Abstract
Background
Carcinoembryonic antigen (CEA) levels are used in conjunction with imaging to monitor response to systemic therapy in metastatic colorectal cancer (mCRC). We sought to identify a threshold for CEA change from baseline to predict progressive disease (PD) in mCRC patients receiving first-line therapy.
Methods
Patients from trials collected in the ARCAD database were included if baseline CEA was at least 10 ng/mL and repeat CEA was available within 14 days of first restaging scan. Optimal cutoffs for CEA change were identified by receiver operating characteristic analysis. Prediction performance of cutoffs was evaluated by sensitivity, specificity, and negative predictive value. Analyses were conducted by treatment class: chemotherapy alone, chemotherapy with anti-VEGF antibody, and chemotherapy with anti-EGFR antibody.
Results
A total of 2643 mCRC patients treated with systemic therapy were included. Median percent change of CEA from baseline to first restaging for patients with complete response, partial response, or stable disease (non-PD) and PD was −53.1% and +23.6% for chemotherapy alone (n = 957) and −71.7% and −45.3% for chemotherapy with anti-VEGF antibody (n = 1355). The optimal area under the curve cutoff for differentiating PD from non-PD on first restaging was −7.5% for chemotherapy alone and −62.0% for chemotherapy with anti-VEGF antibody; chemotherapy alone, adjusted odds ratio = 6.51 (95% CI = 3.31 to 12.83, P < .001), chemotherapy with anti-VEGF antibody, adjusted odds ratio = 3.45 (95% CI = 1.93 to 6.18, P < .001). A 99% negative predictive value clinical cutoff for prediction of non-PD would avoid CT scan at first restaging in 21.0% of chemotherapy alone and 16.2% of chemotherapy with anti-VEGF antibody–treated patients. Among patients with stable disease on first restaging, those with decreased CEA from baseline had statistically significantly improved progression-free and overall survival.
Conclusions
Change in CEA from baseline to first restaging can accurately predict non-progression and correlates with long-term outcomes in patients receiving systemic chemotherapy.
The prognosis of metastatic colorectal cancer (mCRC) has improved over the past 20 years because of improvements in systemic therapies (1). First-line treatment for mCRC includes fluorouracil-based chemotherapy in combination with anti-vascular endothelial growth factor (VEGF) or anti-epidermal growth factor receptor (EGFR) antibodies when appropriate (2). In clinical practice, one of the primary objectives of disease management is to identify disease progression during systemic therapy at the earliest time point and to modify the treatment regimen.
Carcinoembryonic antigen (CEA) is a glycoprotein involved in cell adhesion that is elevated in fetal development but present at low levels in the blood of healthy adults and is elevated in approximately two-thirds of CRC patients (2,3). CEA has low sensitivity and specificity for diagnosis of CRC because other non-malignant conditions and environmental exposures can falsely elevate CEA (2,3). Guidelines recommend against use of CEA as a screening or diagnostic tool for CRC (2–4). However, CEA is often used for prognostication before resection of localized CRC as well as postoperative surveillance (2–4). In conjunction with diagnostic imaging, CEA is also used to indirectly monitor response to systemic therapy in mCRC patients.
Several small studies (n < 140) have addressed correlation between CEA and response to systemic chemotherapy (5–10). These studies suggest that CEA may be enough to evaluate response to chemotherapy and limit the need for imaging. However, no study to date has identified a threshold for change in CEA that can be used to identify patients with mCRC who are progressing on systemic therapy.
Patients and Methods
Patients
Patients were identified from the ARCAD database of pooled individual data from first-line prospective, controlled, randomized clinical trials of systemic chemotherapy with or without targeted therapy in mCRC patients (11). Additional eligibility criteria included baseline CEA of at least 10 ng/mL before initiation of treatment and CEA levels available at time of first restaging scan along with RECIST 1.0 response. Restaging scans were required to be within 3 weeks of protocol-specified schedule and CEA within 14 days of restaging scan. Patients gave written informed consent at time of enrollment on trials. The current retrospective analyses were approved by the Mayo Clinic Institutional Review Board.
Objectives
The primary aim was to investigate whether change in CEA from baseline (before treatment initiation) to time of first restaging scan could predict progressive disease (PD) vs stable disease, partial response (PR), or complete response per RECIST 1.0 criteria on the first restaging scan in patients receiving first-line therapy for mCRC (12). We aimed to identify a threshold for percent change in CEA from baseline to first restaging scan to predict disease control status. We also identified the number of restaging scans that could be avoided using a threshold for CEA increase or reduction to identify progression or non-progression, respectively.
Secondary aims included evaluating the association between CEA change at first restaging and long-term patient outcomes; evaluating CEA change in patients with radiographic stable disease as a predictor of progression-free survival (PFS) or overall survival (OS); and evaluating accuracy of CEA thresholds at predicting stable disease, PR, or complete response (ie, non-PD) on the second restaging scan.
