of cancer. This is a useful framework and as the understanding of the underlying molecular mechanisms of carcinogenesis increases, these steps and new potential areas of mitigation will be better defined ( Gillies et al 2012, McCormick 1999, Vogelstein et al 2013). It is known that cancer is caused by the stepwise accumulation of mutations that affect growth control, differentiation, and survival pathways that intersect and overlap (McCormick 1999). Thus, arguably it is possible to develop interventional strategies at each of these steps. Figure 1 illustrates a multistep carcinogenesis model encompassing ‘initiation’, ‘promotion’ and ‘progression’, and possible risk assessment and mitigation strategies applicable to a radiation mass casualty incident.
Figure 1.
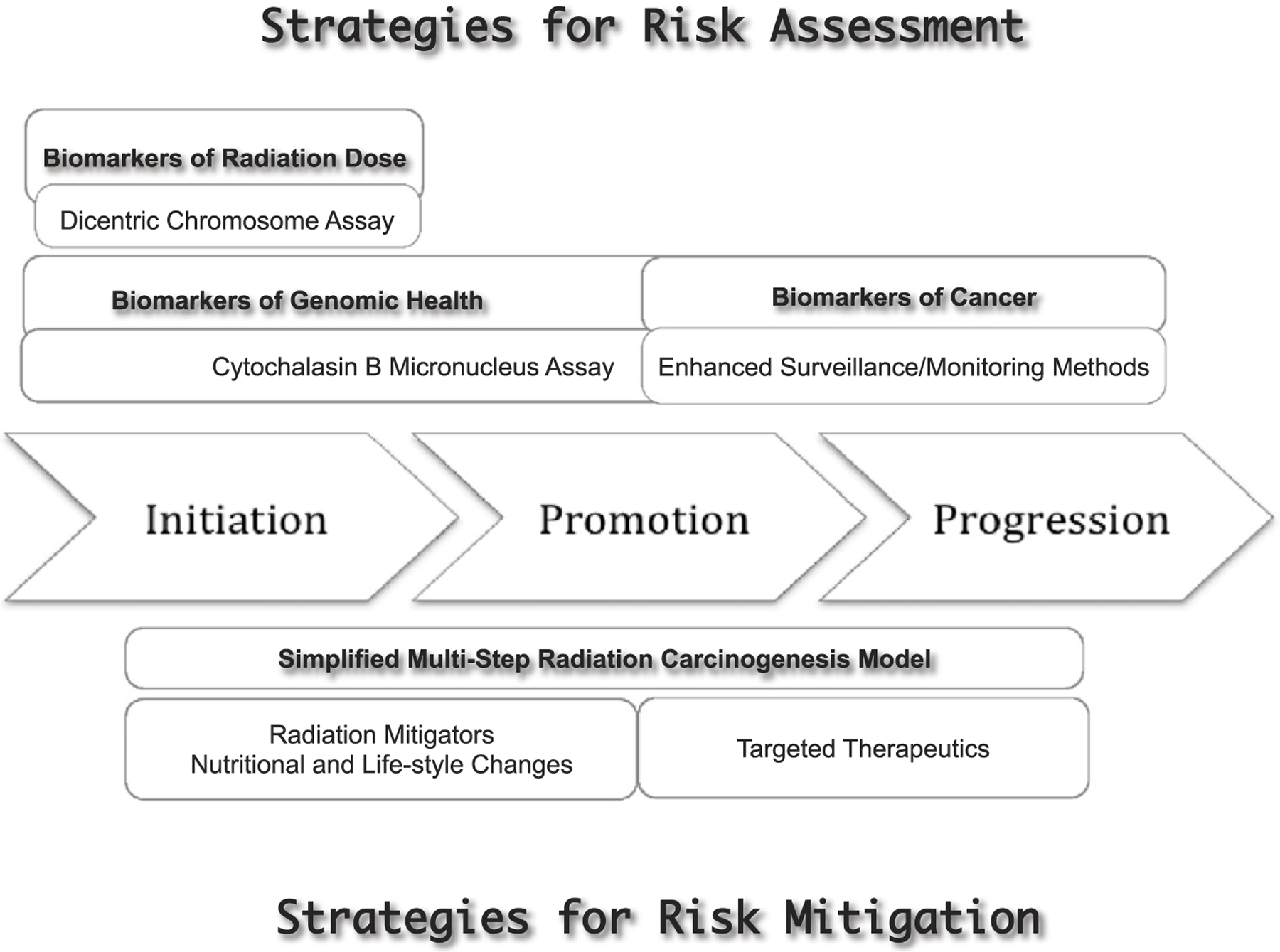
Illustration of a generalised multistep carcinogenesis model involving ‘initiation’, ‘promotion’, and ‘progression’, and possible risk assessment and risk mitigation strategies as applicable to radiation mass casualties. Current understanding of the process of carcinogenesis implicate sequential accumulation of various mutations in specific genes and overcoming distinct microenvironmental proliferation barriers, eventually leading to distinct signalling pathways that regulate cell fate, cell survival, and genome maintenance that intersect and overlap (McCormick 1999, Vogelsteinet al 2013). Radiation exposure will interact with all stages of carcinogenesis: ‘initiation’, ‘promotion’, and ‘progression’. ‘Initiation’ is due to genotoxic insults (e.g. mutations), ‘promotion’ is due to non-genotoxic mechanisms with altered cellular regulatory processes providing selective growth advantage to ‘initiated cells’ eventually leading to ‘progression’ where complex genetic alterations and uncontrolled cell proliferation become evident. Thus, strategies for radiation risk assessment will require biomarkers of radiation dose (e.g. Dicentric Chromosome Assay), biomarkers of genomic health (e.g. Cytokinesis-block micronucleus assay) and biomarkers of cancer (e.g. utilisation of enhanced cancer surveillance/monitoring methods). Similarly, strategies for risk mitigation will involve appropriate changes to lifestyle and cautious use radiation mitigators as well as nutritional changes, and targeted therapeutics with risk versus benefit ratio in view.
4.1. Targeting stages of carcinogenesis
Because radiation interacts with each of the three stages, MCM drug development approaches for radiation-induced cancer have sought to discover and develop inhibitors of each stage. Furthermore, numerous drugs have already been shown to either enhance or suppress the stages of carcinogenesis in both in vitro and in vivo systems (Kennedy 2009). This suggests that identification of a drug that targets one or more stages of the carcinogenic process is possible.
4.1.1. Initiation.
Examples of drugs capable of inhibiting the initiation stage of radiation-induced carcinogenesis in preclinical studies include amifostine, cysteine, and tempol (reviewed in Mettler et al 2011, Weiss and Landauer 2009) and Bowman–Birk inhibitor (Dittman et al 2003). In studies where animals were treated with such compounds prior to irradiation, it has been shown to either increase the latency period and/or reduce the incidence of tumour formation (Grdina et al 1991, Milas et al 1984). Still, no studies to date have evaluated the effectiveness of these drugs as anti-carcinogens when administered after radiation exposure. WR-1065, the active metabolite of amifostine has been demonstrated to be effective in reducing radiation-induced mutations at the hypoxanthine-guanine phosphoribosyltransferase (hprt) locus in V79 Chinese hamster cells when administered up to three hours following radiation exposure (Grdina et al 1985). Amifostine also reduced neutron-induced mutations at the hprt locus in mouse splenocytes when administered both prior to and following irradiation (Grdina et al 1992). Since mutagenesis in these in vitro cellular assays is highly correlated with carcinogenesis, these two drugs may have promise (Compton et al 1991, Grdina et al 2000, Liu et al 1997).
4.1.2. Promotion.
The use of drugs targeting the promotion stage of carcinogenesis builds on data from the chemoprevention literature. Since promotion occurs subsequent to initiation, these drugs might be effectively administered at some time after exposure, but the trade-off is that these drugs would likely need to be taken consistently for the long-term since the promotion stage of carcinogenesis can last for years. This raises the issue of long-term compliance and support by both the exposed individuals and healthcare providers. Additionally, prolonged use of such drugs may reveal toxicities that are not apparent under short-term drug testing regimens, and therefore, development of non-toxic and efficacious mitigator(s), or dietary, and/or lifestyle modifications are advocated. This, of course, speaks to the whole issue of risk versus benefit for using a mitigator(s), thus logically requiring the use of predictive biomarkers or other forms of screening to identify those individuals at a higher genomic risk for cancer. These points are discussed in detail below in the biomarkers section.
4.1.3. Progression.
Progression, which occurs after promotion, can last for decades and is, therefore, the least likely stage of the carcinogenic process to benefit from drug intervention. Therefore, during the progression stage an enhanced cancer surveillance programme may be preferred over drug treatment to individuals at ‘a higher genomic risk’ (similar to the monitoring of progression of cardiovascular disease). Furthermore, the screening would likely be targeted to those who may already be at elevated risk for cancer due to either increased genetic susceptibility or familial predisposition to cancer, because of other carcinogenic exposures (e.g. smoking) or because of a persistent high level of chromosomal instability in apparently normal somatic tissues following the radiation exposure incident. Although genetic testing to identify high cancer risk individuals is still in its infancy and there are no genetic tests to specifically identify individuals at high radiation risk in the general population, even a known family history of cancer might be sufficient to identify genetically based cancer susceptibility that warrants increased surveillance (Boice 2007). It may be likely that such individuals might already be under an appropriate surveillance programme (e.g., breast, lung, or colon cancer screenings).
4.2. Other mechanistic targets for mitigation of cancer risk
In addition to targeting the three stages of carcinogenesis, there are also established mechanistic determinants of cancer risk that might be amenable to intervention. For example, use of pro-apoptotic drugs may enhance the quiescence or removal of DNA-damaged cells and thus preclude their carcinogenic transformation, may be an effective intervention to lower cancer risk. Caution is warranted, however, as clinical use of such drugs might be only theoretically beneficial and even may be ineffective, or even detrimental, in practice. For example, a drug that enhances apoptotic killing of irradiated cells to cull potential transformation may prove deadly to individuals who have sustained a near-lethal radiation dose since the drug may lower the threshold for the haematopoietic or gastrointestinal radiation syndrome.
