Abstract
Background
Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors + endocrine therapy (ET) prolonged progression-free survival as first- or second-line therapy for hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer prognosis. Given the recent publication of overall survival (OS) data for the 3 CDK4/6-inhibitors, we performed a meta-analysis to identify a more precise and reliable benefit from such treatments in specific clinical subgroups.
Methods
We conducted a systematic literature search to select all available phase II or III randomized clinical trials of CDK4/6-inhibitors + ET reporting OS data in first- or second-line therapy of HR+/HER2-negative pre- or postmenopausal metastatic breast cancer. A random effect model was applied for the analyses. Heterogeneity was assessed with I2statistic. Subgroup analysis was performed to explore the effect of study-level factors. The project was registered in the Open Science Framework database (doi: 10.17605/OSF.IO/TNZQP).
Results
Six studies were included in our analyses (3421 patients). A clear OS benefit was observed in patients without (hazard ratio [HR] = 0.68, 95% confidence interval [CI] = 0.54 to 0.85, I2 = 0.0%) and with visceral involvement (HR = 0.76, 95% CI = 0.65 to 0.89, I2 = 0.0%), with at least 3 metastatic sites (HR = 0.75, 95% CI = 0.60 to 0.94, I2 = 11.6%), in an endocrine-resistant (HR = 0.79, 95% CI = 0.67 to 0.93, I2 = 0.0%) and sensitive subset (HR = 0.73, 95% CI = 0.61 to 0.88, I2 = 0.0%), for younger than 65 years (HR = 0.80, 95% CI = 0.67 to 0.95, I2 = 0.0%) and 65 years or older (HR = 0.71, 95% CI = 0.53 to 0.95, I2 = 44.4%), in postmenopausal (HR = 0.76, 95% CI = 0.67 to 0.86, I2 = 0.0%) and pre- or perimenopausal setting (HR = 0.76, 95% CI = 0.60 to 0.96, I2 = 0.0%) as well as in chemotherapy-naïve patients (HR = 0.72, 95% CI = 0.55 to 0.93, I2 = 0.0%).
Conclusions
CDK4/6-inhibitors + ET combinations compared with ET alone improve OS independent of age, menopausal status, endocrine sensitiveness, and visceral involvement and should be preferred as upfront therapy instead of endocrine monotherapy.
Hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer (MBC) represents the most frequent subgroup of advanced breast tumors (1). The most relevant therapeutic improvement of the last few years in this subset has been represented by the introduction of cyclin-dependent kinases (CDK) 4 and 6 (CDK4/6) inhibitors (palbociclib, ribociclib, and abemaciclib) combined with endocrine therapy (ET). These drugs bind to the CDK4 and 6, preventing their correct functioning and leading to cell-cycle arrest and apoptosis. They also seem to induce a broad spectrum of immunological events, which, however, need further investigation to be fully understood (2).
Pivotal trials led to the approval of CDK4/6-inhibitors plus ET combinations after showing very similar statistically significant and clinically meaningful improvements in progression-free survival (PFS) in a first- or second-line setting of both premenopausal (3–5) and postmenopausal (3,4,6–9) patients with HR+/HER2-negative MBC. The median PFS of all the comparison arms roughly doubled, as well as overall response rates, compared with standard ET (3–9). Notably, a recent network meta-analysis confirmed the superiority of CDK4/6-inhibitor regimens over single agent ET, showed a substantial equivalence among the 3 inhibitors and no difference with chemotherapy (CT) (10). However, all these studies were based on PFS as their primary endpoint and, until recently, overall survival (OS) data were available only for palbociclib-containing phase II PALOMA 1 and phase III PALOMA 3 trials (11,12) and the ribociclib-containing MONALEESA 2 trial (7). Previous studies had observed a statistically significant association between PFS and OS in MBC (13), in general and specifically in HR+/HER2-negative disease, overall suggesting that the first might be a good surrogate endpoint for the latter (14). Nevertheless, the prediction of OS based on PFS is still matter of debate, because the number of subsequent treatment lines, cross-over from the control arm to active treatment, and nonrandomized use of second-line agents might interfere with this association (13,15). Finally, OS results for the pivotal phase III trials MONARCH 2, MONALEESA 3, and MONALEESA 7 were recently published, providing additional data regarding abemaciclib- and ribociclib-based regimens (16–18). Considering all available results, a 4- to 10-month improvement in median survival with a 19%-29% relative reduction in the risk of death has been observed so far (7,11,12,16–18). However, results were not statistically significant for each trial or for each subgroup of patients, probably due to the study being substantially under-powered in demonstrating possible OS differences (19). For these reasons and also given the current lack of effective biomarkers capable of identifying patients that might benefit most from these novel therapeutic agents, we decided to perform this meta-analysis in different clinically relevant subgroups of HR+/HER2-negative MBC.
