Abstract
Cases of chronic neutrophilic leukemia (CNL) are extremely rare and easily missed. The World Health Organization revised criteria in 2016 to include evaluation for CSF3R somatic mutations. In this article, we discuss an 84-year-old man who initially presented with leukocytosis, macrocytosis, and mild splenomegaly. The bone marrow biopsy and aspirate revealed hypercellularity (90%) and was comprised primarily of mature neutrophils. There was no genetic rearrangement of PDGFRA, PDGFRB, FGRF1, BCR-ABL, or JAK2. A skin biopsy of a hyperpigmented area on the palm of the patient’s hand proved to be a neutrophilic infiltrate. This patient experience is presented to highlight several issues: the rarity of CNL, the role of molecular testing to confirm diagnosis, and the aggressive nature of this unusual myeloproliferative neoplasm.
CASE STUDY
An 84-year-old man was initially referred to hematology in 2017 for an inpatient consultation for worsening leukocytosis in the setting of a clinically identified cellulitis. The cellulitis improved with antibiotics and his complete blood count (CBC) at the time revealed an elevated white blood cell (WBC) count of 15.4 × 109/L trending down from 17.8 × 109/L, a mean corpuscular volume (MCV) of 91 fL, hemoglobin (Hgb) of 12.1 g/dL, and a platelet count of 321,000/μL. During his hospitalization, a CT of his abdomen revealed a mildly enlarged spleen measuring 14.8 × 9.3 × 12 cm. Leukocytosis at the time of initial consultation was attributed to cellulitis given that it was trending down, and the consultant recommended periodic laboratory surveillance, with a return to the hematology clinic should the leukocytosis worsen.
One year later, the patient’s primary care provider referred him to the hematology clinic for persistent leukocytosis. Laboratory values revealed a WBC count of 80 × 109/L, with an absolute neutrophil count of 66.8 (66,800 mm3) and Hgb of 14.5 g/dL. Flow cytometry of peripheral blood noted granulocytes with decreased CD10 expression and increased CD14 expression with no malignant population identified. Fluorescence in situ hybridization (FISH) was negative for Philadelphia chromosome translocation, and BCR-ABL polymerase chain reaction was negative.
Despite his elderly status and hospitalization in the previous year, he was essentially healthy with no current, regular medications. He was also the primary caregiver for his wife, who had moderate to severe dementia. His initial physical exam was notable for a ruddy complexion, crackles in his left lower lobe, and a palpable spleen 4 cm below the costal margin.
Further labs were ordered, including JAK2 V617F and peripheral smear, as well as a plain film of the chest to evaluate the crackles and an ultrasound to establish stability of splenomegaly. His creatinine was 0.8 mg/dL with normal liver functions and a lactate dehydrogenase of 165 U/L. A bone marrow biopsy was performed.
Chronic neutrophilic leukemia (CNL) is one of the myeloproliferative disorders characterized by bone marrow hypercellularity in one or more cell lines, often with defining somatic mutations. These mutations include Janus kinase mutation (JAK2 V617F), calreticulin (CALR), ASXL1, TP53, TET2, and MPL (Lundberg et al., 2014; Tefferi et al., 2009). Initial evaluation for myeloproliferative neoplasms (MPN) includes bone marrow biopsy/aspirate with cytogenetics, BCR-ABL mutation analysis to look for typical chronic myelogenous leukemia, and JAK2 V617F that can be found in polycythemia vera, essential thrombocytosis, and myelofibrosis. Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative neoplasm identified by sustained neutrophilia and the absence of other myeloproliferative disorder–defining mutations. A recent review by Szuber and Tefferi noted that although over 200 instances of CNL have been recorded, the cases that meet the World Health Organization (WHO) diagnostic criteria are closer to 150 (Bain & Ahmad, 2015; Szuber et al., 2018; Szuber & Tefferi, 2018).
The discovery of the colony-stimulating factor 3 receptor (CSF3R) mutation in patients with CNL presented a critical diagnostic tool for the identification and differentiation BCR-ABL–negative MPNs, specifically CNL. The WHO revised their diagnostic criteria for CNL in 2016 (Arber et al., 2016; Table 1). Historically, CNL had been a clinical diagnosis whose existence had been an ongoing debate. The CSF3R mutation analysis for this patient was performed with next-generation sequencing that is commercially available through several laboratories.
