Abstract
Objective
To determine the effectiveness and safety of acupuncture for chemotherapy-induced peripheral neuropathy. The review has been registered on the “PROSPERO” website; the registration number is CRD42020151654.
Methods
A comprehensive literature search was performed on 7 electronic databases from the time of inception to March 2020. RCTs studies on acupuncture for CIPN compared with medication or sham acupuncture were included. Statistical analysis was carried out using RevMan 5.3.
Results
In total, 19 RCTs covering 1174 patients were enrolled. The results showed that acupuncture significantly increased the effective rate of CIPN compared with medicine and sham acupuncture. And acupuncture had a good effect on the recovery of nerve conduction velocity and improving pain. Among the acupoints involved in the treatment of CIPN, LI4, LI11, ST36, EX10 (Bafeng), and EX-UE 9 (Baxie) were the most commonly used.
Conclusion
The use of acupuncture in the management of CIPN is safe and effective. The most used acupoints for CIPN are LI4, LI11, ST36, EX10 (Bafeng), and EX-UE 9 (Baxie).
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) has a severe dose-limiting side effect for cancer patients treated with taxanes, vinca alkaloids, platinum drugs, and proteasome inhibitor bortezomib [1]. It is caused by damage or impairment in the dorsal root ganglia (DRG); that is to say, most of the neuropathy is sensory neuropathy, less involving motor and autonomic nerves [2]. It can manifest as numbness, tingling, and burning pain and mostly present in a stocking-and-glove distribution which reduces patients' quality of life and leads to patients' chemotherapy dose reductions or treatment discontinuation [3–5]. A recent meta-analysis [6] demonstrated a CIPN prevalence of 68% in the first month after chemotherapy and 30% at 6 months or more. Every third patient (37%) with neuropathic symptoms ranked them as the most troublesome symptom [7].
Currently, the clinical prevention and treatment of CIPN has no recognized specific means. The effectiveness of drugs used to treat other types of neuropathy may not extend to CIPN, such as gabapentin [8], tricyclic antidepressant amitriptyline [9], and opioids [10]. The only successful CIPN trial [11] shows that the antidepressant duloxetine at 30 mg daily for 1 week followed by 60 mg daily for 4 more weeks produced a significant reduction in BPI-SF average pain compared with the placebo arm (mean change score, 1.06 vs. 0.34; P=0.03). But it also has grades 2 to 3 adverse events (mainly fatigue and nausea), and 12 patients discontinued treatment due to toxicity. Therefore, acupuncture, a common adjuvant therapy, has been used encouragingly to treat peripheral neuropathy associated with diabetes [12] and HIV [13] and has attracted interest. Acupuncture is widely used in cancer-related diseases such as hot flashes [14],cancer-related fatigue [15],cancer-related insomnia [16],and leukopenia [17], and it can also reduce pain in arthritis [18–20],sciatica [21, 22], and gout [23]. Although several studies [24–42] have suggested that acupuncture was effective for treating CIPN, few systematic reviews have been published. We performed a systematic review and meta-analysis to assess the strength of the current evidence to support the efficacy and safety of acupuncture and electroacupuncture to treat CIPN, which might be a complementary therapy for CIPN.
2. Methods
2.1. Eligibility Criteria
The included studies met the following eligibility criteria. (1) Studies were designed up to randomized controlled trial (RCT) standards. (2) The patients who had malignant tumor received chemotherapy with peripheral neurotoxic drugs and had symptoms related to peripheral nerve injury. (3) The control group was treated with conventional methods, such as the use of neurotransmitters and antiepileptic drugs, or sham acupuncture.
The exclusion criteria were as follows: (1) Studies were nonrandomized controlled trials. (2) The experimental group was treated with pharmacoacupuncture, auricular acupuncture, laser acupuncture, or other uncommon forms of acupuncture. (3) Studies described interventions combined with other TCM therapies such as Chinese herbal medicine, acupoint injection, or herbal extracts. (4) Patients with original nervous system disease or peripheral neuropathy caused by other diseases.
2.2. Data Sources and Search Strategy
The following seven electronic databases were searched to identify eligible trials published from inception to March 15, 2020: Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PubMed, EMBASE, Chinese Biomedical Literature Database, Chinese National Knowledge Infrastructure, and the Wanfang database. The 2 key concepts used in the search, peripheral neuropathy and acupuncture, included their synonyms. Two reviewers (JYX and ZJY) independently screened the titles and abstracts for eligibility and examined the full text of the articles. Any discrepancies were resolved by consensus or after consulting a third party (ZQ). The search keywords used were “Chemotherapy-induced peripheral neuropathy,” “Peripheral neuropathy,” “Neurotoxicity,” “Acupuncture,” “Acupoints,” “Needle,” as well as “random,” “clinical” and there was no restriction on language. More details have been provided on the “PROSPERO” website, and the registration number is CRD42020151654.
2.3. Data Extraction
Two reviewers (JYX and WYX) independently extracted data using an extraction sheet. The extracted data included general trial characteristics (authors and year); baseline patient and disease data (sample size, age, and gender); and interventions and outcomes. We contacted the authors via e-mail or telephone to acquire further information when the information in the literature was incomplete.