Materials and Methods
Search Strategy and Selection Criteria
We conducted a systematic literature search on PubMed at the end of October 2019 to select all available phase II or III randomized controlled trials(RCT) of CDK4/6 inhibitors plus ET showing OS data in the first- or second-line treatment setting of HR+/HER2-negative pre- or postmenopausal MBC. European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology meetings’ and San Antonio Breast Cancer Symposium’ online databases were also consulted. The query included the terms “palbociclib,” “ribociclib,” “abemaciclib,” “breast,” “metastatic,” and “advanced.” Duplicate reports were excluded. No language restriction was adopted. The research and data extraction were conducted by 2 investigators (F Schettini and F Giudici) and a third one (D Generali) was consulted in case of controversy. Details about study design, patient characteristics, interventions, and previous treatments were extracted from each article. The primary outcome was OS measured in various subgroups of interest. Hazard ratios (HR) and associated 95% confidence intervals (CI) were extracted for OS from published articles. Subgroups of interest were the following: visceral disease (yes vs no), bone-only disease (yes vs no), number of metastatic sites (<3 sites vs ≥3), endocrine sensitivity and resistance (yes vs no), previous CT for the metastatic setting (yes vs no), age (<65 vs ≥65 years), and menopausal status (pre-perimenopausal vs postmenopausal). Endocrine resistance and sensitivity were defined according to ESO-ESMO International Consensus Guidelines (20).
Data Analysis
Analyses were performed applying a priori the random-effect model from DerSimonian and Laird (21). Pooled data were presented in forest plots. All study-specific estimates were combined using inverse variance-weighted averages of logarithmic hazard ratios in random-effects models. Statistical significance was set at P less than .05. All tests were 2-sided. The degree of heterogeneity between studies was assessed by visual inspection of the forest plots and I2 statistic estimate (22). Using subgroup analysis, we planned to explore the effect of the following study-level factors: visceral involvement status (no involvement vs involvement), bone-only disease condition (yes vs no), number of metastatic sites (<3 vs ≥3), endocrine sensitive status (resistant vs sensitive), previous CT for the metastatic setting (untreated vs treated), age (<65 years vs ≥65 years), and menopausal status (postmenopausal vs pre/perimenopausal). Subgroup analyses were performed if at least 2 studies for each of the previously mentioned subgroups of interest were available. Q-test of homogeneity (Q Statistic: Q within and Q between) was performed to compare the pooled effect in 2 or more groups.
Publication bias was not assessed due to inadequate numbers of included trials to properly assess a funnel plot or more advanced regression-based assessments. Statistical analyses were performed using R software version 3.5.0 (package meta) (23). The risk of bias for each trial was assessed by using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (24). Internal validity of eligible studies was assessed according to the Cochrane Collaboration’s “Risk of Bias” tool in Review Manager (25). Each domain related to a risk of bias was assessed in each included trial, because there is evidence that these issues are associated with biased estimates of treatment effect. The domains were the following: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias. Review authors’ judgments were categorized as “low risk,” “high risk,” or “unclear risk” of bias.
The project was registered in the Open Science Framework online public database (http://osf.io with doi: 10.17605/OSF.IO/TNZQP).
Results
Included Studies’ Characteristics
Six out of 8 (75.0%) studies reported OS results and were therefore included in the analyses for a total of 3421 patients (7,11,12,16–18). The study selection process is summarized in Supplementary Figure 1 (available online). Three of the 6 (50.0%) included studies enrolled only postmenopausal patients, 1 (17.0%) study exclusively enrolled premenopausal patients, to whom an analogue of gonadotropin-releasing hormone agonist (GnRH) was administered to induce ovarian function suppression, and the 2 (33.0%) remaining studies enrolled both post- and premenopausal patients. For the latter group, a GnRH analogue was administered along with study treatments. Three out of 6 (50.0%) studies were set in first line, while the remaining (50.0%) were set in first or second line. Five (83.0%) studies were phase III RCT and 1 (17.0%) was a phase II trial. The experimental arms included in these trials were fulvestrant plus ribociclib, palbociclib or abemaciclib, letrozole plus palbociclib or ribociclib, and a nonsteroidal aromatase inhibitor or tamoxifen plus ribociclib. Trial characteristics and main outcomes are reported in Table 1 and full results are shown in Table 2. An overall pooled OS benefit was observed for CDK4/6-inhibitor combinations compared with standard ET (HR = 0.76, 95% CI = 0.68 to 0.85, I2 = 0.0%; Supplementary Figure 2 available online).
Table 1.
