Abstract
Bazex syndrome is a rare paraneoplastic dermatosis that precedes diagnosis of cancer. Awareness of this syndrome is important, as it allows early detection of underlying malignancy and may prevent misdiagnosis and delays in cancer treatment.
Keywords: Acrokeratosis paraneoplastica, Bazex syndrome, head and neck cancer, paraneoplastic dermatosis
Bazex syndrome is a rare paraneoplastic dermatosis that precedes diagnosis of cancer. Awareness of this syndrome is important, as it allows early detection of underlying malignancy and may prevent misdiagnosis and delays in cancer treatment.
1. INTRODUCTION
Acrokeratosis paraneoplastica or Bazex syndrome is a paraneoplastic dermatosis that often presents prior to a diagnosis of an internal malignancy. The symptoms can be significant and include dry and thickened skin on the hands, face, and lower extremities as well as brittle, thickened nails on the hands and feet. These symptoms usually result in presentation to the clinic several months before the manifestation of signs or symptoms of malignancy, and they are also typically resistant to dermatologic management. 1 By the time of cancer diagnosis, most patients have endured months of ineffective dermatologic care as their malignancy was left untreated.
The earliest case report of hyperkeratosis of hands and feet with internal malignancy was published in 1922 by Gougerot and Rupp. 2 Acrokeratosis Paraneoplastica or Bazex syndrome, dermatosis associated with internal malignancy, was not recognized until 1965, by Andre Bazex. 3 However, its recognition outside of France was not until the 1980s when it was classically associated with middle‐aged Caucasian male smokers with cancers of the aerodigestive tract. 4 There are currently about 150 published case reports of Bazex syndrome, with more recent reports focusing on atypical presentations such as new or less common malignancy sites, effective skin treatments, or unusual disease course.
A review of the literature was conducted, and data from >150 patients 1 , 5 , 6 , 7 were synthesized into Table 1, which summarizes the clinical, demographic, and pathologic characteristics of the syndrome.
TABLE 1.
Summary of common patients, malignancies, presentations, treatments, and histologic features associated with Bazex syndrome 1 , 5 , 6 , 7
| Common patient characteristics | Middle‐aged (between 40 and 70 y old) |
| Male | |
| Smoking | |
| Alcohol use | |
| Caucasian | |
| Associated malignancy site | Most common: Head and neck (39%‐60%) 1 , 5 , 6 |
| Second most common: Lung (14%‐15%) 1 , 6 | |
| Third most common: Esophagus (8%‐9%) 1 , 6 | |
| Malignancy histology | Squamous cell carcinoma is most common |
| Clinical course of syndrome | Usually onsets prior to malignancy (47%‐67%) 1 , 6 |
| Involves face, hands, feet, ears most often | |
| Responds to cancer treatment (93%) 1 | |
| Nononcologic treatment options | Topical steroids |
| Systemic steroids | |
| Topical Retinoids | |
| Topical Zinc | |
| Ultraviolet light therapy | |
| Topical antibiotics | |
| Dermatopathologic characteristics | Hyperkeratosis |
| Parakeratosis | |
| Acanthosis | |
| Mixed cellular infiltrate | |
| Dyskeratotic keratinocytes |
In an attempt to promote early detection of malignancy and avoid delays in treatment, we present three cases of Bazex syndrome and elucidate both their typical and atypical characteristics. Cases 1‐3 are summarized in Table 2.
TABLE 2.
