Abstract
ESKAPE pathogens, namely, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, are responsible for a majority of all healthcare-acquired infections (HAI). The bacteria cause nosocomial infections in immunocompromised patients. Extracts from Callistemon viminalis have been shown to have antibacterial, antifungal, and anti-inflammatory activities. Tormentic acid congener, a pentacyclic triterpene saponin, was isolated from C. viminalis leaves. This study aimed to investigate the antibacterial effects of tormentic acid congener and leaf extracts on biofilm formation by A. baumannii, S. aureus, S. pyogenes, and P. aeruginosa. The antibacterial effects were determined by the microbroth dilution method, and ciprofloxacin was used as the standard antibacterial drug. Biofilm formation and detachment assays were performed using crystal violet staining. Production of extracellular polymeric DNA and polysaccharides from biofilms was also determined. Tormentic acid congener showed time-dependent antibacterial activity against P. aeruginosa with a MIC of 100 µg/ml and caused significant protein leakage. Antibacterial activity was found when tormentic acid congener was tested against both S. aureus and P. aeruginosa. The MICs were found to be 25 µg/ml and 12.5 µg/ml for P. aeruginosa and S. aureus cells, respectively. S. pyogenes was found to be susceptible to tormentic acid congener and the hydroethanolic extract with an MIC of 100 µg/ml and 25 µg/ml, respectively. A. baumannii was found not to be susceptible to the compound or the extracts. The compound and the extracts caused a significant decrease in the biofilm extracellular polysaccharide content of S. pyogenes. The extracts and tormentic acid congener caused detachment of biofilms and decreased the release of extracellular DNA and capsular polysaccharides from biofilms of P. aeruginosa and S. aureus. Tormentic acid congener and extracts, thus, have significant antibacterial and antibiofilm activities on these selected ESKAPE bacteria and can act as source lead compounds for the development of antibacterial triterpenoids.
1. Introduction
ESKAPE pathogens are responsible for two-thirds of all healthcare-associated infections [1]. The Infectious Diseases Society of America (ISDA) formulated an acronym ESKAPE to emphasize the group of pathogens that cause hospital infections and effectively “escape” the effects of antibacterial drugs [2]. Gram-positive pathogens, vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA), and Gram-negative pathogens, Pseudomonas aeruginosa and Acinetobacter baumannii as well as extended spectrum-lactamase producing (RSBL) or carbapenem-resistant Enterobacteriaceae (CRE) were used [2]. Approximately 10–15% of the nosocomial infections on a worldwide scale are caused by P. aeruginosa [3]. Nosocomial infections are responsible for hospital-acquired infections largely in immunocompromised patients [4].
K. pneumoniae and P. aeruginosa have been found to cause life-threatening hospital infections in critically ill individuals [5]. These two pathogens have acquired resistance against some of the common antibacterial drugs [6]. K. pneumoniae belongs to the family Enterobacteriaceae [7], and it is one of the most common pathogens associated with hospital-acquired infections [8]. K. pneumoniae naturally inhabits the gastrointestinal tract microbiome of healthy humans and animals [9]. S. aureus is a Gram-positive bacterium carried in the nostrils of approximately 30% of the people [10]. In most cases, S. aureus does not cause any harm; however, in hospitalised immunocompromised people, it may cause infections. These bacteraemia invasions affect people with underlying lung disease, including those on mechanical ventilators and endocarditis, and this can lead to heart failure or stroke [10]. S. pyogenes is a Gram-positive, nonmotile, nonspore forming, catalase-negative cocci that occur in pairs or chains [11]. S. pyogenes infections cause pharyngitis and are responsible for up to 33% of diagnosed cases of sore throat in children and up to 10% in adults [12]. ESKAPE pathogens have become a major cause of morbidity and mortality all over the world [13]. A. baumannii has the ability of surviving for long periods on hospital surfaces and equipment. This is aided by its ability to develop resistance to multiple antibiotics [14] leading to outbreaks in clinical settings [15].
Biofilm formation is a bacterial strategy to survive under adverse conditions [16]. Bacteria develop resistance to antimicrobial agents as well as thrive in seemingly severe conditions, and this has been attributed to their ability to form biofilms [17]. The production of extracellular polymeric substance (EPS) protects bacterial cells from environmental damage, which leads to these cells developing resistance to antibiotics [18]. There is a continued evolution of dangerous multidrug-resistant bacteria that has led to significant increases in morbidity and mortality due to bacterial infections. Many of the currently prescribed antibacterial drugs have significant adverse side effects [19]. There is urgency for the development of new highly effective and safe antibacterial agents from natural products specifically the complementary and alternative medicines (CAM). The use of different plant sources to search for antibacterial agents has been shown to be a promising approach [20].
Plants of the Callistemon genus are known to possess antifungal, antioxidant, antithrombin, anti-inflammatory, antidiabetic, antibacterial, and herbicidal activities [21]. Callistemon viminalis, also known as bottlebrush, is an ornamental plant which belongs to Myrtaceae family. Extracts from C. viminalis have been reported to have various medicinal properties including antibacterial, antifungal, and antioxidant activities [22]. Compounds in C. viminalis have shown antibacterial activity against S. aureus and E. coli with an inhibitory zone diameter about 16–20 mm [23]. Phytochemical analysis of C. viminalis leaves has demonstrated the presence of phenolic, triterpenoid, flavonoid, saponin, steroid, alkaloid, tannin, carbohydrate, amino acid, and protein compounds [24]. Terpenoids compounds in C. viminalis have shown antimicrobial properties for some bacteria [25]. Triterpenoids are chemical compounds from a class of terpenoids, with chemically characterised six isoprene units and a total of 30 carbon atoms [26]. Triterpenoids are widely distributed secondary metabolites found in many genera of plants and other living organisms, and they exist in different states [27]. Several triterpenes have been isolated from fungi and plant species for investigation into antibacterial agents against P. aeruginosa [26]. Several studies have found triterpenes to possess antibacterial effects on K. pneumoniae [26].
Tormentic acid can be isolated from several plants which include C. viminalis, Sarcopoterium spinosum [27], and Potentilla chinensis [28] among others. The main objective of the study was to evaluate the antibacterial activity of tormentic acid congener, a triterpenoid isolated from C. viminalis, on K. pneumoniae, P. aeruginosa, S. aureus, S. pyogenes, and A. baumannii. In addition, we investigated the effects of tormentic acid congener on biofilm production of S. aureus, S. pyogenes, P. aeruginosa, and A. baumannii.
2. Materials and Methods
2.1. Reagents
The chemicals and solvents used in this study were all obtained from Sigma-Aldrich Chemicals Company (Munich, Germany). Dichloromethane, ethanol, methanol, and water were used for extraction of Callistemon viminalis crude extracts. Dimethyl sulfoxide (DMSO) was used for dissolving the extracts, ciprofloxacin (the standard antibiotic drug), and tormentic acid congener, and 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) was used as an indicator of cell viability after carrying out assays. The bacterial species P. aeruginosa (NCTC 10662), S. pyogenes, K. pneumoniae (ATC700603), A. baumannii (CECT(R) 911), and S. aureus (NCTC 6571) were obtained from Merck (Darmstadt, Germany). Before resuscitation, the cells were kept in a −80°C freezer as 1 ml stock strains in 50% glycerol. Luria agar base, Miller, was used to plate cells on agar plates, and all the cells were cultured in Luria broth base, Miller. The tormentic acid congener used was isolated from Callistemon viminalis and characterised by NMR and mass spectrometry. All assays were performed in the biological safety cabinet Bioflow-II Labotec, Model 650. The incubator shaker, Model number S1-300 (Jeiotech Co., Korea), and the incubator SI-300 were used for all incubations carried out. For analysis of cell viability using absorbance, a Tecan Genios Pro microplate reader (Tecan Group Ltd, Männedorf, Austria) was used to record the results from a 96-well microwell plate. GraphPad Prism6 (San Diego California, USA) was used to record and analyse the results. For centrifugation, the centrifuge, Rotafix-32 Hettich Zentrifugen Microcent 94–2 Eppendorf centrifuge 541(Sigma-Aldrich Co. Darmstadt, Germany) was used.
