Abstract
Aims
The 2018 ESC/ESH guidelines for hypertension recommend differential management of patients who are <65, 65–79, and ≥80 years of age. However, it is unclear whether intensive blood pressure lowering is well-tolerated and modifies risk uniformly across the age spectrum.
Methods and results
SPRINT randomized 9361 high-risk adults without diabetes and age ≥50 years with systolic blood pressure 130–180 mmHg to either intensive or standard antihypertensive treatment. The primary efficacy endpoint was the composite of acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes. The primary safety endpoint was composite serious adverse events. We assessed whether age modified the efficacy and safety of intensive vs. standard blood pressure lowering using Cox proportional-hazards regression and restricted cubic splines. In all, 3805 (41%), 4390 (47%), and 1166 (12%) were <65, 65–79, and ≥80 years. Mean age was similar between the two study groups (intensive group 67.9 ± 9.4 years vs. standard group 67.9 ± 9.5 years; P = 0.94). Median follow-up was 3.3 years. In multivariable models, age was linearly associated with the risk of stroke (P < 0.001) and non-linearly associated with the risk of primary efficacy events, death from cardiovascular causes, death from any cause, heart failure, and serious adverse events (P < 0.001). The safety and efficacy of intensive blood pressure lowering were not modified by age, whether tested continuously or categorically (P > 0.05).
Conclusion
In SPRINT, the benefits and risks of intensive blood pressure lowering did not differ according to the age categories proposed by the ESC/ESH guidelines for hypertension.
Trial Registration
SPRINT (Systolic Blood Pressure Intervention Trial); ClinicalTrials.gov Identifier: NCT01206062, https://clinicaltrials.gov/ct2/show/NCT01206062.
Keywords: Age, Blood pressure, Hypertension, Safety
Introduction
Hypertension is the leading contributor to excess cardiovascular deaths and disability-adjusted life-years globally.1,2 The prevalence of hypertension, defined as a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, increases with age and currently exceeds 20% among adult men and women.3,4 In addition, the same degree of blood pressure elevation is associated with greater cardiovascular event rates among older individuals,5 underscoring the importance of appropriately treating this subgroup.1 However, it is unclear whether intensive blood pressure lowering is well-tolerated and modifies risk uniformly across the age spectrum. We leveraged data from the Systolic Blood Pressure Intervention Trial (SPRINT) to assess whether age modified the efficacy and safety of intensive vs. standard blood pressure lowering. Since the 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) guidelines for the management of arterial hypertension recommend differential management of patients who are <65, 65–79, and ≥80 years of age,6 we also examined all associations stratified according to these age categories.
Methods
Study design
The rationale, protocol, and primary results of SPRINT have been previously published.7,8 The SPRINT primary outcome paper dataset was obtained from the National Heart, Lung, and Blood Institute’s Biologic Specimen and Data Repository Information Coordinating Center after having received a waiver for secondary use by the institutional review board at Brigham and Women’s Hospital. In brief, SPRINT was a randomized, controlled, open-label trial conducted in the USA. A total of 9361 persons ≥50 years of age, at high cardiovascular risk but without diabetes, who had a systolic blood pressure 130–180 mmHg at screening, were randomized to receive either intensive (target systolic blood pressure <120 mmHg; n = 4678) or standard antihypertensive treatment (target systolic blood pressure <140 mmHg; n = 4683).8 High cardiovascular risk was defined as clinical or subclinical cardiovascular disease (except stroke), chronic kidney disease with an estimated glomerular filtration rate 20–59 mL/min/1.73 m2, a 10-year risk of cardiovascular disease ≥15% based on the Framingham risk score, or age ≥75 years. Patients with a 1-min standing systolic blood pressure <110 mmHg were excluded.
Study endpoints
The primary efficacy endpoint was the composite of myocardial infarction, non-infarction acute coronary syndrome, stroke, acute decompensated heart failure, or death from cardiovascular causes. Secondary efficacy endpoints in the present study included specific individual components of the primary endpoint (stroke, acute decompensated heart failure, and death from cardiovascular causes) and death from any cause. The primary safety endpoint was the composite of serious adverse events (hypotension, syncope, electrolyte abnormalities, acute kidney injury or failure, or injurious falls).