2.4. Quality Assessment and Data Analysis
Two reviewers (JYX and XX) independently assessed the methodological quality of the RCTs using the Cochrane Collaboration Risk of Bias tool. The risk of bias was assessed according to the Cochrane Handbook, which consists of six items: random sequence generation (i.e., selection bias), allocation concealment (i.e., selection bias), blinding of participants and personnel (i.e., performance bias), blinding of outcomes assessment (i.e., detection bias), incomplete outcomes data (i.e., attrition bias), selective reporting (i.e., reporting bias), and other biases. Discrepancies in this interpretation were resolved by consensus or after discussion with a third party (ZQ).
The data were analyzed using Review Manager 5.3 software (Cochrane Collaboration, Oxford, UK). The results are presented as the risk ratios (RR) or mean differences (MD) with the 95% confidence interval (95% CI). I2 statistics were used to assess heterogeneity.
3. Results
The search results are displayed in Figure 1. 788 publications were identified in the literature search, and 442 duplicated citations were excluded. After screening the titles and abstracts, 285 irrelevant publications were excluded. After reading the full text of the remaining studies, 41 records were excluded. Finally, 19 RCTs [24–42] met the eligibility criteria and were included in the systematic review.
Figure 1.
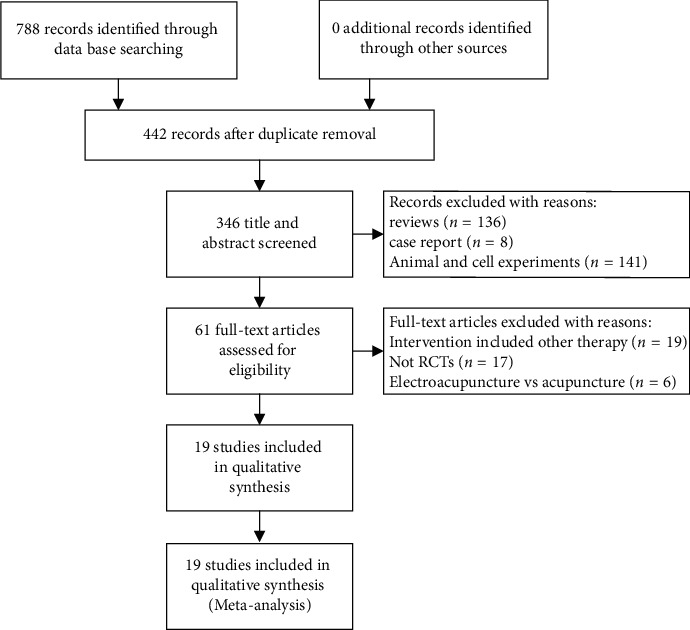
Flowchart of study selection.
3.1. Characteristics of Included Trials
19 RCTs met the inclusion criteria: 1 study was conducted in Germany [42], 2 in the United States [36, 41], 1 in Brazil [40], and the other 16 studies in China. In total, 1174 patients (588 in the acupuncture or electroacupuncture group, 586 in the control group) participated. The characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of the included RCTs.
| Study ID | Sample size (T/C) | Age (year) | Sex (M/F) | Intervention | Outcome measure | Acupoints | |
|---|---|---|---|---|---|---|---|
| Treatment group | Control group | ||||||
| Hou, 2011 | 40 (20/20) | T: 59.20 ± 9.80 | T: 11/9 | Acupuncture | Cobaltine adenosine | NCI-CTC | LI11, PC6, LI4, ST36, SP6, and GB34 |
| C: 62.95 ± 10.85 | C: 8/12 | ||||||
| Mai, 2008 | 60 (30/30) | T: 50.35 | T: 20/10 | Acupuncture | VB1 + VB12 | PNQ | GB20, TE5, ST36, BL40, GB34, ST40, SP6, LR3, and SP10 |
| C: 51.12 | C: 21/9 | ||||||
| Yan, 2012 | 75 (36/39) | T: 52.5 ± 5.63 | T: 20/16 | Acupuncture | VB1 + VB12 | PNQ | LI4, LI11, EX-UE9, LR3, ST36, SP10, and EX-LE10 |
| C: 51.3 ± 6.17 | C: 28/11 | ||||||
| Xu, 2010 | 64 (32/32) | T: 38∼77 | T: 17/15 | Acupuncture | VB1 + VB12 | PNQ | LI4, LR3, ST36, CV6, LI11, SP3, EX-UE9, and EX-LE10 |
| C: 41∼83 | C: 16/16 | ||||||
| Liu, 2009 | 60 (30/30) | T: 64.8 | T: 16/14 | Acupuncture | Cobaltine adenosine | Levi | LI11, LI4, ST36, SP6, GB34, KI16, CV6, and CV4 |
| C: 62.6 | C: 17/13 | ||||||
| Cui, 2011 | 62 (32/30) | T: 59.6 | T: 19/13 | Acupuncture | Cobaltine adenosine | Levi | LI11, TE5, SI3, LI4, SJ3, SP9, ST36, GB34, SP6, GB39, and KI6 |
| C: 58.8 | C: 20/10 | ||||||
| Chen, 2018 | 80 (40/40) | T: 49.02 ± 8.63 | T: 24/16 | Acupuncture | Mecobalamin | RECIST | ST36, CV6, SP3, LI4, LI11, LR3, EX-UE9, and EX-LE10 |
| C: 48.75 ± 8.67 | C: 25/15 | ||||||
| Wang, 2011 | 60 (30/30) | T: 63.7 ± 9.8 | T: 16/14 | Acupuncture | Cobaltine adenosine | PNQ | LI4, LI10, LI1, ST32, ST36, ST40, GB30, GB31, GB34, EX-UE9, and EX-LE10 |