2.2. Plant Collection and Preparation
The leaves of Callistemon viminalis were collected in Harare, the University of Zimbabwe, 17.7840°S, 31.0530°E. The plants had been previously authenticated by a taxonomist from the National Herbarium and Botanic Gardens in Harare, Zimbabwe. The leaves of C. viminalis were separated from the branches and predried using a Labcon orbital incubator (Labotech Co., Cape Town, SA) at 60°C. The dried leaves were ground using a pestle and mortar to produce approximately 4 g of a powdered sample.
2.3. Preparation of Plant Extracts
The leaves were washed with distilled water then dried in an oven (Memmert, Model 400, D06060) at 60°C. The dried leaves were pounded in a traditional mortar and pestle and sieved to obtain a powder. Two solvents were prepared, one with 50% v/v DCM: MeOH and the other one with 50% v/v EtOH:H2O. The powdered leaves and solvents were mixed in the ration 1 : 10, respectively. The leaves were left soaked in solvents for 3 days after which filtration was performed to obtain the extract. Filtration was done twice, first using cotton wool and using a filter paper thereafter. After filtration, the excess solvent was evaporated using a Buchi RII rotavapor (BÜCHI Labortechnik AG, Postfach, Switzerland), and the crude extract was dried under a fan.
2.4. Column Chromatography on Silica Gel
The leaf extract was run on a column with 100% hexane initially as the eluting solvent. Batch gradient system column elution was employed starting with a solvent of low polarity to that with high polarity. The batch gradient system was a 20-step gradient elution with a gradual increase of polarity with 100% ethyl acetate. Methanol was then added to 100% EA up to 90% EA and 10% methanol. Fractions of 250 ml were collected and concentrated using a Buchi RII rotary evaporator (BÜCHI Labortechnik AG, Postfach, Switzerland). Thin-layer chromatography was used for the analysis of the collected fractions. The fractions with similar retardation factor values were pooled. The pooled fractions were left to evaporate to dryness for crystals to form. Single spots observed on the developed chromatograms under UV (354 and 365 nm) after staining with sulphuring acid were deemed pure and were subjected to NMR analyses.
2.5. NMR Analyses and Determination of the Mass of the Isolated Compound
1H NMR spectra were recorded at 400 MHz and 13C NMR spectra at 100 MHz. The chemical shifts for 1H NMR and 13C NMR were referenced to TMS via residual solvent signals (1H, CDCl3 at 7.26 ppm; 13C, CDCl3 at 77.36 ppm; 1H, DMSO-d6 at 2.45 ppm; 13C, DMSO-d6 at 39.43 ppm, 1H, CD3OD at 3.31 ppm; and 13C, CD3OD at 49.0 ppm). Two-dimensional (2D) NMR experiments were run using standard pulse sequences. Molecular formulae were determined by electrospray ionization with a 7T hybrid ion trap and a TOF detector running in a positive or negative mode. Tormentic acid congener was identified as a pure compound.
2.6. Antibacterial Susceptibility Tests
Bacteria were grown in Luria broth base, Miller (Sigma-Aldrich), supplemented by casein acid hydrolysate 10 g/L, yeast extract 5 g/L, and sodium chloride 5 g/L. The antibacterial effects of the compound tormentic acid congener as well as those for the DCM:MeOH and EtOH:H2O leaf extracts of C. viminalis were investigated by adapting the microbroth dilution procedure used by Vipra et al. [29]. Briefly, the tormentic acid congener as well as the DCM:MeOH and EtOH:H2O leaf extracts of C. viminalis were dissolved to give a final concentration of 1% DMSO and 100 µg/ml. The resultant mixtures were serially diluted in a 2-fold manner with media containing 1% DMSO up to a minimum concentration of 12.5 µg/ml. For all experiments, bacterial cells exponentially growing were standardised using 0.5 McFarland's standard solution to give a cell suspension with a concentration of 1 × 106 CFU/ml.
Tormentic acid congener, extracts, cells, and Luria broth were diluted into the 96-well microplate wells (Greiner Bio-One, Sigma-Aldrich, St. Louis, MO, USA). Each well contained a final volume of 200 µl. A positive control containing ciprofloxacin (highest concentration of 10 µg/ml) was set up in each well-containing media and bacterial growth culture. Relevant sterility and negative controls were set up containing media only and media with extract, respectively. Preincubation and postincubation cell density measurements were determined. After postincubation measurements, visualisation of viable cell growth on the plate was investigated by adding 20 µl of 3-(4, 5-dimethylthiazol-2)-2, 5-diphenyltetrazolium bromide (MTT) solution (1 mg/ml) to each well. The 96-well microplate was then covered and placed in an incubator for 2 hours.
2.7. Time-Kill Assays of Tormentic Acid Congener on P. aeruginosa
The time-kill assays for tormentic acid congener on P. aeruginosa were performed in LB culture medium after carrying out serial dilutions starting from 100 to 12.5 µg/ml. After standardisation of cells using the 0.5 McFarland solution, ciprofloxacin was used as a control standard and untreated cells were used as a positive control. Aliquots of diluted cell suspension and tormentic were added to a microplate wells plate in equal volumes to produce a total volume of 200 µl. The microplate was incubated, and then, the absorbance was measured under suitable conditions for varied time intervals (0, 8, 10, 24, 26, 28, 30, and 32 hours). The results of the absorbance representing cell densities were used to plot time-kill graphs for 100 µg/ml, 50 µg/ml, 25 µg/ml, 12.5 µg/ml, and the control. MTT was then added to the 96-well microplate to determine cell viability. The bactericidal effect was obtained from observing the graph with a lethality percentage of 90% for 6 hours, which is equivalent to 99.9% of lethality for 24 hours. This method was used to further confirm the MIC of tormentic acid congener on P. aeruginosa.
2.8. Effects of Combining Ciprofloxacin and Tormentic Acid Congener on P. aeruginosa
The checkerboard assay was used to determine the effects of combining ciprofloxacin and tormentic acid congener on P. aeruginosa in consideration of their individual effects against this bacterium. This assay was aimed at determining and observing the presence of antagonism, synergism, and zero interaction between tormentic acid congener and ciprofloxacin [30]. Since there was 88% agreement between time-kill and checkerboard assay, this method was used to confirm the effects of tormentic acid congener on P. aeruginosa as shown by time-kill assays [31] and to calculate the fractional inhibitory concentration. After standardisation of cells using the 0.5 McFarland scale, 100 µl of the inoculum was added to each well in a sterile 96-well microplate. Equal volumes of 50 µl tormentic acid congener and 50 µl ciprofloxacin were added to the wells. The plate was incubated at 37°C, and absorbance of the cells was measured at varied time intervals (0, 8, 10, 24, 26, 28, 30, and32 hours). The results of the absorbance were used to plot time-kill graphs for 100 µg/ml, 50 µg/ml, 25 µg/ml, 12.5 µg/ml, and control. The fractional inhibitory index (FICI) was calculated with the concentrations in the first nonturbid well found in each row and column of the microplate after extrapolating line graphs [32]. The different combinations are categorised as synergistic if FICI is ≤ 0.5, antagonistic when FICI > 4, and indifferent if 0.5 < FICI ≤ 4 [32]. The FICI was calculated using the following equation [32]:
| (1) |
2.9. Effects of Tormentic Acid Congener on Protein Leakage
From precultured P. aeruginosa cell suspension, a volume of 200 µl was subcultured in 200 ml of Luria broth base in a 1-litre container, and the cells were incubated overnight at 37°C at 100 rpm. The subcultured cells were centrifuged at 3500 rpm for 4 minutes, and the supernatant was removed. The pellet of P. aeruginosa cell suspension was diluted with 0.9% normal saline to produce absorbance of 1.5 OD using a centrifuge (Rotafix-32 Hettich Zentrifugen Microcent 94–2 Eppendorf centrifuge 541). To triplicates of labelled tubes A, B, C, D, and E, 6 ml of diluted cells was added. To tube A, 150 µl of tormentic acid congener was added to make a final concentration of 100 µg/ml. To tube B, 2.4 µl of ciprofloxacin was added to make up a final concentration of 0.16 µg/ml. To the control tube C, 1200 µl of SDS was added, to tube D, 180 µl of DMSO was added, and untreated cells were added to tube E. The tubes were centrifuged at 3500 rpm in the centrifuge for 4 minutes, and the pellets were discarded; the supernatants were used for protein determination assay. An exact volume of 150 µl of the supernatants was separately added to a labelled sterile 96-well plate. A calibration curve of concentration 0–50 µg/ml of the BSA stock was made. To each well, 150 µl of Bradford's reagent was added, and the optical densities of the wells were read at 590 nm using a Tecan Genios Pro microplate reader (Tecan Group Ltd, Männedorf, Austria).