Statistical Analysis
Analyses were conducted separately by treatment class: chemotherapy alone; chemotherapy + anti-VEGF antibody; and, chemotherapy + anti-EGFR antibody. Within each treatment class, box plots were used to visualize the correlation between percentage change in CEA and RECIST response and logistic regression with smoothing splines. The cutoff for percentage change in CEA was selected using two approaches. Optimal cutoff was determined by receiver operating characteristic analysis to maximize the Youden index (ie, sensitivity + specificity − 1). In addition, a clinical cutoff associated with 99% negative predicted value (NPV) was determined. NPV measures the percentage of true non-progressions among those who are predicted to have non-PD. Using each of the above two criteria, the prediction performance of the selected cutoffs was evaluated by sensitivity, specificity, positive predicted value, and NPV. Moreover, adjusted odds ratios (ORadj) and the corresponding 95% confidence intervals (CIs) were estimated using the logistic regression model. Analysis results were validated using the bootstrapping method. Multivariable logistic models were used to assess associations after adjusting for age, sex, Eastern Cooperative Oncology Group performance status, and prior chemotherapy. OS was defined as time from randomization to death due to any cause. PFS was defined as time from randomization to progression or death due to any cause, whichever occurred first. The distributions of time-to-event outcomes were estimated using Kaplan-Meier methods and compared between CEA change below and above the cut point using stratified log-rank test by treatment arm. Hazard ratios and 95% confidence intervals were estimated using Cox proportional hazards model. Multivariable Cox proportional hazards models were used to assess adjusted associations, with adjustment for the same confounding factors indicated above in the multivariable logistic models. All analyses were conducted using two-sided tests with a statistical significance level of .05.
Results
Descriptive Statistics
This is a pooled analysis of 2643 patients identified from seven clinical trials completed between January 10, 2000, and August 19, 2008 (Table 1; Supplementary Figure 1) (13–19). Classification of the study population based on treatment type is shown in Table 1. Timing of first restaging scan from time of randomization varied across trials (6 weeks: AGITG, HORIZON II; 8 weeks: OPTIMOX 1, OPTIMOX 2, PRIME, HORIZON III; 12 weeks: PACCE). Results from patients treated with chemotherapy in combination with anti-EGFR antibody are shown in Supplementary Figures 2–4 and Supplementary Tables 1–3. Results from patients treated with chemotherapy with anti-VEGF plus anti-EGFR antibodies are not included because of small sample size and because this combination is not recommended.
Table 1.
Baseline patient demographics and clinical characteristics (n = 2643 patients)*
| Characteristics | Chemo+anti-EGFR (n = 331) | Chemo+anti-VEGF (n = 1355) | Chemo (n = 957) | Total (n = 2643) |
|---|---|---|---|---|
| Age at enrollment, years | ||||
| Median | 63.0 | 59.0 | 62.0 | 61.0 |
| Range | 27.0–81.0 | 20.0–86.0 | 24.0–82.0 | 20.0–86.0 |
| ECOG performance status, n (%) | ||||
| Unknown | 0 | 1 | 0 | 1 |
| 0 | 179 (54.1) | 776 (57.3) | 513 (53.6) | 1468 (55.6) |
| 1 | 135 (40.8) | 569 (42.0) | 396 (41.4) | 1100 (41.6) |
| 2+ | 17 (5.1) | 9 (0.7) | 48 (5.0) | 74 (2.8) |
| Sex, n (%) | ||||
| Female | 115 (34.7) | 564 (41.6) | 380 (39.7) | 1059 (40.1) |
| Male | 216 (65.3) | 791 (58.4) | 577 (60.3) | 1584 (59.9) |
| CEA at baseline, ng/mL | ||||
| Median | 113.5 | 94.8 | 99.8 | 98.5 |
| Range | 10.3–19 000.0 | 10.0–61 250.0 | 10.0–23 800.0 | 10.0–61 250.0 |
| CEA at first restaging, ng/mL | ||||
| Median | 23.7 | 24.4 | 46.0 | 32.0 |
| Range | 0.2–5660.4 | 0.0–27 800.0 | 0.7–13 610.0 | 0.0–27 800.0 |
| RECIST at first restaging, n (%) | ||||
| CR | 1 (0.3) | 4 (0.3) | 1 (0.1) | 6 (0.2) |
| PR | 144 (43.5) | 420 (31.0) | 352 (36.8) | 916 (34.7) |
| Stable disease | 168 (50.8) | 877 (64.7) | 537 (56.1) | 1582 (59.9) |
| PD | 18 (5.4) | 54 (4.0) | 67 (7.0) | 139 (5.3) |
| Study, n (%) | ||||
| OPTIMOX1 | 0 (0.0) | 0 (0.0) | 274 (28.6) | 274 (10.4) |
| OPTIMOX2 | 0 (0.0) | 0 (0.0) | 115 (12.0) | 115 (4.4) |
| PACCE (C249) | 0 (0.0) | 186 (13.7) | 0 (0.0) | 186 (7.0) |
| PRIME (C203) | 331 (100.0) | 0 (0.0) | 333 (34.8) | 664 (25.1) |
| AGITG (MAX) | 0 (0.0) | 117 (8.6) | 52 (5.4) | 169 (6.4) |
| HORIZON II | 0 (0.0) | 351 (25.9) | 183 (19.1) | 534 (20.2) |
| HORIZON III | 0 (0.0) | 701 (51.7) | 0 (0.0) | 701 (26.5) |
CEA = carcinoembryonic antigen; CR = complete response; ECOG = Eastern Cooperative Oncology Group; EGFR = epidermal growth factor receptor; PD = progressive disease; PR = partial response; VEGF = vascular endothelial growth factor.