Table 1. 2016 World Health Organization Revised Diagnostic Criteria for Chronic Neutrophilic Leukemia.
| 1. Peripheral blood WBC ≥ 25 × 109/L |
| • Segmented neutrophils plus band forms > 80% of WBC |
| • Neutrophil precursors < 10% of WBC |
| • Rare myeloblasts |
| • Monocyte < 1 × 109/L |
| 2. Hypercellular bone marrow |
| • Neutrophil granulocytes increased (in % and number) |
| • Neutrophil with normal maturation |
| • Myeloblasts < 5% of nucleated cells |
| 3. Not meeting WHO criteria for BCR-ABL, CML, PV, ET, or PMF |
| 4. No rearrangement of PDGFRA, PDGFRB, or FGFR1, or PCM1-JAK2 |
| 5. Presence of CSF3RT6181 or other activating CSF3R mutation, OR |
| In the absence of a CSF3R mutation, persistent neutrophilia (at least 3 months), splenomegaly, and no identifiable cause of reactive neutrophilia including absence of a plasma cell neoplasm or, if present, demonstration of clonality of myeloid cells by cytogenetic or molecular studies. |
Note. WBC = white blood cells; CML = chronic myeloid leukemia; PV = polycythemia vera; ET = essential thrombocythemia; PMF = primary myelofibrosis. Adapted from Szuber and Tefferi (2018).
Chronic neutrophilic leukemia has a poor prognosis, with a mean survival of approximately 2 years (Böhm & Schaefer, 2002; Cui et al., 2017). There are currently no standard-of-care guidelines for CNL; anecdotal data have suggested that some patients may have a response to hydroxyurea (Szuber et al., 2018), ruxolitinib (Jakafi; Maxson et al., 2013), imatinib (Elliott et al., 2005), interferon-α (Yassin et al., 2015), and stem cell transplantation (Lee et al., 2015). Cases of CNL in the literature demonstrated a higher mean age (62.5 years), shorter survival times compared with other myeloproliferative neoplasms, and an increased ratio of males diagnosed compared with females.
DIAGNOSTIC ASSESSMENT
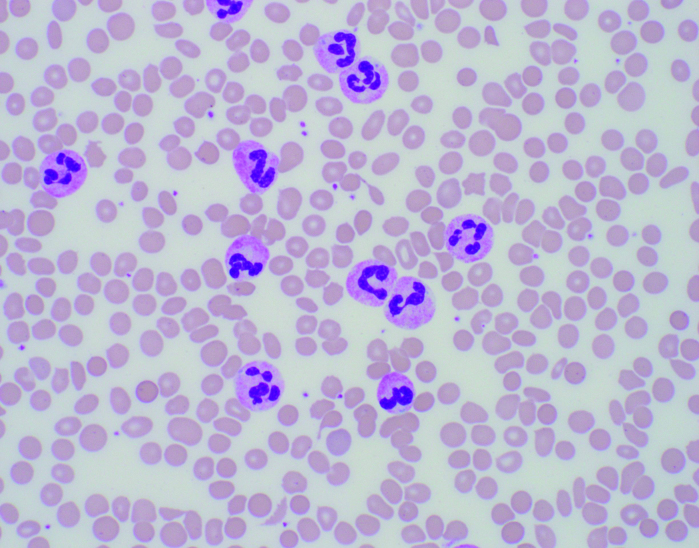
An ultrasound of the patient’s spleen measured 16.0 × 10.0 × 10.6 cm, slightly larger compared with the year prior (although prior imaging was CT). Plain film of his chest revealed no abnormalities. The peripheral smear revealed a predominance of mature neutrophils with rare blasts (< 1%), red blood cells and occasional teardrops, elliptocytes, and schistocytes (Figure 1A ).
