Abstract
Background
Current US Preventive Services Task Force (USPSTF) lung cancer screening guidelines are based on smoking history and age (55–80 years). These guidelines may miss those at higher risk, even at lower exposures of smoking or younger ages, because of other risk factors such as race, family history, or comorbidity. In this study, we characterized the demographic and clinical profiles of those selected by risk-based screening criteria but were missed by USPSTF guidelines in younger (50–54 years) and older (71–80 years) age groups.
Methods
We used data from the National Health Interview Survey, the CISNET Smoking History Generator, and results of logistic prediction models to simulate lifetime lung cancer risk-factor data for 100 000 individuals in the 1950–1960 birth cohorts. We calculated age-specific 6-year lung cancer risk for each individual from ages 50 to 90 years using the PLCOm2012 model and evaluated age-specific screening eligibility by USPSTF guidelines and by risk-based criteria (varying thresholds between 1.3% and 2.5%).
Results
In the 1950 birth cohort, 5.4% would have been ineligible for screening by USPSTF criteria in their younger ages but eligible based on risk-based criteria. Similarly, 10.4% of the cohort would be ineligible for screening by USPSTF in older ages. Notably, high proportions of blacks were ineligible for screening by USPSTF criteria at younger (15.6%) and older (14.2%) ages, which were statistically significantly greater than those of whites (4.8% and 10.8%, respectively; P < .001). Similar results were observed with other risk thresholds and for the 1960 cohort.
Conclusions
Further consideration is needed to incorporate comprehensive risk factors, including race and ethnicity, into lung cancer screening to reduce potential racial disparities.
Lung cancer (LC) is the leading cause of cancer-related death in the United States. Current US Preventive Services Task Force (USPSTF) guidelines recommend annual low-dose computed tomography screening of persons aged 55–80 years with at least 30 pack-years of smoking and within 15 years since cessation (1,2). However, concerns have been raised that these guidelines might miss individuals who do not meet the pack-year (3–6) or age criteria (7,8) but who may be at high LC risk because of factors such as race, low education, history of chronic obstructive pulmonary disease (COPD), or family history of LC (FHLC). For example, a recent study based on the US Southern Community Cohort Study (n = 48 364 ever-smokers) showed that a statistically significantly smaller proportion of black LC patients (17%) vs whites (31%) meet the current smoking criteria set by the USPSTF because of lower smoking pack-years in blacks (3).
Recent studies demonstrated that LC risk models provide a promising approach for identifying high-risk individuals for screening by incorporating comprehensive risk factors, including race, education, FHLC, COPD, and smoking history (9–12). For example, Tammemagi et al. (10) applied their PLCOm2012 risk model and USPSTF criteria to data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial intervention arm and found that risk-based screening (based on >1.51% threshold for a 6-year LC risk) identified 12.4% more LC cases, had fewer false positives, and had a higher positive predictive value compared with USPSTF criteria .
However, studies evaluating risk-based screening for LC are often based on specific trial populations, such as the National Lung Screening Trial or PLCO[9–11] with limited applicability to the general population. Although some studies used more general population data based on national survey data (12), the use of a single data source restricts the study design to a cross-sectional analysis, lacking longitudinal follow-up of the population. In evaluating smoking and age-based screening strategies in the general population, the Cancer Intervention and Surveillance Modeling Network (CISNET) Lung Working Group used the Smoking History Generator (SHG) to simulate lifetime longitudinal smoking histories for each birth cohort in the United States (2,13–15). However, population data with comprehensive risk factors for LC are currently unavailable, which makes the population-level evaluation of risk-based screening challenging.
In this study, we evaluate the performance of risk-based screening compared with the USPSTF criteria in the general US population by utilizing data from the CISNET SHG, the National Health Information Survey (NHIS), the US Census Bureau, and logistic prediction models for nonsmoking risk factors. We characterize the demographic and clinical profiles of those eligible for screening by risk-based criteria but not by the USPSTF criteria in younger (50–54 years), middle (55–70 years), and older (71–80 years) ages.
Methods
Target US Populations and CISNET SHG
We used the US 1950 birth cohort as our target study population, which has also been evaluated in prior USPSTF reports (1,2,16). Additionally, we considered the 1960 birth cohort for sensitivity analyses. We considered the following well-established risk factors for LC: race, education, FHLC, COPD, personal history of cancer, body mass index, and smoking history.