Precise replication of the genome and continuous surveillance of its integrity ensuring ‘error-free’ repair of the damage is critical not only for survival but also for avoidance of carcinogenesis (Aziz et al 2012). Thus, another mechanistically based approach might be to utilise drugs that facilitate an enhanced fidelity of DNA repair; for example, strategies for promoting homologous recombinational error-free repair and/or suppressing error-prone non-homologous end joining. The rationale is that ‘error-prone’ DNA repair pathways are thought to promote cell survival at the expense of introducing mutations into the primary DNA sequence (Bunting and Nussenzweig 2013) because the DNA polymerases involved in error-prone DNA repair pathways tend to have lower fidelity than the DNA polymerases dedicated to replication. Radiomitigators might work by stabilising the DNA enzymatic repair complexes or by shuttling repair to one of the ‘error-free’ repair pathways that are known to introduce fewer mutations. It might also be possible to reduce mutations by prolonging radiation-induced cell cycle arrest thereby allowing more time for ‘error-free repair’ mechanisms to operate. Unfortunately, all approaches based on enhancing DNA repair would likely have a limited time window for use since DNA repair processes typically run their course soon after irradiation (Jeggo et al 2011). Several micronutrients are required for DNA replication and repair to occur accurately (a) either as cofactors for DNA polymerases (e.g. magnesium and zinc) or for the synthesis of nucleotides required for DNA repair (e.g. folate), (b) as an integral component of a DNA repair enzyme (e.g. Zn in hOGG1 (human 8-oxoguanine glycosylase), which is required to remove oxidised guanine), or (c) a precursor of substrates to generate NAD (nicotinamide adenine dinucleotide) for poly-adenosine diphosphate ribosylation (e.g. niacin), which is essential in the detection of strand breaks in DNA and recruitment of the required DNA machinery (Fenech 2010a, Ferguson and Fenech 2012, Hageman and Stierum 2001, Sharif et al 2012). Micronutrient strategies are discussed below in section 5.
5. Chemoprevention products
As mentioned above, products that target the tumour promotion stage of a multistep carcinogenesis model have largely come from the field of chemoprevention. Some cancer preventive products that can modify radiation transformation within in vitro systems have been reported. These include the following classes of compounds: (a) vitamins (or vitamin-like compounds), their precursors (e.g., vitamin A, its precursor β-carotene and other carotenoids), and derivatives (e.g. retinoids), (b) protease inhibitors (e.g. the soybean-derived protease inhibitor, Bowman–Birk inhibitor), (c) hormones (e.g. glucocorticoid hormones, testosterone, dihydrotestosterone), (d) modifiers of arachidonic acid metabolism (eicosanoids), (e) inhibitors of protein kinase C, and (f) antioxidants, including numerous products that scavenge free radicals.
Retinoids, which have been evaluated extensively as cancer preventive products, have been shown to prevent radiation transformation in vitro (Borek 1981, Harisiadis et al 1978). Antioxidants have also been shown to decrease radiation-induced carcinogenesis in both in vivo and in vitro systems (Kennedy 2009). Combinations of antioxidants have been used to inhibit radiation-induced carcinogenesis (Kennedy 2009). Numerous constituents of vitamin pills (i.e., vitamins, minerals and non-vitamin micronutrients) have been shown to prevent DNA damage (Ames 2001). In the nutrition intervention trials in Linxian, China, supplementation of the diet with beta-carotene, vitamin E and selenium together was associated with a statistically significant lower total mortality rate in a nutritionally deficient population with low dietary vitamin intake (Blot et al 1993). In this population, the reduction was mainly due to lower cancer rates; the reduced risk became apparent in about 1–2 years after the start of supplementation with vitamins and minerals. As cancer was the leading cause of death in this population, these results indicate that dietary antioxidant vitamin supplementation was effective at suppressing mortality, primarily from cancer. A recent report indicates a reduction in the radiation-induced formation of γ-H2AX foci in HPBL cultures in vitro by the addition of a mixture of antioxidants and glutathione-elevating compounds (Kuefner et al 2012) suggesting that prior administration of micronutrients might mitigate radiation risks, although evidence is not entirely convincing (Brink and Boice 2012).
Some of the chemoprevention products have shown activity when treatments began after the exposure to a carcinogenic agent. In rats treatment with a soy isoflavone mixture and soy derivative protease inhibitor (Bowman–Birk Inhibitor Concentrate; BBIC), started one week after the end of treatment with a carcinogen, effectively prevented prostate carcinogenesis (McCormick et al 2007). There is also evidence from in vitro and in vivo models that some cancer preventive products may reduce cancer risk even when administered after radiation exposure (Kennedy et al 2011). Many cancer preventive products recognised by Division of Cancer Prevention (DCP, National Cancer Institute) also may be also useful as mitigators of carcinogenesis after exposures to carcinogens implying their potential to intervene in radiation-induced carcinogenesis at the promotion stage as exemplified by a curcumin study in which dietary administration significantly reduced the incidence of mammary tumours in rats (Inano et al 1999).
6. Intervention of radiation-induced thyroid cancer
Relevant to a nuclear power plant accident the release of a complex mixture of radionuclides consisting of radioactive iodine (131I) and caesium (137Cs) can pose a significant health hazard through ingestion and inhalation. While radioactive caesium poses a risk to the lungs by inhalation and to the whole body through ingestion, radioiodine presents a unique radiation hazard since iodine is readily concentrated in the thyroid gland. Upon accumulation from consuming contaminated food and water, 131I can deliver a substantial local tissue dose, significantly increasing the risk of thyroid cancer among people who are exposed when young. 131I has a short half-life (8 days) and usually presents an appreciable health risk only from ingestion of contaminated food or water. It may also pose a more significant threat in individuals who happen to be iodine deficient. While interdiction of potentially contaminated food and water is the primary and preferred means of protection, for those who potentially internalise radioactive iodine by continued consumption of contaminated food and water, the approach to reduce radioactive iodine uptake by thyroid is to saturate it promptly with non-radioactive (i.e. ‘cold’) iodine by ingesting potassium iodide (KI) tablets prior to or within a few hours of 131I exposure (CDC 2013).
It has been reported that providing KI in the form of dietary supplements (as antistrumin, multivitamins containing iodine and iodised salt) at several months to years after the Chernobyl accident to the children from exposed areas reduced the risk of thyroid cancer by a factor of approximately three (Cardis et al 2005). In these studies, the KI could not have reduced the uptake of 131I into the thyroid gland since the 131I had decayed to insignificant doses by the time of drug treatment. It is more likely that the dietary supplements acted by a mechanism other than blocking uptake of 131I by thyroid. Iodide could have been the active chemopreventive drug in the KI supplements, but the vitamins and minerals in which KI was administered to at least some of the children also could have contributed to the observed cancer preventive activity of the dietary supplements (Cardis et al 2005). An alternative explanation could be that KI supplementation may have also corrected local iodine deficiency, which may independently influence the risk of thyroid cancer initiation or its progression (Boice 2005, Shakhtarin et al 2003).
7. Possible medical countermeasures
There are several drugs that are used as human cancer chemopreventive or ‘risk reduction’ products, such as exemestane for breast cancer (Goss et al 2011) and aspirin in several common cancers (Rothwell et al 2011). It is possible that some of these products may also have the ability to prevent radiation-induced human cancer. NCI’s DCP has funded numerous studies to determine the effectiveness of these products in chemoprevention. Several of these drugs have been tested in clinical trials, which are reviewed elsewhere (Greenwald 2002, Kelloff et al 2006, O’Shaughnessy et al 2002). The findings show that in addition to anti-cancer vaccines there are four major classes of compounds that show promise as chemopreventive products in randomised controlled trials (RCT), which are discussed below.
7.1. Vitamins and other essential micronutrients
Vitamin A, its precursor beta-carotene, and vitamin E have been used in clinical trials as chemoprevention products based on laboratory rationale (reviewed in Gallicchio et al 2008 and Huang et al 2006). Paradoxically alcohol drinkers showed an increase in lung cancer risk (Ratnasinghe et al 2000) despite increased serum carotenoid levels. Vitamin E, an antioxidant, also showed a slightly increased risk for developing prostate cancer (Klein et al 2011). These findings are counterintuitive and warrant caution when recommending interventions of ‘off the shelf’ food supplements or vitamins. While there may be a theoretical reason to consider using such an approach, there may be a dose–effect and/or other mechanisms by which antioxidants could actually increase cancer risk suggesting that we may not yet fully understand the clinical biological activities of antioxidants. Several retinoids have shown activity in clinical studies in treatment of pre-cancers and prevention of second primary cancers, but are less effective in early carcinogenesis (Kitareewan 2004).
An example of a non-vitamin essential micronutrient that may influence cancer risk is methionine. The amino acid, methionine is a precursor required for polyamine synthesis which accelerates cell division (Cavuoto and Fenech 2012). Several cancers are methionine-dependent for their growth; frequent co-deletion of the methylthioadenosine phosphorylase (MTAP) gene with p16, a tumour suppressor that is silenced or deleted, is observed in irradiated cells (Belinsky et al 2004, Yamada et al 2010). MTAP codes for a key enzyme in the methionine salvage pathway (methylthioadenosine phosphorylase), which if deleted or silenced, makes cells unable to regenerate methionine (Cavuoto and Fenech 2012). Methionine restriction has been shown to prevent cancer and prolong lifespan in rodents (Komninou et al 2006, Orentreich et al 1993, Richie et al 1994). Methionine restriction may therefore help to control radiation-induced methionine-dependent cancers and could prove to be one of the possible strategies of preventing progression of such cancers. Similarly, long-term dietary supplementation with the thiol-containing antioxidant, N-acetyl-L-cysteine (NAC) appears to suppress carcinogenesis-associated biomarkers, such as DNA deletions and oxidative DNA damage in an ATM-deficient mouse model (Relience et al 2004). Further NAC significantly increased the lifespan and reduced both the incidence and multiplicity of lymphoma in this model (Relience and Schiestl 2006). Thus, understanding which nutrition-relevant genes may be commonly deleted by ionising radiation exposure could inform dietary restriction strategies for prevention of the growth of such cancers.
7.2. Inhibitors of hormone actions
Tamoxifen citrate (tamoxifen) was the first drug to receive US FDA approval a s a cancer preventive drug for breast cancer in 1998. It was shown to prevent cancer recurrence in a randomised, double blind breast cancer prevention trial known as the breast cancer prevention P-1 trial (Fisher et al 1998). While widely acknowledged to be a human breast cancer preventive drug, it has not been widely used in non-cancer populations for the prevention of breast cancer primarily due to its potential side effects (e.g. increased rates of endometrial cancer and thrombotic events, as shown in the original National Surgical Adjuvant Breast and Bowel Project). A subsequent trial of tamoxifen and raloxifene hydrochloride (raloxifene) indicated that raloxifene was as effective as tamoxifen for breast cancer prevention in postmenopausal women but with less toxicity (Vogel et al 2006); the FDA for breast cancer risk reduction has also approved raloxifene. An important aspect of these trials is that reduction of recurrence persisted after discontinuation of the drug (Jordan et al 2011).
Proscar (finasteride)—an α-reductase inhibitor—has been shown to prevent or delay the development of human prostate cancer (Thompson 2003) but there was evidence in this initial study that finasteride treatment l ed to sexual side effects and an increased risk for the development of high-grade prostate cancer. Findings with finasteride and a related drug, Avodart (dutasteride) (Andriole et al 2010), revealed an increase in high-grade tumours despite the major overall decreased incidence in cancer and led the FDA (see product labels at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/) not to approve α-reductase inhibitors for prostate cancer prevention (Hamilton et al 2010, Logothetis and Schellhammer 2008, Lucia et al 2007, Redman et al 2008, Theoret et al 2011).