| C: 61.6 ± 8.9 | C: 17/13 | ||||||
| Tian, 2016 | 60 (30/30) | T: 60.9 ± 10.7 | T: 18/12 | Acupuncture | Cobaltine adenosine | PNQ, NCV, and QOL | LI10, LI11, LI4, TE5, EX-UE9, GB34, ST40, SP6, ST36, GB40, LR3, EX-LE10, CV4, CV6, and GV20 |
| C: 62.7 ± 10.4 | C: 17/13 | ||||||
| Han, 2017 | 98 (49/49) | — | T: 27/22 | Acupuncture | Cobaltine adenosine | VAS, NCV | LR3, ST43, GB4, SP6, ST36, SP10, ST25, GV14, GV12, GV11, GV9, and BL13 (BL17, BL58) |
| — | C: 29/20 | FACT/GOG-NTX | |||||
| Sun, 2012 | 66 (34/32) | T: 64 | T:24/10 | Electroacupuncture | Reduced glutathione | Levi, NCV | ST36, SP10, CV4, LI11, LI4, SJ10, TE4, LI5, SI4, EX-UE9, SI3, ST35, ST34, SP9, SP6, ST41, KI3, BL60, BL62, KI6, LR3, and EX-LE10 |
| C: 61 | C: 22/10 | ||||||
| Tian, 2011 | 76 (38/38) | T: 54.9 | T: 24/14 | Acupuncture | Neurotropin | Levi, KPS | LI4, TE5, LI5, LI10, LI11, LR3, ST36, GB34, CV6, ST40 |
| C:52.7 | C: 20/18 | ||||||
| Zhang, 2018 | 30 (15/15) | T: 52.46 ± 10.06 | T: 10/5 | Electroacupuncture | VB1 + VB12 | Levi, KPS | LI11, LI10, LI4, ST36, ST40, ST44 |
| C: 56.80 ± 8.06 | C: 8/7 | ||||||
| Greenlee, 2016 | 63 (31/32) | T:51.8 | — | Electroacupuncture | Sham acupuncture | BPI-SF, FACT-NTX | GB34, ST36, LI4, LI10, L3, L5, C5, and C7 |
| C:48.3 | — | ||||||
| Molassistis, 2019 | 87 (44/43) | — | T: 9/35 | Acupuncture | VB6 + VB12 | BPI, FACT-NTX | If upper limbs were involved, we used LI4, LI11, PC7, TE5, and/or Ex-UE9; if lower limbs were affected (most common), we used SP6, ST36, LV3, ST41, and/or Ex-LE10 |
| — | C: 15/28 | Neuropathy score | |||||
| Rostock, 2013 | 29 (14/15) | T:49.9 ± 9.6 | T: 4/10 | Electroacupuncture | VB6 + VB1 | NCI-CTC, NRS | LV3, SP9, GB41, GB34, LI4 , LI11 , SI3 , and HT3 |
| C:56.3 ± 11.1 | C: 5/10 | Neuropathy score, NCV, and QLQ-C30 | |||||
| Wu, 2018 | 40 (20/20) | T:57.10 | T: 13/7 | Acupuncture | Mecobalamin | Levi | LI11, PC6, LI4, ST36, SP6, and SP10 |
| C:57.63 | C: 14/5 | ||||||
| Lu, 2019 | 40 (20/20) | T:54.0 | T: 0/20 | Acupuncture | Usual care | PNQ, FACT-NTX, BPI-SF, and EORTC QLQ-C30 | Yin Tang, LI11, TW5, Ex-UE9, SP3, ST36, SP6, K3, and LR3 |
| C:53.5 | C: 0/20 | ||||||
| Alessandro, 2019 | 21 (12/9) | — | — | Acupuncture | Usual care | NCI-CTC, EORTC QLQ-C30, FIM, and VAS | Yuan points of the hands and feet (LR3, SP3, KI3, HT7, PC7, and LU9) plus SP9 and wrist-ankle technique (areas 1–3) |
| — | — | ||||||
Abbreviations: T: Treatment Group; C: Control Group; NCI-CTC: National Cancer Institute Common Toxicity Criteria; PNQ: Patient Neurotoxicity Questionnaire; RECIST: Response Evaluation Criteria in Solid Tumors; NCV: Nerve Conduction Velocity; QOL: Quality of Life; VAS: Visual Analogue Scale; KPS: Karnofsky; MDASI: MD Anderson Symptom Inventory: BPI-SF, Brief Pain Inventory-short form; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30; FACT-NTX, Functional Assessment of Cancer Therapy-Neurotoxicity subscale; FIM: Functional Independence Measure.
3.2. Risk of Bias and Methodological Quality
The assessment of risk of bias is summarized in Figure 2. All studies were described as “randomized” and 10 studies [24, 27, 28, 31, 33, 34, 36, 38, 40, 42] described appropriate methods for sequence generation. The remaining RCTs did not report specific random methods. One study [36] conducted allocation concealment.
Figure 2.