2.10. Effect of Extracts and Tormentic Acid Congener on Biofilm Formation
2.10.1. Biofilm Formation Procedure
For this assay, precultured cells were then centrifuged at 3500 rpm for 15 minutes in a Rotafix-32 Hettich Zentrifugen. The supernatant of the centrifuged cell culture was discarded, and 20 ml of 0.5 M phosphate-buffered saline prepared from sodium dihydrogen phosphate as the acidic salt and disodium hydrogen phosphate as the basic salt at pH 7.20 was used to wash the cells. The cell suspension was centrifuged again at 3500 rpm for 15 minutes, the supernatant was discarded, and 5 ml of sterile media was added and hand shaken to the dissolve the cell pellet in the media. A 2 ml volume of standardised cells was added to each well of a 24-well plate (Sigma-Aldrich Co. Darmstadt, Germany). Thereafter, the cells were allowed to adhere to the wells by incubating the cells at 37°C for 2 hours. During the incubation period, stock concentrations of test solutions were prepared to give a final concentration of 100 µg/ml and 10 µg/ml for ciprofloxacin. After the incubation, 500 µl of antibacterial test solutions was added to the wells and left for further incubation for 72 hours at 37°C in a humid nonshaking incubator. After incubation, the contents of each well were gently discarded, and the plate was gently washed three times with 0.5 M phosphate buffer saline at pH 7.20. After washing, the plate was inverted on a paper towel for 15 minutes to drain off excess liquid, and the biofilms were subsequently fixed by incubating the plate at 60°C for 1 hour in an oven. After incubation, the biofilms were quantified as follows: 2.5 ml of 0.1% crystal violet was added to each well, the plate was further incubated at room temperature for 20 minutes before the crystal violet was discarded, and the plate was gently washed with sterile water. The plate was left to dry for overnight; then, 2.5 ml of absolute alcohol 99.9% was added to the wells of the plate. From each well, 200 µl was transferred to a 96-well plate, and optical density readings of the plate were measured at 590 nm using a Tecan Genios Pro microplate reader (Tecan Group Ltd, Männedorf, Austria).
2.10.2. Effect of Extracts and Tormentic Acid Congener on Biofilm Detachment
Precultured S. pyogenes and A. baumannii cells were centrifuged at 3500 rpm for 15 minutes. The supernatant of the centrifuged cell culture was discarded, and 20 ml of 0.5 M phosphate-buffered saline prepared from sodium dihydrogen phosphate as the acidic salt and disodium hydrogen phosphate as the basic salt at pH 7.20 was used to wash the cells. The cell suspension was centrifuged again at 3500 rpm for 15 minutes, the supernatant was discarded, and 5 ml of sterile media was added and hand shaken so as the dissolve the cell pellet in the media. A 2 ml volume of standardised cells were added to each well of a 24-well plate (Sigma-Aldrich Co. Darmstadt, Germany). The cells were allowed to adhere to the wells of a 24-well microplate by incubating the cells in a shaking incubator at 37°C for 2 hours. After 2 hours of incubation, the microplate is incubated in a nonshaking incubator for 72 hours. After incubation, 1.2 ml of 100 µg/ml of antibacterial test solutions and 1.2 ml of Tween 20 detergent solution were added to the plates. The plate was incubated in a nonshaking incubator, at 37°C for 2 hours; thereafter, the biofilm was quantified.
2.10.3. Effect of Extracts and Tormentic Acid Congener on Capsular Polysaccharide Content of Biofilms
The extracellular polysaccharide of the extra polymeric substance was quantified using the phenol-sulphuric acid method. For the capsular polysaccharide analysis, precultured S. pyogenes and P. aeruginosa cells were standardised and added as 4 ml volumes to 5 sterilised 50 ml centrifuge tubes with 100 µl of 100 µg/ml of C. viminalis extracts and tormentic acid congener as well as 0.64 µg/ml of the positive control, ciprofloxacin. The cells were further incubated in a shaking incubator at 37°C for 4 hours. After the incubation period, cells were separated by centrifugation at 4000 rpm for 15 minutes. The procedure was repeated thrice with chilled phosphate-buffered saline of pH 7.2. After centrifugation, the cells were suspended in 450 µl of autoclaved distilled water, and an equal volume of 450 µl of saturated phenol was added and then heated in a waterbath at 65°C for 20 minutes. For each sample, 300 µl was taken in triplicate and added to sterile Eppendorf tubes. To each tube, 150 µl chloroform was added, and the solution was mixed intensely by vortexing. The cell suspension mixture was then centrifuged, and 50 µl of the supernatant was collected and distributed into their respective wells of a flat-bottomed microplate. A standard curve, using 50 µl mannose at concentrations from 100 µg/ml to 6.30 µg/ml as well as a control with no mannose, was prepared. In each well, 150 µl of concentrated sulphuric acid and 30 µl of 5% phenol were also added, and the microtitre plate was put in a static waterbath of 90°C for 5 minutes. After heating, the plate was allowed to cool at room temperature for a further 5 minutes and wiped dry; absorbance was read at 492 nm using a Stat Fax 2 100 microplate reader (Awareness Technologies Inc, Westport, United States of America).
2.10.4. Effects of Tormentic acid Congener on Extracellular DNA Content in Static Biofilms
The effect of tormentic acid congener, DCM:MeOH extract, and EtOH:H2O extracts on extracellular DNA production in static biofilm formation was studied using a method developed by Hawser and Douglas [33]. Briefly, precultured P. aeruginosa cells were centrifuged at 3500 rpm for 15 minutes. The pellet was washed using 0.5 M phosphate-buffered saline pH 7.2. The tormentic acid congener, DCM:MeOH extract, and EtOH:H2O extract were dissolved in 1% DMSO and Luria broth to give a final concentration of 12.5 µg/ml. Of the resulting solution, 500 µl was taken and added to the wells of a 24-well microplate. Positive control was set up using ciprofloxacin at a concentration of 0.80 µg/ml. Negative control of media and cells was also run in parallel.
3. Results and Discussion
3.1. Isolation and of Identification of Tormentic Acid Congener
The following spectral characteristics were obtained: white powder; 1H-NMR (DMSO, 400 MHz) δ (ppm): 5.14 (1H, br s, H-12), 3.49 (1H, m), 2.74 (1H, d, J = 9.2, H-3), 2.12 (1H, d, J = 11.2, H-18), 2.00–1.20 (CH2 and CH region), 1.04 (3H, s, H-27), 0.93 (3H, s, H-23), 0.93 (3H, s, H-25), 0.92 (3H, s, H-30), 0.83 (3H, d, J = 6.4, H-29), 0.77 (3H, d, H-5), 0.75 (3H, s, H-26), and 0.72 (3H, s, H-24). 13C NMR (DMSO, 100 MHz) δ (ppm): 178.7 (C-28), 138.7 (C-13), 124.9 (C-12), 82.7 (C-3), 70.2 (C-20), 67.6 (C-2), 55.2 (C-5), 52.8 (C-18), 47.5 (C-17), 47.5 (C-1), 47.4 (C-9), 46.4 (C-14), 40.5 (C-10), 39.4 (C-8), 39.3 (C-4), 38.9 (C-21), 38.8 (C-19), 36.8 (C-22), 33.0 (C-7), 30.6 (C-16), 29.3 (C-23), 27.9 (C-15), 23.7 (C-27), 23.4 (C-11), 21.5 (C-30), 18.6 (C-6), 17.6 (C-24), 17.5 (C-26), 17.4 (C-24), 16.9 (C-29), and 16.9 (C-25).