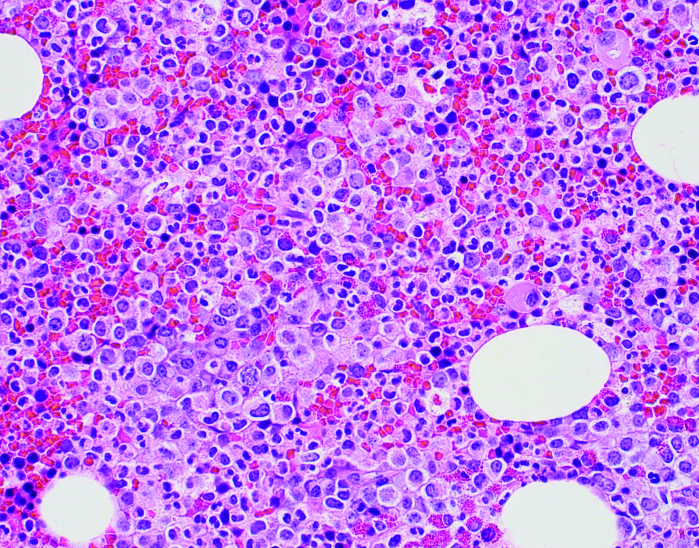
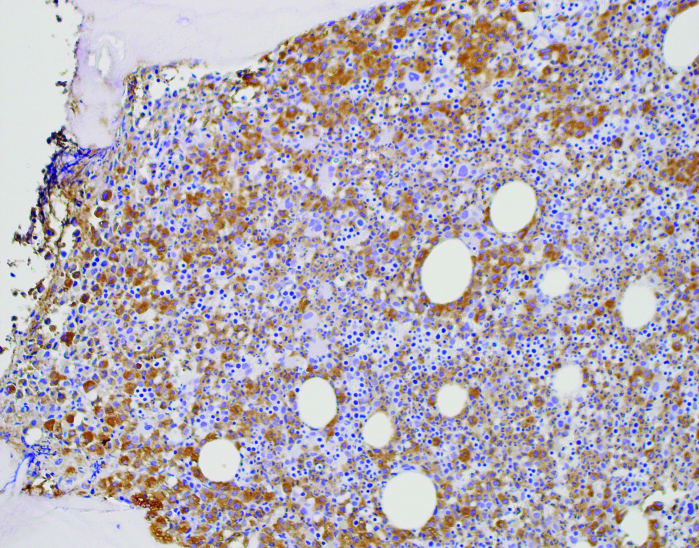
Figure 1.
(A) Peripheral blood smear. Note the predominance of mature neutrophils, the overall morphology, lobation, and how cytoplasmic granules appear “normal.” (B) Bone marrow biopsy at 200x, H & E. Demonstrates increased cellularity with mixed population of cells, increased myeloid precursors. (C) Bone marrow biopsy at 100x with myeloperoxidase staining. Same biopsy in (B) after staining confirms most cells are myeloid (brown); remaining population consists of erythroid cells and a few megakaryocytes.
The bone marrow biopsy was hypercellular (90%), with myeloid cells markedly increased with left-shift maturation (Figures 1B and 1C). There was no evidence of increased reticulin fibrosis, no increase in blasts, and monocytes were decreased. Cytogenetics were normal, and no abnormal rearrangement of PDGFRA, PDGFRB, FGRF, or JAK2 V617F was identified. Because of the presentation, including mature neutrophilia with the bone marrow biopsy and aspirate results, CNL was suspected, and a CSF3R analysis was sent but took several weeks for a result to be returned.
INTERVENTIONS
Our patient was initiated on 2 g of hydroxyurea daily, and initially his WBC count decreased to 70 × 109/L. Although there is debate regarding prophylaxis for tumor lysis syndrome in hematologic malignancies, specifically chronic leukemia, generally, WBC counts > 50 × 109/L are regarded as intermediate risk (Belay, Yirdaw, & Enawgaw, 2017). Consequently, allopurinol was initiated with daily hydration on an outpatient basis to accommodate the patient’s desire to remain at home with his wife.
FOLLOW-UP AND OUTCOMES
Two weeks after the initial consultation, and 1 week after the initiation of hydroxyurea with allopurinol, the patient went to the emergency department with diarrhea and one episode of vomiting. Laboratory results at that time revealed normal creatinine, normal liver function tests, and a persistently elevated WBC of 85 × 109/L. Given his persistently normal uric acid, lactate dehydrogenase, and creatinine levels, the allopurinol was discontinued. He required temporary pressor support in the intensive care unit with an echocardiogram that revealed an ejection fraction of 40% to 45%, but no pericardial effusion, evidence of sepsis, or a gastrointestinal infection. His prior echocardiogram revealed an ejection fraction of 80%. He received 5 days of antibiotics with mildly elevated troponins.