Predicting Response to First-Line Treatment at Time of First Restaging Using CEA
Because of known differences in response kinetics between chemotherapy and targeted therapies, we analyzed outcomes based on treatment type. We found that CEA shows predicted value of PD vs non-PD among patients treated with chemotherapy alone (AUC = 0.79) or chemotherapy with anti-VEGF antibody (AUC = 0.72) (Table 2).
Table 2.
PD prediction by percent change in CEA at first restaging scan for patients treated with chemotherapy alone or chemotherapy + anti-VEGF antibody
| PD prediction by CEA | Change in CEA, % | AUC | Sensitivity, % | Specificity, % | NPV, % | CT scans avoided, % |
|---|---|---|---|---|---|---|
| Cutoff by optimal AUC | ||||||
| Chemo | −7.5 | 0.79 | 71.6 | 76.2 | 97.3 | 72.8 |
| Chemo+anti-VEGF | −62.0 | 0.72 | 66.7 | 63.6 | 97.9 | 62.4 |
| Cutoff by 99% NPV | ||||||
| Chemo | −79.4 | 0.79 | 97.0 | 22.4 | 99.0 | 21.0 |
| Chemo+anti-VEGF | −88.7 | 0.72 | 96.3 | 16.7 | 99.1 | 16.2 |
AUC = area under curve; CEA = carcinoembryonic antigen; CT = computed tomography; NPV = negative predicted value; PD = progressive disease; VEGF = vascular endothelial growth factor.
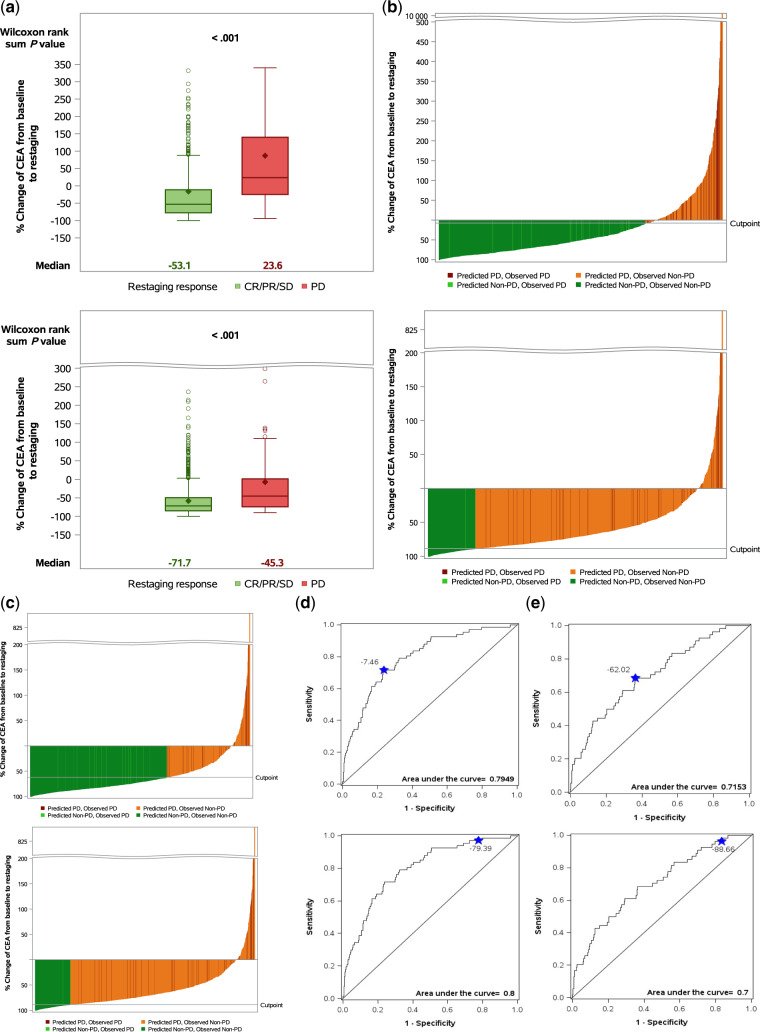
For patients receiving chemotherapy alone (n = 957), median percent change in CEA from baseline to first restaging scan was −53.1% for patients with non-PD and +23.6% for patients with PD (Figure 1A, top). Based on AUC cutoff, CEA reduction by at least 7.5% differentiated between PD and non-PD with sensitivity of 71.6% (95% CI = 59.3% to 82.0%) and specificity of 76.2% (95% CI = 73.2% to 78.9%) (Figure 1B, top; Figure 1D, top; Table 2). Using this cutoff, PD could be excluded in 97.3% (95% CI = 95.8% to 98.4%) of patients and 72.8% of CT scans could be avoided. Patients with an increase in CEA or CEA decrease less than 7.5% from baseline had a 4.7% probability of PD compared with 2.3% in patients with a decrease from baseline greater than 7.5% (ORadj = 6.51, 95% CI = 3.31 to 12.83, P < .001). Based on 99% NPV clinical cutoff, with a CEA decrease of at least 79.4%, PD could be excluded in 99.0% (95% CI = 96.5% to 99.9%) of patients and 21.0% of CT scans could be avoided (Figure 1B, bottom; Figure 1D, bottom; Table 2). Patients with an increase in CEA or CEA decrease less than 79.4% from baseline had a 6.6% probability of PD compared with 0.3% in patients with a decrease from baseline greater than 79.4% (ORadj = 4.98, 95% CI = 1.18 to 21.02, P = .03).