Bazex syndrome case details
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Patient | 1 | 2 | 3 |
| Age/Race/Sex | 60 yo/Caucasian/Male | 55 yo/Caucasian/Female | 48 yo/African American/Male |
| Risk factors | Alcohol, 80 py smoking history | 7.5 py smoking history | 1.25 py smoking history |
| Disease site | Hypopharynx | Oropharynx (p16 negative) | Hypopharynx |
| Histology | Squamous Cell Carcinoma | Squamous Cell Carcinoma | Squamous Cell Carcinoma |
| Clinical presentation |
Figure 1 Skin: hands, feet, and ears with burning and itching Nails: Dryness, flaking, and thickening of fingernails Stress worsened symptoms |
Figure 2 Skin: Raynaud's‐like symptoms with pallor and numbness and focal necrosis of the tip of the right third finger Nails: Raised, brittle yellow, and thickened |
Figure 3 Skin: Diffuse thickening and scaling of palms and soles Dry hyperpigmented patches on the anterior shins Nails: Not affected |
| Skin biopsy | Hyperkeratosis, horn cysts with focal intracorneal acute inflammation, psoriaform dermatitis | Not performed | Not performed |
| Timing of symptoms | Oncologic and dermatologic sx's developed around same time | Dermatologic sx's preceded oncologic sx's by ~6 mo | Oncologic sx's preceded dermatologic sx's by ~1 mo |
| Treatment |
Oncologic: Definitive chemoradiation to 70Gy with concurrent cisplatin Dermatologic: Acitretin |
Oncologic: Definitive chemoradiation to 70 Gy with concurrent cisplatin Dermatologic: Acitretin, clotrimazole/betamethasone, and nitroglycerin |
Oncologic: Definitive chemoradiation to 70Gy with concurrent cisplatin/carboplatin Dermatologic: Triamcinolone and topical urea |
| Cancer outcome | Patient remains NED 46 mo post‐treatment | Patient remains NED 40 mo post‐treatment | Died 7 mo post‐treatment due to disease progression and metastases |
| Bazex symptom outcome |
Figure 1 Complete resolution with intermittent flare ups responsive to acitretin |
Figure 2 Complete resolution with no flare ups |
Figure 3 Complete resolution seen in hands but only partial resolution in legs and foot |
Abbreviations: Gy, gray; NED, no evidence of disease; py, pack‐year; Sx's, symptoms; yo, year old.
2. CASE 1
A 60‐year‐old man with risk factors of alcohol use and 80 pack‐year (PY) smoking history presented with a 4 month history of hoarseness which progressed to intermittent dry cough, dysphagia, weight loss, and an expanding left‐sided neck mass. During that time, he also noticed dryness, flaking, and thickening of his fingernails, hands, feet, and ears with an associated constant severe burning and itching of those areas, worsened by stress or fatigue. These skin findings were diagnosed by a dermatologist as eczema and treated with oral Benadryl, topical Benadryl, and topical hydrocortisone. When the skin findings failed to improve, they were attributed to possibly a separate medication reaction. The neck mass was eventually biopsied by an Otolaryngologist utilizing endoscopy to visualize the lesion and pathology revealed a poorly differentiated squamous cell carcinoma.
At presentation to oncology clinic, physical examination revealed bilateral lymphadenopathy with a prominent left‐sided 4 × 5 cm fixed mass at level III/IV. His dermatologic examination findings (Figure 1) included bilateral fingernail and toenail dystrophy, extreme dryness, and hyperkeratosis of the hands and feet, as well as similar findings on both ear helices, mildly on the nose, along with numbness of those affected areas. Positron emission tomography/computed tomography (PET/CT) confirmed known physical examination findings, along with increased uptake in the bilateral axillary, right infraclavicular, and mediastinal lymph nodes concerning for metastatic disease.
FIGURE 1.
