Abstract
We report a case of an encapsulated fat necrosis without significant medical history. To differentiate from liposarcoma, it should be recognized that a half of abdominal encapsulated fat necrosis cases have a history of inflammation and surgery.
Keywords: abdominal, fat necrosis, liposarcoma, surgery
We report a case of an encapsulated fat necrosis without significant medical history. To differentiate from liposarcoma, it should be recognized that a half of abdominal encapsulated fat necrosis cases have a history of inflammation and surgery.
1. INTRODUCTION
Encapsulated fat necrosis is a rare inflammatory disorder of fat tissue, presenting as an intra‐abdominal mass after pancreatitis, trauma, or abdominal surgery. 1 Encapsulated fat necrosis was first reported in the breast in 1978. 2 It is most likely to occur in the extremities such as the feet, knees, thighs, arms, the hip, chest, and finally the abdomen. 3 In addition, fat necrosis is benign and usually asymptomatic and therefore may be difficult to differentiate from liposarcoma using the clinical history and imaging alone. 4 Although the clinical features of encapsulated fat necrosis in subcutaneous lesions are well described, its features in the abdomen have not fully been summarized to date. 3
Herein, we report a case of laparoscopic resection of an encapsulated fat necrosis without significant medical history other than ureterolithiasis and review the relevant literature.
2. CASE PRESENTATION
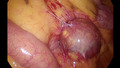
A 69‐year‐old man was referred to our hospital for evaluation of a painless abdominal mass within his left abdomen that was incidentally discovered during assessment for ureterolithiasis. However, according to the patient's medical documents, there was no abdominal mass 4 years ago on computed tomography (CT) scan. He had no history of pancreatitis, trauma, or abdominal surgery. The physical examination did not reveal any abdominal tenderness or a palpable mass. Laboratory tests were normal, including hepatobiliary function, renal function, inflammatory response, and urinalysis. CT scan showed a round and encapsulated 4 × 4 cm mass without any contrast enhancement in the upper left abdomen (Figure 1). There was no evidence of lymphadenopathy or distant metastasis of liver and lungs. Magnetic resonance imaging showed that the tumor on the descending mesocolon had high‐intensity (SI) portions on T1‐weighted images (WIs) and iso‐intense portions on T2‐WIs compared to the fat. Comparison of iso‐SI to the fat showed fat suppression on T2‐weighted short tau inversion recovery images. There was a thin rim at the periphery of the mass showing low SI on both T1‐ and T2‐WIs and revealing contrast enhancement (Figure 2). Imaging findings could not positively suspect malignant signs, but a 4‐cm‐sized tumor that suddenly occurred during the last 4 years with no history of pancreatitis, trauma, or abdominal surgery could not completely rule out liposarcoma. Close follow‐up was also offered to patients as an alternative to surgeries; however, liposarcoma was undeniable and the surgery was conducted for definitive diagnosis and treatment. The tumor on the descending mesocolon was removed laparoscopically (Figure 3). The adhesion around the tumor was peeled and removed without breaking the capsule. There were no adhesion and inflammation in the appendix, appendices epiploicae, omentum, and retroperitoneum around the kidney and ureter. Histopathological findings revealed that the tumor wall comprised hyalinized fibrous connective tissue and the inside of the tumor consisted of degenerated and necrotic fatty tissue of the same size; it was diagnosed as encapsulated fat necrosis (Figures 4 and 5). The patient's postoperative course was uneventful, and he was discharged 5 days later. Presently, about 1 year after surgery, the patient remains healthy and has no signs of recurrence or complications.
FIGURE 1.

Computed tomographic image of the abdomen, demonstrating a round and encapsulated 4 × 4 cm mass without any contrast enhancement
FIGURE 2.

Left: Magnetic resonance imaging T1W and Right: Magnetic resonance imaging T2W showed a round lesion with a thick hypointense wall and a central fatty focus
FIGURE 3.

Laparoscopic image of the tumor on the descending mesocolon
FIGURE 4.

Macroscopic findings
FIGURE 5.