Characteristics and results of published randomized phase II or III trials of CDK4/6-inhibitors combined with ET in HR+/HER2-negative MBC
| Features | Published randomized Phase II or III trials |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PALOMA 1 | PALOMA 2 | PALOMA 3 | MONALEESA 2 | MONALEESA 7 | MONALEESA 3 | MONARCH 3 | MONARCH 2 | MONARCH plus | |
| Phase | II | III | III | III | III | III | III | III | III |
| No. of patients | 165 | 666 | 521 | 668 | 672 | 726 | 493 | 669 | 463 |
| Treatment | Palbociclib + letrozole vs letrozole | Palbociclib + letrozole vs letrozole - | Palbociclib + fulvestrant vs fulvestrant (+ GnRHa in pre/peri pts) | Ribociclib + letrozole vs letrozole | Ribociclib + tamoxifen or AI + GnRHa vs tamoxifen or AI + GnRHa | Ribociclib + fulvestrant vs fulvestrant | Abemaciclib + NSAI vs NSAI | Abemaciclib + fulvestrant vs fulvestrant (+ GnRHa in pre/peri pts) | Abemaciclib + NSAI or fulvestrant vs NSAI or fulvestrant |
| Menopausal status at moment of trial enrollment | Post | Post | Pre/post | Post | Pre | Post | Post | Pre/post | Post |
| Setting | 1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC | 1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC | ≥1st line HR+ HER2− MBC |
| Median PFS, mo | 20.2 vs 10.2 | 24.8 vs 14.5 | 9.5 vs 4.6 | 25.3 vs 16.0 | 23.8 vs 13.0 | 20.5 vs 12.8 | NR vs 14.7 | 16.4 vs 9.3 | NR and 11.5 vs 14.7 and 5.6 |
| PFS HR (95% CI) | 0.49 (0.32 to 0.75) | 0.58 (0.46 to 0.72) | 0.46 (0.36 to 0.59) | 0.57 (0.46 to 0.70) | 0.55 (0.44 to 0.69) | 0.59 (0.48 to 0.73) | 0.54 (0.41 to 0.72) | 0.55 (0.45 to 0.68) | 0.50 (0.35 to 0.72) and 0.38 (0.24 to 0.59) |
| ORRa | 43% vs 33% | 42% vs 35% | 25% vs 11% | 43% vs 29% | 51% vs 36% | 41% vs 9% | 59% vs 44% | 48% vs 21% | 56% and 39% vs 30% and 8% |
| Median OS, mo | 37.5 vs 33.3 | NM | 35.0 vs 28.0 | NR | NR vs 40.9 | NR vs 40.0 | NM | 46.7 vs 37.3 | NM |
| OS HR (95% CI) | 0.81 (0.49 to 1.35) | NM | 0.81 (0.64 to 1.03) | 0.75 (0.52 to 1.08) | 0.71 (0.54 to 0.95) | 0.72 (0.57 to 0.92) | NM | 0.76 (0.61 to 0.95) | NM |
| Journal/Congressb | Lancet Oncol/J Clin Oncol | N Engl J Med | New Engl J Med | Ann Oncol | New Engl J Med | N Engl J Med | J Clin Oncol | JAMA Oncol | Ann Oncol |
| First authorb | Finn RS | Finn RS | Turner NC | Hortobagyi G | Im S-A | Slamon DJ | Goetz MP | Sledge GW | Jiang Z |
| Yearb | 2014/2017 | 2016 | 2018 | 2018 | 2019 | 2019 | 2017 | 2019 | 2019 |
Values are rounded. AI = aromatase inhibitor; CI = confidence interval; ET = endocrine therapy; GnRHa = gonadotropin-releasing hormone agonist; HER2- = human epidermal growth factor receptor 2 negative; HR = hazard ratio; HR+ = hormone receptor positive; MBC = metastatic breast cancer; NM = not mature; NR = not reached; NSAI = nonsteroidal aromatase inhibitor; ORR = overall response rate; OS = overall survival; peri = perimenopausal; PFS = progression-free survival; post = postmenopausal; pre = premenopausal; ESMO = European Society for Medical Oncology; ASCO = American Society of Clinical Oncology.
The citations refer to manuscripts with available OS results unless they have not been published yet.
Table 2.
Full subgroup analyses resultsa
| Variables | No. of Pts | No. of studies | Pooled HR (95% CI) | I2, % | P pooled | P H | P sub. diff. |
|---|---|---|---|---|---|---|---|
| Age, y | 1916 | 3 | 0.77 (0.66 to 0.88) | 12.0 | <.001 | .34 | .49 |
| <65 | 1203 | 3 | 0.80 (0.67 to 0.95) | 0.0 | .01 | .45 | |
| ≥65 | 713 | 3 | 0.71 (0.53 to 0.95) | 44.4 | .003 | .17 | |
| Menopausal status | 3417 | 6 | 0.75 (0.67 to 0.84) | 0.0 | <.001 | .95 | .99 |
| Pre- or perimenopausal | 894 | 3 | 0.76 (0.60 to 0.96) | 0.0 | .02 | .41 | |
| Postmenopausal | 2523 | 5 | 0.76 (0.67 to 0.86) | 0.0 | <.001 | .89 | |
| Bone-only disease | 1577 | 3 | 0.74 (0.62 to 0.89) | 0.0 | <.001 | .61 | .47 |
| Yes | 492 | 3 | 0.82 (0.60 to 1.13) | 0.0 | .23 | .45 | |
| No | 1085 | 2 | 0.71 (0.58 to 0.88) | 0.0 | .002 | .47 | |
| Metastatic sites | 1600 | 3 | 0.77 (0.65 to 0.91) | 0.0 | .002 | .63 | .74 |
| <3 | 891 | 2 | 0.79 (0.62 to 1.01) | 0.0 | .06 | .63 | |
| ≥3 | 709 | 3 | 0.75 (0.60 to 0.94) | 11.6 | .02 | .32 | |
| Previous CT in metastatic setting | 979 | 2 | 0.77 (0.62 to 0.94) | 0.0 | .01 | .74 | .42 |
| Yes | 271 | 2 | 0.85 (0.61 to 1.18) | 0.0 | .34 | .45 | |
| No | 708 | 2 | 0.72 (0.55 to 0.93) | 0.0 | .01 | .82 | |
| Visceral involvement | 2291 | 4 | 0.73 (0.65 to 0.83) | 0.0 | <.001 | .89 | .91 |
| No | 901 | 3 | 0.68 (0.54 to 0.85) | 0.0 | <.001 | .96 | |
| Yes | 1390 | 4 | 0.76 (0.65 to 0.89) | 0.0 | <.001 | .69 | |
| Endocrine sensitivity status | 2834 | 5 | 0.77 (0.68 to 0.86) | 0.0 | <.001 | .73 | .55 |
| Resistance | 1331 | 4 | 0.79 (0.67 to 0.93) | 0.0 | .004 | .45 | |
| Sensitive | 1503 | 4 | 0.73 (0.61 to 0.88) | 0.0 | .001 | .70 |
CI = confidence interval; CT = chemotherapy; HR = hazard ratio; PH = P value for heterogeneity test; Ppooled = P value for the pooled analysis; Psub.diff. = P value for subgroup differences; Pts = patients.
