The coronavirus disease 2019 (COVID-19) pandemic has disrupted established pathways for conducting research worldwide, and its fractious effects could be long-lasting. Cardiovascular drug and device development, which has historically suffered from relative underinvestment, may be particularly susceptible to these system-wide perturbations. Although therapeutic development pipelines are often planned years in advance, trialists and sponsors have been forced to contend with “adapt-or-abandon” decisions over weeks to months during COVID-19 (1). The global nature of the pandemic, together with the dynamic and unpredictable forecast of subsequent waves, has pushed some sponsors to make challenging decisions to prematurely terminate programs. Other trials that were near completion at the start of the pandemic have evaluated potentially important therapies; COVID-19 may lead to delays in their regulatory review. Although many aspects of the COVID-19 pandemic have directly threatened the success of programs from initial target identification to sequential testing to ultimate regulatory approval, innovative solutions and potentially disruptive opportunities have also surfaced that may have a lasting and positive impact on future trial efficiency.
Here, we discuss the central role of regulatory authorities, including the U.S. Food and Drug Administration (FDA), in supporting research adaptations to the realities of the pandemic. Positioned at the heart of the clinical trial enterprise and often viewed as the final arbitrators of therapeutic access, regulatory decision-making has a powerful impact on behavior and responses of multiple stakeholders. Charged with protecting the health of the public, the FDA has been integrally and longitudinally involved in communicating their position and guiding a broad range of efforts during these uncertain times.
With increasing political pressures, heightened public scrutiny, and building anticipation around potential therapies and vaccines targeting COVID-19, the role of regulatory authorities in guiding the evaluation of non–COVID-19 therapeutics has been less discussed. In this report, we specifically examine how COVID-19 has affected regulatory strategy and the pipeline of cardiovascular drugs and devices.
FDA Remains “Open for New Business”
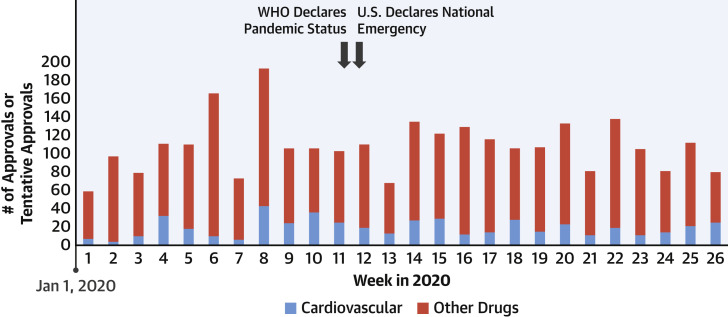
The FDA continues to remain engaged in the reviewing and processing of applications for therapeutics. Compared with the first half of 2019 (n = 13), impressively, the FDA’s Center for Drug Evaluation and Research approved nearly twice the number of new molecular entities or new therapeutic biological products during the first half of 2020 (n = 25), including bempedoic acid (a non-statin cholesterol-lowering therapy) (2). U.S. device regulation has been equally active; in the first half of 2020, 2 automated external defibrillator systems and 5 stent systems (or related components) were cleared (3). More broadly, even beyond the approval of novel drugs and devices, the FDA has actively reviewed labeling changes to existing therapies. Thus far in 2020, approximately 500 cardiovascular therapies have received approvals or tentative approvals, inclusive of labeling updates (4). The pace of these approvals has been relatively steady even during the initial phases of COVID-19 (Figure 1 ). The work for many of these approvals, clearances, and labeling changes were far advanced by the time the pandemic began. New applications are anticipated to slow, resulting in a potential reduction in future cardiovascular therapeutics in the pipeline.
Figure 1.
Approvals and Tentative Approvals Processed by the FDA in 2020
These estimates encompass approvals (and tentative approvals) of New Drug Applications, Biologics License Applications, and Abbreviated New Drug Applications (together with their approved supplements) (6). Approvals related to cardiovascular therapies are identified in blue. Key public health events are identified with black arrows. FDA = U.S. Food And Drug Administration; WHO = World Health Organization.
Ensuring Access to Existing Therapies
In anticipation of supply chain disruptions in access to existing therapies, the FDA is actively working with manufacturers to monitor product availability. As of July 13, 2020, 503 therapies with cardiovascular, endocrinology/metabolism, or hematology indications were in shortage (5). However, only 67 were newly posted since the United States declared a public health emergency on March 13, 2020. Across the United States, patients appeared to stockpile therapies early, while prescription fills of cardiovascular therapies may be modestly lower in later phases of the pandemic (6).
Regulatory Flexibility in Supporting a Sustainable Therapeutic Pipeline
Hazards of face-to-face interactions, shutdowns of clinical research sites, interruptions in institutional review board workflows, reductions in cardiovascular health care utilization, and competing risks of COVID-19 illness have all culminated in distinct challenges to continuing trials. Although temporary pauses may be possible in select situations, these delays may not be financially tenable for many sponsors. COVID-19 has presented particular challenges for select high-risk cardiovascular populations, where patients and trialists cannot afford delays in research, and in certain cases (such as cardio-oncology) access to novel treatments may hinge on trial enrollment. As such, most trials have already begun adapting protocols. As outlined in a general FDA guidance document (7) and a detailed statistical document (8), regulatory authorities appear supportive of promoting study flexibility, while ensuring the safety of participants and clinical research teams, preserving trial integrity, and maintaining a robust evidentiary bar.