Statistical analysis
Baseline characteristics were assessed across the three ESC/ESH-defined age categories. The relationship between age and clinical endpoints was evaluated using Cox proportional-hazards regression and restricted cubic splines, adjusted for treatment group, sex, smoking status, number of antihypertensive agents, history of clinical cardiovascular disease, total cholesterol, high-density lipoprotein cholesterol, serum creatinine, and urine albumin-creatinine ratio. We further determined if the effects of intensive vs. standard blood pressure lowering varied across the age spectrum using interaction analyses for primary efficacy and safety endpoints. The number of knots in the spline models was selected to minimize the Akaike’s information criterion. We have previously used this statistical approach on the SPRINT cohort.9,10 All analyses were performed with Stata/IC 15 (StataCorp LP, College Station, TX, USA).
Results
Descriptive characteristics
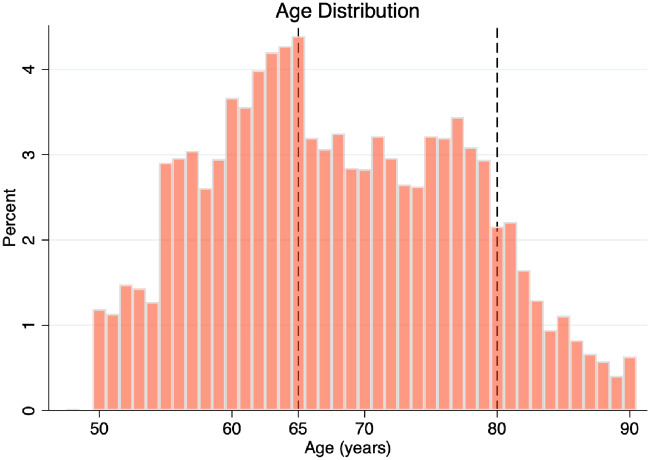
Mean (±standard deviation) age was similar between patients assigned to intensive and standard treatment (67.9 ± 9.4 years vs. 67.9 ± 9.5 years; P = 0.94). A total of 3805 (41%), 4390 (47%), and 1166 (12%) were <65 years, 65–79 years, and ≥80 years, respectively. The age distribution is depicted in Figure 1.
Figure 1.
Age distribution in the Systolic Blood Pressure Intervention Trial (SPRINT).
Older patients enrolled in SPRINT were more often women, white, and met enrolment criteria for clinical cardiovascular disease (compared with other high-risk eligibility features) (P < 0.001). Furthermore, older patients had higher baseline systolic blood pressures, pulse pressures, serum creatinine, urine albumin-to-creatinine ratio, and high-density lipoprotein cholesterol (P < 0.001). Conversely, body mass index, diastolic blood pressure, estimated glomerular filtration rate, total cholesterol, triglycerides, and current smoking status were lower across age categories (P < 0.001). Older patients were more often treated with aspirin and statin (P < 0.001). Baseline characteristics stratified according to age category are shown in Tables 1 and2.
Table 1.