For projecting and simulating correlated risk factor data that change over time, we used smoking history data generated from the CISNET SHG for each birth cohort (15). The SHG is a shared precursor model that utilizes US population data to produce cohort-specific smoking histories and smoking-related death rates at the individual level. This generator is based on the NHIS, the Cancer Prevention studies I and II, and the Human Mortality Database (13,15).
LC Risk Factor Simulator
We simulated cohort-specific nonsmoking risk factor data for 100 000 men and women in each birth cohort using smoking histories from the SHG and logistic and linear regression-based prediction models for nonsmoking risk factors, conditioned on inputted smoking data. In simulating these data, we aimed to keep the correlation among risk factors as observed in real-world settings and to incorporate observed prevalences of relevant risk factors for each birth cohort. Our approach was to estimate the joint distribution of risk factors, conditioning on smoking history, by exploiting large epidemiologic data that include comprehensive risk factor information and to calibrate the parameters of the estimated joint distribution using external prevalence data. For estimating the joint distribution, we used baseline risk factor data from the PLCO trial. The estimation of the joint distribution of risk factors was based on a set of conditional distributions that were derived by a multiplication chain rule approach (see Supplementary Methods and Supplementary Figures 1–2, available online). For calibration of the parameters of the estimated joint distribution, we used US Census Bureau data to calibrate the race and education, the National Health and Nutrition Examination Survey for body mass index (17), and NHIS for COPD, FHLC (18), and personal history of cancer (19). More detailed information is provided in Supplementary Materials (available online). We implemented our lung cancer risk factor simulator in an R package (LC risk factor simulator) that is available on request.
Data Sources
The major source of prevalence data for risk factors came from the 2000 NHIS, a national cross-sectional interview survey. Various cancer-related variables such as self-reported personal and family history of cancer (18) and comorbidity variables (such as COPD) are included in the survey. Race and education distributions for a given cohort were obtained from the US Census Bureau (https://www.census.gov/). Supplementary Table 1 (available online) provides the prevalence information used for simulating the risk factor data for each cohort.
The PLCO is a randomized screening trial in which participants ages 55–74 years were enrolled from 10 US centers between 1993 and 2001 (20). Our study included 154 897 participants who were randomly assigned in PLCO (total n = 154 901) and who completed a baseline risk factor questionnaire. These baseline data were used to generate logistic and linear model-based prediction models for nonsmoking risk factors (see Supplementary Materials, available online).
Risk Calculation Using the PLCOm2012 Model and Evaluation of Screening Eligibility
For each individual in a given birth cohort, we calculated age-specific 6-year LC risks for ages 50–90 years using the PLCOm2012 model (9). This model predicts 6-year LC risks based on demographic, environmental, and clinical risk factors, which was fitted using the PLCO control and intervention arm data (see Supplementary Table 3, available online). We then evaluated age-specific eligibility for screening (yes or no) for each individual using the USPSTF criteria and using the risk-based criteria. For calculating the eligibility based on risk-based criteria, we used a baseline threshold of 6-year LC risk of r equal to 1.51%. This threshold was initially suggested by Tammemagi et al. (10) and is used as selection criteria in several existing screening programs (21). As a sensitivity analysis, we used different risk thresholds: 1.3%, 1.7%, 2.0%, and 2.5%. Supplementary Figure 3 (available online) shows example trajectories of calculated LC risk and eligibility.
Demographic and Clinical Profiles of Those Missed by the USPSTF-Criteria
To project and quantify differences in screening eligibility between the two selection criteria, we examined the lifetime risk factor trajectories of a given cohort and identified those who would be eligible for screening by the risk-based criteria but not by USPSTF for each age from 50 to 80 years. To characterize the demographic and clinical profiles of those who have different eligibility outcomes across different criteria, we stratified each birth cohort by race, education, FHLC, or COPD and calculated the proportion of those eligible by risk-based screening (r ≥ 1.51%) but not by USPSTF. A proportion test based on a two-sided χ2 test statistic was applied to assess the statistical difference in the proportions across different subgroups. P value less than .05 was considered statistically significant.
