Abstract
The incidence and mortality from colorectal cancer in younger adults (younger than 55 years) is increasing. We reviewed the complete database of a gene-expression test, Oncotype DX Colon Recurrence Score test, to determine age-related differences in recurrence score (RS) and single-gene results (7 cancer-related of the 12-gene assay). We included 20 478 stage II and III A and B colon cancer patients submitted to Genomic Health. RS results were grouped by low-, intermediate-, and high-risk groups. Single-gene scores were described using median and interquartile range. Of all patients 72.5% and 72.6% of those younger than 40 years had low-risk RS. Comparing older with younger patients, RS or single-gene expression did not differ by age group or stage. Young-onset colon cancer does not differ by expression of the RS component genes. Most patients with stage II and III colon cancer have low-risk disease as measured by the 12-gene assay, regardless of age.
Colorectal cancer (CRC) incidence and mortality have been decreasing from 1975 to 2006; however, these trends are influenced mainly by older age groups. Between 2009 and 2013, CRC incidence decreased by 4.6% per year in individuals aged 65 years and older, yet increased in individuals younger than 50 years by 1.6% per year (1). Similarly, CRC death rates for adults younger than 50 years increased by approximately 1% per year from 2005 to 2014 while declining 1–3% per year for older patients. For those aged 20–34 years, when compared with 2010, increasing incidence of 90% by 2030 is predicted (2).
The Oncotype DX Colon Recurrence Score (RS) test (12-gene assay; Genomic Health, Inc., Redwood City, CA) examines individual tumor biology via quantitative expression of three stromal (FAP, INHBA, BGN), three cell cycle (MKI67, cMYC, MYBL2), GADD45B, and five reference genes. It was validated in four prospective clinical trials for stage II and two in stage III colon cancer (3–6). We investigated age-specific biological differences using the 12-gene assay in stages II and III colon cancer.
Samples from patients with stage II or III A and B colon cancer were submitted to the Genomic Health clinical laboratory for the 12-gene assay from January 2010 to February 2018 and analyzed retrospectively. Quantitative expression of RNA extracted from formalin-fixed, paraffin-embedded tissue was determined for the 12 genes via reverse transcription polymerase chain reaction (7). Standard cut-points for low-, intermediate-, and high-recurrence risk groups were less than 30, 30 to 40, and no less than 41. The study cohort (n = 20 478) included 10 316 men and 10 162 women with reported RS results and quantitative single-gene scores (determined by the 12-gene assay), patient age, sex, and disease stage. Patients with known mismatch repair deficiency were not tested or included.
The primary objective was to assess age-specific differences in the RS distributions by comparing results by age group (younger than 40, 40–54, 55–64, 65 years and older) and disease stage (stage II and stage III, younger than 55 and 55 years and older); 95% confidence intervals were calculated using Clopper-Pearson method. Two-sided χ2 tests assessed differences in distribution across age and disease stage. The secondary objective was to assess age-specific differences in component single-gene expression. Single-gene scores were compared for patients aged younger than 55 years vs 55 years and older, stratified by disease stage using the two-sided Wilcoxon rank sum test. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC). A P value of less than .05 was considered statistically significant. Study analysis was deemed minimal risk and approved with waiver of informed consent by the Aspire institutional review board.
Among 20 478 specimens analyzed, 19 018 (92.9%) were stage II and 1460 (7.1%) were stage III; 24.3% (n = 4968) were younger than 55 years and 3.2% (n = 664) younger than 40 years (Supplementary Table 1). In comparison, patients younger than 40 years across the four validation studies with the 12-gene assay ranged from approximately 1–7% (3–6). Overall, 50.4% (n = 10 316) were male, but females younger than 40 years (52.9%; n = 351) were overrepresented.