Baseline characteristics stratified according to age category
| Age <65 years | Age 65–79 years | Age ≥80 years | P-value | |
|---|---|---|---|---|
| Study population | 3805 (41) | 4390 (47) | 1166 (12) | |
| Chronic kidney disease | 645 (17) | 1409 (32) | 592 (51) | <0.001 |
| Clinical cardiovascular disease | 466 (12) | 818 (19) | 278 (24) | <0.001 |
| Age (years) | 59 ± 4 | 72 ± 4 | 83 ± 3 | <0.001 |
| Female sex | 1247 (33) | 1634 (37) | 451 (39) | <0.001 |
| Race or ethnic group | <0.001 | |||
| Non-Hispanic black | 1647 (43) | 967 (22) | 188 (16) | |
| Hispanic | 508 (13) | 396 (9) | 80 (7) | |
| Non-Hispanic white | 1585 (42) | 2928 (67) | 886 (76) | |
| Other | 65 (2) | 99 (2) | 12 (1) | |
| Smoking status | <0.001 | |||
| Never smoked | 1625 (43) | 1923 (44) | 574 (49) | |
| Former smoker | 1247 (33) | 2158 (49) | 568 (49) | |
| Current smoker | 925 (24) | 294 (7) | 21 (2) | |
| Missing data | 8 (0) | 15 (0) | 3 (0) | |
| Body mass index (kg/m2) | 31.3 ± 6.2 | 29.3 ± 5.2 | 27.1 ± 4.7` | <0.001 |
| Serum creatinine (mg/dL) | 1.05 ± 0.35 | 1.07 ± 0.32 | 1.17 ± 0.38 | <0.001 |
| Estimated GFR (mL/min/1.73 m2) | 78 ± 21 | 69 ± 19 | 60 ± 18 | <0.001 |
| Urine albumin to creatinine ratio (mg/g) | 37 ± 164 | 42 ± 167 | 61 ± 169 | <0.001 |
| Fasting blood glucose (mg/dL) | 99 ± 15 | 99 ± 12 | 98 ± 12 | 0.02 |
| Total cholesterol (mg/dL) | 198 ± 43 | 186 ± 39 | 180 ± 38 | <0.001 |
| HDL cholesterol (mg/dL) | 51 ± 14 | 54 ± 15 | 56 ± 15 | <0.001 |
| Triglycerides (mg/dL) | 139 ± 117 | 119 ± 66 | 107 ± 58 | <0.001 |
| Statin use | 1261 (33) | 2202 (51) | 591 (51) | <0.001 |
| Aspirin use | 1555 (41) | 2499 (57) | 702 (60) | <0.001 |
| Antihypertensive agents (n) | 1.7 ± 1.0 | 1.9 ± 1.0 | 1.9 ± 1.0 | <0.001 |
| Not using antihypertensive agents | 462 (12) | 330 (8) | 90 (8) | <0.001 |
Continuous variables are presented as means and standard deviations. Categorical variables are presented as counts and corresponding percentages. P-values are calculated using the non-parametric test for trend (Wilcoxon-type test for trend).
GFR, glomerular filtration rate; HDL, high-density lipoprotein.
Table 2.
Baseline and achieved blood pressures and number of antihypertensive agents stratified according to age category
| Age <65 years | Age 65–79 years | Age ≥80 years | P-value | ||
|---|---|---|---|---|---|
| Study population, n (%) | 3762 (41) | 4339 (47) | 1147 (12) | ||
| Baseline | Systolic blood pressure (mmHg) | 139 ± 16 | 140 ± 15 | 143 ± 16 | <0.001 |
| Diastolic blood pressure (mmHg) | 84 ± 11 | 75 ± 11 | 70 ± 11 | <0.001 | |
| Pulse pressure, mmHg | 55 ± 12 | 64 ± 14 | 73 ± 15 | <0.001 | |
| Antihypertensive agents, n | 1.7 ± 1.0 | 1.9 ± 1.0 | 1.9 ± 1.0 | <0.001 | |
| Achieved in follow-up | Systolic blood pressure (mmHg) | 126 ± 15 | 127 ± 16 | 127 ± 17 | <0.001 |
| Diastolic blood pressure (mmHg) | 75 ± 11 | 68 ± 11 | 62 ± 11 | <0.001 | |
| Pulse pressure (mmHg) | 51 ± 11 | 59 ± 13 | 65 ± 14 | <0.001 | |
| Antihypertensive agents, n | 2.3 ± 1.2 | 2.3 ± 1.2 | 2.3 ± 1.2 | 0.37 |
Variables are presented as means and standard deviations. P-values are calculated using the non-parametric test for trend (Wilcoxon-type test for trend).
Achieved systolic blood pressures and number of antihypertensive agents were numerically similar across age categories, while diastolic blood pressures were lower (and pulse pressures higher) among older participants (Table 2).