Figure 1.
Using carcinoembryonic antigen (CEA) to predict response to first-line treatment at time of first restaging. A) Percent change from baseline CEA to first restaging scan in patients treated with chemotherapy alone (top) and chemotherapy + anti-vascular endothelial growth factor (VEGF) antibody (bottom). B) Waterfall plots demonstrating predicted and observed response using CEA cutoffs identified with area under the curve (AUC) method (top) and the 99% negative predicted value (NPV) method (bottom) in patients treated with chemotherapy alone. C) Waterfall plots demonstrating predicted and observed response using CEA cutoffs identified with AUC method (top) and the 99% NPV method (bottom) in patients treated with chemotherapy + anti-VEGF antibody. D) Receiver operating characteristic (ROC) curves with AUC method (top) and the 99% NPV method (bottom) in patients treated with chemotherapy alone. E) ROC curves with AUC method (top) and the 99% NPV method (bottom) in patients treated with chemotherapy + anti-VEGF antibody. All tests were two-sided. CR = complete response; PD = progressive disease; PR = partial response; SD = stable disease.
For patients receiving chemotherapy in combination with anti-VEGF antibody (n = 1355), median percent change in CEA from baseline to first restaging was −71.7% for patients with non-PD and −45.3% for patients with PD (Figure 1A, bottom). Based on AUC cutoff, a CEA reduction by at least 62% differentiated between PD and non-PD with a sensitivity of 66.7% (95% CI = 52.5% to 78.9%) and specificity of 63.6% (95% CI = 60.9% to 66.2%) (Figure 1C, top; Figure 1E, top; Table 2). Using this cutoff, PD could be excluded in 97.9% (95% CI = 96.7% to 98.7%) of patients and 62.4% of CT scans could be avoided. Patients with an increase in CEA or CEA decrease less than 62% from baseline had a 2.7% probability of PD compared with 1.3% in patients with a decrease from baseline greater than 62% (ORadj = 3.45, 95% CI = 1.93 to 6.18, P < .001). Based on 99% NPV clinical cutoff, with a CEA decrease of at least 88.7%, PD could be excluded in 99.1% (95% CI = 96.7% to 99.9%) of patients and 16.2% of CT scans could be avoided (Figure 1C, bottom; Figure 1E, bottom; Table 2). Patients with an increase in CEA or CEA decrease less than 88.7% from baseline had a 3.8% probability of PD compared with 0.2% in patients with a decrease from baseline greater than 88.7% (ORadj = 4.62, 95% CI = 1.11 to 19.20, P = .04). These findings were validated using the bootstrapping method; results are summarized in Supplementary Tables 2 and 3. Moreover, the impact of the baseline CEA level was evaluated as a covariate in multivariable analysis and was not statistically significant for any of the treatments (data not shown).