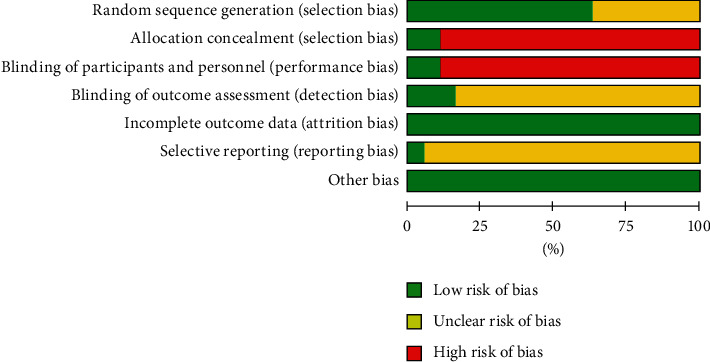
Risk of bias graph.
3.3. Outcomes
3.3.1. Total Effective Rate
Use the nimodipine scoring method to determine whether it is markedly effective, effective, or ineffective: markedly effective—the assessment of peripheral neurotoxicity is reduced by 2 levels or higher; effective—reduced by 1 level; and ineffective—the peripheral neurotoxicity is not reduced or increased; total effective rate = markedly effective rate + effective rate. Although all studies used the scale to evaluate patients, the results of some scales are expressed in the “I/II/III;/IV;” levels, which is not convenient to compare the scores as a continuous variable. Therefore, the change of patients' grades is used to show whether the treatment is effective, and by comparing the effective rate, the improvement degree of the disease is reflected.
Only 8 studies [25, 26, 28, 29, 30, 33–35] (n = 375) were evaluated in this way. These studies showed no heterogeneity; thus, a fixed-effects model was used for statistical analysis. As shown in Figure 3, there is a significant difference between the experimental groups and the control groups in total effective rate (RR, 1.63; 95% CI, 1.38 to 1.93; P < 0.00001; and I2 = 0%). In the subgroup analysis, we found that acupuncture improved the sensory function significantly (RR, 1.62; 95% CI, 1.37 to 1.91; P < 0.00001; and I2 = 0%), while the improvement of motor function was not obvious (RR, 1.82; 95% CI, 0.76 to 4.34; P=0.18; and I2 = 0%).
Figure 3.
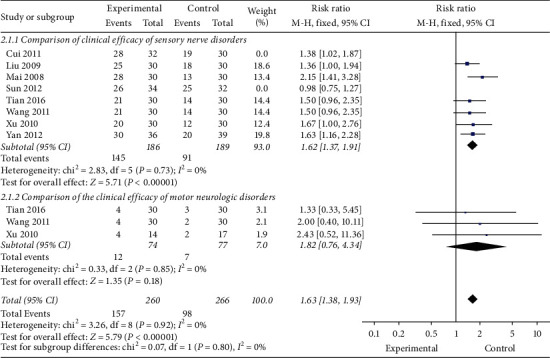
Total effective rate.
As shown in Figure 4, 3 studies [28, 33, 34] compared the improvement of sensory and motor nerve disorders. The results showed that the recovery of sensory function was better than that of motor function (RR, 4.07; 95% CI, 2.41 to 6.86; P < 0.00001; and I2 = 7%).
Figure 4.
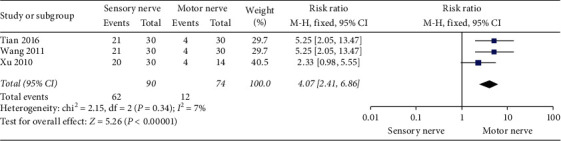
Sensory nerve vs. motor nerve.
3.3.2. Nerve Conduction Velocity (NCV)
(1) Sensory Nerve Conduction Velocity (SCV). As shown in Figure 5, 4 studies [34, 35, 37, 42] (n = 250) were included. A significant improvement in SCV in the experimental group (MD = 2.25, 95% CI 2.06 to 2.44; and P < 0.00001), with high heterogeneity (I2 = 95%). And acupuncture is effective for both upper (MD = 1.08, 95% CI 0.80 to 1.36; and P < 0.00001) and lower (MD = 3.22, 95% CI 2.96 to 3.47; andP < 0.00001) extremities. Because of the high heterogeneity, sensitivity analysis was performed on the results. The results showed that the heterogeneity decreased significantly (I2 = 51%)when Han's results was removed.
Figure 5.
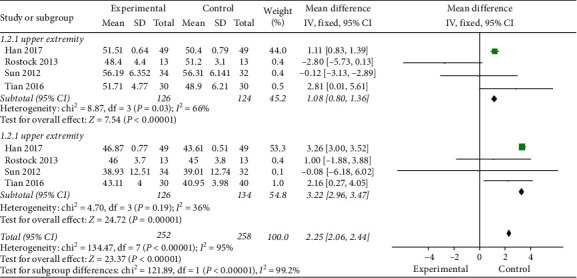
SCV.
(2) Motor Nerve Conduction Velocity (MCV). As shown in Figure 6, 3 studies [34, 35, 37] (n = 205) were included. A significant improvement in MCV in the experimental group (MD = 2.41, 95% CI 2.15 to 2.68; and P < 0.00001), with high heterogeneity (I2 = 90%). And acupuncture is effective for both upper (MD = 1.70, 95% CI 0.79 to 2.62; and P=0.0003) and lower (MD = 2.48, 95% CI 2.20 to 2.76; and P < 0.00001) extremities.
Figure 6.
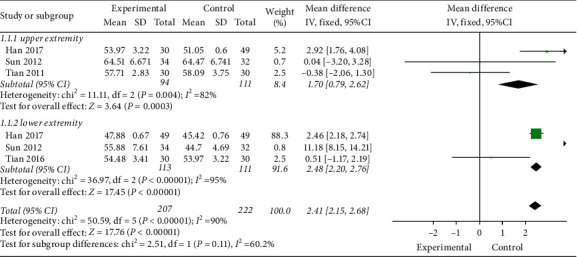
MCV.