Visceral Involvement Status
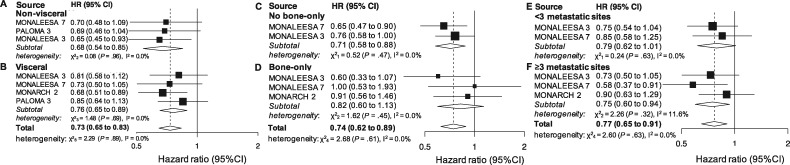
A subgroup analysis in patients without visceral involvement was provided in 3 studies (901 patients). The cumulative effect was statistically significant (HR = 0.68, 95% CI = 0.54 to 0.85, P < .001, I2 = 0.0%; Figure 1A). Four studies (1390 patients) reported OS results for patients with visceral involvement. The cumulative effect was statistically significant (HR = 0.76, 95% CI = 0.65 to 0.89, P < .001, I2 = 0.0%; Figure 1B). When combining all the patients involved in the subgroup analyses, the result was overall statistically significant (HR = 0.73, 95% CI = 0.65 to 0.83, P < .001, I2 = 0.0%; Figure 1) and the 2 groups did not statistically significantly differ (P = .91).
Figure 1.
Pooled overall survival (OS) according to metastatic sites and tumor burden. Pooled OS in nonvisceral (A), visceral (B), no bone-only (C), or bone-only (D) disease and in case of less than 3 (E) and 3 or more metastatic sites (F). CI = confidence interval; HR = hazard ratio.
Bone-Only Status
Two studies (1085 patients) reported OS results for patients without bone-only disease. The cumulative effect was statistically significant (HR = 0.71, 95% CI = 0.58 to 0.88, P = .002, I2 = 0.0%; Figure 1C). Three studies reported results for bone-only disease (492 patients). A non-statistically significant cumulative benefit was observed (HR = 0.82, 95% CI = 0.60 to 1.13, P = .23, I2 = 0.0%; Figure 1D). When combining all the patients involved in the subgroup analyses, the result was overall statistically significant (HR = 0.74, 95% CI = 0.62 to 0.89, P < .001, I2 = 0.0%; Figure 1) and there was no statistically significant difference between the 2 groups (P = .47).
Number of Metastatic Sites
Two studies reported results for patients with less than 3 metastatic sites (891 patients). An almost statistically significant cumulative effect was observed (HR = 0.79, 95% CI = 0.62 to 1.01, P = .06, I2 = 0.0%; Figure 1E). Three studies reported results for patients with at least 3 metastatic sites (709 patients). The cumulative effect was statistically significant (HR = 0.75, 95% CI = 0.60 to 0.94, P = .02, I2 = 11.6%; Figure 1F) as well as the result obtained when joining the 2 subpopulations (HR = 0.77, 95% CI = 0.65 to 0.91, P = .002, I2 = 0.0%; Figure 1), with no statistically significant between-group difference (P = .74).
Endocrine Sensitivity Status
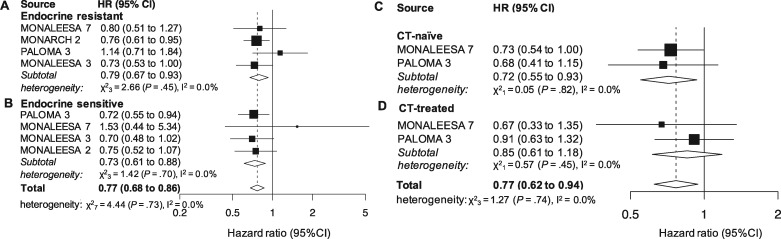
Four studies provided results for the endocrine resistant subset (1331 patients). The effect in the subgroup was statistically significant (HR = 0.79, 95% CI = 0.67 to 0.93, P = .004, I2 = 0.0%; Figure 2A).
Figure 2.
Pooled overall survival (OS) according to endocrine resistance status and previous chemotherapy (CT). Pooled OS in patients younger than 65 years (A), 65 years or older (B), postmenopause (C) and pre- or perimenopause (D). CI = confidence interval; HR = hazard ratio.