Results
Projecting Screening Eligibility Results by Race and Ethnicity
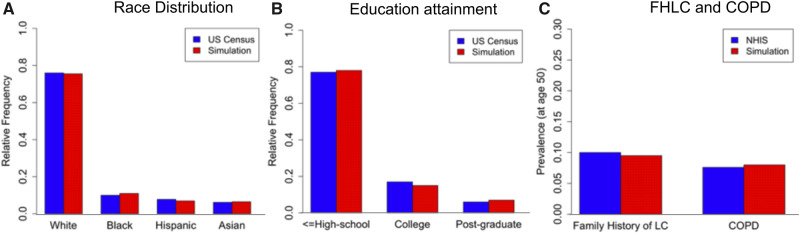
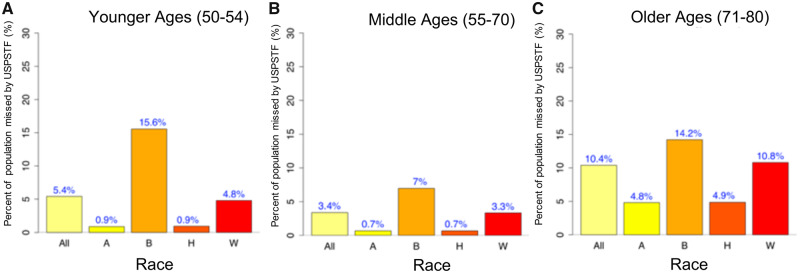
Figure 1 presents simulated risk factor prevalence versus the reference data (US Census Bureau and NHIS), showing that the simulation algorithm provides LC risk factor data that adequately reproduce risk factor for the 1950 birth cohort. Comparisons using more risk factor data are shown in Supplementary Figures 4 and 5 (available online). Figure 2A shows the projected proportion of individuals from the 1950 cohort eligible for screening by the risk-based screening criteria (r ≥ 1.51%) but not eligible by USPSTF criteria in the younger ages (50–54 years), which was obtained by taking the average of the age-specific proportions estimated for the age range of 50–54 years. Overall, 5.4% of the 1950 cohort would be eligible for screening by risk-based criteria but not by USPSTF in their younger ages. When we examine this proportion by race, a statistically significantly higher proportion of blacks (15.6%) would be missed for screening by USPSTF compared with whites (4.8%; P < .001). We also investigated the proportion of those missed by USPSTF in the older ages (71–80 years) (using r ≥ 1.51%), which often occurs among former smokers who ceased smoking longer than 15 years ago, despite having elevated LC risks according to the PLCOm2012 model. Overall, 10.4% of the cohort would be ineligible for screening by USPSTF in older ages. Similar to the younger ages, a higher proportion of blacks would be missed for screening in their older ages compared with whites (14.2% vs 10.8%, respectively; P < .001; Figure 2C). We also investigated whether the eligibility difference between the two screening criteria shown in younger and older ages is observed in ages 55–70 years. Figure 2B shows that although the overall proportion missed by USPSTF in ages 55–70 years is lower compared with ages 50–54 years (Figure 2A), the gap between the two racial groups (blacks and whites) is still observed.
Figure 1.
Model-based vs observed distributions of race, education, FHLC, and COPD for the 1950 birth cohort (male and female combined). The distributions of the remaining risk factors and the bivariate distributions of risk factors are shown in Supplementary Figures 4 and 5 (available online). Data sources: Race and education data from US Census Bureau; FHLC and COPD from the 2000 NHIS. We note that the risk factor simulator algorithm was calibrated to these reference data (see Supplementary Methods, available online). COPD = chronic obstructive pulmonary disease; FHLC = family history of lung cancer; LC = lung cancer; NHIS = National Health Interview Survey.
Figure 2.
Percent of the population missed for screening by the USPSTF criteria vs risk-based criteria (>1.51% threshold) in (A) younger ages 50–54 years, (B) middle ages 55–70 years, and (C) older ages 71–80 years in the 1950 birth cohort. All = all racial and ethnic group combined; A = Asians; B = blacks; H = Hispanic; W = non-Hispanic white; USPSTF = US Preventive Services Task Force.