During his hospitalization course, the patient developed a hyperpigmented, tender area on the ventral aspect of his hand (Figure 2). A skin biopsy did not identify a thromboembolic process; the amount of leukocytoclasia was small, with most neutrophils appearing intact and mature. Pathology concluded that it was a neutrophilic reaction pattern consistent with Sweet syndrome and pyoderma gangrenosum. (Fungal and bacterial stains were negative.)
Figure 2.
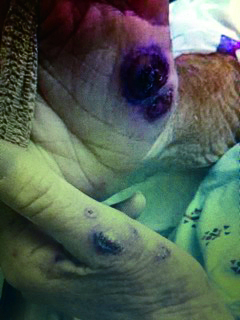
Hyperpigmented nodules on patient’s hands; biopsy was suggestive of Sweet syndrome.
Sweet syndrome is an uncommon skin presentation of erythematous plaques or nodules infiltrated by neutrophils, often occurring with hematologic malignancies (Raza, Kirkland, Patel, Shortridge, & Freter, 2013). Tissue infiltration by neutrophils is felt to be the predominant process of organomegaly with skin findings that may include Sweet syndrome and pyoderma gangrenosum. During his hospitalization, the patient’s overall health and function declined rapidly. As a result of his rapid decline, the patient felt that hospice care would best serve his goals of care, and he died a week after discharge.
DISCUSSION
The patient initially presented with mature leukocytosis without BCR-ABL or an abnormal number of blasts. The bone marrow biopsy and aspirate were suggestive of CNL; however, the CSF3R mutation results took almost 3 weeks to confirm the diagnosis. In the interim, his overall health declined rapidly. He developed a nodular neutrophil infiltrated dermatosis consistent with Sweet syndrome and refractory hyperleukocytosis minimally responsive to hydroxyurea. The patient and his clinical course represent a somewhat common outcome for elderly patients; the most common causes of death in patients with CNL have been intracranial hemorrhage, disease progression including blastic transformation, or as a result of treatment (Böhm & Schaefer, 2002; Elliott et al., 2005; Pardanani et al., 2013).
Next-generation sequencing revealed that the patient had the canonical T618I mutation associated with CNL. In a retrospective review at the Mayo Clinic, Szuber and colleagues identified the clinical and laboratory characteristics, finding those with a T618I mutation to have an older median age, are more likely to be male, have a frequent history of thrombosis, and often presented with palpable splenomegaly. In their review, this mutation evolved to acute myeloid leukemia in 20% of cases.
CONCLUSIONS
The purpose of this article is to increase clinician awareness of this unusual cause of persistent leukocytosis and to encourage the utilization of molecular analysis for this rare disorder, including testing for CSF3R mutations when BCR-ABL and other MPN markers are negative. Outcomes appear to be worse than in other MPNs: A recent case report of 14 patients identified that 75% were deceased within 2 years (Böhm and Schaefer, 2002). This is consistent with the patient’s course.
Unfortunately, there is no current standard-of-care treatment for CNL. The majority of patients in the literature received hydroxyurea as a first-line treatment. Second-line treatment approaches for refractory patients have included imatinib, interferon-α (Yassin et al., 2015), ruxolitinib (Maxson et al., 2013), and hematopoietic stem cell transplantation (HSCT; Szuber et al., 2018). Because most CNL cases occur in the elderly, there are only a few case reports documenting the sustained remission achieved with HSCT (4.5 to 6 years; Böhm & Schaefer, 2002). A prospective multicenter clinical trial is currently underway to evaluate the benefit of ruxolitinib in patients with CNL. Hopefully, greater awareness of CNL will result in improved enrollment of patients into clinical trials, ultimately yielding improved patient outcomes.
Acknowledgment
Peripheral smears and biopsy images appear courtesy of Luke Perkocha, MD, Pathology Department, Kaiser Permanente. The author would also like to acknowledge Brad Lewis, MD, who contributed to the editing of this manuscript.
Footnotes
The author has no conflicts of interest to disclose. The writing of this manuscript was partially supported by the National Institutes of Health under award number T322NR013456.