Four studies (1503 patients) reported results for the endocrine sensitive subset, which was statistically significant as well (HR = 0.73, 95% CI = 0.61 to 0.88, P = .001, I2 = 0.0%; Figure 2B). The overall effect in the joint analysis of the 2 subgroups was also statistically significant (HR = 0.77, 95% CI = 0.68 to 0.86, P < .001, I2 = 0.0%; Figure 2), whereas the between-group difference for the endocrine resistance and sensitive setting was not (P = .55).
Previous CT for Metastatic Disease
Two studies reported results for CT-naïve (708) and CT-pretreated (271) patients in a metastatic setting. A statistically significant cumulative effect was demonstrated for the first (HR = 0.72, 95% CI = 0.55 to 0.93, P = .01, I2 = 0.0%; Figure 2C) but not for the latter group (HR = 0.85, 95% CI = 0.61 to 1.18, P = .34, I2 = 0.0%; Figure 2D). The joint analysis of the 2 subpopulations was statistically significant (HR = 0.77, 95% CI = 0.62 to 0.94, P = .01, I2 = 0.0%; Figure 2), and there was no statistically significant difference between the 2 groups (P = .42).
Age
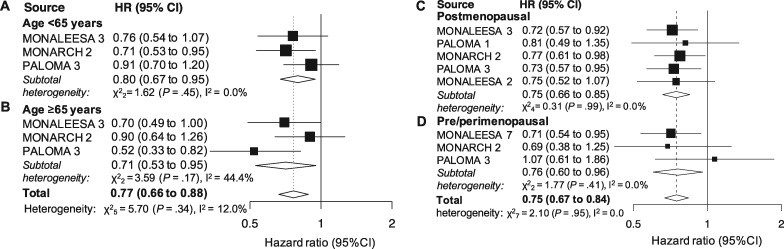
Three studies reported results for patients younger than 65 years and those 65 years and older (1203 and 713 patients, respectively). A statistically significant effect was demonstrated in both subgroups (HR = 0.80, 95% CI = 0.67 to 0.95, P = .01, I2 = 0.0%; and HR = 0.71, 95% CI = 0.53 to 0.95, P = .003, I2 = 44.4%; Figure 3, A and B, respectively). The cumulative effect observed in the joint analysis was statistically significant (HR = 0.77, 95% CI = 0.66 to 0.88, P < .001, I2 = 12.0%; Figure 3), whereas the between group difference was not (P = .49).
Figure 3.
Pooled overall survival (OS)according to age and menopausal status. Pooled OS in patients younger than 65 years (A), 65 years or older (B), postmenopause (C) and pre- or perimenopause (D). CI = confidence interval; HR = hazard ratio.
Menopausal Status
Five studies (2523 patients) provided results for postmenopausal patients. The cumulative effect was statistically significant (HR = 0.76, 95% CI = 0.67 to 0.86, P < .001, I2 = 0.0%; Figure 3C). Three studies (894 patients) provided results for the pre- or perimenopausal setting. The pooled result was statistically significant as well (HR = 0.76, 95% CI = 0.60 to 0.96, P = .02, I2 = 0.0%; Figure 3D). The overall effect in the joint analysis was statistically significant (HR = 0.75, 95% CI = 0.67 to 0.84, P < .001, I2 = 0.0%; Figure 3) with no difference observed between subgroups (P = .99).
Risk of Bias Analysis
The studies included in our analyses did not show any relevant risk of bias within the 7 domains considered. Risk of bias pooled results are reported in Supplementary Figure 3 (available online). A detailed assessment for each single study is reported in Supplementary Figure 4 (available online).
Discussion
We focused our meta-analysis on specific subgroups of clinical relevance. Results show for the first time, to our knowledge, that CDK4/6-inhibitors plus ET combinations, compared with ET monotherapy, improve OS in HR+/HER2-negative MBC as first- or second-line treatment independent of age (<65 vs ≥65 years), menopausal status (pre- or peri- vs postmenopausal), endocrine sensitivity (sensitive vs resistant), and visceral involvement. More specifically, we observed a 24% and 32% relative reduction in the risk of death for patients with or without visceral metastasis, respectively, which was also accompanied by a statistically significant 29% risk reduction in patients without bone-only disease, irrespective of other metastatic sites. The OS benefit was comparable in both pre- or peri- and postmenopausal settings, with statistically significant 24% relative decreases in the risk of death in both cases. Notably, CDK4/6-inhibitor–based therapies produced a statistically significant relative reduction in the risk of death of 20% and 29% for patients younger than 65 years and those 65 years and older, respectively. Given the acceptable and manageable toxicity profile, it is reassuring that CDK4/6-inhibitor combinations also proved to be statistically significantly effective in older patients, confirming and strengthening results from a previous meta-analysis of targeted agents combined with standard ET in elderly patients based on PFS as survival endpoint (26). Importantly, CDK4/6-inhibitor–based treatments were also able to statistically significantly reduce the risk of death by 21% in an endocrine resistance setting and 27% in endocrine sensitive setting. Notably, in the endocrine-resistant subgroup, the only statistically significant individual result was the one obtained within the MONARCH 3 study, which specifically enrolled endocrine-resistant patients to be treated with an abemaciclib-based combination. In this trial, endocrine resistance was defined according to the previously mentioned ESO-ESMO definition (20). Differently, in the endocrine-sensitive setting, only the palbociclib-containing PALOMA 3 trial was associated with a statistically significant result, and no abemaciclib-containing study was available for this analysis. Additionally, palbociclib and ribociclib proved, overall, to produce a statistically significant relative decrease in the risk of death for CT-naïve patients in the metastatic setting (28% death risk reduction) and also in patients with at least 3 metastatic sites (25% death risk reduction).