7.3. Cyclooxygenase-2 (COX-2) inhibitors and other nonsteroidal anti-inflammatory drugs (NSAIDs)
Celecoxib (Celebrex), a COX-2 selective inhibitor, and other NSAIDs have received FDA approval as cancer prevention products and/or drugs used for treating advanced premalignancy. The US FDA approved Celebrex in December 1999 for a reduction of incidence rate in polyps in familial adenomatous polyposis (FAP). However, this indication was removed from the approved labelling in 2011 due to a want of a confirmatory trial. COX-2 inhibitors were widely used in numerous human cancer prevention trials but many of these trials were stopped when results from the Adenomatous Polyp Prevention on Vioxx (APPROVe) (Bresalier et al 2005) and Adenoma Prevention with Celecoxib (Bertagnolli et al 2006) trials indicated that COX-2 inhibitors were associated with serious cardiovascular adverse events. This information is publicly available in the approval letters and the drug labels, which can be accessed at the FDA website (http://www.fda.gov/Drugs/default.htm—accessed 18 October 2013). Some of the NCI/DCP studies on the use of celecoxib for prevention and treatment for human cancer were continued and have shown positive results (Meyskens et al 2011). It has been proposed that COX-2 inhibitors could be used to prevent human cancer without cardiovascular adverse effects under certain conditions such as using low to moderate doses of the drug, reduced frequency of drug administration, and/or use in combination with other cancer risk reduction drugs (Meyskens and McLaren 2010).
Thus, there are several drugs that have already been shown to be useful for human cancer prevention and which might, in theory, be useful to prevent radiation-induced cancer in human populations. The key issue of balancing of benefits versus risks remains; therefore, the drugs should best be targeted to those who could benefit the most. As noted earlier, this might include those who are already at intrinsically high risk of developing cancer either due to their genetics or susceptibility or undue exposure to other carcinogens. One approach could be to identify people with premalignant lesions (e.g., IEN or benign breast disease) who may be already at increased cancer risk that might be further increased due to a ‘significant dose’ of radiation (Meyskens and McLaren 2010, Meyskens et al 2008). What is considered a ‘significant dose’ would be somewhat subjective (perhaps >500 or 750 mSv). Consequently, a role for active chemoprevention is likely to apply to only a very small proportion of people exposed in a radiological or nuclear incident (Meyskens and Gerner 2011) and identification of this cohort is imperative.
7.4. Dietary-related compounds
There are a wide variety of dietary compounds that have been shown to prevent carcinogenesis induced by chemical carcinogens (Wattenberg 1985, 1990, 1992, Wattenberg et al 1985) but only a few classes of agents that have been shown to prevent radiation-induced cancers in animal models. The dietary agents such as protease inhibitors (Kennedy 1998a, 1998b), Vitamin A (Burns et al 2007), a retinoid (Burns et al 2002), other various dietary antioxidant vitamins (Kennedy et al 2008, 2011), and a soybean diet (Troll et al 1980) have been shown to inhibit radiation-induced carcinogenesis in vivo.
Protease inhibitors such as the soybean-derived Bowman–Birk inhibitor have been shown to inhibit radiation-induced cancer in animals and to have anti-carcinogenic effects in humans (Kennedy 2006). Protease inhibitors have been observed to suppress carcinogenesis when administered to cells or animals at long periods of time after exposure to the carcinogenic agent(s) (Kennedy 1998a,b). This phenomenon has also been observed for drugs that prevent radiation-induced cancer in animals (e.g. cortisone suppresses radiation-induced leukaemogenesis when administered to animals months after the radiation exposure; Kaplan et al 1951).
It is known that there are compounds with strong cancer preventive activities in the normal human diet(s) (Wattenberg et al 1985, Bode and Dong 2009, Wattenberg 1985) but their role in human cancer prevention by taking higher than the normal dietary amount is uncertain. However, both in human populations and animal studies, vitamin and micronutrient deficiencies are known to increase cancer incidence and mortality rates ( Blot et al 1993, Fairfield and Fletcher 2002, Li et al 1993, NA/NRC 1982, Nelson 1987, Newberne and Rogers 1985). Americans do, in fact, have significant dietary deficiencies (Ames 2001) that may put them at an increased risk of cancer. Dietary deficiencies of agents that have a Recommended Dietary Allowances (RDAs; generally those with a maximum allowances per day) should be avoided as therapeutics due to dose-limiting toxicities and potentially narrow therapeutic index (TI), but this should be measured and patient-specific as taking amounts beyond the RDA could be beneficial to some. The American Medical Association (AMA) recommended that everyone take a daily multivitamin pill for the prevention of numerous adverse health conditions, which includes carcinogenesis (Fletcher and Fairfield 2002), although there are examples of adverse effects from dietary supplements (Mursu et al 2011). There have been some studies suggesting a negative health impact from dietary supplements that utilise analytical methods that are controversial (Kennedy and Wan 2011). A very recent report by the US Preventive Services Task Force Evidence Syntheses (formerly Systematic Evidence Reviews), found no evidence confirming a health benefit resulting from vitamin, mineral, or mutivitamin supplements (Fortmann et al 2013). A recommendation (editorial) was published recently supporting this position in the annals of internal medicine (Guallar et al 2013). Moreover, recent in vitro studies with HPBL show that both deficiency or excess of certain micronutrients (e.g. zinc) may increase chromosomal DNA damage and moderate folate deficiency can increase micronuclei counts to a similar extent as an exposure of 0.2 Gy from low-LET radiation (Fenech 2010a, Ferguson and Fenech 2012, Sharif et al 2011). As a general conservative approach, deficiencies should be avoided but use of supplementation beyond the RDA remains debatable. Given such constraints, there is a pressing need to define dietary reference values based on DNA damage prevention and optimal DNA repair. Defining a generalised ‘optimal nutrition’ status may likely be a useful recommendation to minimise both ‘spontaneous’ and ‘radiation-induced’ genome mutations.
8. Drug development programmes
Development of drugs and biologics as MCMs for treatment of radiation injuries has been largely the purview of the United States government (BARDA 2013). As such, to date, research and development has focused on developing MCMs against ARS and also against DEARE such as lung fibrosis. MCMs used f or ARS are discussed here because of their discovery, development, regulatory and translational challenges share many features as those agents which may potentially be developed as mitigators of DEARE, and health risks, specifically cancer. However, similarities with molecular pathways of damage are to be explored, as many exist.
The MCM programmes include basic radiation biology studies supported through NCI, scientific studies supported by the National Institute of Allergy and Infectious Disease (NIAID; www.niaid.nih.gov—accessed December 29, 2013) and the Armed Forces Radiobiology Research Institute (AFRRI; www.afrri.usuhs.mil—accessed 29 December 2013), advanced drug development and biodosimetry studies by Biomedical Advanced Research and Development Authority (BARDA; www.phe.gov/about/barda/Pages/default.aspx—accessed 20 January 2014), and drug development by Department of Defense Chemical Biologic Medical Systems—Medical Identification Treatment Systems (CBMS/MITS; www.jpeocbd.osd.mil—accessed 29 December 2013).
These above government programmes have identified several MCM drugs with promise of preventing the acute and intermediate health consequences of high-dose exposures. It is not known whether these agents can also prevent late health effects among those exposed to below threshold doses for ARS development as well as those who survive ARS. The focus of ARS studies is primarily on the haematopoietic depression, gastrointestinal tract pathologies, pneumonitis and delayed fibrosis, and cutaneous damage that follow shortly after total body acute exposures in the 2–10 Gy range as well as combined injuries (operationally defined as trauma plus radiation of >2 Gy; DiCarlo et al 2011). The delayed fibrosis seen in the lung and other tissues following doses of >6–8 Gy are considered to be in the DEARE category as they arise months to years post-exposure. Countering ARS will require administration of MCM drugs within hours or possibly within days of an incident to be effective since ARS can occur quickly (days to weeks). By mitigating an acute injury, or by other mechanisms of action, these drugs might also mitigate DEARE pathologies that manifests months or years later. Conceivably, mitigation of cancer risks of radiation might follow a similar treatment paradigm in that early intervention might affect initial underlying mechanisms that may mitigate late event risks. The intervention could be targeted against cellular damage, tissue loss and/or the inflammatory responses as well as many signalling networks that are involved in carcinogenesis.
Testing MCM drugs for effectiveness against any of the above radiation-induced health conditions is problematic since human studies to induce ARS or DEARE are unethical. The animal rule provides a regulatory mechanism to test MCM’s efficacy in animal models when testing in human subjects is neither ethical nor feasible. MCM drug developers can conduct their experiments in animal models with the assumption that the effect seen in the animal models will be predictive of the response in humans. Thus, the Animal Rule provides the opportunity to evaluate the efficacy of candidate radiation protectors/mitigators in animals.
Neupogen® (Filgrastim) a granulocyte colony stimulating factor (G-CSF), has been approved by the FDA ‘to decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a significant incidence of severe n eutropenia with fever’ (www.accessdata.fda.gov/scripts/cder/drugsatfda/—accessed 20 January 2014). Neupogen may be used in the event of a radiation emergency for treatment of radiation-induced neutropenia. The FDA has not approved the indication of radiation-induced neutropenia. In a joint meeting in May 2013, the Medical Imaging Advisory Committee and Oncologic Drug Advisory Committee reviewed and discussed animal data and clinical experience with Neupogen, and were supportive of the use of Neupogen in radiation-induced neutropenia. (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM363898.pdf—accessed December 29,2013).
DEARE are deterministic effects and, therefore, distinct from the sporadic health effects typified by radiation-induced cancer. Nevertheless, the appearances of both D EARE and radiation-induced cancers are significantly delayed after exposure. Whether or not there are mechanisms in common, such as inflammation, tissue haling, immunological effects, and others needs to be explored. While speculative, given the current state of knowledge, assessment of MCM drugs for efficacy against D EARE m ay thus allow assessment of the concomitant long-term endpoint of cancer as well. It is not clear what aspects of the animal models appropriate to both ARS and DEARE endpoints and would be equally appropriate for cancer endpoints, but it would be desirable to employ such animal models whenever possible since the potential exists to concurrently evaluate the efficacy of MCM drugs for all early and DEARE, including cancer, endpoints.
