Abstract
[Purpose]
Epidemiological evidence has shown that leisure-time physical activity and structured exercise before and after breast cancer diagnosis contribute to reducing the risk of breast cancer recurrence and mortality. Thus, in this review, we aimed to summarize the physical activity-dependent regulation of systemic factors to understand the biological and molecular mechanisms involved in the initiation, progression, and survival of breast cancer.
[Methods]
We systematically reviewed the studies on 1) the relationship between physical activity and the risk of breast cancer, and 2) various systemic factors induced by physical activity and exercise that are potentially linked to breast cancer outcomes. To perform this literature review, PubMed database was searched using the terms “Physical activity OR exercise” and “breast cancer”, until August 5th, 2020; then, we reviewed those articles related to biological mechanisms after examining the resulting search list.
[Results]
There is strong evidence that physical activity reduces the risk of breast cancer, and the protective effect of physical activity on breast cancer has been achieved by long-term regulation of various circulatory factors, such as sex hormones, metabolic hormones, inflammatory factors, adipokines, and myokines. In addition, physical activity substantially alters wholebody homeostasis by affecting numerous other factors, including plasma metabolites, reactive oxygen species, and microRNAs as well as exosomes and gut microbiota profile, and thereby every cell and organ in the whole body might be ultimately affected by the biological perturbation induced by physical activity and exercise.
[Conclusion]
The understanding of integrative mechanisms will enhance how physical activity can ultimately influence the risk and prognosis of various cancers, including breast cancer. Furthermore, physical activity could be considered an efficacious non-pharmacological therapy, and the promotion of physical activity is probably an effective strategy in primary cancer prevention.
Keywords: exercise, breast cancer, metabolic hormones, inflammatory markers, myokines, adipokines, stress hormones, ROS
INTRODUCTION
Breast cancer is one of the most common malignant tumors worldwide, accounting for 30% of all new cases of female cancers [1]. The incidence rate of breast cancer has risen slightly by approximately 0.3% per year, whereas the 5-year survival rate of female breast cancer patients is 90%, which is higher than that of all cancer patients (67%, in average). Approximately 5%–10% of breast cancer cases can be attributed to an inherited genetic predisposition with a family history, such as mutations in two high-penetrance tumor suppressor genes, breast cancer gene 1 and 2 [2]. However, breast cancer is more frequently associated with environmental, reproductive, and lifestyle factors, including nutrition and physical activity [3], that may play an essential role in the pathogenesis of breast cancer.
In recent years, epidemiological studies have been conducted on the relationship between physical activity and cancer outcomes, demonstrating a protective role of physical activity in breast cancer [4-8]. Leisure-time physical activity is associated with lower risks of 13 types of cancer [5], and exercise-associated reduction in breast cancer risk has been apparent in early-stage breast cancer patients [6]. In addition, high-risk breast cancer patients meeting the minimum Physical Activity Guidelines for Americans [9] experienced 50% reduced hazards of recurrence and mortality [7]. Therefore, epidemiological evidence supports that leisure-time physical activity and structured exercise before and after breast cancer diagnosis contribute to reducing the risk of breast cancer recurrence and mortality.
Exercise can substantially influence whole-body homeostasis by affecting multiple organ systems, and the integrative biology of exercise in skeletal muscle adaptation has been extensively studied using omics approaches aiming to decipher the molecular basis of exercise responses [10-12]. In addition, multiple biological mechanisms at systemic levels are hypothesized to mediate the potential protective effects of physical activity and exercise on cancer prevention [13-16]. The current review summarizes the physical activity-dependent regulation of systemic factors that may influence breast cancer progression and clinical outcomes (Figure 1; Table 1).
Figure 1.