Correlating CEA Change at First Restaging With Long-Term Outcomes
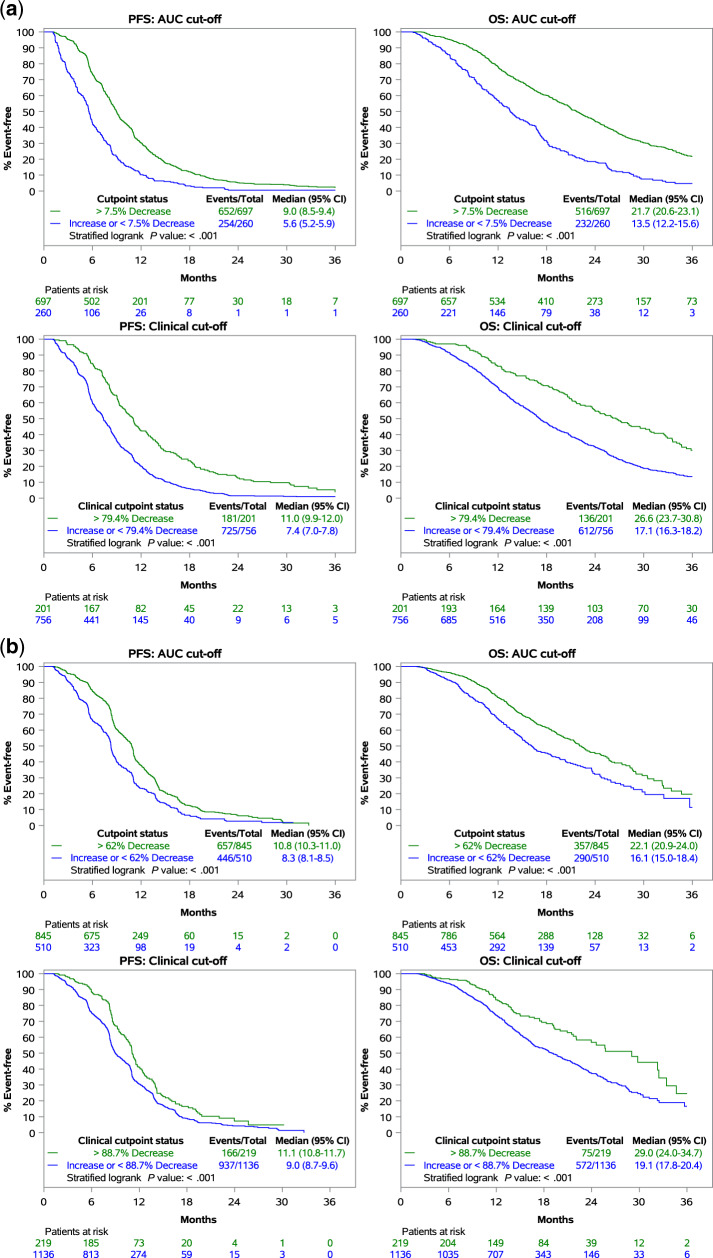
To better understand the prognostic implications of kinetics of CEA change, we analyzed the association between magnitude of change in CEA from baseline to first restaging with long-term outcomes of mCRC patients treated with first-line systemic therapy. For patients receiving chemotherapy alone (n = 957), those with a CEA decrease by at least 7.5% (based on AUC cutoff) had statistically significantly improved PFS (9.0 months, 95% CI = 8.5 to 9.4 months vs 5.6 months, 95% CI = 5.2 to 5.9 months, P < .001) and OS (21.7 months, 95% CI = 20.6 to 23.1 months vs 13.5 months, 95% CI = 12.2 to 15.6 months, P < .001) compared with those with an increase in CEA or CEA decrease less than 7.5% (Figure 2A, top). Patients with a CEA decrease by at least 79.4% (based on 99% NPV clinical cutoff) had statistically significantly improved PFS (11.0 months, 95% CI = 9.9 to 12.0 months vs 7.4 months, 95% CI = 7.0 to 7.8 months, P < .001) and OS (26.6 months, 95% CI = 23.7 to 30.8 months vs 17.1 months, 95% CI = 16.3 to 18.2 months, P < .001) compared with those with an increase in CEA or CEA decrease less than 79.4% (Figure 2A, bottom). For patients receiving chemotherapy with anti-VEGF antibody (n = 1355), those with a CEA decrease by at least 62% (based on AUC cutoff) had statistically significantly improved PFS (10.8 months, 95% CI = 10.3 to 11.0 months vs 8.3 months, 95% CI = 8.1 to 8.5 months, P < .001) and OS (22.1 months, 95% CI = 20.9 to 24.0 months vs 16.1 months, 95% CI = 15.0 to 18.4 months; P < .001) compared with those with an increase in CEA or CEA decrease less than 62% (Figure 2B, top). Patients with a CEA decrease of at least 88.7% (based on 99% NPV clinical cutoff) from baseline had statistically significantly improved PFS (11.1 months, 95% CI = 10.8 to 11.7 months vs 9.0 months, 95% CI = 8.7 to 9.6 months, P < .001) and OS (29.0 months, 95% CI = 24.0 to 34.7 months vs 19.1 months, 95% CI = 17.8 to 20.4 months, P < .001) compared with those with an increase in CEA or CEA decrease less than 88.7% (Figure 2B, bottom).
Figure 2.
Correlating carcinoembryonic antigen (CEA) change at first restaging with long-term outcomes. A) Association between magnitude of change in CEA at first restaging with long-term outcomes of patients treated with chemotherapy alone. B) Association between magnitude of change in CEA at first restaging with long-term outcomes of patients treated with chemotherapy + anti-vascular endothelial growth factor (VEGF) antibody .