Variation of Risk Thresholds and Analysis by Sex to Examine Racial Disparity
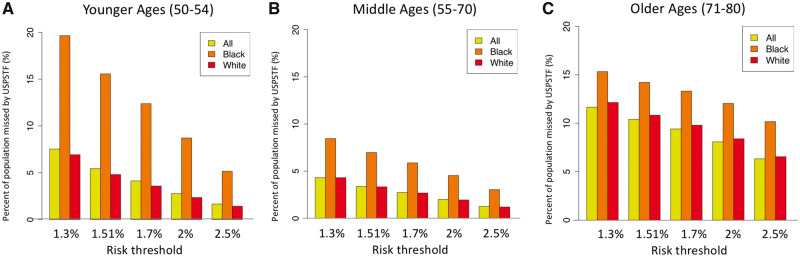
To examine how different risk thresholds affect screening eligibility differences between the two screening criteria, we evaluated risk thresholds between 1.3% and 2.5%. Figure 3 shows the decreasing pattern in the proportions of those missed by USPSTF vs risk-based criteria as the threshold increases, where the proportion missed by USPSTF is 7.5% for the threshold of 1.3% and decreases to 1.7% for the threshold of 2.5% (ages 50–54 years). This reflects the fact that higher risk thresholds select fewer individuals for screening, and hence the gap between the two criteria decreases. The proportion of individuals ineligible for screening by USPSTF is consistently higher in blacks compared with whites (Figure 3, yellow bars) independently of risk threshold.
Figure 3.
Impact of varied 6-year LC risk threshold (1.3–2.5%) on the percent of the population missed by the USPSTF criteria vs risk-based criteria in (A) younger ages 50–54 years, (B) middle ages 55–70 years, and (C) older ages 71–80 years in the 1950 birth cohort. USPSTF = US Preventive Services Task Force.
Subgroup analyses examining the racial gap by sex (Supplementary Figure 6, available online) show that racial disparities in screening eligibility are more distinct among males than females in both younger and older ages, which is related to racial differences in smoking behaviors (eg, prevalence) being larger among males than females (22). The result in Supplementary Figure 7 (available online) shows that overall proportions of the population missed by USPSTF vs the risk-based criteria is lower for the 1960 birth cohort relative to the 1950 cohort. In the 1960 cohort analysis, similar racial disparity patterns are still observed as in the 1950 cohort, where blacks have a higher proportion (5.8%) missed by USPSTF compared with whites (2.3%; P < .001). However, the magnitude of this disparity is smaller in the 1960 cohort, reflecting the observed convergence in smoking behavior differences between blacks and whites in more recent birth cohorts (6).
Difference in Eligibility by Other Risk Factors: Education, Family History of LC, and COPD
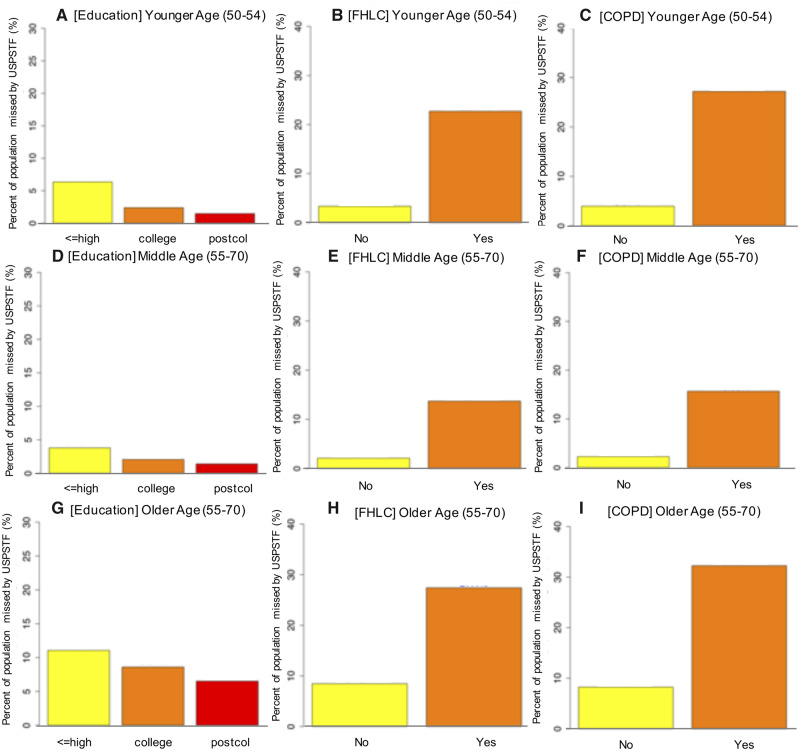
To characterize the demographic and clinical profiles of those eligible for screening by risk-based criteria but not by USPSTF, we projected the proportion of the population missed by USPSTF stratified by different subgroups such as education, FHLC, and COPD (see Figure 4). Figure 4A shows that 6.4% of those who have less than a high school education or high school graduates have 6-year LC risk greater than 1.51% but would be ineligible for screening by USPSTF at the younger ages of 50–54 years. This proportion was statistically significantly higher compared with those with a college education (2.4%; P < .001) or with postgraduate or professional degrees (1.5%; P < .001). Figure 4, B and C, shows the results for FHLC and COPD. Similar results were observed for middle ages 55–70 years and older ages 71–79 years (Figure 4, D–I) and in the 1960 birth cohort (Supplementary Figure 8, available online).