(3) SCV vs. MCV. As shown in Figure 7, 3 studies [34, 35, 37] (n = 226) compared the SCV and MCV. The results showed that the recovery of SCV was worse than MCV (MD = −1.39, 95% CI −1.59 to −1.19; and P < 0.00001) with high heterogeneity (I2 = 97%).
Figure 7.
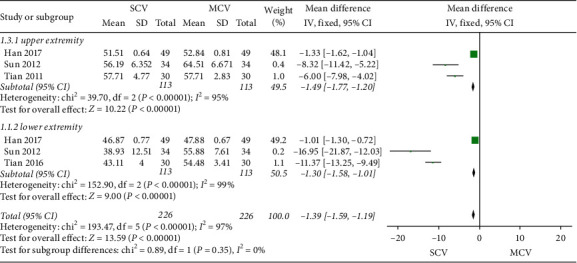
SCV vs. MCV.
3.3.3. FACT-NTX Scores
As shown in Figure 8, 3 studies [36–38] (n = 233) compared the FACT-NTX scores. A significant reduction in FACT-NTX scores in the experimental group (MD = −1.82, 95% CI −2.03 to −1.62; and P < 0.00001), with high heterogeneity (I2 = 99%).
Figure 8.
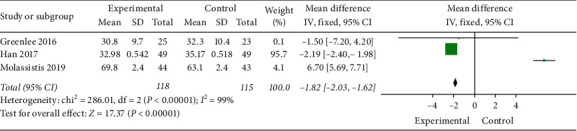
FACT-NTX scores.
3.3.4. BPI Scores
As shown in Figure 9, 4 studies [36–38, 42] (n = 262) compared the BPI scores. A significant reduction in the experimental group (MD = −1.64, 95% CI −1.71 to −1.58; and P < 0.00001), with high heterogeneity (I2 = 99%).
Figure 9.
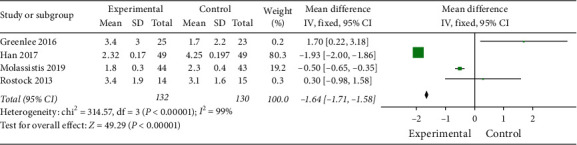
BPI scores.
3.3.5. Adverse Events
One trial [36] reported mild adverse events which were discomfort, minor swelling, and bruising after acupuncture needle withdrawal. Three trials reported [24, 33, 41] that no adverse events occurred. The remaining trials did not mention any adverse events.
4. Discussion
4.1. Analysis of Efficacy
This systematic review shows that the overall effectiveness of acupuncture treatment of CIPN is superior to that of neurotrophic drugs (Figure 3). Meanwhile, the recovery of sensory function is significantly better than motor function (Figure 4). It is interesting to see that many non-CIPN symptoms [26, 28, 31, 38] caused by chemotherapy, like nausea, appetite change, and drowsiness, had also improved,suggesting that acupuncture for CIPN can impact on a wider range of symptoms. However, on the other hand, the results [26, 40] show that acupuncture for mild and moderate limb numbness, pain, and other sensory disorders is significantly effective, while the curative effect for severe CIPN patients was poor. And measurable results can only be found after a longer period of treatment (up to 10 weeks) [43]. While the mechanism of action of acupuncture is not completely understood,several animal studies [44, 45] have reported that acupuncture can stimulate C nerve fibers which can be damaged from chemotherapy and enhance spinal/central GABA receptors, serotoninergic, and adrenergic neurotransmission [46]. Other hypotheses including “de qi” sensation,the principal of acupuncture,can increase blood flow in the capillaries,release local opioid peptides, stimulate specific areas of the brain [47], and participate in signal integration of central nervous system [48].
4.2. Analysis of NCV
The result shows that acupuncture has a good effect on the recovery of nerve conduction velocity (Figures 5 and 6), and the recovery of motor nerve function is better than that of sensory nerve function (Figure 7), but this is contrary to the results of the above questionnaire survey (Figure 4). This may be related to the fact that the motor nerve recovery is covered up by the decrease of myodynamia due to reduced exercise. The evidence shows that long-term aerobic exercise training can prevent the onset or modify the natural history of diabetic peripheral neuropathy [49]. This conclusion has also been confirmed in an animal experiment [50]. More favorable outcomes of peripheral neuropathy and psychosocial functioning are also reported in ovarian cancer survivors who were meeting physical activity guidelines [51]. These articles suggest that moderate exercise may play an important role in the treatment of CIPN. On the other hand, questionnaires and self-reports are the preferred measure of CIPN [52], but they can be highly susceptible to subjective factors. Significant statistical difference is found in the change of mean values of the NCI-CTCAE Scale in Alessandro et al.'s experiment [40], and some literatures [53] think that the assessment modalities for neuropathy may overestimate the presence of motor neuropathy and misdiagnose CIPN. So, current scales may not be appropriate to measure CIPN in a valid way. More objective outcome measurements and a combination of scales for the standardization of CIPN are advisable.