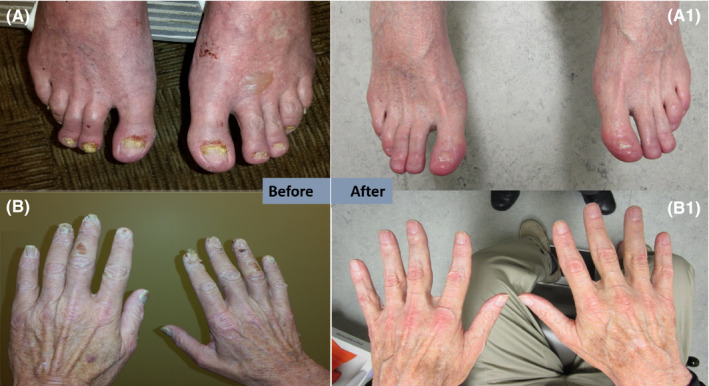
Images of clinical manifestations of Bazex syndrome in Patient 1 before (A, B) and after (A1, B1) oncologic treatment and oral acitretin. Resolution can be seen in hands and feet
Biopsy of the suspicious axillary lymph nodes on PET/CT was benign. The patient was staged as cT2N2cM0, overall stage IVA squamous cell carcinoma of the left hypopharynx. He was treated with definitive chemoradiation with 70 Gray(Gy) and weekly cisplatin over 7 weeks.
The patient's dermatologic findings, burning, and itching improved significantly after 2 months of acitretin. However, his acitretin was stopped due to elevated liver function enzymes. Then, his cutaneous findings, burning, and itching became more severe and progressed to include the elbows and knees. He was later restarted on acitretin, at a lower dose of 10 mg three times per week, which resulted in gradual resolution of his dermatologic symptoms. Currently, the patient remains without evidence of cancer for 46 months after completion of chemoradiation.
3. CASE 2
A 55‐year‐old woman with a 7.5 PY smoking history and a history of hypercalcemia noted a right neck mass and was treated with two courses of antibiotics by her primary care provider (PCP) without resolution. Six months after initial presentation for the neck mass she began receiving dermatologic care for what was believed to be a possible fungal infection resulting in lifting and brittle yellow thickening of the nails on her bilateral hands and feet.
When the treatments were continually ineffective, the dermatologist suspected underlying malignancy, and an ultrasound of the neck was done, showing enlarged bilateral cervical lymph nodes. She presented to the Medical Oncologist at our institution with significant cervical lymphadenopathy and a base of tongue mass associated with mild sore throat and dysphagia. At this time, physical examination (Figure 2) revealed Reynaud's‐like symptoms resulting in focal necrosis of the tip of the right third finger with pallor and numbness on the right fourth fingertip. After imaging and pathologic workup, she was concluded to be Stage IVA (T2N2cM0) poorly differentiated squamous cell carcinoma positive for human papilloma virus and negative for p16. Moving forward with treatment, she received definitive chemoradiation to the sites of her disease consisting of definitive radiation therapy (70 Gy in 35 fractions) and concurrent cisplatin.
FIGURE 2.
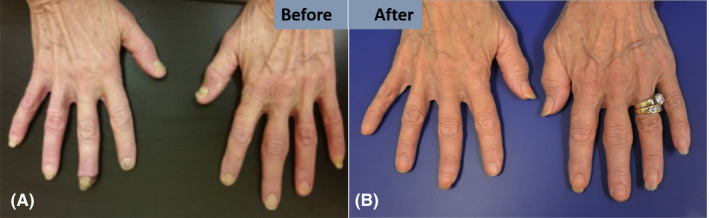
Images of clinical manifestations of Bazex syndrome in Patient 2 before (A) and after (B) oncologic treatment concurrent oral acitretin with topical clotrimazole/betamethasone, and nitroglycerin. Resolution is evident in both hands
She met with a dermatologist who diagnosed her with Bazex syndrome and initiated treatment with acitretin, nitroglycerin, and clotrimazole/betamethasone as she was beginning her radiation treatment. She tolerated oncologic treatment with expected side effects, and her recovery was smooth. Following treatment, her Bazex symptoms decreased in severity, and 1 month postchemoradiation, acitretin, and nitroglycerin were stopped. She is currently following with her Otolaryngologist 40 months post‐treatment with no evidence of disease.
4. CASE 3
A 48‐year‐old man with a 1.25 PY smoking history noticed 1 month of globus sensation. Initially, he was treated by PCP for presumed upper respiratory tract infection. Following two courses of antibiotics without resolution, he was started on antireflux medications which also did not alleviate his symptoms. One month later, the patient noticed some changes of his extremities, including diffuse thickening and scaling of his palms and soles bilaterally. He also developed dry hyperpigmented patches of skin on his bilateral anterior shins. There was no involvement of his finger or toe nails.