Figure 4.
Percent of the population missed for screening by the USPSTF criteria vs risk-based criteria (>1.51% threshold) in younger ages 50–54 years (first rows), middle ages 55–70 years, and older ages 71–80 (second rows) in the 1950 birth cohort. COPD = chronic obstructive pulmonary disease; FHLC = family history of lung cancer; Postcol = post-college; USPSTF = US Preventive Services Task Force.
Discussion
We compared risk-based criteria with the USPSTF selection criteria by projecting lifetime screening eligibility for the US 1950–1960 birth cohorts. We examined the demographic and clinical profiles of those missed for screening by the USPSTF criteria but screen eligible by risk-based criteria (>1.51%). Our analysis showed that high proportions of blacks were ineligible for screening by USPSTF criteria at younger (15.6%) and older (14.2%) ages, which were Statistically significant greater than those of whites (4.8% and 10.82%, respectively). Similar results were observed for other risk thresholds (1.3–2.5%) for the 1960 cohort and for both sexes.
It is notable that the projected racial disparity in screening eligibility is more prominent in younger ages than in middle or older ages. Several epidemiologic studies showed that blacks have a higher LC risk compared with whites at young ages (23,24). However, the racial difference in LC risk was smaller among heavy smokers and in older age groups (24). Previous epidemiologic studies show that increased LC risk among blacks vs whites in younger age groups is more consistently observed among males, whereas the evidence is less clear among females (23,24). Similarly, our analysis showed that the projected racial difference in screening eligibility was smaller among females compared with males across different birth cohorts. The results for the 1960 birth cohort showed that the racial disparity in screening eligibility is reduced compared with the 1950 birth cohort, which may be related to the fact that differences in smoking behavior between blacks and whites are decreasing in younger cohorts (6). In addition, we note that the overall proportion of the population missed by USPSTF was lower in the 1960 cohort relative to the 1950 cohort. This could be partly due to the decreasing prevalences of risk factors (eg, COPD and low education) in more recent birth cohorts.
Several recent studies raised the issue of racial disparity regarding national lung cancer screening guidelines (5,7,8). A study from the Southern Community screening program showed that a statistically significantly smaller proportion of blacks (32%) who are diagnosed with LC meets the 30 pack-year criteria compared with whites (55%; P < .001), suggesting that different pack-years criteria should be applied to blacks. Pinsky and Kramer (4) made a similar point by using the PLCO data to show that the 30 pack-year limit may artificially exclude proportionally more racial and ethnic minorities than non-Hispanic whites. Although adjusting pack-years criteria based on race could be one solution to reduce potential disparities, our study indicates that the disparities induced by applying uniform screening criteria by USPSTF can also arise in other risk subgroups. For example, disparities were observed across different education levels or by comorbidity status (eg, COPD), which cannot be reduced by adjusting pack-years criteria. Our study supports the hypothesis that risk-based screening that utilizes comprehensive LC risk factors can prevent the increase of existing LC disparities by race, socioeconomic status, comorbidities, or FHLC.