In general, adaptations should be individualized to meet the specific trial circumstances and practicalities. Regulators have emphasized that adaptations should be well-documented and communicated to both regulators and patients in a timely manner. These may require formal protocol amendments, investigational device exemption supplements, and changes to statistical analysis plans. Global trials face unique challenges in that protocol adaptations must be acceptable across regional regulatory authorities; reassuringly, the European Medicines Agency and other regulators worldwide have expressed similar flexibility (9).
Protocol adaptations should preferably be performed prospectively on the basis of knowledge of only blinded trial data. Complex decisions about whether to prematurely terminate the study sooner than planned may be informed by projected disruptions to participant enrollment, investigational drug supply, and study monitoring procedures. As in original study protocols, methods to handle increased anticipated data missingness or premature study discontinuation rates during the pandemic should be adapted as needed.
The regional variation in the spread of COVID-19 also introduces unique challenges. Ongoing prospective efforts are underway to harmonize capture of common patient-level and site-level data elements. For instance, the Heart Failure Collaboratory, a public–private partnership with the FDA, is creating structured, streamlined, and standardized case report forms to capture COVID-19–related information (10). In global trials, as sites will be anticipated to be affected during different time periods, this information may guide sensitivity analyses to dissect regional, temporal, and treatment interactions.
Other Regulatory Considerations
-
•
Informed consent: The FDA has offered several alternative approaches to troubleshoot and navigate complex trial scenarios when traditional methods of obtaining informed consent may not be feasible or safe (9). Certain high-risk cohorts, such as those enrolled in cardio-oncology trials, further complicate in-person contact. In many such cases, electronic consent may be acceptable if appropriately accompanied by proper documentation. To promote a secure and accessible electronic consent process, the FDA has recently made their COVID MyStudies application freely available to investigators. This downloadable, open source application can be used to review, sign, and transfer forms, and further allows trial-specific branding and is supported by a dedicated technical assistance team.
-
•
Data collection: Depending on the trial-specific procedures, ongoing data collection during the pandemic is preferred if it does not pose excess risks to participants or research personnel. Data collection even outside of protocol-designated windows may maximize interpretability of affected data and inform decision-making.
-
•
Endpoint ascertainment: Many traditional cardiovascular endpoints may be feasibly ascertained remotely without the need for in-person physical examination. Collection of extensive adverse events and serious adverse events may be curtailed when appropriate, especially for drugs that are already approved for other indications.
-
•
Clinical trial adjudication: Although traditional committees apply stringent definitions to ensure high-fidelity identification of clinical events, endpoint definitions may need to be adapted to allow for adjudication even with incomplete source documentation. COVID-19 cases and hospitalizations should be prospectively ascertained. Adjudication may not always be necessary, and investigator-defined events are often sufficient.
-
•
Flexible target sample sizes and timelines: It is anticipated that COVID-19 will introduce statistical noise and uncertainty given potential competing risks of nonmodifiable events, excess premature drug discontinuation, missing or incomplete outcome data, and/or lapses in study protocol adherence. Sponsors may consider expanding target sample sizes or extending follow-up (in event-driven trials) to account for this potential loss of statistical power. They may also consider shifting resources and enrollment targets to geographic areas with lower population burden of COVID-19.
-
•
Statistical analysis plans: COVID-19 may unpredictably affect data quality, expected endpoint accrual, cardiovascular disease trajectory, as well as care patterns worldwide. As such, planned analyses may require amendment to account for data collected before, during, and after COVID-19 (adjusted for varying timing of affected communities).
-
•
Transparent communication: Regulatory bodies must continue to provide explicit guidance and clinical trialists are encouraged to share best practices to optimize clinical research during the pandemic. Reassuringly, the FDA has been responsive in delivering webinars and virtual town hall meetings and putting forth over 60 specific guidance documents related to various regulatory positions.
Translating Current Learnings to Durable Innovation
Many of these adaptations may be appropriate even during nonpandemic times. In the recently launched COVID-19 Pandemic Recovery and Preparedness Plan, the FDA outlines ways in which traditional regulatory approaches may be modernized to effectively apply learnings from COVID-19 to future public health emergencies. This structured program will identify and support strategies that have been effective and efficient during COVID-19 for long-term retention and implementation.
However, even with the best efforts to preserve data quality, it is expected that regulators will face challenging decisions in the post-pandemic period in reviewing these data as each trial may have slightly varied adaptations or unique considerations. Disruptions in the usual pathways of evidence generation are likely to result in imperfect data in many cases emphasizing the need for continued evaluation as the pandemic evolves with robust phase IV post-marketing studies. COVID-19 has accelerated the FDA’s consideration of diverse data sources including real-world evidence (such as the Sentinel System). Although the role of these ancillary data sources in supporting therapeutic approval remains less well defined, commitment to evaluation of broad streams of data may be particularly important in post-marketing surveillance.