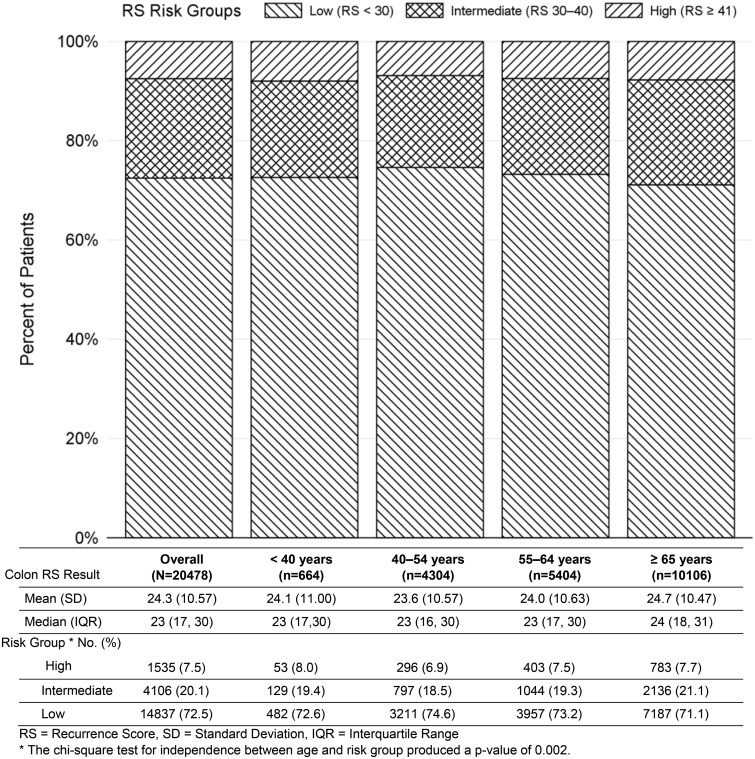
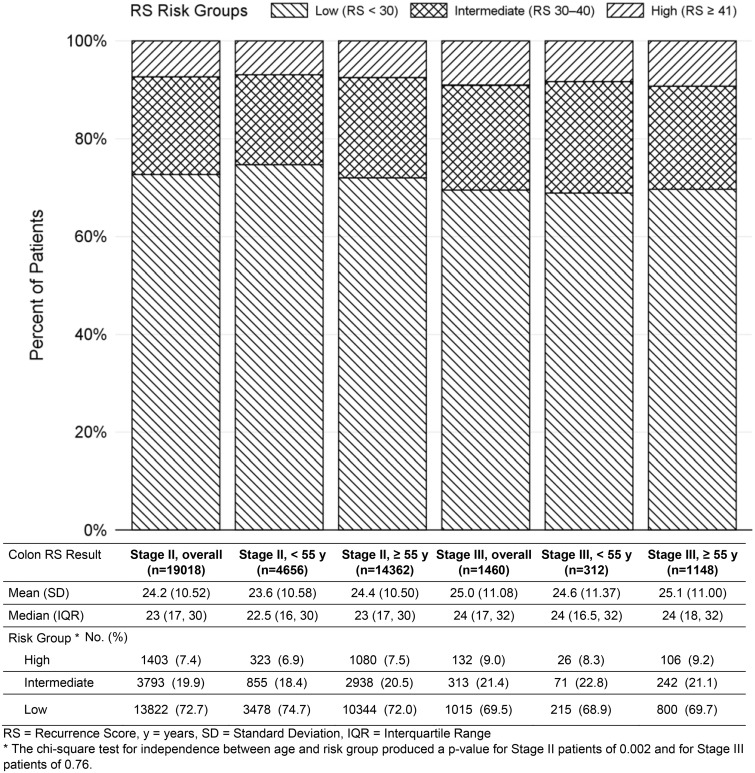
The distribution of RS results was similar across age groups, as shown for patients with RS less than 30, 30–40, and at least 41 and by age group (younger than 40 years, 40–54 years, 55–64 years, and 65 years and older; Figure 1). Overall, 72.5% (n = 14 837) of all patients and 72.6% of patients younger than 40 years were low risk. Across all age groups, 71–75% were low, 19–21% intermediate, and 7–8% high risk of recurrence. The χ2 test for independence between age and risk group produced a P value of .002. Although statistically significant, the minor differences were not clinically meaningful. The median RS overall was 23 (interquartile range [IQR] = 17–30), signifying low risk, and had little variation between groups. When factored by stage and age (younger than 55 years vs 55 years and older), the distribution of RS results remained similar (Figure 2); the median for stage II was 23 (IQR = 17–30) and stage III was 24 (IQR = 17–32). There were no clinically meaningful differences for stage II patients younger than 55 years (median 22.5, IQR = 16–30) compared with 55 years and older (median = 23, IQR = 17–30) or in stage III patients younger than 55 years (median = 24, IQR = 16.5–32) vs 55 years and older (median = 24, IQR = 18–32).
Figure 1.
Distribution of colon recurrence score risk groups by age. The percentage of patients with low risk (<30), intermediate risk (30–40), and high risk (≥41) based on the recurrence score (RS) result are graphically represented in stage II and stage III overall and by stage and age group (stage II younger than 55 years, stage II 55 years and older, stage III younger than 55 years, stage III 55 years and older) as well as the total number of patients within each group represented in the table below. The mean (standard deviation) and median (interquartile range) RS result overall and within each stage and age group are also represented in the table. All statistical tests were two-sided. IQR = interquartile range; SD = standard deviation. *The χ2 test for independence between age and risk group produced a P value for stage II patients of .002 and for stage III patients of .76.
Figure 2.