Event risk
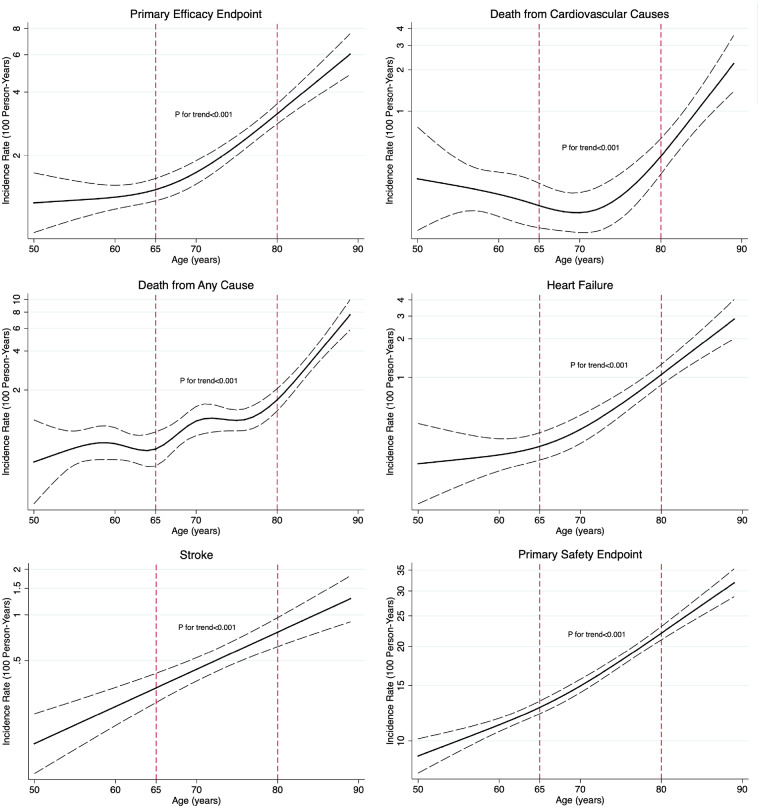
Median follow-up was 3.3 years (range 0–4.8 years), with 562 primary efficacy events (6%) and 3529 primary safety events (38%) observed during the study period. Age was linearly associated with the risk of stroke (test for overall trend, P < 0.001) and non-linearly associated with the risk of primary efficacy events, death from cardiovascular causes, death from any cause, heart failure, and serious adverse events (test for non-linearity, P < 0.05; test for overall trend, P < 0.001) (Figure 2). The incidence rate of primary events increased over ESC/ESH guideline-recommended age-categories, as did the rate of serious adverse events (Table 3).
Figure 2.
The association between age and efficacy and safety endpoints. The solid lines represent the incidence rate (per 100 person-years) at each age interval. The dashed lines represent the upper and lower bounds of the 95% confidence interval. P-values are for adjusted trends (adjusted for treatment group, sex, number of antihypertensive drug classes, smoking status, clinical cardiovascular disease, urine albumin-to-creatinine ratio, total cholesterol, high-density lipoprotein cholesterol, and creatinine).
Table 3.
Incidence rates, unadjusted and adjusted hazard ratios, and treatment effects (absolute risk reductions, numbers needed to treat, and hazard ratios for intensive vs. standard blood pressure lowering) for efficacy and safety endpoints stratified according to age category
| Age <65 years (n = 3805) | Age 65–79 years (n = 4390) | Age ≥80 years (n = 1166) | Age category × treatment interaction | |
|---|---|---|---|---|
| Primary efficacy endpoint | ||||
| Incidence rate per 100 person-years (intensive treatment) | 1.1 | 1.8 | 2.9 | |
| Incidence rate per 100 person-years (standard treatment) | 1.5 | 2.1 | 5.0 | |
| Hazard ratio (unadjusted) | Reference | 1.52 (1.25–1.85) | 3.02 (2.39–3.81) | |
| Hazard ratio (adjusted) | Reference | 1.65 (1.34–2.04) | 3.17 (2.44–4.11) | |
| Absolute risk reduction | 1.3% | 0.9% | 5.6% | |
| Number needed to treat | 81 | 108 | 18 | |
| Hazard ratio (treatment effect) | 0.73 (0.53–1.00) | 0.86 (0.68–1.09) | 0.58 (0.40–0.82) | 0.17 |
| Death from cardiovascular causes | ||||
| Incidence rate per 100 person-years (intensive treatment) | 0.2 | 0.2 | 0.7 | |
| Incidence rate per 100 person-years (standard treatment) | 0.3 | 0.4 | 1.1 | |
| Hazard ratio (unadjusted) | Reference | 1.12 (0.70–1.79) | 3.65 (2.22–6.01) | |
| Hazard ratio (adjusted) | Reference | 1.14 (0.69–1.89) | 3.46 (1.98–6.06) | |
| Absolute risk reduction | 0.4% | 0.6% | 1.3% | |