8.1. Is a prospective clinical trial even possible for MCM drugs?
A short answer at present is ‘no’. To test an MCM for reducing cancer risk from radiation for a general population would be a challenge based on the heterogeneity of risk factors (stochastic in nature) and the length of study needed. For a ‘time-to-event’ analysis (e.g. Cox proportional hazard model or Kaplan–Meier model) of a clinical response to a hypothetical mitigator/protector, the ‘event’ is a confirmed cancer diagnosis. This is problematic in a clinical trial as cancer per se is not radiation-specific and because long periods (decades) of observation are required to accumulate a sufficient number of ‘events’. There may be certain populations who were treated with radiation therapy and are at elevated risk of developing cancer, and for which such studies could be conducted regarding mechanisms and possible biomarkers of radiation injury. The mechanisms and biomarkers remain to be explored but availability of some populations at increased risk of treatment-induced cancer may facilitate the design and approach to define their roles in cancer. For example, patients treated with total body irradiation for bone marrow transplants are at high risk of developing cancer (Rizzo et al 2009). Large numbers of survivors of childhood cancer, currently under study, are at higher risk of radiation-induced cancers of the thyroid, breast, bone and brain as well as heart disease (Armstrong et al 2010). Patients treated with radiotherapy for Hodgkin’s lymphoma are at higher risk of radiation-induced cancers of the breast and lung (Boice 2007, Gilbert et al 2003, Travis et al 2003). Young women given radiotherapy to treat breast cancer are at two-fold risk of developing a second breast cancer (Boice et al 1992, Darby et al 2010, Stovall et al 2008). Persons irradiated as children for enlarged thymus glands are at an elevated risk of radiation-induced cancers of the breast and thyroid (Adams et al 2010a, 2010b). Occupational studies of underground uranium miners exposed to radon and its decay products have found high risk (>5-fold) increases in lung cancer (Lubin et al 2005).
Thus, at present the need to explore a range of potential strategies and endpoints, the lack of complete knowledge of the radiobiological mechanisms of radiation-induced cancer, the resultant lack of a biomarker (see below), the heterogeneity of risk factors and uncertainty of lifetime exposure to ionising radiation of individuals, and the likely need for a defined mechanism for an effective MCM, makes it almost impossible to conduct a prospective efficacy study of an MCM using cancer incidence as an endpoint.
9. Biomarkers
Mitigating the risk of radiation-induced cancers will require the use of three categories of biomarkers: (i) radiation dose assessment (biodosimeters), (ii) genomic health prediction, and (iii) cancer surveillance. We have provided a rationale in preceding sections for using and/or developing suitable diagnostic biomarkers for determining the radiation dose of individuals and assigning a population at risk for developing delayed health risks including cancer. In so doing, we seek to identify individuals from the at-risk pool who may benefit from a reduction in their cancer risk from a mitigator-based therapy. Similarly, there is also a need for assessing the overall ‘genomic health risk’ of individuals in such an exposed population and also to monitor the efficacy of mitigator intervention by possibly using appropriate biomarkers. Further, we have also argued for a need for using an enhanced cancer surveillance programme for selected high-risk radiation-exposed cohort following a radiological mass casualty, specifically for radiogenic cancers such as breast, respiratory, digestive system, thyroid and other solid cancers, and leukaemia among children. The Early Detection Research Network of Division of Cancer Prevention (EDRN, NCI, http://edrn.nci.nih.gov—accessed 29 December 2013), a consortium of laboratories, is focused on identifying, testing, and validating methods to noninvasively and accurately detect cancers at their earliest stages. Biomarker-based treatment decisions may eventually drive personalised medicine (Hamburg and Collins 2010), however, the definitive utility of these biomarkers requires conducting large R CTs f or validation. Issues related to designing such RCTs with biomarkers have been discussed elsewhere (Freidlin et al 2010).
There are many sources of genotoxic damage but our focus is on radiological and nuclear incidents, primarily a nuclear detonation and mass casualty response. In this context, how we can distinguish the sources of carcinogenic insults and detect added health risks due to such exposure to radiation and develop biomarker-based approaches to assess a reduction of such risks due to intervention—pharmacological, nutritional or other?Comprehensive strategies for determining ‘total genomic health risk’ assessment and companion radiation dose diagnostic tools to predict the radiation component of such genomic risks becomes a critical need. Such tools could serve the dual purpose of assessing MCM efficacy (table 1) for use at doses below the assigned threshold doses for ARS and also for monitoring survivors of ARS. Furthermore, if it is possible to distinguish the source of ‘genomic insult’ between radiogenic or not, that would be useful in determining the ‘probability of cause of late effects’ and help improve resolution of medico/legal compensation issues (DHHS 2002).
Identifying an at-risk population cohort for increased health risk due to radiation exposure is critical in addressing risk–benefit issues and the potential need for a prolonged mitigator treatment and/or enhanced cancer screening. In this context, consideration is given to two well-established/validated cytogenetic assays performed using HPBL, cytokinesis-block micronucleus (CBMN) for assessing ‘total genomic health risk’ and the dicentric chromosome assay (DCA) to determine the risk related to radiation component. We recognise the need for both validated assays and logistics/resources to conduct such an analysis on an appropriate at-risk population, which could be defined based on their exposure during an incident above a certain dose (e.g., >0.5 Gy). Biodosimetry techniques, their capability, window of utility, and the concept of operations (CONOPs) needed for deployment of various tests are under consideration by the Office of the Assistant Secretary for Preparedness and Response (ASPR) (Sullivan et al 2013).
9.1. Cytokinesis-block micronucleus (CBMN) assay
The CBMN assay is well validated as a biomarker for radiation biodosimetry and inter-laboratory studies have shown it to be highly reproducible both when scored visually or automatically (Fenech 2010b, IAEA 2011, Romm et al 2013). It is useful for rapidly triaging individuals exposed to doses above 1 Gy of radiation with high degree of accuracy and specificity ( McNamee et al 2009, Romm et al 2013). However, at doses less than 0.5 Gy, a substantial proportion of assessed damage may not be very specific to ionising radiation due to a variety of other contributors to genomic damage such as age, gender, smoking, diet, genetic predisposition, etc, but it, nonetheless, reflects ‘total genomic damage’ (Bonassi et al 2003, Fenech 1999, Fenech and Bonassi 2011, Fenech and Morley 1985). The CBMN assay performed using HPBL obtained from children from the Chernobyl radiation accident cohort showed an increase in their ‘total genomic risk’ (Fenech et al 1997, Zotti-Martelli et al 1999). However, because nutritional deficiency or excess can also cause chromosomal aberrations and micronuclei in children or adults (Fenech and Bonassi 2011) it is not possible to exclude malnutrition as a contributing variable affecting MN frequency at exposure doses less than 0.5 Gy. Furthermore, the observation that MN frequency can be reduced by nutritional supplementation (e.g. folic acid and vitamin B12) (Thomas et al 2011) indicates the possibility of using nutritional strategies for DNA damage prevention after a radiation incident that could complement and further enhance countermeasures against radiation-induced DNA damage.
Evidence of a direct link between increased genomic damage and elevated risks for adverse health outcome is becoming stronger. For example, an increase in chromosomal damage as determined by the CBMN assay is predictive of cancer risk, cardiovascular disease mortality and pregnancy complications such as pre-eclampsia and intrauterine growth restriction (Bonassi et al 2007, Federici et al 2008, Furness et al 2010). Furthermore a high MN frequency is associated with the pathogenesis of neurodegenerative disorders such as Parkinson’s and Alzheimer’s disease, metabolic syndrome, and cardiovascular diseases (Andreassi et al 2011, Migliore et al 2011). Using a biomarker of chromosomal instability it has also been shown that folate is an important determinant of chromosomal stability and a modifying factor of cellular sensitivity to radiation (Beetstra et al 2005).
Mechanistic, theoretical, and empirical evidence accumulated over the last several decades supports the role of chromosomal abnormalities in the etiology of human cancer and the CBMN assay has emerged as a powerful tool for measuring chromosomal damage and total genomic health, especially in selected populations. A recent large international study assembling data from over 6000 individuals from 10 countries, showed a significant association between MN frequency in healthy subjects and cancer risk (Bonassi et al 2011). This study supports the idea that MN frequency in HPBL is predictive of cancer risk suggesting that it is associated with early events in carcinogenesis and potential for prospective use of the CBMN assay in cancer screening programmes. To investigate the translational feasibility of the above observations, Fenech and colleagues have begun a Genome Health Clinic aimed at diagnosis and mitigation against DNA damage using dietary and lifestyle interventions (Fenech 2005, 2013).
Given that this paper aims to stimulate potential new approaches, one speculative idea is to use the CBMN assay as a low cost minimally invasive predictive biomarker that is also responsive to various other genomic health determinants in the aftermath of a nuclear event. In theory, the lymphocyte MN frequency index could become a very useful medical test result available in each person’s medical record so that a recent baseline value is available, which could, in theory, be used to obtain a more accurate assessment of radiation exposure particularly at doses less than 0.5 Gy rather than resorting to a statistical estimate as done in current practice (Tucker et al 2013). That a CBMN assay should become a routine medical test is not implausible because the use of MN in haematological clinical practice is already accepted via the measurement of Howell–Jolly bodies (also MN) in erythrocytes as a biomarker of folate and/or vitamin B12 deficiency (Dawson and Bury 1961, Jolly 1905, Howell 1890–1891). It was in fact this development that eventually led to the use of micronuclei in erythrocytes as an in vivo biomarker of DNA damage in rodents (mouse micronucleus test, MMT; an in vivo standardised genetic test used in drug development for drug-induced micronuclei) and then eventually to the measurement of MN in lymphocytes in humans (Heddle et al 2011).
9.2. Dicentric chromosome assay (DCA)
The DCA is the current ‘gold standard’ method for radiation dose assessment. For many years, the DCA performed using HPBL has been used as a biological dosimeter for specifically assessing radiation dose to individuals. DCA shows a high degree of radiation specificity, high sensitivity, and robust dose dependency in the range 0.15–5.0 Gy. Furthermore, a sampling delay of up to six weeks after radiation exposure does not interfere with dose assessment and the effects of many confounders have already been well characterised. Dose estimation can be made independent of radiation type and dose rate (IAEA 2011). Well-defined laboratory protocols and quality control standards are already available (IAEA 2011, ISO 2004, 2008). Estimated doses using the DCA correlate well with the severity of ARS. The assay can diagnose partial-body exposures and indicate the presence of surviving bone marrow, as this may be helpful in managing partial-body exposures (Lloyd 1997). The DCA is validated in several inter-laboratory comparison studies (Beinke et al 2013, Rothkamm et al 2013, Wilkins et al 2008, 2011). While precise dose estimation for health risk assessment requires analysis of 500 or more metaphase spreads, analysis of approximately 50 metaphase spreads per person is adequate for initial mass casualty triage and management of people at risk for the ARS (Prasanna et al 2010, Romm et al 2011). Since predicted doses by different laboratories were in good agreement a network of laboratories can improve throughput during a mass casualty incident. Several national and international collaborative networks are already in place (e.g. WHO BioDoseNet, Canadian Network, European MULTIDose and Japanese Networks). Both sample preparation and dicentric analysis are time-consuming and laborious. Laboratory automation is essential to further increase throughput and to enhance DCA’s practical applications in mass casualties. Serving as a radiation-specific diagnostic tool to assess risks of developing ARS, DCA can also serve as a means to estimate dose and help identify a populations at high risk for delayed health risks.