He underwent an esophagogastroduodenoscopy 3 months after initial presentation which showed a mass‐like lesion involving the posterior wall of the hypopharynx. Upon presentation to an Oncology clinic, he underwent physical examination (Figure 3), further imaging and pathologic workup and was staged as IVa/IVb (cT3/T4b N2c N0 M0) Squamous Cell Carcinoma of the posterior wall of the hypopharynx, with the uncertainty in staging resulting from uncertainty surrounding the involvement of the prevertebral fascia with disease.
FIGURE 3.
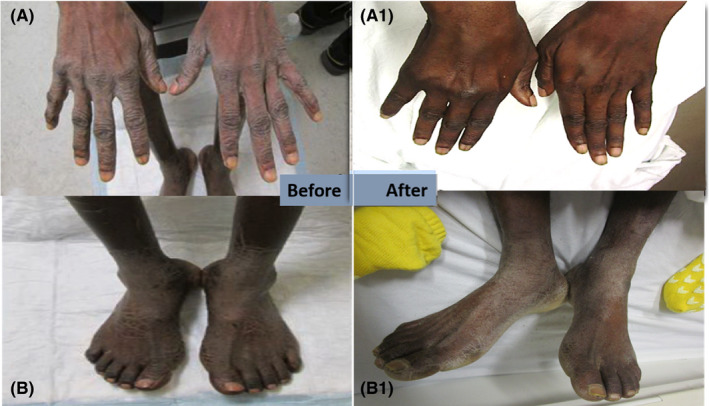
Images of clinical manifestations of Bazex syndrome in Patient 3 before (A, B) and after (A1, B1) oncologic treatment and oral acitretin. Resolution can be seen in hands but symptoms persisted in feet
During the course of his oncologic treatment, he required a feeding tube and experienced grade 3 dermatitis managed with topical remedies and Silvadene and grade 2 mucositis managed with oxycodone and Magic mouthwash. While receiving treatment, he met with a dermatologist for his acro‐keratotic symptoms that had been present 2 months prior to diagnosis but had decreased in intensity following the initiation chemotherapy.
One month after treatment, he met with radiation oncology after receiving a CT scan of the neck showing bilateral lung base nodules concerning for metastasis despite improvement of the disease in the irradiated fields. This prompted a PET/CT which unfortunately revealed multiple hypermetabolic bilateral pulmonary nodules, multiple hepatic lesions, a right posterior iliac bone lytic lesion, and lymph nodes in the right hilar, paraesophageal and gastrohepatic regions compatible with new widely metastatic disease. He passed away 6 months later.
5. DISCUSSION
These cases illustrated both typical and atypical features of Bazex syndrome. Typical features include being middle‐aged smokers who developed head and neck cancers with dermatological features. 4 However, certain elements of the cases (dermatologic symptoms after cancer resolution, female sex, African American race, and sporadic cutaneous flares after eradication of both cancer and dermatologic manifestations) are relatively uncommon. In Bolognia et al's review, 89/93 (95.7%) case reports were of men, and 48/93 (51.6%) involved a head and neck primary. 1 Although the majority of the dermatologic signs and symptoms in our cases were typical, vesicular formation and pruritus were atypical, with severe burning and Raynaud's‐like features being extremely rare.