| Number needed to treat | 277 | 156 | 81 | |
| Hazard ratio (treatment effect) | 0.63 (0.31–1.30) | 0.48 (0.25–0.93) | 0.62 (0.30–1.29) | 0.83 |
| Death from any cause | ||||
| Incidence rate per 100 person-years (intensive treatment) | 0.6 | 1.0 | 2.7 | |
| Incidence rate per 100 person-years (standard treatment) | 0.8 | 1.2 | 4.0 | |
| Hazard ratio (unadjusted) | Reference | 1.56 (1.21–2.02) | 4.78 (3.62–6.30) | |
| Hazard ratio (adjusted) | Reference | 1.73 (1.30–2.29) | 4.93 (3.59–6.76) | |
| Absolute risk reduction | 0.7% | 0.7% | 4.3% | |
| Number needed to treat | 130 | 136 | 24 | |
| Hazard ratio (treatment effect) | 0.71 (0.46–1.08) | 0.82 (0.60–1.12) | 0.65 (0.45–0.95) | 0.63 |
| Heart failure | ||||
| Incidence rate per 100 person-years (intensive treatment) | 0.2 | 0.4 | 1.3 | |
| Incidence rate per 100 person-years (standard treatment) | 0.3 | 0.7 | 2.0 | |
| Hazard ratio (unadjusted) | Reference | 1.93 (1.28–2.91) | 6.23 (4.05–9.59) | |
| Hazard ratio (adjusted) | Reference | 2.11 (1.36–3.27) | 6.14 (3.79–9.95) | |
| Absolute risk reduction | 0.3% | 0.9% | 1.8% | |
| Number needed to treat | 277 | 104 | 57 | |
| Hazard ratio (treatment effect) | 0.65 (0.32–1.30) | 0.55 (0.34–0.89) | 0.68 (0.40–1.16) | 0.84 |
| Stroke | ||||
| Incidence rate per 100 person-years (intensive treatment) | 0.2 | 0.4 | 0.9 | |
| Incidence rate per 100 person-years (standard treatment) | 0.3 | 0.5 | 1.1 | |
| Hazard ratio (unadjusted) | Reference | 1.85 (1.21–2.83) | 4.01 (2.47–6.51) | |
| Hazard ratio (adjusted) | Reference | 2.13 (1.34–3.39) | 4.48 (2.61–7.70) | |
| Absolute risk reduction | 0.05% | 0.2% | 0.6% | |
| Number needed to treat | 2167 | 541 | 183 | |
| Hazard ratio (treatment effect) | 0.93 (0.46–1.89) | 0.89 (0.55–1.44) | 0.81 (0.42–1.58) | 0.96 |
| Primary safety endpoint | ||||
| Incidence rate per 100 person-years (intensive treatment) | 11.4 | 16.6 | 24.2 | |
| Incidence rate per 100 person-years (standard treatment) | 11.1 | 15.2 | 27.7 | |
| Hazard ratio (unadjusted) | Reference | 1.41 (1.31–1.52) | 2.26 (2.05–2.49) | |
| Hazard ratio (adjusted) | Reference | 1.37 (1.26–1.49) | 2.03 (1.82–2.26) | |
| Absolute risk increase | 1.0% | 2.5% | 3.1% (absolute risk reduction) | |
| Number needed to harm | 97 | 40 | 32 (number needed to treat) | |
| Hazard ratio (treatment effect) | 1.03 (0.92–1.16) | 1.09 (0.99–1.20) | 0.91 (0.78–1.06) | 0.14 |
Multivariable analyses were adjusted for treatment group, sex, smoking status, the number of antihypertensive agents, history of clinical cardiovascular disease, total cholesterol, high-density lipoprotein cholesterol, serum creatinine, and urine albumin-creatinine ratio.