Distribution of colon recurrence score risk groups by age and stage. The percentage of patients with low risk (<30), intermediate risk (30–40), and high risk (≥41) based on the recurrence score (RS) result are graphically represented in stage II and stage III overall and by stage and age group (stage II younger than 55 years, stage II 55 years and older, stage III younger than 55 years, stage III 55 years and older) as well as the total number of patients within each group represented in the table below. The mean (standard deviation) and median (interquartile range) RS result overall and within each stage and age group are also represented in the table. All statistical tests were two-sided. IQR = interquartile range; SD = standard deviation. *The χ2 test for independence between age and risk group produced a P value of .002.
We quantitated the expression levels of the seven colon cancer-related genes of the 12-gene assay, comparing median single-gene expression in younger (younger than 55 years) and older (55 years and older) patients and by disease stage. P values from the Wilcoxon rank sum test ranged from less than .001 to .96. However, there were no meaningful differences in gene expression in all seven genes for younger vs older patients. The gene expression was similar in stage II and stage III patients and by age (Supplementary Figure 1, available online).
Young-onset CRC is increasing in incidence and mortality for uncertain factors. Although some molecular alterations, including increased rates of P53 and CTNNB1 alterations with decrease of APC, KRAS, BRAF, and FAM123B, have been identified, much is still unknown regarding the role of age in colorectal carcinogenesis and treatment outcomes (8–10). In this study, Oncotype DX Colon RS results and single-gene expression were analyzed within specified age groups (younger than 40, 40–54, 55–64, 65 years and older) and by stage (stage II and stage III younger than 55 years and 55 years and older) identifying no differences in recurrence-associated gene expression.
These data represent a select subset of stage II and III colon cancer, as referred by their clinicians; if biased, younger-aged and higher-risk colon cancer would be overrepresented. Although outcome data are not collected for this lab test, the data represent the complete, very large sample of the Genomic Health clinical laboratory experience. We show that RS distribution and individual cancer-related gene expression within the 12-gene assay are similar between younger and older individuals, regardless of stage. The large size of this sample would be sufficient to show clinically meaningful age-related differences in recurrence-associated genes, if present. The small magnitude differences of statistical significance comparing across all groups (Supplementary Figure 1, available online) result from the large sample size, not clinically meaningful differences. This large sample of RS gene-expression with well-documented demographics and rigorous controls does not demonstrate differences based on age and stage using the 12-gene assay.
In summary, young age-at-onset mismatch repair-proficient colon cancer is not associated with worse tumor biology in the RS result or individual gene expression using the 12-gene assay. Treatment decisions for younger colon cancer populations should not be based solely on age but risk of recurrence based on tumor biology, in conjunction with the patient’s personal values and preferences.
Funding
This work was supported by Genomic Health, Inc.
Notes
The funder did not have a role in the design, collection, analysis, interpretation of the data, writing of the manuscript, and decision to submit for publication. CAR, MBT, MT, JPB, and AL are employed by, and own stock in, Genomic Health, Inc. GJC, YNY, and HSH declare no relevant conflicts.
This work was previously presented at the American Society of Clinical Oncology: Gastrointestinal Cancers Symposium 2018 and the Society of Surgical Oncology Annual Cancer Symposium 2018.
Supplementary Material
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER 18 Regs Research Data, Nov 2015 Sub (2000-2013) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.seer.cancer.gov. Published 2016. Accessed March 3, 2018.
- 2. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29(35):4611–4619. [DOI] [PubMed] [Google Scholar]
- 4. Venook AP, Niedzwiecki D, Lopatin M, et al. Biologic determinants of tumor recurrence in stage II colon cancer: validation study of the 12-gene recurrence score in cancer and leukemia group B (CALGB) 9581. J Clin Oncol. 2013;31(14):1775–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamanaka T, Oki E, Yamazaki K, et al. 12-gene recurrence score assay stratifies the recurrence risk in stage II/III colon cancer with surgery alone: the SUNRISE study. J Clin Oncol. 2016;34(24):2906–2913. [DOI] [PubMed] [Google Scholar]
- 6. Yothers G, O’Connell MJ, Lee M, et al. Validation of the 12-gene colon cancer recurrence score in NSABP C-07 as a predictor of recurrence in patients with stage II and III colon cancer treated with fluorouracil and leucovorin (FU/LV) and FU/LV plus oxaliplatin. J Clin Oncol. 2013;31(36):4512–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark-Langone KM, Sangli C, Krishnakumar J, et al. Translating tumor biology into personalized treatment planning: analytical performance characteristics of the Oncotype DX Colon Cancer Assay. BMC Cancer. 2010;10(1):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 2015;150(5):402–409. [DOI] [PubMed] [Google Scholar]
- 9. Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg. 2017;152(5):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy CC, Sanoff HK, Stitzenberg KB, et al. Patterns of sociodemographic and clinicopathologic characteristics of stages II and III colorectal cancer patients by age: examining potential mechanisms of young-onset disease. J Cancer Epidemiol. 2017;2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.