5.1. Pathophysiology
Analysis of skin lesions in Bazex reveals typical findings of hyperkeratosis and psoriasiform dermatitis. Epidermal findings tend to be nonspecific with hyperkeratosis or parakeratosis mimicking psoriasis. Unlike psoriasis however, Bazex findings can also include acanthosis, dyskeratotic keratinocytes, and vacuolar degeneration. Dermal histologic findings generally reveal perivascular, lymphocytic, or histiocytic infiltrate. 1 Immunofluorescence studies are usually negative. 8 , 9 The pathophysiology of Bazex syndrome remains unclear, although there are several hypotheses. The first proposes tumor‐directed antibodies cross‐react with epidermal or basement membrane antigens. 10 The second hypothesis proposes tumors secrete growth factors, such as TGF‐α, EGF, or IGF‐1, that stimulate epidermal and epithelial growth, comparable to other paraneoplastic skin conditions or melanoma, and other hypotheses include various vitamin deficiencies. 1
5.2. Staging
As defined by Bazex and Griffiths, the first stage typically appears before any signs or symptoms of malignancy, limited to the nails, digits, bridge of nose, and ear helices. During the second stage, involvement spreads to the hands, feet, and/or upper lip, and signs and symptoms of malignancy are usually present. Stage three is the spread to arms, legs, scalp, and/or extensive facial involvement. 4 Cutaneous symptoms typically develop before oncologic symptoms in Bazex patients, at a mean of 11 months, 1 but this was not the case in our case series. Patients 1, 2, and 3 presented in stages two, one, and three, respectively.
5.3. Response to therapy
Bazex syndrome typically responds to treatment of the underlying malignancy, rather than treatment of the skin findings. 1 In all of our cases, chemoradiation coincided with significant improvement of skin findings, and patients 1 and 2 also found relief with acitretin use. Interestingly, when acitretin use was discontinued after cancer treatment and symptom improvement in patient 1, the cutaneous findings and burning sensations not only returned, but progressed despite the patient being cancer‐free. Restarting acitretin led to improvement. Together, the timeline of dermatologic recurrence and regression indicates that Patient 1’s case of Bazex syndrome responded to both acitretin and the cancer treatment. The recurrence of patient 1’s dermatologic symptoms in the absence of oncologic recurrence as well as the subsequent sensitivity of her dermatologic symptoms to acitretin is atypical, but reports of Bazex syndrome effectively treated with retinoids, 11 , 12 as well as other treatments, 1 , 13 have been documented. However, there are also case reports of Bazex syndrome refractory to acitretin 14 or other retinoids. 15
5.4. Diagnostic challenge
Oftentimes, Bazex is misdiagnosed as psoriasis, eczema, or some atypical or fungal infection. Two of our patients spent significant time receiving dermatologic treatment prior to discovering their underlying malignancies. Valdivielso et al 16 have proposed an algorithm that involves a comprehensive examination and workup including laboratory work, procedures, and imaging in the setting of Bazex‐like skin findings. This method would likely increase the early detection of the associated malignancy; however, because of the rarity of Bazex syndrome relative to refractory dermatologic conditions, 17 costly and potentially morbid workup of benign conditions would occur. Thus, it is important to recognize major Bazex syndrome risk factors such as alcohol/smoking history in setting of symptoms associated with head and neck cancer.
6. CONCLUSION
Acrokeratosis paraneoplastica or Bazex syndrome is a rare paraneoplastic dermatologic condition that often, but not always, precedes an internal malignancy. It typically begins in fingers and toes and spreads proximally as the syndrome progresses. Bazex syndrome usually improves with oncologic treatment, but may require specific dermatologic remedies for symptom management. Awareness of the typical Bazex syndrome presentation and its variations by providers, including oncologists and those in primary care, could allow for early detection of underlying malignancy. This will help minimize misdiagnosis, allow early initiation of therapy for the underlying cancer condition, and ultimately may improve prognosis.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
JE, ADB: wrote manuscript, reviewed charts, designed figures, and processed revisions. EH, AA, EO, RR, VMD, and AJ: wrote, reviewed and edited manuscript. Daniel Hawkins: wrote manuscript and designed figures. Q‐AH: wrote manuscript and reviewed charts.
ETHICAL APPROVAL
This study was conducted in accordance with established guidelines and ethical standards.
ACKNOWLEDGMENTS
No acknowledgements outside of the authorship are necessary. Consent statement: Published with written consent of the patient.