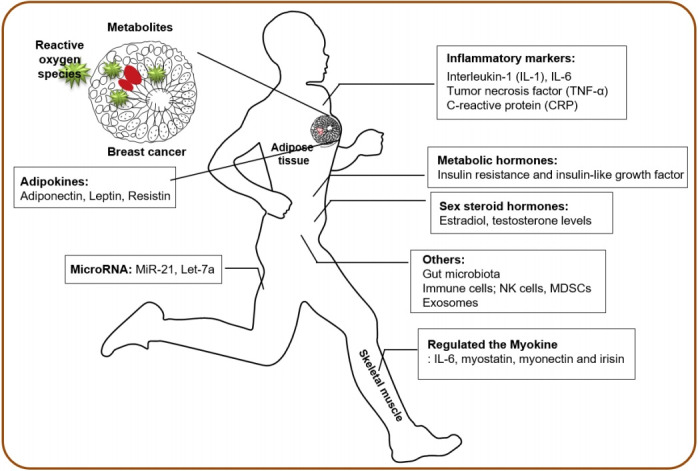
Physical activity-induced systemic factors associated with breast cancer outcomes
Table 1.
Summary of physical activity-dependent regulators of systemic factors involved in breast cancer risk, progression, and recurrence
| Mediators affected by physical activity* | Ref |
|---|---|
| Sex steroid hormones | |
| Estrogen, androgen, testosterone | 17-23 |
| Metabolic hormones | |
| Insulin, leptin, IGF | 24-31 |
| Inflammatory markers | |
| CRP, TNF-α, IL-6, IL-1b, IL-7, IL-15 | 32-35 |
| Adipokines | |
| Adiponectin, leptin, resistin | 36-40 |
| Myokines | |
| Myostatin, myonectin, irisin, IL-6, ANGPTL-4, MCP-1, CX3CL1, IL-8, IL-15 | 41-43 |
| Stress hormones | |
| Catecholamines; epinephrine, norepinephrine, cortisol | 44-46 |
| Others | |
| Metabolites | 47, 48 |
| Reactive oxygen species | 49, 50 |
| microRNAs; miR-21, let-7a | 51-53 |
| Exosomes | 54-57 |
| Immune cells; NK cells, MDSCs | 58-60 |
| Gut microbiomes | 61-63 |
IGF, insulin-like growth factor; CRP, C-reactive protein; TNF, tumor necrosis factor; IL, interleukin; ANGPTL-4, angiopoietin-like 4; MCF-1, monocyte chemoattractant protein-1; CX3CL1, C-X3-C motif chemokine ligand 1; NK, natural killer cells; MDSCs, myeloid-derived suppressor cells.
The biological mechanisms linking physical activity and breast cancer
Sex steroid hormones
Women with elevated systemic levels of estrogens and androgens have increased risks of breast cancer incidence and development [17,18]. In premenopausal women, physical activity is inversely correlated with sex hormones, estradiol, and testosterone level [19,20]. As sex hormones in postmenopausal women are primarily produced in the adipose tissue, physical activity has been associated with decreased estrone and estradiol levels after adjustment for Body Mass Index [21,22], indicating that weight loss and lower adiposity are linked to controlled sex hormone levels and lower risk of breast cancer. A meta-analysis of the effect of physical activity on sex hormones demonstrated that physical activity produced protective effects against breast cancer by decreasing the levels of circulating sex hormones, regardless of menopausal status and weight loss [23].
Metabolic hormones
Several studies have shown that elevated plasma insulin levels are associated with an increased incidence of various cancers [24] and higher recurrence in breast cancer survivors [25,26]. Insulin resistance and insulin-like growth factor (IGF) may increase the risk of breast cancer by increasing the level of circulating estrogen [27]. Exercise can reduce insulin levels and insulin resistance, thereby decreasing fasting glucose, total IGF-1, and increasing IGF binding proteins [28]. In addition, after exercise interventions, breast cancer patients have reduced fasting insulin levels due to the reductions in body weight, anticipating better prognosis of breast cancer [29]. However, most of the benefits of exercise-induced modulation might be associated with body weight loss and comorbidities, such as type 2 diabetes and metabolic syndrome [30,31].