The principle active showed the molecular ion (M+) at m/z 488, which agrees with the molecular formula C30H48O5. The 1H-NMR spectrum showed the presence of six singlet methyls and two doublet methyls, which were characteristic of the ursene skeleton, and exhibited signals of an olefinic proton (δ 5.14). The spectrum also showed a singlet at δ 3.49 (m) and two oxygen-bearing methine protons suggestive of the 2α, 3β, 19α-trihydroxy structure. These results indicated that the compound was an ursane-type triterpene. Furthermore, 13C NMR data substantiated the presence of a pair of olefinic carbons (δ 138.7 (C-13) and δ 124.9 (C-12)), a carboxylic acid group (δ 178.7 (C-28)), and three hydroxylated carbons (δ 82.7 (C-3), 70.2 (C-20), and 67.6 (C-2)) on the ursene structure. The structure was determined to be 2α, 3β, 20 β-trihydroxyurs-12-en-28-oic acid [34].
3.2. Antibacterial Susceptibility Tests
The antibacterial effect of C. viminalis leaves was first screened by testing the effects of methanolic and ethanolic crude extracts on all five bacteria using the microbroth dilution assay. After incubation with MTT, the number of viable cells was determined spectrophotometrically. The antibacterial susceptibility tests showed S. aureus and K. pneumoniae (Figure 1) to be more susceptible to both extracts of C. viminalis than P. aeruginosa. These results are consistent with those of other studies which suggest that Gram-negative bacteria are less susceptible to xenobiotics than Gram-positive bacteria [35]. The DCM:MeOH extract and EtOH:H2O extract of C. viminalis may, however, have a broader spectrum of antibacterial activity as they exhibited activity against both Gram-positive and Gram-negative bacterial species.
Figure 1.
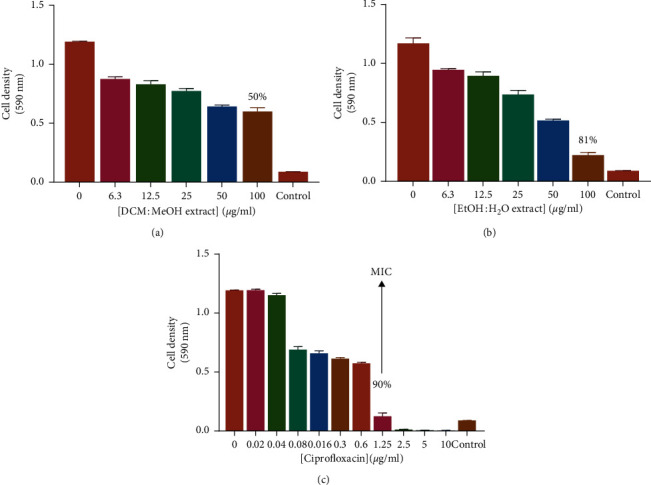
The effects of exposing on K. pneumoniae to extracts from Callistemon viminalis. Results of the microbroth dilution assay results are shown for (a) DCM:MeOH extract, (b) EtOH:H2O extract, and (c) ciprofloxacin. The error bars represent standard deviation from the mean of 4 repeat measurements.
Another important finding was that all extracts of C. viminalis had a minimal antibacterial effect against P. aeruginosa matching those observed in an earlier study by Chitemerere and Mukanganyama [36]. P. aeruginosa has a thick outer membrane that is highly hydrophobic, and this membrane provides a permeability barrier to the extract. The determination of MIC using C. viminalis crude extracts was an indicator of the effect of various triterpenes, flavonoids, and alkaloids that have antibacterial activity which are present in the plant leaves. At the highest concentration of tormentic acid congener (100 µg/ml), there was complete inhibition of the bacterial activity of P. aeruginosa.
Extracts of C. viminalis were also tested for antibacterial activity against S. pyogenes and A. baumannii. The 50% v/v EtOH:H2O extract of C. viminalis and tormentic acid congener proved were shown to have the most potent antibacterial effects against S. pyogenes at 100 µg/ml. Extracts of C. viminalis and tormentic acid congener did not have much inhibitory effects against A. baumannii, indicating intrinsic resistance of this bacterial strain. Antimicrobial resistance of A. baumannii is mainly due to reduced permeability of the outer membrane caused by loss or low porin expression, increased expression of multidrug efflux pumps, and mutations altering targets or different cellular functions [37]. The antibacterial effects of β-naphthoflavone on A. baumannii were also tested and were shown to have an inhibitory effect of 68%. Flavonoids are formed as antimicrobial barriers in plants response to microbial infection [38]. Flavonoids target the bacterial cytoplasmic membranes causing membrane fusion between microorganisms, resulting in leakage of intramembranous materials which promotes aggregation. Also, large bacterial aggregate clumps are more easily detected by the innate immune system compared to those bacteria in biofilm [39]. The poor activity of cell membrane active flavones against Gram-positive S. pyogenes might be because of poor penetration to the cell membrane of the bacteria due to presence of a thick layer of peptidoglycan in their cell walls which act as a barrier. Tiwari and colleagues showed that herbal compounds are generally antibacterial agents but show better antimicrobial activity when used in synergy with other antibiotics [37].
3.3. Time-Kill Kinetics of Tormentic Acid Congener on P. aeruginosa
The time-kill assay was carried out to determine the capacity of tormentic acid congener to kill the bacterium P. aeruginosa in relation to time. A range of five concentrations (100–0 µg/ml) were used, together with ciprofloxacin as a control. The antibacterial effect of tormentic acid congener on P. aeruginosa was shown to be time-dependent. At the highest concentration of tormentic acid congener 100 µg/ml, there was a complete inhibition of the bacterial activity of P. aeruginosa (Figure 2(a)). At 100 µg/ml, tormentic acid congener completely inhibited the growth of P. aeruginosa cells. The antibacterial activity of tormentic acid congener was efficient with 50 µg/ml inhibiting the growth of P. aeruginosa during the first 10 hours. The positive control ciprofloxacin showed an inhibition of the growth of P. aeruginosa cells from a minimum concentration of 0.32 µg/ml–10 µg/ml. There was a subsequent increase in cell growth in the wells with 0.16, 0.08, 0.04, and 0.02 µg/ml of tormentic acid congener, although the rate of cell growth was lower than that of the standard control. The time-kill assay of ciprofloxacin further confirmed the minimum inhibitory concentration of 0.32 µg/ml against P. aeruginosa (Figure 2(b)).
Figure 2.

Time-kill curves of P aeruginosa cells after exposure to tormentic acid congener (a) and ciprofloxacin (b). Cells were standardised to 1 × 106 cfu/ml. They were incubated with the compounds at 2-fold increasing concentrations for 32 hours and incubated at 37°C.
3.4. Effects of Combining Tormentic Acid Congener and Ciprofloxacin on the Growth of P. aeruginosa
Antibacterial combinations have been extensively researched to determine the synergistic combination and interactions between potential antibacterial agents to optimise the efficacy and potency of antibacterial drugs [40]. Synergism occurs when there is a decrease in the viable organism as an outcome of combining two antibiotics when compared to the effect of using the most effective antibiotic alone [41]. The checkerboard combination assay was carried out to determine the effect of combining ciprofloxacin and tormentic acid congener. For tormentic acid congener, the MIC, ½ MIC, ¼ MIC, and ⅛ MIC was used. The concentrations of ciprofloxacin used were MIC 0.32, 0.16, 0.08, 0.04, and 0.02. As shown in Figure 3, tormentic acid congener decreased the MIC of ciprofloxacin from 0.3 µg/ml to 0.16 µg/ml at the MIC concentration of 100 µg/ml. The other concentrations of tormentic acid congener did not have a significant effect on the MIC of ciprofloxacin.