CEA Dynamics as a Predictor of Long-Term Outcomes in Patients With Stable Disease on First Restaging
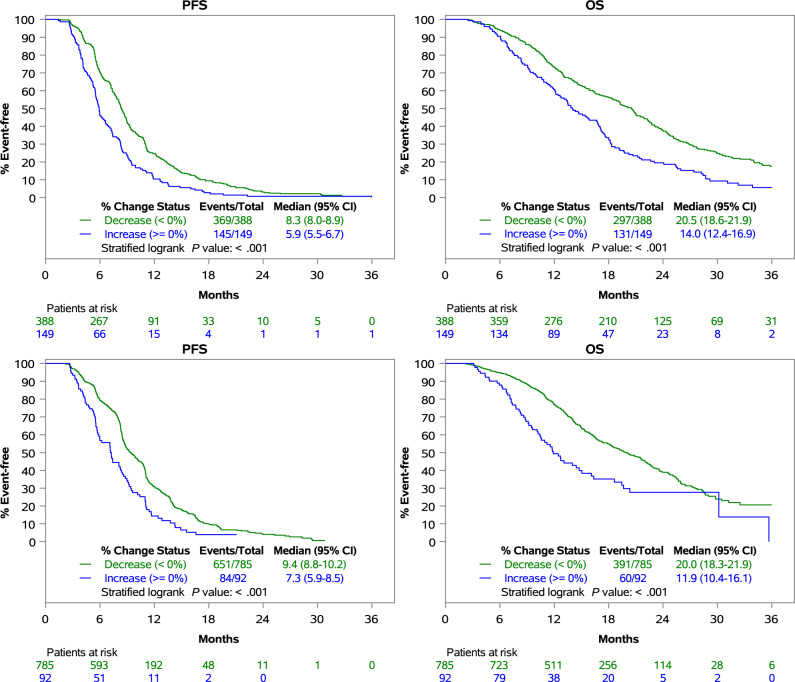
To better understand the prognostic implications of discordant CEA responses in patients with identical RECIST responses, we analyzed the association between CEA dynamics and long-term outcomes of mCRC patients with stable disease on first restaging scan during first-line treatment. For patients receiving chemotherapy alone (n =537), those with a CEA decrease from baseline had statistically significantly improved PFS (8.3 months, 95% CI = 8.0 to 8.9 months vs 5.9 months 95% CI = 5.5 to 6.7 months, P < .001) and OS (20.5 months, 95% CI = 18.6 to 21.9 months vs 14 months, 95% CI = 12.4 to 16.9 months, P < .001) compared with patients with a CEA increase from baseline (Figure 3, top). For patients receiving chemotherapy with anti-VEGF antibody (n = 877), those with a CEA decrease from baseline had statistically significantly improved PFS (9.4 months, 95% CI = 8.8 to 10.2 months vs 7.3 months, 95% CI = 5.9 to 8.5 months, P < .001) and OS (20.0 months, 95% CI = 18.3 to 21.9 months vs 11.9 months, 95% CI = 10.4 to 16.1 months, P < .001) compared with patients with an increase in CEA from baseline (Figure 3, bottom).
Figure 3.
Carcinoembryonic antigen (CEA) dynamics as a predictor of long-term outcomes in patients with stable disease on first restaging. Association between CEA change at first restaging and long-term outcomes of patients treated with chemotherapy alone (top) or chemotherapy + anti-vascular endothelial growth factor (VEGF) antibody (bottom) who had stable disease on the first restaging scan.
Predicting Response to First-Line Treatment at Time of Second Restaging Using CEA
To further validate the identified CEA thresholds, we evaluated the AUC CEA cutoffs at the time of the second restaging. Because of lack of longitudinal data availability, data from 215 patients were analyzed at the second restaging. The comparison of baseline patient demographics between patients who were included and excluded (due to lack of data availability) for the second scan analysis is summarized in Supplementary Table 4 (for patients receiving chemotherapy alone) and Supplementary Table 5 (for patients receiving chemotherapy with anti-VEGF antibody); there were no statistically significant differences between the two populations. We found that CEA showed a predicted value of PD vs non-PD among patients treated with chemotherapy alone (AUC = 0.83) or chemotherapy with anti-VEGF antibody (AUC = 0.77) (Supplementary Table 6).
For patients receiving chemotherapy alone (n = 115), based on AUC cutoff, CEA reduction by at least 51.3% differentiated between PD and non-PD with a sensitivity of 81.1% (95% CI = 68.0% to 90.6%) and specificity of 79.0% (95% CI = 66.8% to 88.3%) (Supplementary Table 6). Using this cutoff, PD could be excluded in 83.1% (95% CI = 71.0% to 91.6%) of patients and 51.3% CT scans could be avoided. Patients with an increase in CEA or CEA decrease less than 51.3% from baseline had a 41.5% probability of PD compared with 9.2% in patients with a decrease of at least 51.3% from baseline (ORadj = 61.69, 95% CI = 8.56 to 444.52, P < .001). Meanwhile, for patients receiving chemotherapy in combination with anti-VEGF antibody (n = 100), based on AUC cutoff, a CEA reduction by at least 60.9% differentiated between PD and non-PD with a sensitivity of 66.0% (95% CI = 50.7% to 79.1%) and specificity of 73.6% (95% CI = 59.7% to 84.7%) (Supplementary Table 6). Using this cutoff, PD could be excluded in 70.9% (95% CI = 57.1% to 82.4%) of patients and 55% CT scans could be avoided. Patients with an increase in CEA or CEA decrease of less than 60.9% from baseline had a 31% probability of PD compared with 16% in patients with a decrease from baseline of greater than 60.9% (ORadj = 6.06, 95% CI = 2.44 to 15.06, P < .001).