Inflammatory markers, cytokines, and adipokines
Cancer-related inflammation is one of the hallmarks of cancer [32], and interleukin-1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP) are widely recognized as biomarkers of systemic inflammation related to breast cancer [33]. Studies have shown that increased levels of pro-inflammatory cytokines and CRP have been linked to increased cancer risk and reduced overall survival of breast cancer [34,35]. Physical activity generally has an anti-inflammatory effect and reduces systemic inflammation in healthy individuals without cancer diagnosis. The impact of physical activity on the levels of IL-6, TNF-α and CRP varies and has its limitations. Further, a meta-analysis of exercise intervention showed no effect on the levels of CRP, IL-6, or TNF-α [31].
Adipose tissues, bearing one of the multiple cell types in the mammary gland, secrete adiponectin, leptin, resistin, and other cytokines. The modulation of circulating adipokines by physical activity has also been demonstrated [36]. Leptin stimulates growth, migration, and invasion of breast cancer via its pro-inflammatory effect, whereas adiponectin is an anti-inflammatory factor and is inversely associated with adiposity. Decreased levels of adiponectin are associated with higher body mass indices and higher fat percentages, whereas the ratio of adiponectin to leptin is a key determinant of the effect of adipokines on the pathological process of breast cancer [37,38]. Significant elevations in serum adiponectin levels and reductions in serum leptin levels have been observed with physical activity interventions by directly lowering the amount of body fat [39,40]. Therefore, physical activity may regulate inflammatory cytokines and adipokines; however, the reduced risk of breast cancer is strongly associated with fat mass and weight loss.
Myokines and stress hormones as exercise factors
Skeletal muscle, the largest organ in our body, secretes numerous myokines, such as IL-6, myostatin, myonectin, and irisin, and the circulating myokines levels are regulated by physical exercise [41]. A large-scale omics-based approach is aimed at elucidating the entire secretome secreted by muscle cells to understand the molecular basis of exercise adaptation. Preclinical studies have demonstrated that irisin, which increases with physical activity, can inhibit breast cancer viability due to increased caspase activity and suppressed NF-κB activity [42].
The stress hormones catecholamines are exercise factors responsible for breast cancer progression inhibition. Plasma epinephrine and norepinephrine rapidly increase by exercise [43]; in contrast, cortisol levels are dependent on the duration and intensity of exercise [44]. Exercise-induced catecholamines mediate breast cancer suppressive effects by activating the tumor suppressor Hippo signaling pathway [45]. However, data on the role of stress hormones as exercise factors have not been studied directly in breast cancer patients, and they could induce an opposite effect on breast cancer protection [46].
Other systemic factors
In addition to hormones, inflammatory markers, and myokines, several other circulating systemic factors are regulated by physical activity and exercise. Various plasma metabolites are related to physical activity, and these metabolites may play an essential role in the protective effect of cancer progression. Several studies have shown that plasma metabolites are associated with the risk of breast cancer and have potential as biomarkers for the early diagnosis of breast cancer [47,48].
Oxidative stress and reactive oxygen species have been implicated in a number of diseases as well as the initiation and progression of cancer [49]. Interestingly, acute exercise produces pro-oxidant environments; however, repeated exercise stimulates antioxidant defenses, resulting in a greater capacity to resist oxidative environments [50]. In this context, the effect of repeated physical activity on oxidative stress may be beneficial for preventing the progression and metastasis of breast cancer.
Recent studies have shown that the expression levels of circulating microRNAs are modulated by physical activity and exercise in healthy individuals and patients with various diseases [51-53]. Because of their pivotal role in controlling cell proliferation, microRNAs may be important regulators of exercise adaptation and potential biomarkers of exercise response [51,52]. The expression of several breast cancer-related microRNAs, such as miR-21 and let-7a, is altered by exercise [53], and thereby circulating microRNAs may be mediators of the association between physical activity and breast cancer.
In addition to various soluble mediators, extracellular vesicles containing functional molecules such as proteins, lipids, mRNA, and microRNAs can exert systemic biological effects by providing the means for inter-tissue crosstalk during physical exercise [54]. Physical exercise triggers a rapid release of extracellular vesicles into the circulation [55], and their compositions via delivery of myokines play an essential role in exercise adaptation throughout the body [56]. Therefore, extracellular vesicles could mediate the beneficial effects of exercise [57] and potentially affect breast cancer progression.