4.3. Analysis of FACT-NTX Score
We can see the positive effect of acupuncture in the analysis of FACT-NTX score (Figure 8), but the results are highly heterogeneous. It may be due to the fact that FACT-NTX score is a subjective index, and results are easily affected by artificial errors. The different therapeutic effects caused by different acupoints may also be the cause of differences. We found that the acupoints in Yangming Meridian are generally used for the treatment of CIPN because “treatment for flaccidity aims at Yangming meridian” is a therapeutic principle of acupuncture in traditional Chinese medicine, which means that the acupoints on Yangming Meridian like LI4, LI11, and ST36 are often used in the treatment of muscle atrophy and peripheral neuropathy, such as CIPN, diabetic peripheral neuropathy, stroke, and myasthenia gravis. Yangming Meridian is also closely related to the gastrointestinal system, so it can explain that in the treatment of CIPN, gastrointestinal related symptoms like nausea, loss of appetite, and fatigue are also improved. Another characteristic of the treatment of CIPN is using local points on the extremes like EX10 (Bafeng) and EX-UE 9 (Baxie) to stimulate the peripheral nerves and improve the local symptoms of the limbs.
4.4. Analysis of BPI Score
Although the result shows that acupuncture has a certain effect on improving pain, the heterogeneity of the results is high. In Rostock et al.'s study [42], there is no therapeutic advantage of electroacupuncture over an orally administered placebo control. They attribute it to a floor effect that their study design is based on patients with more severe CIPN symptoms and higher pain scores,so there are no large margins for many patients to improve considerably. Alessandro et al. and Yan et al.'s studies [26, 40] also show that the benefits of acupuncture for pain are moderate and appear to be more significant for patients with recent neuropathy symptoms. In Greenlee et al.'s study [36],the pain symptoms become worse after the postintervention in the electroacupuncture arm,which is similarly reported in Chen et al.'s RCT [54] of electroacupuncture in reducing pancreatic cancer pain. The mechanism is not clear, but we found that both Rostock et al. and Greenlee et al.'s studies use electroacupuncture. The strong stimulation of electroacupuncture may aggravate the pain. Therefore, electroacupuncture may not be as effective and safe as traditional acupuncture in CIPN.
4.5. Limitations of Study
We only searched the studies from the English and Chinese databases, so some studies may be omitted, which may lead to the sampling bias. The sample size included in the study is small and because there is no generally agreed-upon placebo control, the control group is inconsistent, which may affect the accuracy of the effectiveness evaluation. Lack of blinding of both subjects and investigators is a common methodological flaw of the selected studies, which means positive expectation may amplify acupuncture's effect. Therefore, the findings of this study should be interpreted cautiously.
5. Conclusion
In conclusion, our analysis indicates that the use of acupuncture in the management of CIPN is safe and effective. The most used acupoints for CIPN are LI4, LI11, ST36, EX10 (Bafeng), and EX-UE 9 (Baxie). However, considering the methodological limitation of the studies, caution needs to be taken in applying the conclusions of this review. The existing studies lack long-term observation. Furthermore, none of the included studies compared acupuncture with duloxetine [3], a drug which is currently the most effective treatment in CIPN. Thus, we would recommend that future studies use this medication as a comparator and observe the long-term curative effect of acupuncture.
Data Availability
All the data were taken from the published studies.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
References
- 1.Argyriou A., Kyritsis A., Makatsoris T., Kalofonos H. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Management and Research. 2014;6:135–147. doi: 10.2147/cmar.s44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyritsis S. B., Goldstein D., Krishnan A. V., et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA: A Cancer Journal for Clinicians. 2013;63(6):419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein N., Obaid A. Management of peripheral neuropathy induced by chemotherapy in adults with cancer: a review. International Journal of Palliative Nursing. 2017;23(1):13–17. doi: 10.12968/ijpn.2017.23.1.13. [DOI] [PubMed] [Google Scholar]
- 4.André T., Boni C., Mounedji-Boudiaf L., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. New England Journal of Medicine. 2004;350(23):2343–2351. doi: 10.1056/nejmoa032709. [DOI] [PubMed] [Google Scholar]
- 5.Boni J. A., Wang M., Martino S., et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. New England Journal of Medicine. 2008;358(16):1663–1671. doi: 10.1056/nejmoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M., Currie G. L., Sena E. S., et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Currie A.-L., Haanpaa M., Kautiainen H., Kalso E., Saarto T. Burden of chemotherapy-induced neuropathy-a cross-sectional study. Supportive Care in Cancer. 2011;19(12):1991–1996. doi: 10.1007/s00520-010-1043-2. [DOI] [PubMed] [Google Scholar]
- 8.Haanpää R. D., Michalak J. C., Sloan J. A., et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy. Cancer. 2007;110(9):2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 9.Kautio A.-L., Haanpää M., Leminen A., Kalso E., Kautiainen H., Saarto T. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Research. 2009;29(7):2601–2606. [PubMed] [Google Scholar]