Our study has several strengths. First, we evaluated risk-based screening eligibility compared with the USPSTF criteria by following the lifetime smoking and risk factor changes for the 1950–1960 US birth cohorts. To do this, we simulated birth cohort-specific risk factor data by conditioning on smoking history information generated by the CISNET SHG and by utilizing external prevalence data to take into account the prevalence variation of smoking and nonsmoking risk factors by cohort, period, and age. Second, the cohorts and the age range we examined are highly relevant for current screening programs. In addition to younger ages (50–54 years), we evaluated screening eligibility at ages 55–80 years of the 1950 birth cohort (which will be years 2005–2030). In particular, this cohort will be aged 70–80 years in 2020–2030. Given that the median age of LC diagnosis is 70 years (SEER-21, years 2012–2016), this analysis is applicable to inform current screening. Third, a recent study by Landy et al. (25) showed that the risk threshold of 1.51% chooses millions more individuals for screening compared with the USPSTF guidelines. Landy et al. suggested that an alternative threshold should be chosen to select a similar number of individuals as USPSTF. However, we note the choice of a specific threshold does not impact our main findings. Our analysis based on the range of risk thresholds (1.3–2.5%) showed that independently of the threshold chosen, we consistently observe the racial disparities across all age groups.
Our study also has limitations. First, in evaluating risk-based criteria compared with USPSTF, we focused on screening eligibility. However, not all eligible individuals go through screening, because recent studies reported low uptake rates of LC screening (2–4%) (26). Also, it is possible that screening adherence can vary by different factors, and the disparities estimated from our study could be larger or smaller, when directly taking into account different uptake rates across different subgroups. Our previous study showed that varying assumptions on adherence by different factors heavily influences screening outcomes (27). Second, we applied the PLCOm2012 model for calculating LC risks under a wide range of ages that included younger ages 50–54 years. However, we note that this model was developed based on individuals aged 55–74 years, and we extrapolated this model to ages younger than 55 years. However, a recent study by Tammemagi et al. (28) validated the PLCOm2012 model to the Pan-Canadian cohort that is aged 50–75 years. The results show that the PLCOm2012 model has similar or better overall prediction and calibration performance compared with the PanCan model, a precursor to PLCOm2012 that was developed based on this cohort that includes participants aged younger than 55 years. Third, our study is based on a modeling approach that has several underlying assumptions. We assumed that LC risk factors are correlated with smoking behaviors, and we used a sequence of conditional probabilities in predicting nonsmoking risk factors based on this assumption. Also, we assumed that the structure of correlations among risk factors (including smoking and nonsmoking) that we estimated from the PLCO data does not change much across different birth cohorts. Although this is a strong assumption, we captured the variation of risk factors across different birth cohorts by incorporating cohort-specific prevalence of nonsmoking risk factors and also by exploiting cohort, period, and age-specific smoking history information based on the SHG. In estimating the correlation among risk factors, we used the PLCO data because it provides high-quality environmental, clinical, and demographic risk factor data directly relevant to cancer, based on that which several lung cancer risk prediction models have been developed and validated (9,11). Although PLCO is not general population data, the data from NHIS and US Bureau census were incorporated into our analysis through the calibration of birth cohort–specific risk factor prevalence so that the risk factor data well represents the US population.
We note that our current study examines the impact of risk-based screening on the eligibility of screening, and our future plan includes evaluating the impact of risk-based screening on long-term effects such as LC mortality. This evaluation requires a comparative microsimulation modeling approach based on the input data generated by the risk factor simulator we present in this paper. Although risk-based criteria can expand screening eligibility by including those with low socioeconomic status and high comorbidity, it is possible that long-term benefit and harm ratios may be different in the expanded populations. For example, individuals with COPD, low socioeconomic status, or older ages may be at a high risk for LC, but they also may have a reduced life expectancy, which may lead to generally less life-years gained and more overdiagnosis (29).
In conclusion, further consideration is needed to incorporate comprehensive risk factors for LC screening to reduce potential disparities related to race, socioeconomic status, and comorbidities. Personalized screening based on risk prediction models has the potential to reduce disparities in screening compared with national lung screening guidelines that recommend uniform screening criteria, which are likely to exclude high-risk individuals from disadvantaged groups.