Physical activity has an impact on the level and activity of circulating immune cells, such as natural killer cells, which are the most responsive immune cells to exercise-dependent mobilization to the circulation [58]. The mobilization of IL-6-sensitive natural killer cells was significantly increased by epinephrine in mice that experienced voluntary wheel running, resulting in the reduction of tumor incidence and growth [59]. In addition, myeloid-derived suppressor cells, as regulators of the immune system in the tumor microenvironment, could regulate tumor growth and metastasis by controlling inflammatory markers with exercise and weight loss [60].
Finally, emerging evidence suggests that the gut microbiome may confer susceptibility to several cancers and may influence the therapeutic responses, suggesting that the microbiome has been implicated in increased risks of certain malignancies, including breast cancer [61]. Exercise alters the composition and derived metabolic products in the human gut microbiota by reducing the inflammatory signaling pathway induced by obesity [62,63]. Therefore, the disease-related deleterious gut microbiota profile could be modified by physical activity intervention. Overall, complex systemic changes during physical activity and structured exercise may directly inhibit breast cancer progression and improve the overall survival outcome.
DISCUSSION
In this review, we summarize the current literature supporting various biological mechanisms whereby physical activity and exercise may influence the initiation, progression, and growth of tumors, mainly breast cancer (Figure 1; Table 1). There is strong evidence that physical activity reduces the risk of breast cancer, and the protective effect of physical activity on this type of cancer has been achieved by long-term regulation of various circulatory risk factors, such as sex hormones, metabolic hormones, and inflammatory factors. In addition, physical activity substantially alters whole-body homeostasis by inducing numerous other factors as well as by changing the gut microbiota profile. Ultimately, every cell and organ in the body might be affected by the biological perturbation induced by exercise. The potential intracellular mechanisms underlying the effects of physical activity on breast carcinogenesis include the phosphatidylinositol-3-kinase/protein kinase B and mammalian target of rapamycin signaling pathways as well as cell cycle and apoptosis [64]. However, most health-promoting benefits induced by exercise are associated with body mass index and weight loss, indicating that the level of adiposity and the percentage of fat mass are critical indicators in determining the effect of exercise.
Current physical activity recommendations for breast cancer survivors encourage following aerobic exercise routines that include 150 min per week of moderate or 75 min per week of high intensity exercise, and resistance exercise for at least 2 days per week [65-67]. However, as breast cancer is generally considered as a heterogeneous disease, the impact of physical activity may differ depending on the clinicopathologic features (e.g., tumor stage and hormone receptor status) and body composition (e.g., fat and skeletal muscle mass), eliciting diverse biological and molecular mechanisms and the varied and limited outcomes of breast cancer. In addition, breast cancer progression is influenced by the integrity and composition of the tumor microenvironment; the efficacy of exercise intervention is probably dependent on the modulation of host-tumor interaction. Moreover, even though the evidence for the benefits of physical activity in breast cancer continues to grow, most studies have not applied all the components of exercise prescription (frequency, intensity, time, and type). Recently, guidelines for academic researchers have been published for reporting exercise programs to increase clinical uptake and improve patient outcomes [68]. Therefore, further investigations should follow the Consensus on Exercise Reporting Template to understand a detailed mechanistic explanation for physical activity-dependent suppression of breast cancer growth for the transparency, consistency, and implementation of effective exercise interventions in clinical practice.
Physical activity could be considered an efficacious non-pharmacological therapy, and its promotion is probably an effective strategy in primary cancer prevention [69]. Exercise training has a significant physiological effect on IGF-1 in postmenopausal breast cancer survivors [28], and high-intensity interval training shows a remarkable effect on the expression of microRNAs in breast cancer patients undergoing hormone therapy. Although the expression of microRNAs does not change in healthy women after a 12-week exercise program, emerging evidence suggests that exercise is also directly linked to cancer progression by affecting tumor-intrinsic factors [14]. Therefore, not only exercise itself is a medicine in oncology, but it is also a critical synergistic medicine with conventional anti-cancer therapies, such as chemotherapy or hormone therapy. However, more research is needed to fully understand the direct and synergistic effects of exercise on breast cancer progression.