Eckstein J, Healy E, Jain A, et al. A series of typical and atypical cases of Bazex syndrome: Identifying the red herring to avoid delaying cancer treatment. Clin Case Rep. 2020;8:2259–2264. 10.1002/ccr3.3133
REFERENCES
- 1. Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (Acrokeratosis Paraneoplastica) an analytic review. Medicine. 1991;70:269‐280. [DOI] [PubMed] [Google Scholar]
- 2. Gougerot H, Grupper C. Dermatose érythémato‐squameuse avec hyperkératose palmoplantaire, porectasies digitales et cancer de la langue latent. Paris Med. 1922;43:234‐237. [Google Scholar]
- 3. Bazex A, Salvador R, Dupré A, Christol B. Syndrome paranéoplasique à type d’hyperkératose des extrémités. Guérison après le traitement de l’épithélioma laryngé. Bull Soc Fr Dermatol Syphiligr. 1965;72:182. [Google Scholar]
- 4. Bazex A, Griffiths A. Acrokeratosis paraneoplastica—a new cutaneous marker of malignancy. Br J Dermatol. 1980;103(3):301‐306. [DOI] [PubMed] [Google Scholar]
- 5. Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84‐89. [DOI] [PubMed] [Google Scholar]
- 6. Räßler F, Goetze S, Elsner P. Acrokeratosis paraneoplastica (Bazex syndrome) ‐ a systematic review on risk factors, diagnosis, prognosis and management. J Eur Acad Dermatol Venereol. 2017;31(7):1119‐1136. [DOI] [PubMed] [Google Scholar]
- 7. Gill D, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42(4):278‐280. [DOI] [PubMed] [Google Scholar]
- 8. Estrela F, Pinto GM, Pinto LM, Afonso A. Acrokeratosis paraneoplastica (Bazex syndrome) with oropharyngeal squamous cell carcinoma. Cutis. 1995;55:233‐236. [PubMed] [Google Scholar]
- 9. Buxtorf K, Eugen H, Panizzon RG. Bazex syndrome. Dermatology. 2001;202:350‐352. [DOI] [PubMed] [Google Scholar]
- 10. Pecora AL, Landsman L, Imgrund SP, Lambert C. Acrokeratosis paraneoplastica (Bazex' syndrome): Report of a case and review of the literature. Archiv Dermatol. 1983;119:820‐826. [DOI] [PubMed] [Google Scholar]
- 11. Wishart JM. Bazex paraneoplastic acrokeratosis: a case report and response to Tigason. Br J Dermatol. 1986;115:595‐599. [DOI] [PubMed] [Google Scholar]
- 12. Esteve E, Serpier H, Cambie MP, et al. Acrokératose paranéoplasique de Bazex: traitement par acitrétine. Ann Dermatol Vénéréol. 1995;122:26‐29. [PubMed] [Google Scholar]
- 13. David G, Fergin P, Kelly J. Bullous lesions in Bazex syndrome and successful treatment with oral psoralen phototherapy. Australas J Dermatol. 2001;42:278‐280. [DOI] [PubMed] [Google Scholar]
- 14. Zhao J, Zhang X, Chen Z, Wu JH. Case report: Bazex syndrome associated with pulmonary adenocarcinoma. Medicine. 2016;95:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juhlin L, Baran R. Abnormal amino acid composition of nails in Bazex's paraneoplastic acrokeratosis. Acta Dermato Venereol. 1984;64:31. [PubMed] [Google Scholar]
- 16. Valdivielso M, Longo I, Suárez R, Huerta M, Lázaro P. Acrokeratosis paraneoplastica (Bazex syndrome). J Eur Acad Dermatol Venereol. 2005;19:340‐344. [DOI] [PubMed] [Google Scholar]
- 17. Blanchet F, Leroy D, Deschamps P. Acrokératose paranéoplasique de Bazex. A propos de 8 cas. J Fr Otorhinolaryngol. 1980;29:165‐172. [PubMed] [Google Scholar]