On the other hand, the observed OS benefits in patients with less than 3 metastatic sites (21% death risk reduction) or bone-only disease (18% death risk reduction) and in CT-pretreated patients in an advanced setting (15% death risk reduction) were not statistically significant. However, these data must be put into context. Firstly, it is important to point out that each of the clinical subsets examined had a different sample size and biological plausibility for a different effect size (as reflected by different HR). This also translates in a different power to identify a statistically significant treatment effect. In fact, it is highly likely that the analysis regarding the CT-pretreated subgroup was negatively affected by the low number of patients (94 of 572 and 177 of 521 from MONALEESA 7 and PALOMA 3 trials, respectively), probably insufficient to demonstrate a clear benefit in terms of OS. Additionally, when putting together CT-naïve and CT-pretreated patients, the cumulative effect observed was a statistically significant 23% relative reduction in the risk of death, with no statistically significant difference between the 2 groups, suggesting that CDK4/6-inhibitor–based treatments are effective in both subsets, albeit a more pronounced effect could be obtained in CT-naïve patients. Furthermore, a posthoc subgroup analysis of the recently published Young-PEARL phase II trial comparing palbociclib + exemestane or fulvestrant vs capecitabine in premenopausal patients similarly showed a statistically significantly improved PFS for the CDK4/6-inhibitor arm in patients pretreated with CT for metastatic disease (HR = 0.62, 95% CI = 0.38 to 0.99) with a more uncertain benefit for CT-pretreated patients (HR = 0.82, 95% CI = 0.36 to 1.92) (27). Taken together, these results clearly support the recommendation of international guidelines concerning the need to delay CT in HR+/HER2-negative MBC, except in case of visceral crisis (20,28), and support the use of CDK4/6-inhibitor–based treatments as upfront therapy.
When considering the subgroup of patients with less than 3 metastatic sites, the result was only marginally non-statistically significant (P = .06, 95% CI = 0.62 to 1.01), reflecting a clear trend for improved survival. Additionally, when joining together patients with less than 3 and at least 3 metastatic sites, the pooled effect was statistically significant, with a meaningful 31% relative reduction in the risk of death and a statistically non-significant test for subgroup differences (P = .74), suggesting a potentially more pronounced effect in patients with higher tumor burden compared with patients with a low tumor burden metastatic disease. Similarly, the OS benefit obtained with CDK4/6-inhibitor–based combinations in bone-only disease was not statistically significant. In fact, patients with bone-only metastatic tumors usually show a more indolent and less rapidly evolving disease, with an improved survival over patients with other metastatic sites (29). Therefore, it is highly likely that more patients, longer follow-up, and more events might be needed to obtain more conclusive results. Moreover, within pivotal trials the interaction tests between treatment effect and metastatic sites were not statistically significant. Additionally, another meta-analysis demonstrated a statistically significantly improved PFS for CDK4/6-inhibitor–based therapies as first line in bone-only metastatic disease (29), further confirmed by a patient-level pooled analysis from the US Food and Drug Administration (30). Furthermore, when taken together with the result obtained in the subset of patients with no bone-only tumors, the benefit was clinically meaningful (26% risk reduction) and statistically significant (P < .001), with no statistically significant subgroup difference (P = .55). Overall, this result should thus be interpreted carefully and be updated in the near future with still unpublished OS subgroup data from other CDK4/6-inhibitors trials in order to draw more definitive conclusions.
This meta-analysis has some limitations that need to be addressed. Firstly, some of the subgroups from published trials were not totally identical. More specifically, it was not possible to extract a clean result concerning all the patients untreated with CT in metastatic setting due to different subgroup characterization, which led to a potential underestimation of the number of patients untreated with CT specifically in the metastatic setting (this happened for the PALOMA 3 study). Similarly, for visceral and nonvisceral disease, both MONALEESA 3 and 7 studies used the categorization “liver or lung involvement” instead of visceral and nonvisceral. Secondly, data about crossover after progression in the mono-ET arms have not been reported, except for MONALEESA 2 trial, where crossover was explicitly not admitted. Therefore, its impact on survival outcomes could not be clearly elucidated. Furthermore, subgroup analyses differed among trials; thus, for each of the subgroups considered, not all of the RCT could be included. In fact, for some of the trials included (7,16,17), OS data were published before final analysis, possibly because more mature results for worse subgroups (patients with visceral metastases, primary endocrine resistance) provided more OS events that drove the early stopping rules data. This might have produced a theoretical risk of “enriched” meta-analysis in positive trials because a negative trial is only published at the time of the final analysis. Therefore, intention-to-treat and subgroup OS data from the remaining PALOMA 2 and MONARCH 3 trials and final OS results from the MONALEESA 2, and MONARCH 2 trials are awaited. Nevertheless, based on PFS data and current results, we expect a substantial confirmation of the effects already observed, specifically in the subgroups with non-statistically significant results (e.g. tumors with very limited tumor burden and bone-only disease) for which current data might not be sufficiently mature. In fact, more patients and longer follow-up might be needed to observe a statistically significant effect. At the same time, due to the potentially more indolent disease course, it cannot be excluded that patients with bone-only metastases or very small tumor burden may experience prolonged PFS and OS even when receiving ET alone initially.