In conclusion, the biology of physical activity and exercise is highly complex and variable, and affects multiple organ systems via autocrine, paracrine, and endocrine factors within a crosstalk network. Thus, an understanding of these integrative mechanisms will enhance how physical activity can ultimately influence the risk and prognosis of various cancers, including breast cancer.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2019R1H1A1035588, and NRF-2020R1F1A1049665).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 3.Iacoviello L, Bonaccio M, de Gaetano G, Donati MB. Epidemiology of breast cancer, a paradigm of the "common soil" hypothesis. Semin Cancer Biol. 2020:1–7. doi: 10.1016/j.semcancer.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 5.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Gonzalez AB, Hartge P, Adami HO, Blair C, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Dusen RV, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–25. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones LW, Kwan ML, Weltzien E, Chandarlapaty S, Sternfeld B, Sweeney C, Bernard PS, Castillo A, Habel LA, Kroenke CH, Langholz BM, Queensbeerry Jr CP, Dang C, Weigelt B, Kushi LH, Caan B. Exercise and prognosis on the basis of clinicopathologic and molecular features in early-stage breast cancer: the LACE and pathways studies. Cancer Res. 2016;76:5415–22. doi: 10.1158/0008-5472.CAN-15-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannioto RA, Hutson A, Dighe S, McCann W, McCann SE, Zirpoli GR, Barlow W, Kelly KM, DeNysschen CA, Hershman DL, Unger JM, Moore JA, Isaacs C, Hobday TJ, Salim M, Hortobagyi GN, Gralow JR, Albain KS, Budd GT, Ambroson CB. Physical activity before, during and after chemotherapy for high-risk breast cancer: relationships with survival. J Natl Cancer Inst. 2020;113:1–10. doi: 10.1093/jnci/djaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Fensom GK, Reeves GK, Key TJ. Physical activity and breast cancer risk: results from the UK Biobank prospective cohort. Br J Cancer. 2020;122:726–32. doi: 10.1038/s41416-019-0700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320:2020–8. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley JA, Hargreaves M, Joyner MJ, Zierath J. Integrative biology of exercise. Cell. 2014;159:738–49. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–84. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Zierath JR, Wallberg-Henriksson H. Looking ahead perspective: where will the future of exercise biology take us? Cell Metab. 2015;22:25–30. doi: 10.1016/j.cmet.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 13.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 14.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27:10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 15.de Boer MC, Worner EA, Verlaan D, van Leeuwen PAM. The mechanisms and effects of physical activity on breast cancer. Clin Breast Cancer. 2017;17:271–8. doi: 10.1016/j.clbc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Christine D, Katrine SP, Pernile H. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res Treat. 2017;162:399–408. doi: 10.1007/s10549-017-4129-4. [DOI] [PubMed] [Google Scholar]
- 17.Key T, Appleby P, Barnes I, Reeves G, Endogenous H, Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 18.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Travis RC, Alberg AJ, Barricarte A, Berrino F, Krogh V, Sieri S, Brinton LA, Dorgan JF, Dossus L, Doweet M, Eliassen AH, Fortner RT, Hankinson SE, Helzlsouer KJ, Hoffman-Bolton J, Comstock GW, Kaaks R, Kahle LL, Muti P, Overvad K, peeters PHM, Riboil E, Rinaldi S, Rollison DE, Stanczyk FZ, Trichopoulos D, Tworoger SS, Vineis P. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14:1009–19. doi: 10.1016/S1470-2045(13)70301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emaus A, Veierod MB, Furberg AS, Espetvedt S, Friedenreich C, Ellison PT, Jasienska G, Andersen LB, Thune I. Physical activity, heart rate, metabolic profile, and estradiol in premenopausal women. Med Sci Sports Exerc. 2008;40:1022–30. doi: 10.1249/MSS.0b013e318167411f. [DOI] [PubMed] [Google Scholar]