Figure 3.

The effects of combining tormentic acid congener and ciprofloxacin on P aeruginosa. The effect of ciprofloxacin on P aeruginosa together with a concentration of (a) 0 µg/ml, (b) 25 µg/ml, (c) 50 µg/ml, and (d) 100 µg/ml of tormentic acid congener.
Combining concentrations of 100 µg/ml of tormentic acid congener and 0.016 µg/ml of ciprofloxacin was indifferent at 37°C for 3 hours incubation because the calculated FICI was 0.5016. Some in vitro tests have indicated that terpenes show ineffective antimicrobial activity when used as a single compound but are more effective when used in combination with other antibacterial agents [42]. Another study showed synergism when triterpenes were combined with ciprofloxacin and tested on E. coli [43]. Several diterpenoids, terpenoids, and sesquiterpenoids have been found to act synergistically with different antibiotics [44]. Similarly, tormentic acid congener might be able to work in synergy with other antibiotics regardless of its inability to work in synergy with ciprofloxacin. Some of the antibacterial drugs that show synergistic or additive effects when combined with triterpenoids include methicillin and vancomycin [45].
3.5. Determination of the Effects of Tormentic Acid Congener on Membrane Integrity Using the Protein Leakage Determination Assay
The proposed mechanism of action of tormentic acid congener was protein leakage, and this was determined by exposing the bacteria to the compound and determining the amount of protein using the Bradford assay. The mechanism of action of ciprofloxacin against bacteria is not cell lysis, and therefore, ciprofloxacin did not cause significant cell lysis in P. aeruginosa in comparison to the unexposed cells. As shown in Figure 4, tormentic acid congener caused cell lysis in P. aeruginosa, and this exposure resulted in significant protein leakage. The positive controls were 1% SDS that caused significant protein leakage in P. aeruginosa. Exposure to ciprofloxacin did not result in protein leakage, and this was in correlation with the research that was performed by Jedrey et al. [46]. SDS caused notable cell lysis in P. aeruginosa cells [47], which resulted in protein leakage.
Figure 4.

The effect of exposing P aeruginosa to ciprofloxacin (0.016 µg/ml) and tormentic acid congener (100 µg/ml) on release of intracellular protein in P. aeruginosa. The absorbance intensities of treated P aeruginosa cells were measured at 590 nm. Results are presented as mean (n = 3) ± standard deviations. Asterisk denotes a significant difference (∗∗P < 0.01).
Studies have shown that some phytochemicals including terpenes affect the stability of the cell membrane in bacterial cells, and these include p-cymene, carvacrol, and thymol [43]. Carvacrol for instance causes functional and structural damage and disruption of the membrane Gram-negative pathogens [43]. Thymol merges to the polar headgroups that make up the lipid bilayers, and it induces alterations to the cell membrane affecting its permeability [43]. Other terpenes that have effectively damaged bacterial cells of S. aureus and E. coli, respectively, are citronellol and citronellal, and their permeabilisation to the membrane or cell wall are related to alterations on their physicochemical properties [48].
3.6. Effects of Extracts and Tormentic Acid Congener on Species on Biofilm Production
Biofilm formation is a major resistance mechanism displayed by bacteria. Both S. pyogenes and A. baumannii can form biofilms [49, 50]. The extracellular polymeric substance is composed of polysaccharide, proteins, and extracellular DNA [51]. Biofilm formation activities were determined using 0.1% crystal violet staining of adherent biofilm, and the results are shown in Figure 5. To evaluate the effect of C. viminalis extracts, tormentic acid congener, and ciprofloxacin against biofilm formation, the antibiofilm agents were incubated together with the bacterial strains in a 24-well plate for 72 hours at 37°C. Ciprofloxacin inhibited biofilm formation by 80% and 85% in S. pyogenes and A. baumannii, respectively. Tormentic acid congener and C. viminalis extracts had no significant effect on biofilm formation inhibition for both test bacterial strains. Biofilms account for over 80% of microbial infections in the human body [52]. Bacterial cells in biofilms are 10–1000 times less susceptible to antimicrobial agents compared to their planktonic counterparts due to the physical impedance leading to poor diffusion of the drugs into the biofilm [53]. Also, bacteria embedded in a biofilm can evade the host immune system, therefore, contributing to its resistance mechanism [54]. Most bacteria exist within biofilms encased in an extracellular polymeric substance made up of biopolymers [55].
Figure 5.

The effect of ciprofloxacin, tormentic acid congener, and C. viminalis extracts on S pyogenes (a) and A. baumannii (b) biofilm production, respectively. The bacterial strains were incubated together with the test agents for 72 hours, and the formed biofilm was quantified using 0.1% crystal violet. Ciprofloxacin inhibited the formation of biofilms in both bacteria, whilst tormentic acid congener and C. viminalis extracts showed no significant inhibitory effect. Asterisk denotes a significant difference (∗∗∗∗P < 0.0001).
3.6.1. Effects of Extracts and Tormentic Acid Congener Detachment of Preformed Biofilms
The standard antibiotic drug ciprofloxacin showed the least antiadhesion properties on the already formed biofilms in both test bacteria, S. pyogenes and A. baumannii (Figure 6). Ciprofloxacin only caused the detachment of 28% of the biofilm formed in S. pyogenes and had no detachment effect on biofilms formed by A. baumannii. Tormentic acid congener and C. viminalis extracts had no biofilm formation inhibitory effect but rather cause detachment of already formed biofilms in both S. pyogenes and A. baumannii. Lack of inhibitory effect on biofilm formation could be attributed to the low antibacterial activity of the test antimicrobial agents. A. baumannii proliferated at 100 µg/ml of test antibacterial agents and produced biofilms at same concentrations increasing its mechanisms of resistance. Ciprofloxacin showed antibiofilm activity at 10 µg/ml of the drug. The subminimum inhibitory concentration of ciprofloxacin was supposed to be used to ensure bacterial species were not killed by high ciprofloxacin concentrations. Ciprofloxacin had no biofilm detachment activity against test bacterial species as shown by tormentic acid congener and C. viminalis extracts.
Figure 6.
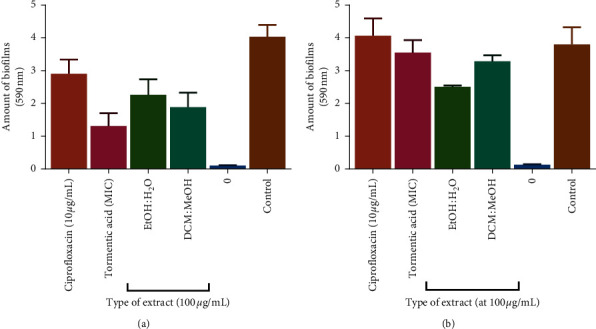
The effect of ciprofloxacin, tormentic acid congener, and C. viminalis extracts on biofilm detachment in S. pyogenes (a) and A. baumannii (b). Ciprofloxacin showed less effect on preformed biofilms than C. viminalis extracts and tormentic acid congener. Tormentic acid congener caused detachment of biofilms in S. pyogenes more than other agents, whilst the EtOH extract of C. viminalis had the greatest effect in A. baumannii.
Cell surface hydrophobicity is a crucial factor for biofilm formation and stabilisation in biofilm-forming bacteria [56]. The hydrophobic property of bacterial surfaces is a major determinant in the adhesion of bacteria and the formation and stabilisation of biofilms by bacteria on animate and inanimate surfaces [56]. Nandu et al., established that fukugiside, a biflavonoid isolated from the leaves of Garcinia travancorica, reduced the cell surface hydrophobicity of S. pyogenes as a mechanism of its antibiofilm activity [55]. Therefore, tormentic acid congener could also be reducing the hydrophobicity of the cell surface of S. pyogenes thus causing the detachment of already formed biofilms. It has also been suggested by Lim and colleagues that the detachment of biofilms could be as a result of reduced adhesive forces in the biofilms due to increased solubility of bacterial exopolysaccharides [56]. Tormentic acid congener and 50% v/v EtOH:H2O extract of C. viminalis disrupted the biofilm architecture S. pyogenes and A. baumannii, respectively.