For patients receiving chemotherapy alone (n = 115), based on a 99% NPV clinical cutoff, CEA reduction by at least 98.5% differentiated between PD and non-PD with a sensitivity of 100% (95% CI = 93.3% to 100.0%) and specificity of 3.2% (95% CI = 0.4% to 11.2%) (Supplementary Table 6). Using this cutoff, PD could be excluded in 100% (95% CI = 15.8% to 100.0%) of patients and 1.7% of CT scans could be avoided. Meanwhile, for patients receiving chemotherapy in combination with anti-VEGF antibody (n = 100), based on a 99% NPV clinical cutoff, CEA reduction by at least 98.9% differentiated between PD and non-PD with a sensitivity of 100% (95% CI = 92.5% to 100.0%) and specificity of 1.9% (95% CI = 0.0% to 10.1%) (Supplementary Table 6). Using this cutoff, PD could be excluded in 100% (95% CI = NA) of patients and 1.0% of CT scans could be avoided.
Discussion
In this study, we demonstrate that change in CEA from baseline to first restaging can accurately predict non-PD in mCRC patients receiving first-line systemic chemotherapy, either alone or in combination with anti-VEGF antibody. Our findings underscore the prognostic implications of CEA change kinetics during treatment. In both treatment groups (chemotherapy and anti-VEGF), patients who met the threshold for CEA decrease based on AUC or 99% NPV clinical cutoffs had statistically significantly improved PFS and OS compared with patients who did not meet the identified thresholds. Furthermore, we identified different long-term outcomes for patients with discordant CEA responses in the setting of identical RECIST stable disease response at first restaging. The utility of CEA to predict non-PD decreased on the second restaging compared with the first restaging, with lower NPV and fewer CT scans avoided.
There have been several smaller studies (n < 140) that have addressed the correlation of CEA with disease response to systemic chemotherapy in mCRC (5–10). Hanke et al. examined the diagnostic accuracy of CEA in 85 mCRC patients compared with objective response by WHO criteria (5). CEA was found to be elevated (≥10 ng/mL) in 51% of patients, and a CEA increase of at least 50% differentiated between PD and disease control with a sensitivity of 76% and specificity of 90%. With CEA decreases from baseline of at least 30%, PD could be excluded in 99% patients. Similarly, Ward et al. measured CEA levels in 33 patients (6). CEA was elevated in 85% patients at baseline, and it correlated with disease control status with positive predicted value of 54% and 100% for PR and PD, respectively. They concluded that although decreasing levels of tumor markers overestimate the number of responses demonstrated by imaging, increasing tumor markers accurately predict PD. Finally, de Haas et al. studied 113 patients with mCRC to the liver undergoing preoperative chemotherapy and found that 94% of patients with radiologic response or stabilization had similar biological evolution for CEA and 95% of patients with radiologic progression had similar biological evolution of CEA (8).
Our findings suggest that CEA reduction performs better for prediction of non-PD in mCRC patients treated with chemotherapy alone compared with anti-VEGF–treated patients. In particular, median change in CEA to first restaging scan in patients with PD was notably different with chemotherapy (23.6%) compared with anti-VEGF–treated patients (−45.3%). This may reflect differences in imaging response characteristics with use of anti-VEGF agents. Prior studies have reported lack of association between outcomes and radiological response in mCRC patients treated with anti-VEGF antibodies (20,21). In addition to size, morphological criteria (including tumor attenuation and interface between normal-tumor tissues) may aid in reliable response assessment (22). Our findings suggest that evaluation of CEA along with the imaging characteristics may be beneficial in the assessment of response to regimens containing anti-VEGF agents. Since chemotherapeutic agents, including 5-fluorouracil, which was part of the chemotherapy backbone for all seven trials included in this study, can increase CEA expression and shedding (23), an alternative explanation for the findings could be that anti-VEGF treatment may attenuate CEA expression or shedding by tumor cells, as proposed previously by others (24). In comparing the use of CEA at first restaging vs second restaging, the NPV of identified AUC CEA cutoffs and the percentage of CT scans that could be avoided favored the first restaging time point. These findings suggest that the utility of CEA to predict non-PD decreases with increasing time on the same treatment regimen.
There are clear advantages of CEA over imaging studies, because it is inexpensive; requires only a blood draw, thus avoiding unnecessary radiation exposure; and does not require subjective interpretation. The proposed cutoffs for identifying non-PD, in whom CT scans can be avoided, represents a value-based approach to disease monitoring by which health-care resources may be more efficiently utilized. To realize its health economic benefit, CEA could be checked at the start of the chemotherapy cycle before the anticipated time of the routine restaging scan to allow sufficient time for oncologists to determine whether the restaging scan can be safely omitted based on CEA change and allow the scanning slot to be reallocated to another patient. The results of this study are applicable only to patients with unresectable metastatic disease; any patients with potentially resectable metastatic disease should undergo routine restaging scans to determine eligibility for local treatment modalities, including surgery, ablation, or radiation. In addition, this analysis, which to the author’s knowledge represents the largest dataset to date correlating CEA levels with disease response for mCRC, provides benchmarks for upcoming clinical trials of novel tumor-monitoring approaches, such as circulating tumor cells or circulating tumor DNA (25).