To our knowledge, this is the first meta-analysis exploring the impact and benefit of CDK4/6-inhibitor–based regimens on OS in specific clinically relevant subgroups. Results from our study address clinically relevant questions that might help the clinicians in better tailoring patients’ treatments. In this perspective, we also would like to point out that a study-level meta-analysis like ours, compared with patient-level meta-analysis, provides more rapid results and does not need large, time-consuming, and potentially more expensive collaborations between major competitors to obtain individual patients’ data from each trial, making it more suitable for addressing clinically relevant questions in a reasonable timeline. What most, results were not affected by significant heterogeneity and, overall, there was no truly relevant risk of bias concerning the included trials. It is also noteworthy that when the meta-analysis is based on only a few studies (2 or 3), the heterogeneity is difficult to estimate and standard random-effects meta-analysis methods are usually performed even if the obtained results may be influenced by the small number of studies (wide pooled confidence intervals). Nevertheless, it is unclear whether or to what extent small-sample-size behavior could be improved by more sophisticated modeling systems.
In conclusion, CDK4/6-inhibitors plus ET combinations are substantially effective in improving OS in HR+/HER2-negative MBC as first- or second-line treatment in young or adult (<65 years) as well as in older patients independently from visceral involvement, endocrine sensitivity, and menopausal status. Ribociclib-based combinations might be preferred for the premenopausal setting, because the major contribution on the overall positive subgroup analysis result came from the ribociclib-based MONALEESA 7 trial, which specifically enrolled pre- and perimenopausal patients (a total of 672), whereas the other studies included only contributed with relatively small subgroups of the overall patients enrolled (108 and 114 for PALOMA 3 and MONARCH 3, respectively). On the other hand, abemaciclib-based combinations might be preferred for endocrine-resistant tumors, being the only CDK4/6-inhibitor clearly providing a statistically significant effect in this subset. However, it must be considered that this is only speculative, because no currently published data support the superiority of 1 of the 3 molecules, or the same CDK4/6-inhibitor with a different ET companion (AI, fulvestrant or tamoxifen), over the others (10,31). Furthermore, the degree of benefit shown across pivotal trials for the intention-to-treat populations is quite similar (3–9). Standard ET without CDK4/6-inhibitors might still be an option for bone-only and very limited disease given a more uncertain OS benefit. However, a clear PFS benefit demonstrated elsewhere (29,30) and a the current OS pooled analysis being substantially under-powered suggest that more data are needed to draw definitive conclusions. CDK4/6-based regimens should thus be considered in these subsets as upfront therapy, although they could still be used as a second-line option in case of different first-line treatment choice. Finally, it could be preferable avoiding CT as the upfront therapy in the metastatic setting. Apart from toxicity, activity, and efficacy concerns reported elsewhere (10,20,28,29,31), our analysis shows that upfront CT might also reduce the beneficial impact on OS for CDK4/6-inhibitor–based treatments.
Overall, our results strongly support the recommendations from major international treatment guidelines (20,28) and recent pooled analyses (10,30–32).
Funding
There was no funding source for this study.
Notes
Disclosures: FS has declared travel and accommodation expenses paid by Pfizer and Celgene. MG, GA and SDP have declared honoraria from Roche, Pfizer, Astra-Zeneca, Novartis, Celgene, Eli Lilly, Amgen and Eisai. AP has declared an immediate family member being employed by Novartis, personal honoraria from Pfizer, Novartis, Roche, MSD Oncology, Lilly and Daiichi Sankyo, travel, accommodations and expenses paid by Daiichi Sankyo, research funding from Roche and Novartis, consulting/advisory role for NanoString Technologies, Amgen, Roche, Novartis, Pfizer and Bristol-Myers Squibb and patent PCT/EP2016/080056: HER2 AS A PREDICTOR OF RESPONSE TO DUAL HER2 BLOCKADE IN THE ABSENCE OF CYTOTOXIC THERAPY. GJ has reported grants, personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Lilly, personal fees from Celgene, personal fees and non-financial support from Amgen, personal fees and non-financial support from BMS, personal fees from Puma Technology, personal fees and non-financial support from Astra-Zeneca, personal fees from Daiichi Sankyo, personal fees from Abbvie, outside the submitted work. LDM has declared honoraria from Roche, Pfizer, Ipsen, Eli Lilly, Eisai, Novartis, Takeda and MSD, consulting/advisory role for Roche and Eli Lilly, travels, accommodations and expenses from Roche, Pfizer and Celgene. MDL has declared consulting fees from Pfizer, Novartis, Eli Lilly, Roche, Eisai, Celgene. MC has declared consulting fees from Novartis and Pfizer. FP has declared honoraria for advisory boards, activities as a speaker, travel grants, research grants from Amgen, Astra-Zeneca, Celgene, Eisai, Eli Lilly, Ipsen, MSD, Novartis, Pierre-Fabre, Pfizer, Roche and Takeda, and research funding from Astra-Zeneca, Eisai, Roche and Italian Ministry of Health. PFC has declared consultant role for Novartis, Eli Lilly, Astra Zeneca and Tesaro, honoraria from BMS, Roche, Eli Lilly, Novartis and AstraZeneca, research funding from Novartis, Roche, BMS, Merck-KGa, Italian Ministry of Health, Veneto Secretary of Health and University of Padova. IP has declared honoraria for advisory boards, activities as a speaker, and travel grants from Eisai, Eli Lilly, Ipsen, Italfarmaco, Novartis, Pierre-Fabre, Pfizer, Roche, Gentili and Astra-Zeneca. DJ has served as a consultant/advisory board at Novartis, Genentech, Eisai, Ipsen, and EMD Serono. AL-C reports grants, personal fees and non- financial support from Novartis, Roche, AstraZeneca, Eli Lilly, and Pfizer; grants and non-financial support from EISAI, grants and personal fees from Genomic Health, GlaxoSmithKline Tesaro, personal fees from MSD, personal fees and non-financial support from Bristol-Myers Squibb. ADL has reported advisory board, consultant and honoraria from AZ, Bayer, Celgene, Daiichi-Sankyo, Eisai, Genomic Health, Genentech, Ipsen, Lilly, Novartis, Puma Biotechnology, Pfizer and Roche. DG has declared consulting fees from Novartis, Lilly and Pfizer, research funding from LILT, Novartis Astra-Zeneca and University of Trieste. The other authors have nothing to declare.