- 10.Raja S. N., Haythornthwaite J. A., Pappagallo M., et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59(7):1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 11.Smith E. M. L., Pang H., Cirrincione C., et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy. JAMA. 2013;309(13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesbit S. A., Sharma R., Waldfogel J. M., et al. Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Current Medical Research and Opinion. 2019;35(1):15–25. doi: 10.1080/03007995.2018.1497958. [DOI] [PubMed] [Google Scholar]
- 13.Özsoy M., Ernst E. How effective are complementary therapies for HIV and AIDS?-a systematic review. International Journal of STD & AIDS. 1999;10(10):629–635. doi: 10.1258/0956462991913088. [DOI] [PubMed] [Google Scholar]
- 14.Wang X. P., Zhang D.-Z., Zhang D.-J., Wei X.-D., Wang J.-P. Acupuncture for the relief of hot flashes in breast cancer patients: a systematic review and meta-analysis of randomized controlled trials and observational studies. Journal of Cancer Research and Therapeutics. 2018;14(10):S600–S608. doi: 10.4103/0973-1482.183174. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Lin L., Li H., Hu Y., Tian L. Effects of acupuncture on cancer-related fatigue: a meta-analysis. Supportive Care in Cancer. 2018;26(2):415–425. doi: 10.1007/s00520-017-3955-6. [DOI] [PubMed] [Google Scholar]
- 16.Choi T.-Y., Kim J. I., Lim H.-J., Lee M. S. Acupuncture for managing cancer-related insomnia: a systematic review of randomized clinical trials. Integrative Cancer Therapies. 2017;16(2):135–146. doi: 10.1177/1534735416664172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu W., Hu D., Dean-Clower E., et al. Acupuncture for chemotherapy-induced leukopenia: exploratory meta-analysis of randomized controlled trials. Journal of the Society for Integrative Oncology. 2007;05(01):1–10. doi: 10.2310/7200.2006.035. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z., Ma C., Xu L., Wu Z., Wu L. Laser acupuncture for patients with knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Evidence Based Complementary and Alternative Medicine. 2019;2019:10. doi: 10.1155/2019/6703828.6703828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seca S., Miranda D., Cardoso D., et al. Effectiveness of acupuncture on pain, physical function and health-related quality of life in patients with rheumatoid arthritis: a systematic review of quantitative evidence. Chinese Journal of Integrative Medicine. 2019;25(9):704–709. doi: 10.1007/s11655-018-2914-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., Yue J., Golianu B., Sun Z., Lu Y. Updated systematic review and meta-analysis of acupuncture for chronic knee pain. Acupuncture in Medicine. 2017;35(6):392–403. doi: 10.1136/acupmed-2016-011306. [DOI] [PubMed] [Google Scholar]
- 21.Qin Z., Liu X., Wu J., Zhai Y., Liu Z. Effectiveness of acupuncture for treating sciatica: a systematic review and meta-analysis. Evidence Based Complementary and Alternative Medicine. 2015;2015:13. doi: 10.1155/2015/425108.425108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji M., Wang X., Chen M., Shen Y., Zhang X., Yang J. The efficacy of acupuncture for the treatment of sciatica: a systematic review and meta-analysis. Evidence Based Complementary and Alternative Medicine. 2015;2015:12. doi: 10.1155/2015/192808.192808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W. B., Woo S. H., Min B.-I., Cho S.-H. Acupuncture for gouty arthritis: a concise report of a systematic and meta-analysis approach. Rheumatology. 2013;52(7):1225–1232. doi: 10.1093/rheumatology/ket013. [DOI] [PubMed] [Google Scholar]
- 24.Chein T. J., Liu C.-Y., Fang C.-J., Kuo C.-Y. Effect of acupuncture on chemotherapy-ralated periphral neurotoxicity and digestive tract adverse effects of malignant tumor patients: a clinical study on 20 cases. Journal of Traditional Chinese Medicine. 2011;52(23):2031–2033. [Google Scholar]
- 25.Mai Z. Clinical observation of acupuncture in the treatment of Please note that as per the journal style, if there are more than 6 authors/editors, the first 3 author names are listed followed by ‘et al.’ if the author group consists of 6 authors or fewer, all author names should be listed. Therefore, in Ref(s). ; 26; 29; 30; 31; 35; please list all names for up to 6 authors/editors. For more than 6 authors/editors, use ‘et al.’ after the first 3 authors/editors.chemotherapy-ralated periphral neurotoxicity. Modern Preventive Medicine. 2008;35(S1):122–123. [Google Scholar]
- 26.Yan Y., He C., Hu C. Clinical study on acupuncture treatment of peripheral neuropathy induced by chemotherapy. Journal of Liaoning University of TCM. 2012;14(08):230–231. [Google Scholar]
- 27.Zhang W. Clinical Trial of Electroacupuncture Stimulation on Preventionand Treatment of Oxaliplatin Neurotoxicity. Dalian, Liaoning, China: Dalian Medical University; 2018. [Google Scholar]
- 28.Xu W., Hua B., Hou W., Bao Y. Clinical randomized controlled study on acupuncture for treatment of peripheral neuropathy induced by chemotherapeutic drugs. Chinese Acupuncture & Moxibustion. 2010;30(06):457–460. [PubMed] [Google Scholar]
- 29.Bo L., Zhang L., Xu K., Luo H., Li Y. Clinical observation on acupuncture treatment of peripheral nervous system toxicity caused by chemotherapeutic drugs. Hebei Traditional Chinese Medicine. 2009;31(07):1040–1041. [Google Scholar]
- 30.Cui D., Wang L. Clinical observation on the treatment of oxaliplatin neurotoxicity by acupuncture. Gansu Traditional Chinese Medicine. 2011;24(02):45–46. [Google Scholar]
- 31.Tian Y., Zhang Y., Jia Y. Curative effect observation of acupuncture on peripheral neurotoxicity after oxaliplatin chemotherapy. Tianjin Traditional Chinese Medicine. 2011;28(03):212–213. [Google Scholar]