Several other new biodosimetry technologies are also currently under development including biomarkers based on DNA damage while others are based on metabolomics, gene and protein expression changes (Sullivan et al 2013) as well as biophysical methods (Brink et al 1980). The biomarker development and MCM drug development efforts complement one another, similar to companion diagnostics in treatment for a given disease setting. Commercial development and use of radiation risk mitigators will benefit from predictive biomarkers for radiation dose and genomic health concurrently and these would also help in the understanding of the impact of nutrients, repurposed drugs or other interventions. Having a unique set of biomarkers that show an MCM drug is preventing or delaying cancer development will be extraordinarily important for physicians to assess and medically manage at-risk populations. The challenge will be in developing the marker, its validation, and regulatory considerations.
A major caveat in the clinical utility of a predictive biomarker for cancer is inter-individual differences in genetics and radiation responses. The biology underlying these variable responses among individuals to radiation remains poorly understood. Indeed, significant variation in response to radiation-induced chromosome damage is evident even when studying just ten or more randomly chosen healthy individuals (Fenech 2010a, Rothfuss et al 2000). These differences may be partly due to genetic defects in key genes involved in homologous recombinational DNA repair pathways such as the ATM, BRCA1 and BRCA2 genes, and, in fact, the CBMN assay and DCA can be used successfully to identify individuals with such defects (Claes et al 2013, Rothfuss et al 2000, Scott 2000, Vral et al 1996).
There is much to be learned from biomarker development applied to cancer research, as biomarkers can be prognostic (estimate overall disease prognosis) and/or predictive (who may respond to treatment or develop disease). The lack of standardised technologies and assays for verifying and validating candidate biomarkers, as well as a plethora of parameters to consider for validation studies, complicates the development pipeline from biomarker discovery through clinical use. Nonetheless, the impact of radiation on DNA damage and the radiation-induced stress response may enable biomarkers to be developed for early triage in predicting late effects and potentially identifying who might be suitable candidates for an MCM drug trial.
10. Conclusion
In the event of a nuclear detonation or major radiological incident the potential number of people at risk (Knebel et al 2011) for radiation-induced cancers is large. It is important to identify people who are at significant risk and for whom treatment with mitigating agents is worth consideration. While a nuclear detonation is catastrophic, the size of the device modelled for terrorist-related incidents leaves much of the local and regional infrastructures intact so that a response is both possible and mandatory to save lives (US EPA 2013). The adverse psychological impact of prolonged fear of radiation-induced cancer also would be a major public health concern (Bromet 2012, 2014) and psychological stress itself may be related to chromosomal DNA damage (Fischman and Kelly 1999, Fischman et al 1996, Ingel et al 2001, York et al 2013). While managing those with immediate and critical medical needs is the first priority, the large number of people potentially with lower radiation doses deserve, and will ask for, attention. The vast majority of people following a nuclear incident will have very low or no exposure so that the use of any MCM may produce little benefit and could involve the risk of side effects. Furthermore, the additional burden and expense of employing a drug that requires long-term use must be considered. Nevertheless, the availability of an MCM drug that could be used after a radiation incident to lower the risk of radiation-induced cancer is considered desirable. At present, there is no MCM drug for such use.
A logical strategy to identify MCMs that reduce the risk of cancer might be to first establish and validate biomarkers of radiation injury that relate to increased cancer risk and then evaluate potential efficacy in an appropriate laboratory model. Well-known chemopreventive agents, antioxidants, and nutritional supplements might be the prime or at least initial candidates, since many have already been studied in clinical trials and some are already US FDA approved for indications other than radiation-induced cancers. The testing of entirely novel MCM drugs in humans is problematic (or not possible) for scientific, statistical, ethical and logistical reasons, so that MCM development would likely require the use of FDA’s Animal Rule and early discussion with the FDA in project design.
An alternative or complement to MCM drug treatment might be increased surveillance of radiation-exposed people known to be a high risk for cancer and other radiation-associated health effects. Surveillance is already in use for people known to be at high genetic risk for certain types of cancers (e.g., breast cancer in young women with known mutations in breast cancer genes such as BRCA1, or a positive family history of cancer), and an approach would be to ensure that they maintain appropriate surveillance. Surveillance could also be an option for radiation victims if the doses that they received put them at ‘significant’ additional lifetime cancer risk, which would need to be determined but likely at a dose above 0.75 Gy given the high background rate of cancer.
Since cancers associated with behavioural choices typically entail much greater risk than radiation exposure, sound medical advice for victims might be to modify lifestyle activities to reduce general cancer risk—healthy diet and weight, no smoking, exercise and good general health practices. This could be particularly beneficial if the postulated synergistic interaction between some of these and cancer risks and radiation are validated (e.g. smoking and radiation interaction for lung cancer).
In conclusion, there is ongoing individual and societal concern about radiation-induced cancer from accidental exposures as evidenced by the recent nuclear power plant accident in Japan. Addressing this concern requires credible dose information following an incident, risk assessment and mitigation strategies, a better understanding of the impact of radiation and the competing risks for lifetime cancer in general and effective risk communication with the public. For some subset of those exposed to ‘significant’ doses, increased disease surveillance is logical and effective MCM that can reduce cancer risk after radiation exposure is needed. Progress in understanding the molecular mechanisms of cancer and radiation injury, along with progress in establishing biomarkers of cancer risk; make this an opportune time to consider investing in research and development into mitigating the risks of radiation-induced cancers. While solutions will be challenging, the widespread concern and potential positive impact of even a mildly effective compound or cocktail of compounds (polypharmacy approaches) given the large number of people potentially affected by a radiation exposure incident would make a careful investment in this research area important for both science and society. While minimising and mitigating risks of the occurrence of a nuclear incident are critical, preparation and planning are key to response and societal resilience should one occur. Research and development can benefit cancer care so dual-utility drugs, or drug repurposing makes investment in this area applicable to the general public health.
Disclaimer and Acknowledgment
NCI’s Radiation Research Program supported this work. The views expressed are those of the authors; no endorsement by NCI or other agencies has been given or inferred. This article has been reviewed by FDA and determined not to be consistent with Agency’s views or policies. It reflects only the views and opinions of the author. Authors would like to acknowledge and thank the critical reading and review of the manuscript by Drs Rosemary Roberts and Joanne Holmes, Center for Drug Evaluation and Research, FDA, Silver Spring, MD, USA.
List of abbreviations
- Abbreviation
Term
- ARS
Acute radiation syndrome
- ASPR
Office of the Assistant Secretary for Preparedness and Response, DHHS
- CBMN
Cytokinesis-Block Micronucleus assay
- CDC
Centers for Disease Control and Prevention
- COX-2
Cyclooxygenase 2
- DCA
Dicentric chromosome assay
- DCP
Division of Cancer Prevention, National Cancer Institute, NIH
- DEARE
Delayed effects of acute radiation exposure
- DHHS
Department of Health and Human Services
- FAP
Familial adenomatous polyposis
- FDA
Food and Drug Administration
- HPBL
Human peripheral blood lymphocytes
- hprt
Hypoxanthine-guanine phosphoribosyltransferase
- γ-H2AX
Phosphorylation of the histone, H2AX in response to DNA double strand breaks
- ICRP
International Commission on Radiological Protection
- IAEA
International Atomic Energy Agency
- IEN
Intraepithelial neoplasia
- KI
Potassium iodide
- MCM
Medical countermeasure
- MN
Micronucleus or Micronuclei
- mSV
Milli-sievert
- MTAP
Methylthioadenosine phosphorylase
- NCI
National Cancer Institute
- NIAID
National Institutes of Allergy and Infectious Diseases, NIH
- NIH
National Institute of Health
- NSABP
National Surgical Adjuvant Breast and Bowel Project
- NSAID
Nonsteroidal anti-inflammatory drug
- RCT
Randomised clinical trial
- RDA
Recommended daily allowances
- RERF
Radiation Effects Research Foundation
- TI
Therapeutic Index; ratio of toxicity to efficacy doses
References
- ACS (American Cancer Society) 2013. www.cancer.org/cancer/cancerbasics/lifetime-probability-of-developing-or-dying-from-cancer- (accessed 23 September 2013)
- Adams MJ, Dozier A, Shore RE, Lipshultz SE, Schwartz RG, Constine LS, Pearson TA, Stovall M, Winters P and Fisher SG 2010a. Breast cancer risk 55+ years after irradiation for an enlarged thymus and its implications for early childhood medical irradiation today Cancer Epidemiol. Biomarkers Prev 19 48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MJ, Shore RE, Dozier A, Lipshultz SE, Schwartz RG, Constine LS, Pearson TA, Stovall M, Thevenet-Morrison K and Fisher SG 2010b. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann cohort Radiat. Res 174 753–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN 2001. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer Mutat. Res 475 7–20 [DOI] [PubMed] [Google Scholar]