- 20.Verkasalo PK, Thomas HV, Appleby PN, Davey GK, Key TJ. Circulating levels of sex hormones and their relation to risk factors for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom) Cancer Causes Control. 2001;12:47–59. doi: 10.1023/a:1008929714862. [DOI] [PubMed] [Google Scholar]
- 21.McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, Potter JD, Schwartz RS. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64:2923–8. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 22.Liedtke S, Schmidt ME, Becker S, Kaaks R, Zaineddin AK, Buck K, Flesch-Janys D, Wahrendorf J, Chang-Claude J, Steindorf K. Physical activity and endogenous sex hormones in postmenopausal women: to what extent are observed associations confounded or modified by BMI? Cancer Causes Control. 2011;22:81–9. doi: 10.1007/s10552-010-9677-4. [DOI] [PubMed] [Google Scholar]
- 23.Ennour-Idrissi K, Maunsell E, Diorio C. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015;17:139. doi: 10.1186/s13058-015-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 25.Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, Kalan RC, Harris TG, Howard BV, Wylie-Rosett J, Burk RD, Strickler HD. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 27.Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–42. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–7. [PubMed] [Google Scholar]
- 29.Ahn N. Effects of 12-week exercise training on osteocalcin, high-sensitivity C-reactive protein concentrations, and insulin resistance in elderly females with osteoporosis. J Phys Ther Sci. 2016;28:2227–31. doi: 10.1589/jpts.28.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 31.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 34.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Standish LJ, Sweet ES, Novack J, Wenner CA, Bridge C, Nelson A, Martzen M, Torkelson C. Breast cancer and the immune system. J Soc Integr Oncol. 2008;6:158–68. [PMC free article] [PubMed] [Google Scholar]
- 36.Sturgeon K, Digiovanni L, Good J, Salvatore D, Fenderson D, Domchek S, Stopfer J, Galantino ML, Bryan C, Hwang WT, Schmitz K. Exercise-induced dose-response alterations in Adiponectin and Leptin levels are dependent on body fat changes in women at risk for breast cancer. Cancer Epidemiol Biomarkers Prev. 2016;125:1195–200. doi: 10.1158/1055-9965.EPI-15-1087. [DOI] [PubMed] [Google Scholar]
- 37.Ando S, Gelsomino L, Panza S, Giordano C, Bonofiglio D, Barone I, Catalano S. Obesity, leptin and breast cancer: epidemiological evidence and proposed mechanisms. Cancers (Basel) 2019;11 doi: 10.3390/cancers11010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grossmann ME, Ray A, Nkhata KJ, Malakhov DA, Rogozina OP, Dogan S, Cleary MP. Obesity and breast cancer: status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–53. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 39.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 40.de Salles BF, Simao R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Med. 2010;31:441–50. doi: 10.1055/s-0030-1251994. [DOI] [PubMed] [Google Scholar]
- 41.Diaz BB, Gonzalez DA, Gannar F, Perez MCR, de Leon AC. Myokines, physical activity, insulin resistance and autoimmune diseases. Immunol Lett. 2018;203:1–5. doi: 10.1016/j.imlet.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136:E197–202. doi: 10.1002/ijc.29142. [DOI] [PubMed] [Google Scholar]
- 43.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38:401–23. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 44.Budde H, Machado S, Ribeiro P, Wegner M. The cortisol response to exercise in young adults. Front Behav Neurosci. 2015;9:13. doi: 10.3389/fnbeh.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, Pedersen BK, Hojman P. Exercise-induced Catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 2017;77:4894–904. doi: 10.1158/0008-5472.CAN-16-3125. [DOI] [PubMed] [Google Scholar]