3.6.2. Effect of Tormentic Acid Congener on Extracellular DNA Production in Biofilms
Most bacteria exist within biofilms encased in an extracellular polymeric substance made up of biopolymers [57]. This present study investigated the antibacterial effects of tormentic acid congener and different C. viminalis crude extracts as well as the inhibition of extracellular polysaccharide production against S. aureus and P. aeruginosa. The results for the effects of the extracts and tormentic acid congener on the production of extracellular DNA from P. aeruginosa (Figure 7(a)) and S. aureus (Figure 7(b)) are shown. Ciprofloxacin, extracts, and tormentic acid congener reduced extracellular DNA production in the biofilms. As shown in Figure 7(a), ciprofloxacin reduced significantly more the extracellular DNA produced by P. aeruginosa compared to tormentic acid congener and the crude extracts. Tormentic acid congener does, however, significantly reduced extracellular DNA more than the crude extracts. The EtOH:H2O extract was more potent than the DCM:MeOH extract in reducing extracellular DNA in P. aeruginosa biofilms. Data in Figure 7(b) show that ciprofloxacin and tormentic acid congener had similar effects on extracellular DNA formed by S. aureus biofilms as those shown with P. aeruginosa. The DCM:MeOH was, however, more potent than the EtOH:H2O extract in reducing extracellular DNA in S. aureus biofilms.
Figure 7.

The effect of C. viminalis EtOH:H2O extract, DCM:MeOH extract, tormentic acid congener, and ciprofloxacin on extracellular DNA produced by P. aeruginosa (a) and S. aureus (b). Cells were incubated in a medium containing 0.80 µg/ml ciprofloxacin as well as 12.5 µg/ml of the crude extracts and tormentic acid congener for 72 h. The amount of extracellular DNA present was determined by staining the biofilms with propidium iodide and spectrophotometrically measuring the amount of dye. These are results after a 24 well plate was washed with phosphate-buffered saline. ANOVA was carried out using GraphPad Prism6 and Dunnett's multiple comparisons test with a summary of ∗∗∗∗P value < 0.0001. Ciprofloxacin, tormentic acid congener, and extracts reduced extracellular DNA production in P. aeruginosa and S. aureus biofilms.
Extracellular DNA (eDNA) was observed to be abundant in the biofilm matrix as shown in a study by Flemming et al. [58]. A study by Tang et al. showed that eDNA had many functions such as the initial attachment of biofilms of P. aeruginosa, Reinheimera, Microbacterium, and Serratia species [59]. Due to the complex structure of the biofilm, eDNA may provide inherent resistance to antimicrobial agents. The search for an effective drug to eradicate the biofilms or inhibit their growth by targeting the inhibition of eDNA is of importance. Our results showed that the amounts of extracellular DNA produced by S. aureus and P. aeruginosa strains were significantly inhibited by tormentic acid congener and C. viminalis crude extracts.
3.6.3. Effects of Tormentic Acid Congener on Capsular Polysaccharide Production
Polysaccharides act as molecular glue in biofilm formation so the reduction in polysaccharide production implies a reduction in biofilm formation and thus provides a possible alternative to killing or inhibition of the growth of pathogenic bacteria [60]. In order to determine if tormentic acid congener, ciprofloxacin, DCM:MeOH extract, and EtOH:H2O extracts had any effect on the formation of biofilms in P. aeruginosa and S. aureus, the capsular polysaccharide production was quantified after exposure to these samples. The EtOH:H2O extract showed the most significant reduction in polysaccharide formation when compared to the reductions caused by tormentic acid congener and DCM:MeOH extract (Figure 8). The EtOH:H2O extract was more potent than the DCM:MeOH extract in reducing polysaccharide production in P. aeruginosa biofilms (Figure 8(a)). The DCM:MeOH showed more potency in reducing polysaccharide production than the EtOH:H2O extract in S. aureus (Figure 8(b)). All antibacterial test agents reduced the extracellular polysaccharide content in S. pyogenes biofilms (Figure 9).
Figure 8.
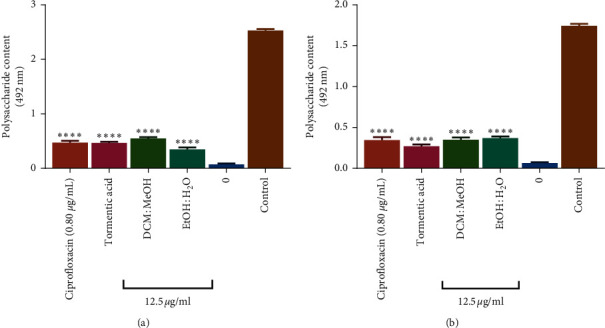
The effect of C. viminalis EtOH:H2O extract, DCM:MeOH extract, tormentic acid congener, and ciprofloxacin on biofilm polysaccharide content. P. aeruginosa (a) and S. aureus (b) were incubated in a medium containing 0.80 µg/ml ciprofloxacin and 12.5 µg/ml of extracts and tormentic acid congener for 16 hours. Mannose was used as the standard for carbohydrate content. Carbohydrate content was read at 492 nm using a Stat Fax model microplate reader. ANOVA was carried out using GraphPad Prism6 and Dunnett's multiple comparisons test with a summary of ∗∗∗∗P value < 0.0001.
Figure 9.

The effect of ciprofloxacin, tormentic acid congener, and C. viminalis extracts on extracellular polysaccharide content in S. pyogenes. Quantification of extracellular polysaccharide content of S. pyogenes was performed using the 5% phenol saturated sulphuring acid test and collection and measuring of the supernatant. All test agents inhibited the formation of capsular exopolysaccharides in S. pyogenes.
Bacteria in biofilms have been shown to produce extracellular polysaccharides (EPS) which help them to bind the biofilm together in a matrix while anchoring it to surfaces [61]. For this study, it is interesting to note that EPS production is significantly reduced for both Gram-positive and Gram-negative pathogens in the presence of tormentic acid congener and different C. viminalis crude extracts. This presents a result that has some importance for the possible use of tormentic acid congener as a biofilm control agent. The regulation of EPS production has been studied well for both Gram-positive and Gram-negative pathogens. In a study by Olofsson et al. [62], it was shown that Gram-negative bacteria extracellular polysaccharide production is inhibited directly or indirectly due to naturally occurring antibacterial agents. Similar findings of reduction of EPS against Gram-positive pathogens were reported for the methanol leaf extract fraction of Mangifera indica [63]. Our results also demonstrate a reduction in EPS production after exposure to plant extract, thus resulting in inhibition of biofilm production. This study showed that exposing in S. aureus and P. aeruginosa to tormentic acid congener and extracts from C. viminalis results in decreases in EPS and eDNA production which may result in decrease in biofilm formation.
4. Conclusion
C. viminalis extracts had antibacterial activity against P. aeruginosa, S. aureus, S. pyogenes, K. pneumoniae, and A. baumannii. Extracts of C. viminalis reduced polysaccharide production and extracellular DNA production in P. aeruginosa and S. aureus biofilms. Tormentic acid congener caused significant cell lysis in P. aeruginosa cells. Exposure to tormentic acid congener produced detachment of S. pyogenes biofilms well as it reduced the extracellular polysaccharide content of S. pyogenes biofilms. However, tormentic acid congener and both C. viminalis extracts did not show significant antibacterial activity and antibiofilm formation activity against A. baumannii. Tormentic acid congener and extracts of C. viminalis, thus, had significant antibacterial and antibiofilm activities on selected ESKAPE bacteria and can act as source lead compounds for the development of antibacterial triterpenoids.