REFERENCES
- Arber D. A., Orazi A., Hasserjian R., Thiele J., Borowitz M. J., Le Beau M. M.,...Vardiman J. W. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127(20), 2391–2405. 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- Bain B. J., & Ahmad S. (2015). Chronic neutrophilic leukaemia and plasma cell-related neutrophilic leukaemoid reactions. British Journal of Haematology, 171(3), 400–410. 10.1111/bjh.13600 [DOI] [PubMed] [Google Scholar]
- Belay Y., Yirdaw K., & Enawgaw B. (2017). Tumor lysis syndrome in patients with hematological malignancies. Journal of Oncology, 2017, Article ID 9684909. 10.1155/2017/9684909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm J., & Schaefer H. E. (2002). Chronic neutrophilic leukaemia: 14 new cases of an uncommon myeloproliferative disease. Journal of Clinical Pathology, 55(11), 862–864. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12401827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y. J., Jiang Q., Liu J. Q., Li B., Xu Z. F., Qin T. J.,...Xiao Z. J. (2017). [The clinical characteristics, gene mutations and prognosis of chronic neutrophilic leukemia]. Zhonghua Xue Ye Xue Za Zhi, 38(1), 28–32. 10.3760/cma.j.issn.0253-2727.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. A., Hanson C. A., Dewald G. W., Smoley S. A., Lasho T. L., & Tefferi A. (2005). WHO-defined chronic neutrophilic leukemia: a long-term analysis of 12 cases and a critical review of the literature. Leukemia, 19(2), 313–317. 10.1038/sj.leu.2403562 [DOI] [PubMed] [Google Scholar]
- Lee S. E., Jo I., Jang W., Kim Y., Han K., & Kim M. (2015). T618I-mutated colony stimulating factor 3 receptor in chronic neutrophilic leukemia and chronic myelomonocytic leukemia patients who underwent allogeneic stem cell transplantation. Annals of Laboratory Medicine, 35(3), 376–378. 10.3343/alm.2015.35.3.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P., Karow A., Nienhold R., Looser R., Hao-Shen H., Nissen I.,...Skoda R. C. (2014). Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood, 123(14), 2220–2228. 10.1182/blood-2013-11-537167 [DOI] [PubMed] [Google Scholar]
- Maxson J. E., Gotlib J., Pollyea D. A., Fleischman A. G., Agarwal A., Eide C. A.,...Tyner J. W. (2013). Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. New England Journal of Medicine, 368(19), 1781–1790. 10.1056/NEJMoa1214514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A., Lasho T. L., Laborde R. R., Elliott M., Hanson C. A., Knudson R. A.,...Tefferi A. (2013). CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia, 27(9), 1870–1873. 10.1038/leu.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza S., Kirkland R. S., Patel A. A., Shortridge J. R., & Freter C. (2013). Insight into Sweet’s syndrome and associated-malignancy: A review of the current literature. International Journal of Oncology, 42(5), 1516–1522. 10.3892/ijo.2013.1874 [DOI] [PubMed] [Google Scholar]
- Szuber N., & Tefferi A. (2018). Chronic neutrophilic leukemia: New science and new diagnostic criteria. Blood Cancer Journal, 8(2), 19 10.1038/s41408-018-0049-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuber N., Finke C. M., Lasho T. L., Elliott M. A., Hanson C. A., Pardanani A., & Tefferi A. (2018). CSF3R-mutated chronic neutrophilic leukemia: Long-term outcome in 19 consecutive patients and risk model for survival. Blood Cancer Journal, 8(2), 21 10.1038/s41408-018-0058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A., Pardanani A., Lim K. H., Abdel-Wahab O., Lasho T. L., Patel J.,...Levine R. L. (2009). TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia, 23(5), 905–911. 10.1038/leu.2009.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin M. A., Kohla S., Al-Sabbagh A., Soliman A. T., Yousif A., Moustafa A.,...Al-Dewik N. (2015). A case of chronic neutrophilic leukemia successfully treated with pegylated interferon alpha-2a. Clinical Medicine Insights: Case Reports, 8, 33–36. 10.4137/CCRep.S22820 [DOI] [PMC free article] [PubMed] [Google Scholar]