Histopathological findings
3. DISCUSSION
Encapsulated fat necrosis can exist as an intra‐abdominal mass without pancreatitis, trauma, or abdominal surgery. Laparoscopic resection of encapsulated fat necrosis is acceptable if liposarcoma cannot be ruled out by clinical history and imaging findings.
Although encapsulated fat necrosis usually occurs after pancreatitis, trauma, or abdominal surgery, the etiology in the patient described is unclear. A PubMed database search was performed using the terms “encapsulated” OR “nodular” AND “fat necrosis” up to February 2020 and nine articles were retrieved. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 The citation lists of the extracted articles were searched through Google Scholar for further identification of additional studies. Table 1 summarizes the characteristics of previous nine patients (seven males and two females) diagnosed with abdominal encapsulated fat necrosis with the average age of 54 years (range, 20‐78 years). Eight patients had some symptoms, four indicating with abdominal pain, and one was incidentally found on CT scan. Two patients had a history of appendectomy, two had appendicitis or colonic diverticulosis, and five had no history of surgery or injury. Most patients underwent laparoscopic resection. The literature review revealed that the encapsulated fat necrosis may occur in traumatic injury and abdominal surgery, but the cause may not be well understood in most cases.
TABLE 1.
Summary of the characteristics of abdominal encapsulated fat necrosis
| Author (ref)/y | Age | Sex | Chief complaint | Cause | Location | Size | Treatment |
|---|---|---|---|---|---|---|---|
| Felipo et al 5 /2004 | 67 | Male | Morbid obesity | Appendectomy | Intraperitoneal | 14.0 cm | Surgery |
| Unal et al 6 /2005 | 20 | Male | Abdominal pain | Appendectomy | Intraperitoneal | 2.0 cm | Surgery |
| Gupta et al 7 /2007 | 45 | Female | Menorrhagia | NR | Ovary | NR | Surgery |
| Lee et al 8 /2010 | 60 | Male | Abdominal pain | Without injury | Retroperitoneum | 33.0 cm | Laparotomy |
| Brooke and Choti 9 /2011 | 56 | Male | Chest pain | NR | Intraperitoneal | 3.2 cm | Laparotomy |
| Oh et al 10 /2015 | 66 | Male | None | Appendicitis | Intraperitoneal | NR | Laparotomy |
| De Kock and Delrue 11 /2016 | 33 | Female | Abdominal pain | NR | Intraperitoneal | NR | NR |
| Magalhães et al 12 /2016 | 78 | Male | Abdominal pain | Diverticulitis | Intraperitoneal | 3.8 cm | Laparotomy |
| Biondi et al 13 /2017 | 59 | Male | Diarrhea and fever | Without surgery | Intraperitoneal | 6.5 cm | Laparotomy |
Abbreviation: NR, not reported.
Laparoscopic resection of encapsulated fat necrosis is acceptable if there are no signs of pancreatitis, trauma, and abdominal surgery, and imaging findings do not rule out liposarcoma. Although encapsulated fat necrosis can be monitored as an alternative to resection due to its nature of shrinking in size over the time, pathological analysis with surgical resection is critical in making the final diagnosis. 14
Because the natural progression is to decrease in size over time, pathological analysis with surgical resection is critical in making the final diagnosis. 14 Septum enhancement and/or thick septa of 2 mm or more in the intra‐abdominal cystic masses containing fat are presumed liposarcomas; however, in this case, the absence of a septum within the mass was difficult to distinguish from liposarcoma. 14 On our radiological findings, well‐differentiated liposarcoma, spindle cell lipoma, and fat necrosis were diagnosed because the tumor mainly consisted of both fat and nonlipomatous components. 15 Macroscopically, liposarcoma is generally well circumscribed but not encapsulated, which may depend on tumor differentiation. 16 Although spindle cell lipoma is more suggestive when the tumor occurs in middle‐aged men, especially localized behind the neck, in this case, the tumor was located in the abdominal cavity of older men. 17 The deep typical lesions, such as retroperitoneum and deep thigh musculature, of liposarcoma require biopsy for definitive diagnosis. 18 However, in the abdominal cavity, the liposarcoma is at risk of intraperitoneal dissemination, so it is more important to remove the tumor without breaking the capsule than to biopsy the mass. In the histopathological findings, liposarcoma showed adipocytes of different sizes and atypical stromal cells in the fibrous septum, but in the present case, the above findings, including lipoblast, were not observed. 16 Therefore, additional immunohistochemical study was not added.