The strengths of this study include its large sample size and high data quality. Study limitations include small size of subsets on second restaging scan because of limited data availability, inability to review imaging, and exclusion of patients with baseline CEA < 10 ng/mL (due to variation in upper limit of normal of CEA across sites). The location of primary tumor (right vs left) has recently been identified as a prognostic and predictive factor in the metastatic setting with regard to response to targeted therapies (26). However, we were unable to assess this factor because tumor sidedness was only noted in a small minority of trials. We believe the trials included in this study remain very relevant because they are currently standard of care. When additional data become available to the ARCAD group, validation of these findings in patients receiving other targeted therapies (regorafenib, TAS-102, trastuzumab, dabrafenib) and immunotherapy will be needed to compare with findings from targeted therapy–treated patients included in our study.
In conclusion, change in CEA from baseline to first restaging can accurately predict non-PD in patients receiving systemic chemotherapy. We identify different long-term outcomes based on the magnitude of CEA change for mCRC patients with identical RECIST stable disease response. Use of CEA is a cost-effective method for predicting non-PD during first-line systemic therapy for mCRC.
Funding
This work was supported by the ARCAD foundation.
Notes
We thank the patients who participated in these studies as well as their family members for their help in advancing our knowledge about this malignancy. The ARCAD Foundation provided the database and is credited with its conception, development, data collection from individual trials, and database maintenance.
The authors have no conflicts of interest to declare.
Presented in part at ASCO Annual Meeting 2018 (abstract 369).
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Z, Zhang Y, Niu Y, et al. A systematic review and meta-analysis of diagnostic and prognostic serum biomarkers of colorectal cancer. PLoS One. 2014;9(8):e103910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network Guidelines: Colon Cancer. www.nccn.org. Accessed December 17, 2019. [Google Scholar]
- 5. Hanke B, Riedel C, Lampert S, et al. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA). Ann Oncol. 2001;12(2):221–226. [DOI] [PubMed] [Google Scholar]
- 6. Ward U, Primrose JN, Finan PJ, et al. The use of tumour markers CEA, CA-195 and CA-242 in evaluating the response to chemotherapy in patients with advanced colorectal cancer. Br J Cancer. 1993;67(5):1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grem JL, Steinberg SM, Chen AP, et al. The utility of monitoring carcinoembyronic antigen during systemic therapy for advanced colorectal cancer. Oncol Rep. 1998;5(3):559–567. [DOI] [PubMed] [Google Scholar]
- 8. de Haas RJ, Wicherts DA, Flores E, et al. Tumor marker evolution: comparison with imaging for assessment of response to chemotherapy in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17(4):1010–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang WS, Lin JK, Lin TC, et al. Carcinoembryonic antigen in monitoring of response to systemic chemotherapy in patients with metastatic colorectal cancer. Int J Colorectal Dis. 2001;16(2):96–101. [DOI] [PubMed] [Google Scholar]
- 10. Trillet-Lenoir V, Chapuis F, Touzet S, et al. Any clinical benefit from the use of oncofetal markers in the management of chemotherapy for patients with metastatic colorectal carcinomas? Clin Oncol. 2004;16(3):196–203. [DOI] [PubMed] [Google Scholar]
- 11. Buyse M, Sargent DJ, Goldberg RM, et al. The ARCAD advanced colorectal cancer database: open for business. Ann Oncol. 2012;23(1):281–282. [DOI] [PubMed] [Google Scholar]
- 12. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 13. Tebbutt NC, Wilson K, Gebski VJ, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28(19):3191–3198. [DOI] [PubMed] [Google Scholar]
- 14. Hoff PM, Hochhaus A, Pestalozzi BC, et al. Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX in patients with previously untreated metastatic colorectal cancer: a randomized, double-blind, phase III study (HORIZON II). J Clin Oncol. 2012;30(29):3596–3603. [DOI] [PubMed] [Google Scholar]
- 15. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer-A GERCOR study. JCO. 2006;24(3):394–400. [DOI] [PubMed] [Google Scholar]
- 16. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 study. JCO. 2009;27(34):5727–5733. [DOI] [PubMed] [Google Scholar]
- 17. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705. [DOI] [PubMed] [Google Scholar]
- 18. Schmoll HJ, Cunningham D, Sobrero A, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol. 2012;30(29):3588–3595. [DOI] [PubMed] [Google Scholar]
- 19. Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27(5):672–680. [DOI] [PubMed] [Google Scholar]
- 20. Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. JCO. 2012;30(36):4566–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grothey A, Hedrick EE, Mass RD, et al. Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107. JCO. 2008;26(2):183–189. [DOI] [PubMed] [Google Scholar]
- 22. Mizard T, Boonsirikamchai P, Overman MJ, et al. Comparison of early radiological predictors of outcome in patients with colorectal cancer with unresectable hepatic metastases treated with bevacizumab. Gut. 2018;67(6):1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aquino A, Formica V, Prete SP, et al. Drug-induced increase of carcinoembryonic antigen expression in cancer cells. Pharmacol Research. 2004;49(5):383–396. [DOI] [PubMed] [Google Scholar]
- 24. Abuqayyas L, Balthasar JP. Pharmacokinetic mAb-mAb interaction: anti-VEGF mAb decreases the distribution of anti-CEA mAb into colorectal tumor xenografts. AAPS J. 2012;14(3):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corcoran RB, Chabner B. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754–1765. [DOI] [PubMed] [Google Scholar]
- 26. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.