Supplementary Material
References
- 1. Schettini F, Buono G, Cardalesi C, Desideri I, De Placido S, Del Mastro L. Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: where we are now and where we are going. Cancer Treat Rev. 2016;46:20–26. [DOI] [PubMed] [Google Scholar]
- 2. Schettini F, De Santo I, Rea CG, et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol. 2018;8:608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. [DOI] [PubMed] [Google Scholar]
- 4. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. [DOI] [PubMed] [Google Scholar]
- 5. Tripathy D, Im S-A, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. [DOI] [PubMed] [Google Scholar]
- 6. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. [DOI] [PubMed] [Google Scholar]
- 7. Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547. [DOI] [PubMed] [Google Scholar]
- 8. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. [DOI] [PubMed] [Google Scholar]
- 9. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35(32):3638–3646. [DOI] [PubMed] [Google Scholar]
- 10. Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20(10):1360–1369. [DOI] [PubMed] [Google Scholar]
- 11. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. [DOI] [PubMed] [Google Scholar]
- 12. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936. [DOI] [PubMed] [Google Scholar]
- 13. Miksad RA, Zietemann V, Gothe R, et al. Progression-free survival as a surrogate endpoint in advanced breast cancer. Int J Technol Assess Health Care. 2008;24(04):371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forsythe A, Chandiwana D, Barth J, Thabane M, Baeck J, Tremblay G. Progression-free survival/time to progression as a potential surrogate for overall survival in HR+, HER2− metastatic breast cancer. Breast Cancer. 2018;10:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sargent DJ, Hayes DF. Assessing the measure of a new drug: is survival the only thing that matters? J Clin Oncol. 2008;26(12):1922–1923. [DOI] [PubMed] [Google Scholar]
- 16. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382(6):514–524. [DOI] [PubMed] [Google Scholar]
- 17. Sledge GW, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Im S-A, Lu Y-S, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316. [DOI] [PubMed] [Google Scholar]
- 19. Tanguy M-L, Cabel L, Berger F, Pierga J-Y, Savignoni A, Bidard F-C. CDK4/6-inhibitors and overall survival: power of first-line trials in metastatic breast cancer. NPJ Breast Cancer. 2018;4(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R-project.org/.
- 24. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. http://handbook.cochrane.org. Accessed March 2011.
- 25.The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan) Copenhagen, Denmark: The Nordic Cochrane Centre;2014.
- 26. Omarini C, Piacentini F, Sperduti I, et al. Endocrine-based targeted combination versus endocrine therapy alone as first-line treatment in elderly patients with hormone receptor-positive advanced breast cancer: meta-analysis of phase II and III randomized clinical trials. J Clin Oncol. 2019;37(15_suppl):1046–1046. [Google Scholar]
- 27. Park YH, Kim T-Y, Kim GM, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15-10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019;20(12):1750–1759. [DOI] [PubMed] [Google Scholar]
- 28. Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor–positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34(25):3069–3103. [DOI] [PubMed] [Google Scholar]
- 29. Toss A, Venturelli M, Sperduti I, et al. First-line treatment for endocrine-sensitive bone-only metastatic breast cancer: systematic review and meta-analysis. Clin Breast Cancer. 2019;19(6):701–716. [DOI] [PubMed] [Google Scholar]
- 30. Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250–260. [DOI] [PubMed] [Google Scholar]
- 31. Wilson FR, Varu A, Mitra D, Cameron C, Iyer S. Systematic review and network meta-analysis comparing palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res Treat. 2017;166(1):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Generali D, Venturini S, Rognoni C, et al. A network meta-analysis of everolimus plus exemestane versus chemotherapy in the first- and second-line treatment of estrogen receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2015;152(1):95–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.