In conclusion, encapsulated fat necrosis can present as an abdominal mass with no significant history and laparoscopic resection is indicated. We should be aware that half of the encapsulated fat necrosis mimicking liposarcoma can manifest as an intra‐abdominal mass with histories of pancreatitis, trauma, and abdominal surgery. Encapsulated fat necrosis with a well‐defined cause may allow for careful follow‐up.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
JW and KE: conceived and designed the study. KG, TO, and TS: revised the manuscript. SN and YH: were involved in overall supervision of the paper. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval was not necessary for this case report. All patient's data and photographs are de‐identified.
INFORMED CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ACKNOWLEDGMENTS
None.
Watanabe J, Osaki T, Tatebe S, et al. Encapsulated fat necrosis mimicking abdominal liposarcoma: A case report and literature review. Clin Case Rep. 2020;8:2255–2258. 10.1002/ccr3.3120
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Hurt MA, Santa Cruz DJ. Nodular‐cystic fat necrosis. A reevaluation of the so‐called mobile encapsulated lipoma. J Am Acad Dermatol. 1989;21(3 Pt 1):493‐498. [PubMed] [Google Scholar]
- 2. Schmidt‐Hermes HJ, Loskant G. Verkalkte Fettgewebsnekrose der weiblichen Brust [Calcified fat necrosis of the female breast]. Med Welt. 1975;26(24):1179‐1180. [PubMed] [Google Scholar]
- 3. Kiryu H, Rikihisa W, Furue M. Encapsulated fat necrosis–a clinicopathological study of 8 cases and a literature review. J Cutan Pathol. 2000;27(1):19‐23. [DOI] [PubMed] [Google Scholar]
- 4. Kransdorf MJ, Bancroft LW, Peterson JJ, et al. Imaging of fatty tumors: distinction of lipoma and well‐differentiated liposarcoma. Radiology. 2002;224(1):99‐104. [DOI] [PubMed] [Google Scholar]
- 5. Felipo F, Vaquero M, del Agua C. Pseudotumoral encapsulated fat necrosis with diffuse pseudomembranous degeneration. J Cutan Pathol. 2004;31(8):565‐567. [DOI] [PubMed] [Google Scholar]
- 6. Unal E, Yankol Y, Sanal T, et al. Laparoscopic resection of a torsioned appendix epiploica in a previously appendectomized patient. Surg Laparosc Endosc Percutan Tech. 2005;15(6):371‐373. [DOI] [PubMed] [Google Scholar]
- 7. Gupta R, Singh DK, Singh S, et al. Bilateral encapsulated adipocytic prosoplasia of ovary with fat necrosis: a case report. Arch Gynecol Obstet. 2007;275(4):307‐309. [DOI] [PubMed] [Google Scholar]
- 8. Lee KR, Seo TJ, Cho JH, et al. A case of large retroperitoneal lipoma mimicking liposarcoma. Korean J Gastroenterol. 2010;55(6):394‐398. [DOI] [PubMed] [Google Scholar]
- 9. Brooke BS, Choti MA. Image of the month: encapsulated fat necrosis. Arch Surg. 2011;146(12):1447‐1448. [DOI] [PubMed] [Google Scholar]
- 10. Oh HB, Arab N, Teo L, et al. Snapshot in surgery: intraperitoneal encapsulated fat necrosis. Clin Case Rep. 2015;3(2):131‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Kock I, Delrue L. Encapsulated mesenteric fat necrosis. J Belg Soc Radiol. 2016;100(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magalhães R, Oliveira J, Caridade S. Encapsulated abdominal fat necrosis. Acta Med Port. 2016;29(10):673. [DOI] [PubMed] [Google Scholar]
- 13. Biondi A, Fico V, Marra AA, Persiani R. Encapsulated fat necrosis mimicking an intra‐abdominal tumor. J Gastrointest Surg. 2017;21(5):918‐919. [DOI] [PubMed] [Google Scholar]
- 14. Kamaya A, Federle MP, Desser TS. Imaging manifestations of abdominal fat necrosis and its mimics. Radiographics. 2011;31(7):2021‐2034. [DOI] [PubMed] [Google Scholar]
- 15. Jo VY, Doyle LA. Refinements in sarcoma classification in the current 2013 World Health Organization classification of tumours of soft tissue and bone. Surg Oncol Clin N Am. 2016;25(4):621‐643. [DOI] [PubMed] [Google Scholar]
- 16. Thway K. Well‐differentiated liposarcoma and dedifferentiated liposarcoma: an updated review. Semin Diagn Pathol. 2019;36(2):112‐121. [DOI] [PubMed] [Google Scholar]
- 17. Bancroft LW, Kransdorf MJ, Peterson JJ, Sundaram M, Murphey MD, O'Connor MI. Imaging characteristics of spindle cell lipoma. AJR Am J Roentgenol. 2003;181(5):1251‐1254. [DOI] [PubMed] [Google Scholar]
- 18. Burkholz KJ, Roberts CC, Lidner TK. Posttraumatic pseudolipoma (fat necrosis) mimicking atypical lipoma or liposarcoma on MRI. Radiol Case Rep. 2015;2(2):56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