- Andreassi MG, Barale R, Iozzo P and Picano E 2011. The association of micronucleus frequency with obesity, diabetes and cardiovascular disease Mutagenesis 26 77–83 [DOI] [PubMed] [Google Scholar]
- Andriole GL et al. 2010. Effect of dutasteride on the risk of prostate cancer N. Engl. J. Med 362 1192–202 [DOI] [PubMed] [Google Scholar]
- Armstrong GT, Stovall M and Robison LL 2010. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study Radiat. Res 174 840–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz K, Nowsheen S, Pantelias G, Iliakis G, Gorgoulis VG and Georgakilas AG 2012. Targeting DNA damage and repair: embracing the pharmacological era for successful cancer therapy Pharmacol. Theor 133 334–50 [DOI] [PubMed] [Google Scholar]
- BARDA (Biomedical Advanced Research and Development Authority) 2013. Assistant Secretary for Preparedness and Response, Washington, DC, USA: www.phe.gov/about/barda/Pages/2011barda-stratplan.aspx (accessed 20 March 2014) [Google Scholar]
- Beetstra S, Thomas P, Salisbury C, Turner J and Fenech MF 2005. Folic acid deficiency increases chromosomal instability, chromosome 21 aneuploidy and sensitivity to radiation-induced micronuclei Mutat. Res 578 317–26 [DOI] [PubMed] [Google Scholar]
- Beinke C et al. 2013. Laboratory intercomparison of the dicentric chromosome analysis assay Radiat. Res 180 129–37 [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Klinge DM, Liechty KC, March TH, Kang T, Gilliland FD, Sotnic N, Adamova G, Rusinova G and Telnov V 2004. Plutonium targets the p16 gene for inactivation by promoter hypermethylation in human lung adenocarcinoma Carcinogenesis 25 1063–7 [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM et al. 2006. Celecoxib for the prevention of sporadic colorectal adenomas N. Engl. J. Med 355 873–84 [DOI] [PubMed] [Google Scholar]
- Blot WJ et al. 1993. Nutritional intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population J. Natl Cancer Inst 85 1483–92 [DOI] [PubMed] [Google Scholar]
- Bode AM and Dong Z 2009. Cancer prevention research—then and now Nature Rev. Cancer 9 508–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boice JD Jr 2005. Radiation-induced thyroid cancer—What’s new? J. Natl Cancer Inst 97 703–4 (editorial) [DOI] [PubMed] [Google Scholar]
- Boice JD Jr 2007. An affair of the heart J. Natl Cancer Inst 99 186–7 [DOI] [PubMed] [Google Scholar]
- Boice JD Jr 2012. Radiation epidemiology: a perspective on Fukushima J. Radiol. Prot 32 N33–40 [DOI] [PubMed] [Google Scholar]
- Boice JD Jr, Harvey EB, Blettner M, Stovall M and Flannery JT 1992. Cancer in the contralateral breast after radiotherapy for breast cancer N. Engl. J. Med 326 781–5 [DOI] [PubMed] [Google Scholar]
- Bonassi S, El-Zein R, Bolognesi C and Fenech MF 2011. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: evidence from human studies Mutagenesis 26 93–100 [DOI] [PubMed] [Google Scholar]
- Bonassi S, Neri M, Lando C, Ceppi M, Lin YP, Chang WP, Holland N, Kirsch-Volders M, Zeiger E and Fenech MF 2003. Effect of smoking habit on the frequency of micronuclei in human lymphocytes: results from the Human MicroNucleus project Mutat. Res 543 155–66 [DOI] [PubMed] [Google Scholar]
- Bonassi S et al. 2007. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans Carcinogenesis 28 625–3 [DOI] [PubMed] [Google Scholar]
- Borek C 1981. Cellular transformation by radiation: induction, promotion, and inhibition J. Supramol. Struct. Cell Biochem 16 311–36 [DOI] [PubMed] [Google Scholar]
- Bresalier RS et al. 2005. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial N. Engl. J. Med 352 1092–102 [DOI] [PubMed] [Google Scholar]
- Brink JA and Boice JD Jr 2012. Science to practice: can antioxidant supplements protect against the possible harmful effects of ionizing radiation from medical imaging? Radiology 264 1–2 [DOI] [PubMed] [Google Scholar]
- Brink JD, Imbus C and Woo-Sam J 1980. Physical recovery after severe closed head trauma in children and adolescents J. Pediatr 97 721–7 [DOI] [PubMed] [Google Scholar]
- Bromet EJ 2012. Mental health consequences of the Chernobyl disaster J. Radiol. Prot 32 N71–5 [DOI] [PubMed] [Google Scholar]
- Bromet EJ 2014. Emotional consequences of nuclear power plant disasters Health Phys 106 206–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfiel G 2013. Fukushima: fallout of fear Nature 493 290–3 [DOI] [PubMed] [Google Scholar]
- Bunting SF and Nussenzweig A 2013. End-joining, translocations and cancer Nature Rev. Cancer 13 443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns FJ, Chen S, Xu G, Wu F and Tang MS 2002. The action of a dietary retinoid on gene expression and cancer induction in electron-irradiated rat skin J. Radiat. Res 43 S229–32 [DOI] [PubMed] [Google Scholar]
- Burns FJ, Tang MS, Frenkel K, Nadas A, Wu F, Uddin A and Zhang R 2007. Induction and prevention of carcinogenesis in rat skin exposed to space radiation Radiat. Environ. Biophys 46 195–9 [DOI] [PubMed] [Google Scholar]
- Cardis E et al. 2005. Risk of thyroid cancer after exposure to 131I in childhood J. Natl Cancer Inst 97 724–32 [DOI] [PubMed] [Google Scholar]
- Cavuoto P and Fenech MF 2012. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension Cancer Treat. Rev 38 726–36 [DOI] [PubMed] [Google Scholar]
- CDC (Center for Disease Control and Prevention) 2013. Radiation and Potassium Iodide (Atlanta, GA: CDC; ) http://emergency.cdc.gov/radiation/ki.asp (accessed 20 March 2014) [Google Scholar]
- Claes K et al. 2013. Variant ataxia telangiectasia: clinical and molecular findings and evaluation of radiosensitive phenotypes in a patient and relatives NeuroMolecular Med 15 447–57 [DOI] [PubMed] [Google Scholar]
- Compton PJ, Hooper K and Smith MT 1991. Human somatic mutation assays as biomarkers of carcinogenesis Environ. Health Perspect 94 135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby SC et al. 2010. Radiation-related heart disease: current knowledge and future prospects Int. J. Radiat. Oncol. Biol. Phys 76 656–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer LT, Thornton RH, Hay JL, Balter R, Williamson MJ and St Germain J 2011. Fears, feelings, and facts: interactively communicating benefits and risks of medical radiation with patients Am. J. Roentgenol 196 756–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DW and Bury HP 1961. The significance of Howell–Jolly bodies and giant metamyelocytes in marrow smears J. Clin. Pathol 14 374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS (Department of Health and Human Services) 2002. Guidelines for determining the probability of causation under the energy employees occupational illness and compensation program act of 2000; Final rule 42 C.F.R. pt. 81 Fed. Regist 67 22296–313 (National Archives and Records Administration, Washington, DC, USA: ) [Google Scholar]
- DHHS (Department of Health and Human Services) 2013. 2012 Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) Strategy (Washington, DC: Assistant Secretary for Preparedness and Response; ) www.phe.gov/Preparedness/mcm/phemce/Documents/2012-PHEMCE-Strategy.pdf (accessed on 20 March 2014) [Google Scholar]
- DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, Bader JL, Coleman CN and Weinstock DM 2011. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation Disaster Med. Public Health Prep 5 S32–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman KH, Mayer G and Rodemann HP 2003. Radioprotection of normal tissue to improve radiotherapy: the effect of the Bowman Birk protease inhibitor Curr. Med. Chem. Anti-Cancer Agents 3 360–3 [DOI] [PubMed] [Google Scholar]
- Fairfield KM and Fletcher RH 2002. Vitamins for chronic disease prevention in adults: a scientific review J. Am. Med. Assoc 287 3116–26 [DOI] [PubMed] [Google Scholar]
- Federici C, Botto N, Manfredi S, Rizza A, Del Fiandra M and Andreassi MG 2008. Relation of increased chromosomal damage to future adverse cardiac events in patients with known coronary artery disease Am. J. Cardiol 102 1296–300 [DOI] [PubMed] [Google Scholar]
- Fenech MF 1999. Micronucleus frequency in human lymphocytes is related to plasma vitamin B12 and homocysteine Mutat. Res 428 299–304 [DOI] [PubMed] [Google Scholar]
- Fenech MF 2005. The genome health clinic and genome health nutrigenomics concepts: diagnosis and nutritional treatment of genome and epigenome damage on an individual basis Mutagenesis 20 255–69 [DOI] [PubMed] [Google Scholar]
- Fenech MF 2010a. Dietary reference values of individual micronutrients and nutriomes for genome damage prevention: current status and a road map to the future Am. J. Clin. Nutr 91 1438S–54S [DOI] [PubMed] [Google Scholar]
- Fenech MF 2010b. The lymphocyte cytokinesis-block micronucleus cytome assay and its application in radiation biodosimetry Health Phys 98 234–43 [DOI] [PubMed] [Google Scholar]
- Fenech MF 2013. A public health genomics approach to improving well-being based on DNA damage prevention using nutritional, life-style, environmental and psycho-social strategies ACNEM J 32 9–16 [Google Scholar]
- Fenech MF and Bonassi S 2011. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes Mutagenesis 26 43–9 [DOI] [PubMed] [Google Scholar]
- Fenech M and Morley AA 1985. The effect of donor age on spontaneous and induced micronuclei Mutat. Res 148 99–105 [DOI] [PubMed] [Google Scholar]
- Fenech MF, Perepetskaya G and Mikhalevich L 1997. A more comprehensive application of the micronucleus technique for biomonitoring of genetic damage rates in human populations— experiences from the Chernobyl catastrophe Environ. Mol. Mutagen 30 112–8 [PubMed] [Google Scholar]
- Ferguson LR and Fenech MF 2012. Vitamin and minerals that influence genome integrity, and exposure/intake levels associated with DNA damage prevention Mutat. Res 733 1–3 [DOI] [PubMed] [Google Scholar]
- Fischman HK and Kelly DD 1999. Chromosomes and stress Int. J. Neurosci 99 201–19 [DOI] [PubMed] [Google Scholar]
- Fischman HK, Pero RW and Kelly DD 1996. Psychogenic stress induces chromosomal and DNA damage Int. J. Neurosci 84 219–27 [DOI] [PubMed] [Google Scholar]
- Fisher B et al. 1998. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study J. Natl Cancer Inst 90 1371–88 [DOI] [PubMed] [Google Scholar]
- Fletcher RH and Fairfield KM 2002. Vitamins for chronic disease prevention in adults: clinical applications J. Am. Med. Assoc 287 3127–9 [DOI] [PubMed] [Google Scholar]
- Fortmann SP, Burda BU, Senger CA, Lin JS, Beil TL, O’Connor E and Whitlock EP 2013. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: a systematic evidence review for the US preventive services task force Evidence Synthesis vol 108 (Rockville, MD: Agency for Healthcare Research and Quality; ) [PubMed] [Google Scholar]