Acknowledgments
The authors acknowledge the assistance of Mr Christopher Chapano, a taxonomist with the National Herbarium and Botanical Gardens, Harare, Zimbabwe, in the authentication of the plant sample names. Support from Swedish International Development Agency through the International Science Programmes (ISP IPICS: ZIM01), Uppsala University, Uppsala, Sweden, is acknowledged. ISP IPICS: ZIM01 supported the research under the title “Biomolecular Interactions Analyses.” Support from the Alliance for Global Health and Science (CEND: small grants, Zimbabwe) University of California, Berkeley, is also acknowledged.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Ramsamy Y., Essack S. Y., Sartorius B., Patel M., Mlisana K. P. Antibiotic resistance trends of ESKAPE pathogens in Kwazulu-Natal, South Africa: a five-year retrospective analysis. African Journal of Laboratory Medicine. 2018;7(2):1–8. doi: 10.4102/ajlm.v7i2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pogue J. M., Kaye K. S., Cohen D. A., Marchaim D. Appropriate antimicrobial therapy in the era of multidrug-resistant human pathogens. Clinical Microbiology and Infection. 2015;21(4):302–312. doi: 10.1016/j.cmi.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Strateva T., Yordanov D. Pseudomonas aeruginosa-a phenomenon of bacterial resistance. Journal of Medical Microbiology. 2009;58(9):1133–1148. doi: 10.1099/jmm.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs in Context. 2018;7 doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riquelme S. A., Ahn D., Prince A. Pseudomonas aeruginosa and Klebsiella pneumoniae adaptation to innate immune clearance mechanisms in the lung. Journal of Innate Immunity. 2018;10(5-6):442–454. doi: 10.1159/000487515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veesenmeyer J. L., Hauser A. R., Lisboa T., Rello J. Pseudomonas aeruginosa virulence and therapy: evolving translational strategies. Critical Care Medicine. 2009;37(5):p. 1777. doi: 10.1097/ccm.0b013e31819ff137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasaikar S., Obi L., Morobe I., Bisi-Johnson M. Molecular characteristics and antibiotic resistance profiles of Klebsiella isolates in Mthatha, Eastern Cape province, South Africa. International Journal of Microbiology. 2017;2017 doi: 10.1155/2017/8486742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarojamma V., Ramakrishna V. Prevalence of ESBL-producing Klebsiella pneumoniae isolates in tertiary care hospital. ISRN Microbiology. 2011;2011 doi: 10.5402/2011/318348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navon-Venezia S., Kondratyeva K., Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiology Reviews. 2017;41(3):252–275. doi: 10.1093/femsre/fux013. [DOI] [PubMed] [Google Scholar]
- 10.Zapotoczna M., O’Neill E., O’Gara J. P. Untangling the diverse and redundant mechanisms of Staphylococcus aureus biofilm formation. PLoS Pathogens. 2016;12(7) doi: 10.1371/journal.ppat.1005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong S. S., Yuen K.-Y. Streptococcus pyogenesand re-emergence of scarlet fever as a public health problem. Emerging Microbes & Infections. 2012;1(1):1–10. doi: 10.1038/emi.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler T., Köller T., Kreikemeyer B. Streptococcus pyogenes biofilms-formation, biology, and clinical relevance. Frontiers in Cellular and Infection Microbiology. 2015;5:p. 15. doi: 10.3389/fcimb.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio-López V., Valdezate S., Álvarez D., et al. Molecular epidemiology, antimicrobial susceptibilities and resistance mechanisms of Streptococcus pyogenes isolates resistant to erythromycin and tetracycline in Spain (1994–2006) BMC Microbiology. 2012;12(1):p. 215. doi: 10.1186/1471-2180-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsan M., Klompas M. Acinetobacter baumannii: an emerging and important pathogen. Journal of Clinical Outcomes Management: JCOM. 2010;17(8):p. 363. [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q., Schultz G. S., Gibson D. J. A surfactant-based dressing to treat and prevent Acinetobacter baumannii biofilms. Journal of Burn Care & Research. 2018;39(5):766–770. doi: 10.1093/jbcr/irx041. [DOI] [PubMed] [Google Scholar]
- 16.Costerton J. W., Geesey G. G., Cheng K.-J. How bacteria stick. Scientific American. 1978;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 17.Mishra B., Wang G. Individual and combined effects of engineered peptides and antibiotics on Pseudomonas aeruginosa biofilms. Pharmaceuticals. 2017;10(3):p. 58. doi: 10.3390/ph10030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zatorska B., Arciola C. R., Haffner N., Segagni Lusignani L., Presterl E., Diab-Elschahawi M. Bacterial extracellular DNA production is associated with outcome of prosthetic joint infections. BioMed Research International. 2018;2018 doi: 10.1155/2018/1067413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu Z., Xing N., Wang Q., et al. Antibacterial and anti-inflammatory activities of Physalis alkekengi var. franchetii and its main constituents. Evidence-Based Complementary and Alternative Medicine. 2016;2016:p. 10. doi: 10.1155/2016/4359394.4359394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramalhete C., Spengler G., Martins A., et al. Inhibition of efflux pumps in meticillin-resistant Staphylococcus aureus and Enterococcus faecalis resistant strains by triterpenoids from Momordica balsamina. International Journal of Antimicrobial Agents. 2011;37(1):p. 70. doi: 10.1016/j.ijantimicag.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Das S., Singh U. Therapeutic potentials of Callistemon lanceolatus DC. International Journal of Advances in Pharmacy, Biology and Chemistry. 2012;1(2):206–210. [Google Scholar]
- 22.Salem M. Z. M., EL-Hefny M., Nasser R. A., Ali H. M., El-Shanhorey N. A., Elansary H. O. Medicinal and biological values of Callistemon viminalis extracts: history, current situation and prospects. Asian Pacific Journal of Tropical Medicine. 2017;10(3):229–237. doi: 10.1016/j.apjtm.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Ramezanian M., Ashraf Mansuri S. J., Minaeifar A. A. Evaluation of the drug synergistic and antibacterial effects of methanol extracts of Callistemon viminalis on some urinary tract infection bacteria. Advanced Herbal Medicine. 2016;2(4):13–21. [Google Scholar]
- 24.Delahaye C. H., Rainford L., Nicholson A., Mitchell S., Lindo J., Ahmad M. Antibacterial and antifungal analysis of crude extracts from the leaves of Callistemon viminalis. Journal of Medical and Biological Sciences. 2009;3(1):1–7. [Google Scholar]
- 25.Evaristo F. F. V., Albuquerque M. R. J. R., dos Santos H. S., et al. Antimicrobial effect of the triterpene 3β, 6β, 16β-trihydroxylup-20 (29)-ene on planktonic cells and biofilms from Gram positive and Gram negative bacteria. BioMed Research International. 2014;2014 doi: 10.1155/2014/729358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco A. G., Alcântara A. F. C., Abreu V. G. C., Corrêa G. M. Relationships between Chemical Structure and Activity of Triterpenes against Gram-Positive and Gram-Negative Bacteria. A Search for Antibacterials Agents. Rijeka, Croatia: InTech; 2012. [Google Scholar]
- 27.Loizzo M. R., Bonesi M., Passalacqua N. G., Saab A., Menichini F., Tundis R. Antiproliferative activities on renal, prostate and melanoma cancer cell lines of Sarcopoterium spinosum aerial parts and its major constituent tormentic acid. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(5):768–776. doi: 10.2174/1871520611313050011. [DOI] [PubMed] [Google Scholar]
- 28.Lin X., Zhang S., Huang R., et al. Protective effect of tormentic acid from Potentilla chinensis against lipopolysaccharide/D-galactosamine induced fulminant hepatic failure in mice. International Immunopharmacology. 2014;19(2):365–372. doi: 10.1016/j.intimp.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Vipra A., Desai S. N., Junjappa R. P., et al. Determining the minimum inhibitory concentration of bacteriophages: potential advantages. Advances in Microbiology. 2013;3(2):p. 10.32359 [Google Scholar]