Funding
This work was supported by National Institutes of Health (R37CA226081 to SSH and U01CA199284 to RM and SKP).
Notes
The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
SP is a consultant for GRAIL. SSH, KTH, IT, PC, MB, JJ, RM, and SP are members of the CISNET Lung Working Group. The other authors have no conflicts or disclosures.
Supplementary Material
References
- 1. Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 2. de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the US Preventive Services Task Force. Ann Intern Med. 2014;160(5):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldrich MC, Mercaldo SF, Sandler KL, et al. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol. 2019;5(9):1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinsky PF, Kramer BS. Lung cancer risk and demographic characteristics of current 20–29 pack-year smokers: implications for screening. J Natl Cancer Inst. 2015;107(11):djv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li C-C, Matthews AK, Rywant MM, et al. Racial disparities in eligibility for low-dose computed tomography lung cancer screening among older adults with a history of smoking. Cancer Causes Control. 2019;30(3):235–240. [DOI] [PubMed] [Google Scholar]
- 6. Holford TR, Levy DT, Meza R. Comparison of smoking history patterns among African American and white cohorts in the United States born 1890 to 1990. Nicotine Tob Res. 2016;18(suppl 1):S16–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aldrich M, Mercaldo S, Sandler K, et al. MA20.05 Who gets screened for lung cancer? A simple adjustment to current guidelines to reduce racial disparities. J Thorac Oncol. 2018;13(10):S426–S427. [Google Scholar]
- 8. Annangi S, Nutalapati S, Foreman MG, Pillai R, Flenaugh EL. Potential racial disparities using current lung cancer screening guidelines. J Racial Ethn Health Disparities. 2019;6(1):22–26. [DOI] [PubMed] [Google Scholar]
- 9. Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever-and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11(12):e1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katki HA, Kovalchik SA, Berg CD, et al. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holford TR, Clark L. Development of the counterfactual smoking histories used to assess the effects of tobacco control. Risk Anal. 2012;32(suppl 1):S39–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeon J, Holford TR, Levy DT, et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med. 2018;169(10):684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moolgavkar SH, Holford TR, Levy DT, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975–2000. J Natl Cancer Inst. 2012;104(7):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McMahon PM, Meza R, Plevritis SK, et al. Comparing benefits from many possible computed tomography lung cancer screening programs: extrapolating from the national lung screening trial using comparative modeling. PLoS ONE. 2014;9(6):e99978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ogden CL, Statistics NCfH. Mean Body Weight, Height, and Body Mass Index: United States 1960-2002 Hyattsville, MD: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2004.
- 18. Ramsey SD, Yoon P, Moonesinghe R, et al. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med. 2006;8(9):571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McClure L, Clarke T, Fernandez C, et al. A report of Florida’s cancer history, risk factors, and screening behaviors: data from the national health interview survey. Florida Public Health Rev. 2012;9(1):67–87. [Google Scholar]
- 20. Oken MM, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality. JAMA. 2011;306(17):1865–1873. [DOI] [PubMed] [Google Scholar]
- 21. Lam S, Myers R, Atkar-Khattra S, et al. MA03.02 prospective evaluation of the clinical utility of the international lung screen trial lung nodule management protocol. J Thorac Oncol. 2018;13(10):S362–S363. [Google Scholar]
- 22. Fiore MC, Novotny TE, Pierce JP, et al. Trends in cigarette smoking in the United States: the changing influence of gender and race. JAMA. 1989;261(1):49–55. [PubMed] [Google Scholar]
- 23. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333–342. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz AG, Swanson GM. Lung carcinoma in African Americans and whites: a population‐based study in metropolitan Detroit, Michigan. Cancer. 1997;79(1):45–52. [DOI] [PubMed] [Google Scholar]
- 25. Landy R, Cheung LC, Berg CD, et al. Contemporary implications of US Preventive Services Task Force and risk-based guidelines for lung cancer screening eligibility in the United States. Ann Intern Med. 2019;171(5):384–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han SS, Erdogan SA, Toumazis I, et al. Evaluating the impact of varied compliance to lung cancer screening recommendations using a microsimulation model. Cancer Causes Control. 2017;28(9):947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18(11):1523–1531. [DOI] [PubMed] [Google Scholar]
- 29. ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5): djz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.