- Freidlin B, Mcshane LM and Korn EL 2010. Randomized clinical trials with biomarkers: design issues J. Natl Cancer Inst 102 152–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DL, Dekker GA, Hague WM, Khong TY and Fenech MF 2010. Increased lymphocyte micronucleus frequency in early pregnancy is associated prospectively with pre-eclampsia and/or intrauterine growth restriction Mutagenesis 25 489–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio L et al. 2008. Carotenoids and the risk of developing lung cancer: a systematic review Am. J. Clin. Nutr 88 372–83 [DOI] [PubMed] [Google Scholar]
- Gilbert ES et al. 2003. Lung cancer after treatment for Hodgkin’s disease: focus on radiation effects J. Radiat. Res 159 161–73 [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Verduzco D and Gatenby RA 2012. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work Nature Rev. Cancer 12 487–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González AJ et al. 2013. Radiological protection issues arising during and after the Fukushima nuclear reactor accident J. Radiol. Prot 33 497–571 [DOI] [PubMed] [Google Scholar]
- Goss PE et al. 2011. Exemestane for breast-cancer prevention in postmenopausal women N. Engl. J. Med 364 2381–91 [DOI] [PubMed] [Google Scholar]
- Grace MB et al. 2011. The US government’s medical countermeasure portfolio management for nuclear and radiological emergencies: synergy from interagency cooperation Health Phys 101 238–47 [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Carnes BA, Grahn D and Sigdestad CP 1991. Protection against late effects of radiation by S-2-(3-aminopropylamino)-ethylphosphorothioic acid Cancer Res 51 4125–30 [PubMed] [Google Scholar]
- Grdina DJ, Kataoka Y, Basic I and Perrin J 1992. The radioprotector WR-2721 reduces neutron-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in mouse splenocytes when administered prior to or following irradiation Carcinogenesis 13 811–4 [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Kataoka Y and Murley JS 2000. Amifostine: mechanisms of action underlying cytoprotection and chemoprevention Drug Metabol. Drug Interact 16 237–79 [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Nagy B, Hill CK, Wells RL and Peraino C 1985. The radioprotector WR1065 reduces radiation-induced mutations at the hypoxanthine-guanine phosphoribosyl transferase locus in V79 cells Carcinogenesis 6 929–31 [DOI] [PubMed] [Google Scholar]
- Greenwald P 2002. Cancer chemoprevention BMJ 324 714–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guallar E, Stranges S, Mulrow C, Appel LJ and Miller ER III 2013. Enough is enough: stop wasting money on vitamin and mineral supplements Ann. Int. Med 159 850–1 [DOI] [PubMed] [Google Scholar]
- Hageman GJ and Stierum RH 2001. Niacin, poly(ADP-ribose) polymerase-1 and genomic stability Mutat. Res 475 45–56 [DOI] [PubMed] [Google Scholar]
- Hamburg MA and Collins FS 2010. The path to personalized medicine N. Engl. J. Med 363 301–4 [DOI] [PubMed] [Google Scholar]
- Hamilton RJ, Kahwati LC and Kinsinger LS 2010. Knowledge and use of finasteride for the prevention of prostate cancer Cancer Epidemiol. Biomarkers Prev 19 2164–71 [DOI] [PubMed] [Google Scholar]
- Harisiadis L, Miller RC, Hall EJ and Borek C 1978. A vitamin A analogue inhibits radiation-induced oncogenic transformation Nature 274 486–7 [DOI] [PubMed] [Google Scholar]
- Heddle JA, Fenech MF, Hayashi M and MacGregor JT 2011. Reflections on the development of micronucleus assays Mutagenesis 26 3–10 [DOI] [PubMed] [Google Scholar]
- Howell WH 1890–1891. The life-history of formed elements of blood, especially the red blood corpuscles J. Morphol 4 57–116 [Google Scholar]
- Huang HY et al. 2006. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference Ann. Intern. Med 145 372–85 [DOI] [PubMed] [Google Scholar]
- IAEA (International Atomic Energy Agency) 2011. Cytogenetic Dosimetry: Applications in Preparedness and Response to Radiation Emergencies (Vienna: IAEA Press; ) [Google Scholar]
- ICRP (International Commission on Radiological Protection) 2007. The 2007 Recommendations of the International Commission on Radiological Protection ICRP Publication 103; Ann. ICRP 37 (2–4) [DOI] [PubMed] [Google Scholar]
- Inano H, Onoda M, Inafuku N, Kubota M, Kamada Y, Osawa T, Kobayashi H and Wakabayashi K 1999. Chemoprevention by curcumin during the promotion stage of tumorigenesis of mammary gland in rats irradiated with gamma-rays Carcinogenesis 20 1011–8 [DOI] [PubMed] [Google Scholar]
- Ingel F, Platonova V and Katosova L 2001. Human emotional stress, dioxin blood content and genetic damage in Chapaevsk town Chemosphere 43 989–98 [DOI] [PubMed] [Google Scholar]
- ISO (International Standardization Organization) 2004. Radiation Protection—Performance Criteria for Service Laboratories Performing Biological Dosimetry by Cytogenetics, ISO 19238 (Geneva: ISO; ) [Google Scholar]
- ISO (International Standardization Organization) 2008. Radiation Protection—Performance Criteria for Laboratories Performing Cytogenetic Triage for Assessment of Mass Casualties in Radiological or Nuclear Emergencies —General Principles and Application to Dicentric Assay ISO 21243 (Geneva: ISO; ) [Google Scholar]
- Jacob P et al. 1999. Childhood exposure due to the Chernobyl accident and thyroid cancer risk in contaminated areas of Belarus and Russia Br. J. Cancer 80 1461–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Ruhm W, Walsh L, Blettner M, Hammer G and Zeeb H 2009. Is cancer risk of radiation workers larger than expected? Occup. Environ. Med 66 789–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo PA, Geuting V and Lobrich M 2011. The role of homologous recombination in radiation-induced double-strand break repair Radiother. Oncol 101 7–12 [DOI] [PubMed] [Google Scholar]
- Jolly JMJ 1905. Sur la formation des globules rouges des mammifères C. R. Soc. Biol. (Paris) 58 528–31 [Google Scholar]
- Jordan VC, Obiorah I, Fan P, Kim HR, Ariazi E, Cunliffe H and Brauch H 2011. The St. Gallen Prize Lecture 2011: evolution of long-term adjuvant anti-hormone therapy: consequences and opportunities Breast 20 S1–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Marder SN and Brown MB 1951. Adrenal cortical function and radiation-induced lymphoid tumors of mice Cancer Res 11 629–33 [PubMed] [Google Scholar]
- Kelloff GJ et al. 2006. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer—a plan to mover forward Clin. Cancer Res 12 3661–97 [DOI] [PubMed] [Google Scholar]
- Kennedy AR 1998a. The Bowman–Birk inhibitor from soybeans as an anticarcinogenic agent Am. J. Clin. Nutr 68 1406S–12S [DOI] [PubMed] [Google Scholar]
- Kennedy AR 1998b. Chemopreventive agents: protease inhibitors Pharmacol. Theor 78 167–209 [DOI] [PubMed] [Google Scholar]
- Kennedy AR 2006. The status of human trials utilizing Bowman–Birk inhibitor concentrate from Soybeans Soy in Health and Disease Prevention ed Sugano M (Boca Raton, FL: CRC Press, Taylor and Francis Group; ) pp 207–23 [Google Scholar]
- Kennedy AR 2009. Factors that modify radiation-induced carcinogenesis Health Phys 97 433–45 [DOI] [PubMed] [Google Scholar]
- Kennedy AR, Davis JG, Carlton W and Ware JH 2008. Effects of dietary antioxidant supplementation on the development of malignant lymphoma and other neoplastic lesions in mice exposed to proton or iron ion radiation J. Radiat. Res 169 615–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AR and Wan XS 2011. Countermeasures for space radiation induced adverse biologic effects Adv. Space Res 48 1460–79 [Google Scholar]
- Kennedy AR, Ware JH, Carlton W and Davis JG 2011. Suppression of the later stages of radiation induced carcinogenesis by antioxidant dietary formulations J. Radiat. Res 176 62–70 [DOI] [PubMed] [Google Scholar]
- Kitareewan S 2004. The Retinoids and Cancer Chemoprevention (Totowa, NJ: Humana Press; ) [Google Scholar]
- Klein EA et al. 2011. Vitamin E and the risk of prostate cancer: the selenium and vitamin E Cancer Prevention Trial (SELECT) J. Am. Med. Assoc 306 1549–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebel AR et al. 2011. Allocation of scarce resources after a nuclear detonation: setting the context Disaster Med. Public Health Prep 5 S20–31 [DOI] [PubMed] [Google Scholar]
- Komninou D, Leutzinger Y, Reddy BS and Richie JP Jr 2006. Methionine restriction inhibits colon carcinogenesis Nutr. Cancer 54 202–8 [DOI] [PubMed] [Google Scholar]
- Kuefner MA, Brand M, Ehrlich J, Braga L, Uder M and Semelka RC 2012. Effect of antioxidants on x-ray-induced gamma-H2AX foci in human blood lymphocytes: preliminary observations Radiology 264 59–67 [DOI] [PubMed] [Google Scholar]
- Li JY et al. 1993. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia J. Natl Cancer Inst 85 1492–8 [DOI] [PubMed] [Google Scholar]
- Liu SC, Murley JS, Woloschak G and Grdina DJ 1997. Repression of c-myc gene expression by thiol and disulfide forms of the cytoprotector amifostine Carcinogenesis 18 2457–9 [DOI] [PubMed] [Google Scholar]
- Lloyd DC 1997. Chromosomal analysis to assess radiation dose Stem Cells 82 107–14 [DOI] [PubMed] [Google Scholar]
- Logothetis CJ and Schellhammer P F 2008. High-grade prostate cancer and the prostate cancer prevention trial Cancer Prev. Res. (Phila) 1 151–2 [DOI] [PubMed] [Google Scholar]
- Lubin JH et al. 2005. Adjusting lung cancer risks for temporal and spatial variations in radon concentration in dwellings in Gansu Province, China J. Radiat. Res 163 571–9 [DOI] [PubMed] [Google Scholar]
- Lucia MS et al. 2007. Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial J. Natl Cancer Inst 99 1375–83 [DOI] [PubMed] [Google Scholar]
- McCormick F 1999. Signalling networks that cause cancer Trends Cell Biol 9 M53–6 [PubMed] [Google Scholar]
- McCormick DL, Johnson WD, Bosland MC, Lubet RA and Steele VE 2007. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman–Birk inhibitor Nutr. Cancer 57 184–93 [DOI] [PubMed] [Google Scholar]
- McNamee JP, Flegal FN, Greene HB, Marro L and Wilkins RC 2009. Validation of the cytokinesis-block micronucleus (CBMN) assay for use as a triage biological dosimetry tool Radiat. Prot. Dosim 135 232–42 [DOI] [PubMed] [Google Scholar]
- Mettler FA Jr, Brenner D, Coleman CN, Kaminski JM, Kennedy AR and Wagner LK 2011. Can radiation risks to patients be reduced without reducing radiation exposure? The status of chemical radioprotectants Am. J. Roentgenol 196 616–8 [DOI] [PubMed] [Google Scholar]