Effect of intensive blood pressure lowering
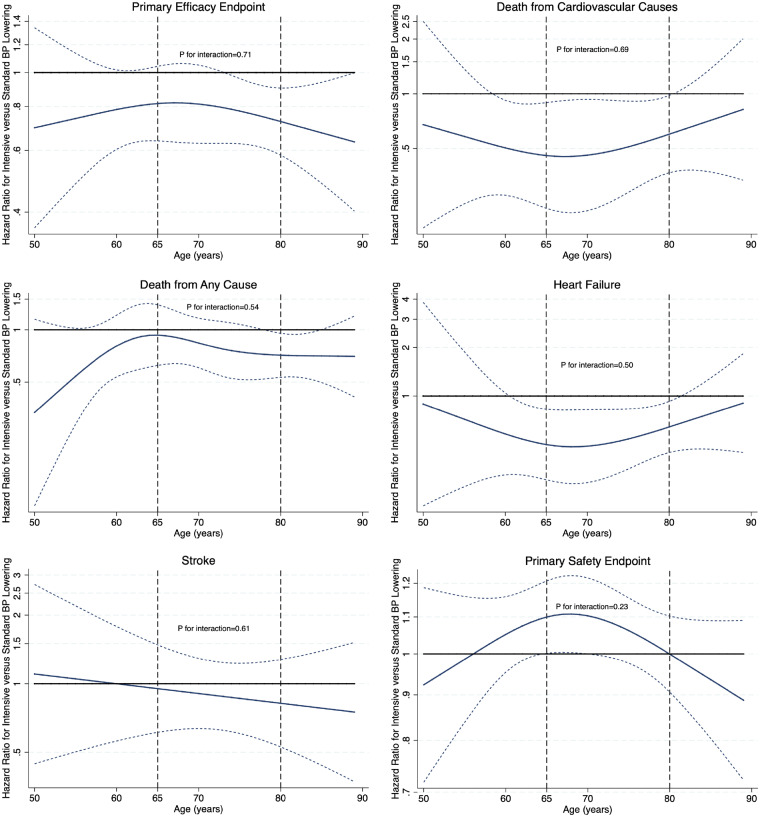
For efficacy endpoints, absolute risk reductions with intensive blood pressure lowering were highest (and corresponding numbers needed to treat lowest) among the oldest participants (Table 3). The absolute increase in the risk of serious adverse events was not greater among individuals ≥80 years of age compared with those <80 years. The safety and efficacy of intensive blood pressure lowering were not modified by age, regardless of whether it was tested continuously (Figure 3) or categorically (Table 3) (P > 0.05).
Figure 3.
The effect of intensive vs. standard blood pressure lowering across the age spectrum. The solid lines represent unity (hazard ratio = 1) and the hazard ratio for intensive vs. standard blood pressure lowering at each age interval, respectively. The dotted lines represent the upper and lower bounds of the 95% confidence interval. P-values are for the continuous interaction between age and treatment effect for each endpoint.
Discussion
In SPRINT, older adults faced high rates of cardiovascular events and serious adverse events, regardless of blood pressure lowering strategy. However, we demonstrated comparable efficacy and safety of intensive blood pressure control across 2018 ESC/ESH guideline-defined age thresholds. These data suggest that selection of optimal candidates for intensive blood pressure lowering should not rely on age alone.