- 30.Martinez-Irujo J. J., Villahermosa M. L., Alberdi E., Santiago E. A checkerboard method to evaluate interactions between drugs. Biochemical Pharmacology. 1996;51(5):635–644. doi: 10.1016/s0006-2952(95)02230-9. [DOI] [PubMed] [Google Scholar]
- 31.Orhan G., Bayram A., Zer Y., Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. Journal of Clinical Microbiology. 2005;43(1):140–143. doi: 10.1128/jcm.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meletiadis J., Pournaras S., Roilides E., Walsh T. J. Defining fractional inhibitory concentration index cutoffs for additive interactions based on self-drug additive combinations, Monte Carlo simulation analysis, and in vitro-in vivo correlation data for antifungal drug combinations against Aspergillus fumigatus. Antimicrobial Agents and Chemotherapy. 2010;54(2):602–609. doi: 10.1128/aac.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hawser S. P., Douglas L. J. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infection and Immunity. 1994;62(3):915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi S., Imayoshi Y., Kobayashi E., et al. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry. 2002;59(3):315–323. doi: 10.1016/s0031-9422(01)00455-1. [DOI] [PubMed] [Google Scholar]
- 35.Stavri M., Piddock L. J. V., Gibbons S. Bacterial efflux pump inhibitors from natural sources. Journal of Antimicrobial Chemotherapy. 2007;59(6):1247–1260. doi: 10.1093/jac/dkl460. [DOI] [PubMed] [Google Scholar]
- 36.Chitemerere T. A., Mukanganyama S. In vitro antibacterial activity of selected medicinal plants from Zimbabwe. The African Journal of Plant Science and Biotechnology. 2011;5(1):1–7. [Google Scholar]
- 37.Tiwari V., Roy R., Tiwari M. Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Frontiers in Microbiology. 2015;6:p. 618. doi: 10.3389/fmicb.2015.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orhan D. D., Özçelik B., Özgen S., Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiological Research. 2010;165(6):496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Green A. E., Rowlands R. S., Cooper R. A., Maddocks S. E. The effect of the flavonol morin on adhesion and aggregation of Streptococcus pyogenes. FEMS Microbiology Letters. 2012;333(1):54–58. doi: 10.1111/j.1574-6968.2012.02598.x. [DOI] [PubMed] [Google Scholar]
- 40.Perumal S., Mahmud R., Mohamed N. Combination of epicatechin 3-gallate from Euphorbia hirta and cefepime promotes potential synergistic eradication action against resistant clinical isolate of Pseudomonas aeruginosa. Evidence-Based Complementary and Alternative Medicine. 2018;2018 doi: 10.1155/2018/5713703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dundar D., Otkun M. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Medical Journal. 2010;51(1):111–116. doi: 10.3349/ymj.2010.51.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6(12):1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penduka D., Gasa N., Hlongwane M., Mosa R., Osunsanmi F., Opoku A. The antibacterial activities of some plant-derived triterpenes. African Journal of Traditional, Complementary and Alternative Medicines. 2015;12(6):180–188. doi: 10.4314/ajtcam.v12i6.19. [DOI] [Google Scholar]
- 44.Wang Y.-L., Sun G.-Y., Zhang Y., He J.-J., Zheng S., Lin J.-N. Tormentic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-κB signaling pathway. Molecular Medicine Reports. 2016;14(4):3559–3564. doi: 10.3892/mmr.2016.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung P., Navaratnam P., Chung L. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Annals of Clinical Microbiology and Antimicrobials. 2011;10(1):p. 25. doi: 10.1186/1476-0711-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jedrey H., Lilley K. S., Welch M. Ciprofloxacin binding to GyrA causes global changes in the proteome of Pseudomonas aeruginosa. FEMS Microbiology Letters. 2018;365(13) doi: 10.1093/femsle/fny134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox S. D., Markham J. L. Susceptibility and intrinsic tolerance ofPseudomonas aeruginosato selected plant volatile compounds. Journal of Applied Microbiology. 2007;103(4):930–936. doi: 10.1111/j.1365-2672.2007.03353.x. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Romero J. C., González-Ríos H., Borges A., Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evidence-Based Complementary and Alternative Medicine. 2015;2015 doi: 10.1155/2015/795435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa T., Terao Y., Okuni H., et al. Biofilm formation or internalization into epithelial cells enable Streptococcus pyogenes to evade antibiotic eradication in patients with pharyngitis. Microbial Pathogenesis. 2011;51(1-2):58–68. doi: 10.1016/j.micpath.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Tiwari V., Tiwari D., Patel V., Tiwari M. Effect of secondary metabolite of Actinidia deliciosa on the biofilm and extra-cellular matrix components of Acinetobacter baumannii. Microbial Pathogenesis. 2017;110:345–351. doi: 10.1016/j.micpath.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez L. M., Cheng A. T., Warner C. J., et al. Biofilm formation and detachment in Gram-negative pathogens is modulated by select bile acids. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0149603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramenium G. A., Vijayakumar K., Pandian S. K. Limonene inhibits streptococcal biofilm formation by targeting surface-associated virulence factors. Journal of Medical Microbiology. 2015;64(8):879–890. doi: 10.1099/jmm.0.000105. [DOI] [PubMed] [Google Scholar]
- 53.Irani N., Basardeh E., Samiee F., et al. The inhibitory effect of the combination of two new peptides on biofilm formation by Acinetobacter baumannii. Microbial Pathogenesis. 2018;121:310–317. doi: 10.1016/j.micpath.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 54.Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. Microbial biofilms. Annual Review of Microbiology. 1995;49(1):711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 55.Nandu T. G., Subramenium G. A., Shiburaj S., et al. Fukugiside, a biflavonoid from Garcinia travancorica inhibits biofilm formation of Streptococcus pyogenes and its associated virulence factors. Journal of Medical Microbiology. 2018;67(9):1391–1401. doi: 10.1099/jmm.0.000799. [DOI] [PubMed] [Google Scholar]
- 56.Lim J. H., Song S.-H., Park H.-S., Lee J. R., Lee S. M. Spontaneous detachment of Streptococcus mutans biofilm by synergistic effect between zwitterion and sugar alcohol. Scientific Reports. 2017;7 doi: 10.1038/s41598-017-08558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thenmozhi R., Nithyanand P., Rathna J., Karutha Pandian S. Antibiofilm activity of coral-associated bacteria against different clinical M serotypes ofStreptococcus pyogenes. FEMS Immunology & Medical Microbiology. 2009;57(3):284–294. doi: 10.1111/j.1574-695x.2009.00613.x. [DOI] [PubMed] [Google Scholar]
- 58.Flemming H.-C., Neu T. R., Wozniak D. J. The EPS matrix: the “house of biofilm cells. Journal of Bacteriology. 2007;189(22):7945–7947. doi: 10.1128/jb.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang L., Schramm A., Neu T. R., Revsbech N. P., Meyer R. L. Extracellular DNA in adhesion and biofilm formation of four environmental isolates: a quantitative study. FEMS Microbiology Ecology. 2013;86(3):394–403. doi: 10.1111/1574-6941.12168. [DOI] [PubMed] [Google Scholar]
- 60.Fuqua C., Parsek M. R., Greenberg E. P. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annual Review of Genetics. 2001;35(1):439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 61.Christensen B. E. The role of extracellular polysaccharides in biofilms. Journal of Biotechnology. 1989;10(3-4):181–202. doi: 10.1016/0168-1656(89)90064-3. [DOI] [Google Scholar]
- 62.Olofsson A.-C., Hermansson M., Elwing H. N-acetyl-L-cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Applied and Environmental Microbiology. 2003;69(8):4814–4822. doi: 10.1128/aem.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Husain F. M., Ahmad I., Al-thubiani A. S., Abulreesh H. H., AlHazza I. M., Aqil F. Leaf extracts of Mangifera indica L. Inhibit quorum sensing–regulated production of virulence factors and biofilm in test bacteria. Frontiers in Microbiology. 2017;8:p. 727. doi: 10.3389/fmicb.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon request.