- Meyskens FL Jr, Curt GA, Brenner DE, Gordon G, Herberman RB, Finn O, Kelloff GJ, Khleif SN, Sigman CC and Szabo E 2011. Regulatory approval of cancer risk-reducing (chemopreventive) drugs: moving what we have learned into the clinic Cancer Prev. Res. (Phila) 4 311–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL Jr and Gerner EW 2011. Back to the future: mechanism-based, mutation-specific combination chemoprevention with a synthetic lethality approach Cancer Prev. Res. (Phila) 4 628–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyskens FL Jr and McLaren CE 2010. Chemoprevention, risk reduction, therapeutic prevention, or preventive therapy? J. Natl Cancer Inst 102 1815–7 [DOI] [PubMed] [Google Scholar]
- Meyskens FL Jr et al. 2008. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial Cancer Prev. Res. (Phila) 1 32–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L, Coppedè F, Fenech M and Thomas P 2011. Association of micronucleus frequency with neurodegenerative diseases Mutagenesis 26 85–92 [DOI] [PubMed] [Google Scholar]
- Milas L, Huner N, Stephens LC and Peters LJ 1984. Inhibition of radiation carcinogenesis in mice by S-2-(3-aminopropylamino-)ehtylphosphorothioic acid Cancer Res 44 5567–9 [PubMed] [Google Scholar]
- Murrain-Hill P et al. 2011. Medical response to a nuclear detonation: creating a playbook for state and local planners and responders Disaster Med. Public Health Prep 5 S89–97 [DOI] [PubMed] [Google Scholar]
- Mursu J, Robien K, Harnack LJ, Park K and Jacobs DR Jr 2011. Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study Arch. Intern. Med 171 1625–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NA/NRC (National Academies/National Research Council) 1982. Diet, Nutrition and Cancer (Washington, DC: National Academies Press; ) [Google Scholar]
- NA/NRC (National Academies/National Research Council) 2006. Health Risks from Exposure to Low Levels of Ionizing Radiation, BEIR VII Phase 2 (Washington, DC: National Academies Press; ) [PubMed] [Google Scholar]
- Nelson RL 1987. Dietary minerals and colon carcinogenesis Anticancer Res 7 259–69 [PubMed] [Google Scholar]
- Newberne PM and Rogers AE 1985. The role of nutrients in cancer causation Princess Takamatsu Symp 16 205–22 [PubMed] [Google Scholar]
- Normile D 2011. Tohoku–Oki earthquake. Fukushima revives the low-dose debate Science 332 908–10 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A and Zimmerman JA 1993. Low methionine ingestion by rats extends life span J. Nutr 123 269–74 [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy JA et al. 2002. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development Clin. Cancer Res 8 314–46 [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H and Kodama K 2012. Studies of the mortality of atomic bomb survivors Report 14, 1950–2003: an overview of cancer and noncancer diseases J. Radiat. Res 177 229–43 [DOI] [PubMed] [Google Scholar]
- Prasanna PG, Moroni M and Pellmar TC 2010. Triage dose assessment for partial-body exposure: dicentric analysis Health Phys 98 244–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnasinghe D et al. 2000. Serum carotenoids are associated with increased lung cancer risk among alcohol drinkers, but not among non drinkers in a cohort of tin miners Alcohol Alcohol 35 355–60 [DOI] [PubMed] [Google Scholar]
- Redman MW, Tangen CM, Goodman PJ, Lucia MS, Coltman CA Jr and Thompson IM 2008. Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach Cancer Prev. Res. (Phila) 1 174–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relience R, Fischer E and Schiestl RH 2004. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in Atm-deficient mice Cancer Res 64 5148–53 [DOI] [PubMed] [Google Scholar]
- Relience R and Schiestl RH 2006. Antioxidant N-acetyl cysteine reduces incidence and multiplicity of lymphoma in Atm deficient mice DNA Repair 5 852–9 [DOI] [PubMed] [Google Scholar]
- Richie JP Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N and Zimmerman JA 1994. Methionine restriction increases blood glutathione and longevity in F344 rats FASEB J 8 1302–7 [DOI] [PubMed] [Google Scholar]
- Rizzo JD et al. 2009. Solid cancers after allogeneic hematopoietic cell transplantation Blood 113 1175–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romm H et al. 2013. Laboratory intercomparison of the cytokinesis-block micronucleus assay J. Radiat. Res 180 120–8 [DOI] [PubMed] [Google Scholar]
- Romm H et al. 2011. Biological dosimetry by the triage dicentric chromosome assay: potential implications for treatment of acute radiation syndrome in radiological mass casualties J. Radiat. Res 175 397–404 [DOI] [PubMed] [Google Scholar]
- Ropeik D 2013. Fear vs. Radiation: the mismatch NY Times, October 1, 2013 www.nytimes.com/2013/10/22/opinion/fear-vs-radiation-the-mismatch.html?r=0 (accessed 28 December 2013) [Google Scholar]
- Rothfuss A, Schütz P, Bochum S, Volm T, Eberhardt E, Kreienberg R, Vogel W and Speit G 2000. Induced micronucleus frequencies in peripheral lymphocytes as a screening test for carriers of a BRCA1 mutation in breast cancer families Cancer Res 60 390–4 [PubMed] [Google Scholar]
- Rothkamm K et al. 2013. Comparison of established and emerging biodosimetry assays Radiat. Res 180 111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP and Meade TW 2011. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials Lancet 377 31–41 [DOI] [PubMed] [Google Scholar]
- Scott D 2000. Chromosomal radiosensitivity, cancer predisposition and response to radiotherapy trahlenther. Onkol 176 229–34 [DOI] [PubMed] [Google Scholar]
- Shakhtarin VV, Tsyb AF, Stepanenko VF, Orlov MY, Kopecky KJ and Davis S 2003. Iodine deficiency, radiation dose, and the risk of thyroid cancer among children and adolescents in the Bryansk region of Russia following the Chernobyl power station accident Int. J. Epidemiol 32 584–91 [DOI] [PubMed] [Google Scholar]
- Sharif R, Thomas P, Zalewski P and Fenech MF 2012. The role of zinc in genomic stability Mutat. Res 733 111–21 [DOI] [PubMed] [Google Scholar]
- Sharif R, Thomas P, Zalewski P, Graham RD and Fenech M 2011. The effect of zinc sulphate and zinc carnosine on genome stability and cytotoxicity in the WIL2-NS human lymphoblastoid cell line Mutat. Res 720 22–33 [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D and Jemal A 2012. Cancer statistics, 2012 CA Cancer J. Clin 62 10–29 [DOI] [PubMed] [Google Scholar]
- Stovall M et al. 2008. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study Int. J. Radiat. Oncol. Biol. Phys 72 1021–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Prasanna PG, Grace MB, Wathen L, Wallace RL, Koerner JF and Coleman CN 2013. Assessment of biodosimetry methods for a mass-casualty radiological incident: medical response and management considerations Health Phys 105 540–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoret MR, Ning YM, Zhang JJ, Justice R, Keegan P and Pazdur R 2011. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention N. Engl. J. Med 365 97–9 [DOI] [PubMed] [Google Scholar]
- Thomas P, Wu J, Dhillon V and Fenech MF 2011. Effect of dietary intervention on human micronucleus frequency in lymphocytes and buccal cells Mutagenesis 26 69–76 [DOI] [PubMed] [Google Scholar]
- Thompson CA 2003. Finasteride may prevent prostate cancer Am. J. Health Syst. Pharm 60 1511–5 [DOI] [PubMed] [Google Scholar]
- Thompson DE et al. 1994. Cancer incidence in atomic bomb survivors. Part II: solid tumors, 1958–1987 J. Radiat. Res 137 17–67 [PubMed] [Google Scholar]
- Travis LB et al. 2003. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease J. Am. Med. Assoc 290 465–75 [DOI] [PubMed] [Google Scholar]
- Troll W, Wiesner R, Shellabarger CJ, Holtzman S and Stone JP 1980. Soybean diet lowers breast tumor incidence in irradiated rats Carcinogenesis 1 469–72 [DOI] [PubMed] [Google Scholar]
- Tucker JD, Vadapalli M, Joiner MC, Ceppi M, Fenech MF and Bonassi S 2013. Estimating the lowest detectable dose of ionizing radiation by the cytokinesis-block micronucleus assay J. Radiat. Res 180 284–91 [DOI] [PubMed] [Google Scholar]
- US EPA (US Environmental Protection Agency) 2013. PAG Manual, Protective Action Guides and Planning Guidance for Radiological Incidents www.epa.gov/radiation/docs/er/pag-manual-interim-public-comment-4-2-2013.pdf (accessed 20 March 2014)
- Vogel VG et al. 2006. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial J. Am. Med. Assoc 295 2727–41 [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr and Kinzler KW 2013. Cancer genome landscapes Science 339 1546–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vral A, Thierens H and De Ridder L 1996. Micronucleus induction by 60Co gamma-rays and fast neutrons in ataxia telangiectasia lymphocytes Int. J. Radiat. Biol 70 171–6 [DOI] [PubMed] [Google Scholar]
- Wattenberg LW 1985. Chemoprevention of cancer Cancer Res 45 1–8 [PubMed] [Google Scholar]
- Wattenberg LW 1990. Inhibition of carcinogenesis by minor anutrient constituents of the diet Proc. Nutr. Soc 49 173–83 [DOI] [PubMed] [Google Scholar]
- Wattenberg LW 1992. Inhibition of carcinogenesis by minor dietary constituents Cancer Res 52 2085s–91s [PubMed] [Google Scholar]
- Wattenberg LW, Hanley AB, Barany G, Sparnins VL, Lam LK and Fenwick GR 1985. Inhibition of carcinogenesis by some minor dietary constituents Princess Takamatsu Symp 16 193–203 [PubMed] [Google Scholar]
- Weiss JF and Landauer MR 2009. History and development of radiation-protective agents Int. J. Radiat. Biol 85 539–73 [DOI] [PubMed] [Google Scholar]
- Wilkins RC, Romm H, Kao TC, Awa AA, Yoshida MA, Livingston GK, Jenkins MS, Oestreicher U, Pellmar TC and Prasanna PG 2008. Interlaboratory comparison of the dicentric chromosome assay for radiation biodosimetry in mass casualty events Radiat. Res 169 551–60 [DOI] [PubMed] [Google Scholar]
- Wilkins RC, Romm H, Oestreicher U, Marro L, Yoshida MA, Suto Y and Prasanna PG 2011. Biological dosimetry by the triage dicentric chromosome assay—further validation of international networking Radiat. Meas 46 923–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Nakata A, Yoshida MA, Shimada Y, Oghiso Y and Poncy JL 2010. Implication of p16 inactivation in tumorigenic activity of respiratory epithelial cell lines and adenocarcinoma cell line established from plutonium-induced lung tumor in rat In Vitro Cell Dev. Biol. Anim 46 477–86 [DOI] [PubMed] [Google Scholar]
- York TP, Brumelle J, Juusola J, Kendler KS, Eaves LJ, Amstadter AB, Aggen SH, Jones KH, Ferreira-Gonzalez A and Jackson-Cook C 2013. Increased frequency of micronuclei in adults with a history of childhood sexual abuse: a discordant monozygotic twin study PLoS One 8 e55337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotti-Martelli L, Migliore L, Panasiuk G and Barale R 1999. Micronucleus frequency in Gomel (Belarus) children affected and not affected by thyroid cancer Mutat. Res 440 35–43 [DOI] [PubMed] [Google Scholar]