Tolerability concerns among the very old, defined in the 2018 ESC/ESH guidelines as individuals ≥80 years of age, may lead to poorer blood pressure control.6,11 Clinicians may be concerned about higher comorbidity burden, number of prescribed drugs, risk of falls, or frailty among older adults. Indeed, antihypertensive drug treatment is an important and modifiable risk factor for falls.12 Accordingly, the 2018 ESC/ESH guidelines for hypertension recommend the following target ranges for systolic blood pressure: 120–129 mmHg for patients <65 years, 130–139 mmHg for patients 65–79 years, and 130–139 mmHg (if tolerated) for patients ≥80 years of age. In contrast, the 2017 American College of Cardiology/American Heart Association guideline for the prevention, detection, evaluation, and management of high blood pressure provide target blood pressure thresholds for initiation or intensification of therapies that do not strictly depend on age.13 Interestingly, drug choice does not seem to matter if treatment targets are reached.14,15
The Hypertension in the Very Elderly Trial (HYVET) included 3845 patients ≥80 years of age with a sustained systolic blood pressure ≥160 mmHg and randomized them to either indapamide (with or without perindopril) or matching placebo.16 Target systolic and diastolic blood pressures were <150 and <80 mmHg. At 2 years, antihypertensive treatment was associated with significant risk reductions ranging from 21% to 64% for various mortality endpoints and heart failure. Frailty did not modify the effect of antihypertensive drug treatment on risk of stroke, cardiovascular events, and mortality.17
SPRINT also evaluated old and frail patients. The investigators oversampled subjects aged ≥75 years (n = 2636) and confirmed the benefits of intensive blood pressure lowering in this subgroup, with an overall rate of serious adverse events that did not differ between the two treatment groups.18 The benefits persisted when stratifying for frailty index, although a higher frailty index was independently associated with a greater risk of falls.19 Similarly, no subgroup heterogeneity for patients <65 vs. ≥65 years was detected in the Action to Control Cardiovascular Risk in Diabetes blood pressure (ACCORD BP) trial of intensive (target systolic blood pressure <120 mmHg) vs. standard blood pressure lowering (target systolic blood pressure <140 mmHg) among 4733 patients with type 2 diabetes mellitus.20 However, patients older than 79 years were not included in ACCORD BP.
Advanced age is tightly linked with pulse pressure, another marker of cardiovascular risk.21 Among patients with or at high risk for cardiovascular disease, there has been concern regarding aggressive blood pressure lowering due to potential effects on limiting coronary perfusion during diastole.22–28 In SPRINT, however, the relative efficacy and safety profile of a strategy of intensive blood pressure lowering was not significantly modified among patients with wide pulse pressures (high systolic and relatively low diastolic blood pressures).9 Indeed, in a recent examination of 1.3 million adults in a general outpatient population, diastolic blood pressure displayed a J-shaped relationship with subsequent cardiovascular events.29 Heightened cardiovascular risk at the low diastolic blood pressure range was partially accounted for by increased age and higher systolic blood pressure among these patients. Taken together, high systolic blood pressure remains an enduring target for cardiovascular risk reduction, even in the presence of low diastolic blood pressure and widened pulse pressure, findings commonly observed among older adults.
Our results both complement and extend prior findings as there does not appear to be an age threshold at which the harms of intensive blood pressure lowering clearly outweigh the observed benefits in this clinical trial setting. Nevertheless, despite the large, well-characterized study population with findings that were consistent over a broad range of endpoints, our study may be limited by its post hoc nature, potential lack of generalizability to patients not satisfying the specific SPRINT inclusion and exclusion criteria, and the possible absence of power to detect significant age-related interactions.
Conclusion
In SPRINT, the benefits and risks of intensive blood pressure lowering did not differ according to the age categories proposed by the ESC/ESH guidelines for hypertension. Decision-making surrounding more intensive blood pressure targets among high-risk older adults should be individualized and move beyond age alone.
Funding
SPRINT was supported by the National Heart, Lung, and Blood Institute. This exploratory analysis was unfunded.
Conflict of interest: M.P. discloses the following relationships—Advisory Board: AstraZeneca; Speaker Honorarium: AstraZeneca, Bayer, and Boehringer Ingelheim. M.V. is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, and Boehringer Ingelheim, and participates on clinical endpoint committees for studies sponsored by Novartis and the NIH. T.B.-S. discloses the following relationships—Steering Committee member of the Amgen financed GALACTIC-HF trial; Advisory Board: Sanofi Pasteur and Amgen; Speaker Honorarium: Novartis and Sanofi Pasteur. A.Q. was supported by the NHLBI T32 postdoctoral training grant (T32HL007604) and the American Heart Association Strategically Focused Research Network in Vascular Disease grant (18SFRN3390085). A.P. was funded by the Texas Health Resources Clinical scholarship. M.H.O. discloses that he has received a part-time clinical research grant from the Novo Nordisk Foundation. D.L.B. discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), and Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda. The other authors have no conflict of interest to declare.