Conclusions
Patients with cardiovascular diseases are particularly vulnerable to the direct and indirect health impacts associated with the COVID-19 pandemic; ensuring stable therapeutic access is thus of high priority. The FDA and global regulators have played a central role in shaping the public health response to the COVID-19 pandemic, particularly with respect to ensuring access to existing therapies and promoting a steady pipeline of novel therapies. The trajectory of COVID-19 remains uncertain and may durably influence clinical trial conduct in the months and years ahead. Regardless, COVID-19 was neither the first nor the last modern pandemic. Clinical trialists and sponsors should actively work with regulatory authorities to mitigate the impact of COVID-19 on the current and future development of cardiovascular therapies. Development of innovative strategies to conduct trials and interpret affected data during this pandemic may yield lasting improvements to the cardiovascular clinical trial enterprise.
Author Relationship With Industry
Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541); has received research support from Amgen; has served on Advisory Boards for Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, and Relypsa; and has participated on clinical endpoint committees for studies sponsored by Galmed, Novartis, and the National Institutes of Health. Dr. Butler has served as a consultant to Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Limited, and Vifor. Dr. Krumholz has received expenses and/or personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing; is an owner of Refactor Health and HugoHealth; and has had grants and/or contracts from the Centers for Medicare & Medicaid Services, Medtronic, the U.S. Food and Drug Administration, Johnson & Johnson, and the Shenzhen Center for Health Information. Dr. Bhatt has served on the Advisory Boards of Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, and Regado Biosciences; has served on the boards of directors of the Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; has served as chair of the American Heart Association Quality Oversight Committee; has served on data monitoring committees for the Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards Lifesciences), Contego Medical (chair of the PERFORMANCE 2 trial), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi-Sankyo), and Population Health Research Institute; has received honoraria from the American College of Cardiology (senior associate editor, Clinical Trials and News, ACC.org; and vice chair of the ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (guest editor; associate editor), K2P (co-chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (chief medical editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (secretary/treasurer), and WebMD (CME steering committees); has other relationships with Clinical Cardiology (deputy editor), NCDR-ACTION Registry Steering Committee (chair), and VA CART Research and Publications Committee (chair); has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Synaptic, and The Medicines Company; has received royalties from Elsevier (editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has been a site coinvestigator for Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), and Svelte; has been a trustee for the American College of Cardiology; and has performed unfunded research for FlowCo, Merck, Novo Nordisk, and Takeda. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
This paper reflects multidisciplinary discussions at the virtual “Cardiovascular Clinical Research in the COVID-19 Era Roundtable” convened by the American College of Cardiology on May 29, 2020. The authors thank the program participants including clinical trialists, public and private sponsors, payers, and patients.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Bagiella E., Bhatt D.L., Gaudino M. The consequences of the COVID-19 pandemic on non-COVID-19 clinical trials. J Am Coll Cardiol. 2020;76:342–345. doi: 10.1016/j.jacc.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration . 2020. Novel Drug Approvals for 2020.https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 Available at: [Google Scholar]
- 3.U.S. Food and Drug Administration . 2020. 2020 Device Approvals.https://www.fda.gov/medical-devices/recently-approved-devices/2020-device-approvals Available at: [Google Scholar]
- 4.U.S. Food and Drug Administration . 2020. Drugs@FDA: FDA-Approved Drugs.https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=reportsSearch.process Available at: [Google Scholar]
- 5.U.S. Food and Drug Administration Drug Shortages. https://www.accessdata.fda.gov/scripts/drugshortages/ Available at: Accessed on July 14, 2020.
- 6.Vaduganathan M., Van Meijgaard J., Mehra M.R., Joseph J., O’Donnell C.J., Warraich H.J. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA. 2020;323:2524–2526. doi: 10.1001/jama.2020.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration FDA Guidance on Conduct of Clinical Trials of Medical Products During COVID-19 Public Health Emergency. March 2020. https://www.fda.gov/media/136238/download Available at:
- 8.U.S. Food and Drug Administration Statistical Considerations for Clinical Trials During the COVID-19 Public Health Emergency. June 2020. https://www.fda.gov/media/139145/download Available at:
- 9.European Medicines Agency Points to Consider on Implications of Coronavirus Disease (COVID-19) on Methodological Aspects of Ongoing Clinical Trials. https://www.ema.europa.eu/en/implications-coronavirus-disease-covid-19-methodological-aspects-ongoing-clinical-trials Available at:
- 10.Abraham W.T., Fiuzat M., Psotka M.A., O’Connor C.M. Heart Failure Collaboratory statement on clinical trials in the landscape of COVID-19. J Am Coll Cardiol HF. 2020;8:423–425. doi: 10.1016/j.jchf.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]