- 32.Chen W. Clinical observation of acupuncture on peripheral neuropathy induced by oxaliplatin chemotherapy in patients with colorectal cancer. World Latest Medical Information. 2018;18(05):144–145. [Google Scholar]
- 33.Wang B. Clinical Study on Acupuncture Treatment of Peripheral Neuropathy after Oxaliplatin Chemotherapy in Patients with Colorectal Cancer. Beijing, China: Beijing University of Traditional Chinese Medicine; 2011. [Google Scholar]
- 34.Tian H. The Clinical Observation of Curative Effect Acupuncture on Chemotherapy-Induced Peripheral Neuropathy Caused by FOL-FOX4 Regimen. Heilongjiang, China: Heilongjiang University of Traditional Chinese Medicine; 2016. [Google Scholar]
- 35.Sun X., He S., Chen H., et al. Clinical study of electroacupuncture in the treatment of oxaliplatin neurotoxicity. Shanghai Journal of Acupuncture. 2012;31(10):727–729. [Google Scholar]
- 36.Greenlee H., Crew K. D., Capodice J., et al. Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Research and Treatment. 2016;156(3):453–464. doi: 10.1007/s10549-016-3759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han X., Sun J., Cai Z., et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer. 2017;17(1):p. 40. doi: 10.1186/s12885-016-3037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molassiotis A., Chan V., Wang C. H., et al. A randomized assessor-blinded wait-list-controlled trial to assess the effectiveness of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Integrative Cancer Therapies. 2019;18 doi: 10.1177/1534735419836501.1534735419836501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y. Clinical Study on the Prevention and Treatment of Oxaliplatin Neurotoxicity by Acupuncture. Hefei, Anhui, China: Anhui University of Traditional Chinese Medicine; 2014. [Google Scholar]
- 40.D’Alessandro E. G., Nagy D. R. N., Brito C. M. M. D., Almeida E. P. M., Cecatto R. B. Acupuncture for chemotherapy-induced peripheral neuropathy: a randomised controlled pilot study. BMJ Support Palliat Care. 2019 doi: 10.1136/bmjspcare-2018-001542. [DOI] [PubMed] [Google Scholar]
- 41.Lu W., Lin N. U., Shin I. H., et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. 2020;25(4):310–318. doi: 10.1634/theoncologist.2019-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rostock M., Jaroslawski K., Guethlin C., Ludtke R., Bartsch H. H. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evidence Based Complementary and Alternative Medicine. 2013;2013:9. doi: 10.1155/2013/349653.349653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder S., Liepert J., Greten J. H. Acupuncture treatment improves nerve conduction in peripheral neuropathy. European Journal of Neurology. 2007;14(3):276–281. doi: 10.1111/j.1468-1331.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 44.Gao X., Qin Q., Yu X., et al. Acupuncture at heterotopic acupoints facilitates distal colonic motility via activating M3 receptors and somatic afferent C-fibers in normal, constipated, or diarrhoeic rats. Neurogastroenterol Motil. 2015;27(12):1817–1830. doi: 10.1111/nmo.12694. [DOI] [PubMed] [Google Scholar]
- 45.Murase K., Kawakita K. Diffuse noxious inhibitory controls in anti-nociception produced by acupuncture and moxibustion on trigeminal caudalis neurons in rats. The Japanese Journal of Physiology. 2000;50(1):133–140. doi: 10.2170/jjphysiol.50.133. [DOI] [PubMed] [Google Scholar]
- 46.Park J. H., Han J. B., Park J.-H., et al. Spinal GABA receptors mediate the suppressive effect of electroacupuncture on cold allodynia in rats. Brain Research. 2010;1322:24–29. doi: 10.1016/j.brainres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Cheng K. J. Neurobiological mechanisms of acupuncture for some common illnesses: a clinician’s perspective. Journal of Acupuncture Meridian Studies. 2014;7(3):105–114. doi: 10.1016/j.jams.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Zhou W., Benharash P. Significance of “Deqi” response in acupuncture treatment: myth or reality. Journal of Acupuncture and Meridian Studies. 2014;7(4):186–189. doi: 10.1016/j.jams.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Balducci S., Lacobellis G., Parisi L., et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. Journal of Diabetes and Its Complications. 2006;20(4):216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Selagzi H., Buyukakillie B., Cimen B., Yilmaz N., Erdogan S. Protective and therapeutic effects of swimming exercise training on diabetic peripheral neuropathy of streptozotocin-induced diabetic rats. Journal of Endocrinological Investigation. 2008;31(11):971–978. doi: 10.1007/bf03345634. [DOI] [PubMed] [Google Scholar]
- 51.Stevinson C., Steed H., Faught W., et al. Physical activity in ovarian cancer survivors: associations with fatigue, sleep, and psychosocial functioning. International Journal of Gynecologic Cancer. 2009;19(1):73–78. doi: 10.1111/igc.0b013e31819902ec. [DOI] [PubMed] [Google Scholar]
- 52.Steed D. L., Lacchetti C., Dworkin R. H., et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology. 2014;32(18):1941–1967. doi: 10.1200/jco.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 53.Molassiotis A., Li Y. C., Lopez V., et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019;19(1):p. 132. doi: 10.1186/s12885-019-5302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H., Liu T.-Y., Kuai L., Zhu J., Wu C.-J., Liu L.-M. Electroacupuncture treatment for pancreatic cancer pain: a randomized controlled trial. Pancreatology. 2013;13(6):594–597. doi: 10.1016/j.pan.2013.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data were taken from the published studies.