References
- 1. Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, Lopez-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 2016;388:2665–2712. [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Barnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catala-Lopez F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME, Murray CJ. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990-2015. JAMA 2017;317:165–182. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 5. Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ 1992;304:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 7. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr, Whelton PK; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014;11:532–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pareek M, Vaduganathan M, Biering-Sorensen T, Byrne C, Qamar A, Almarzooq Z, Pandey A, Olsen MH, Bhatt DL. Pulse pressure, cardiovascular events, and intensive blood pressure lowering in the systolic blood pressure intervention trial (SPRINT). Am J Med 2019;132:733–739. [DOI] [PubMed] [Google Scholar]
- 10. Oxlund CS, Pareek M, Rasmussen BSB, Vaduganathan M, Biering-Sorensen T, Byrne C, Almarzooq Z, Olsen MH, Bhatt DL. Body mass index, intensive blood pressure management, and cardiovascular events in the SPRINT Trial. Am J Med 2019;132:840–846. [DOI] [PubMed] [Google Scholar]
- 11. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018;137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callisaya ML, Sharman JE, Close J, Lord SR, Srikanth VK. Greater daily defined dose of antihypertensive medication increases the risk of falls in older people–a population-based study. J Am Geriatr Soc 2014;62:1527–1533. [DOI] [PubMed] [Google Scholar]
- 13. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KS, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 14. Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Crowley K, Goto S, Ohman EM, Bakris GL, Perlstein TS, Kinlay S, Bhatt DL; REACH Registry Investigators. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J 2013;34:1204–1214. [DOI] [PubMed] [Google Scholar]
- 15. Briasoulis A, Agarwal V, Tousoulis D, Stefanadis C. Effects of antihypertensive treatment in patients over 65 years of age: a meta-analysis of randomised controlled studies. Heart 2014;100:317–323. [DOI] [PubMed] [Google Scholar]
- 16. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 17. Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, Bulpitt C, Peters R. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med 2015;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years. A randomized clinical trial. JAMA 2016;315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pajewski NM, Williamson JD, Applegate WB, Berlowitz DR, Bolin LP, Chertow GM, Krousel-Wood MA, Lopez-Barrera N, Powell JR, Roumie CL, Still C, Sink KM, Tang R, Wright CB, Supiano MA; SPRINT Study Research Group. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci 2016;71:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Selvaraj S, Steg PG, Elbez Y, Sorbets E, Feldman LJ, Eagle KA, Ohman EM, Blacher J, Bhatt DL; REACH Registry Investigators. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH Registry. J Am Coll Cardiol 2016;67:392–403. [DOI] [PubMed] [Google Scholar]
- 22. McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016;68:1713–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhatt DL. Troponin and the J-curve of diastolic blood pressure: when lower is not better. J Am Coll Cardiol 2016;68:1723–1726. [DOI] [PubMed] [Google Scholar]
- 24. Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG; CLARIFY Investigators. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016;388:2142–2152. [DOI] [PubMed] [Google Scholar]
- 25. Mancia G. Should blood pressure reduction be aggressive in patients with hypertension and coronary artery disease? Lancet 2016;388:2061–2062. [DOI] [PubMed] [Google Scholar]
- 26. Bohm M, Schumacher H, Teo KK, Lonn E, Mahfoud F, Mann JFE, Mancia G, Redon J, Schmieder R, Weber M, Sliwa K, Williams B, Yusuf S. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120-140 mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur Heart J 2018;39:3105–3114. [DOI] [PubMed] [Google Scholar]
- 27. Vidal-Petiot E, Greenlaw N, Ford I, Ferrari R, Fox KM, Tardif JC, Tendera M, Parkhomenko A, Bhatt DL, Steg PG. Relationships between components of blood pressure and cardiovascular events in patients with stable coronary artery disease and hypertension. Hypertension 2018;71:168–176. [DOI] [PubMed] [Google Scholar]
- 28. Vidal-Petiot E, Sorbets E, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG. Potential impact of the 2017 ACC/AHA guideline on high blood pressure in normotensive patients with stable coronary artery disease: insights from the CLARIFY registry. Eur Heart J 2018;39:3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, Melles RB, Bhatt DL. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med 2019;381:243–251. [DOI] [PubMed] [Google Scholar]