- 46.Perez Pinero C, Bruzzone A, Sarappa MG, Castillo LF, Luthy IA. Involvement of alpha2- and beta2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166:721–36. doi: 10.1111/j.1476-5381.2011.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J, Shin Y, Kim TH, Kim DH, Lee A. Plasma metabolites as possible biomarkers for diagnosis of breast cancer. PLoS One. 2019;14:e0225129. doi: 10.1371/journal.pone.0225129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.His M, Viallon V, Dossus L, Gicquiau A, Achaintre D, Scalbert A, Ferrari P, Romieu I, Onland-Moret NC, Weiderpass E, Dahm CC, Overvad K, Olsen A, TJonneland A, Fournier A, Rothwell JA, Severi G, Kuhn T, Fortner RT, Boeing H, Trichopoulou A, Karakatsani A, Martimianaki G, Masala G, Sieri S, Tumino R, Vineis P, Panico S, Gils CH, Nost TH, Sandanger TM, Skeie G, Quiros R, Agudo A, Sanchez MJ, Amiano P, Huerta JM, Ardanaz E, Schmidt JA, Travis RC Riboli E, Tsilidis KK, Christakoudi S, Gunter MJ, Rinaldi S. Prospective analysis of circulating metabolites and breast cancer in EPIC. BMC Med. 2019;17:178. doi: 10.1186/s12916-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang D. Oxidative stress, DNA damage, and breast cancer. AACN Clin Issues. 2002;13:540–9. doi: 10.1097/00044067-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010;42:1448–53. doi: 10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polakovicova M, Musil P, Laczo E, Hamar D, Kyselovic J. Circulating microRNAs as potential biomarkers of exercise response. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva GJJ, Bye A, El Azzouzi H, Wisloff U. MicroRNAs as important regulators of exercise adaptation. Prog Cardiovasc Dis. 2017;60:130–51. doi: 10.1016/j.pcad.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Alizadeh S, Isanejad A, Sadighi S, Khalighfard S, Alizadeh AM. Effect of a high-intensity interval training on serum microRNA levels in women with breast cancer undergoing hormone therapy. A single-blind randomized trial. Ann Phys Rehabil Med. 2019;62:329–35. doi: 10.1016/j.rehab.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, Febbraio MA. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27:237–51. doi: 10.1016/j.cmet.2017.12.001. e234. [DOI] [PubMed] [Google Scholar]
- 55.Fruhbeis C, Helmig S, Tug S, Simon P, Kramer-Albers EM. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles. 2015;4:28239. doi: 10.3402/jev.v4.28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trovato E, Di Felice V, Barone R. Extracellular vesicles: delivery vehicles of Myokines. Front Physiol. 2019;10:522. doi: 10.3389/fphys.2019.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Han C, Wang J, Zhou J, Liang C, Ranganna K, Song YH. Exosomes mediate the beneficial effects of exercise. Adv Exp Med Biol. 2017;1000:333–53. doi: 10.1007/978-981-10-4304-8_18. [DOI] [PubMed] [Google Scholar]
- 58.Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–81. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehi J, Pedersen BK, Straten PT, Hojman P. Voluntary running suppresses tumor growth through Epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–62. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–20. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–88. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 62.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–57. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 63.Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, Loyttyniemi E, Salminen S, Nuutila P, Kalliokoski KK, Hannukainen JC. Exercise training modulates gut microbiota profile and improves endotoxemia. Med Sci Sports Exerc. 2020;52:94–104. doi: 10.1249/MSS.0000000000002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61:895–901. doi: 10.1002/iub.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611–35. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, Gruenigen E, Schwartz AL. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 67.Kushi LH, Doyle C, McCullough M, Rock CL, DemarkWahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 68.Slade SC, Dionne CE, Underwood M, Buchbinder R, Beck B, Bennell K, Brosseau L, Costa L, Cramp F, Cup E, Feehan L, Ferreira M, Forbes S, Glasziou P, Habets B, Harris S, HaySmith J, Hillier S, Hinman R, Holland A, Hondras M, Kelly G, Kent P, Lauret GJ, Long A, Maher C, Morso L, Osteras N, Peterson T, Ouinlivan R, Rees K Regnaux JP, Rietberg M, Saunders D, Skoetz N, Sogaard K, Takken T, Tulder M, Voet N, Ward L, White C. Consensus on Exercise Reporting Template (CERT): modified Delphi study. Phys Ther. 2016;96:1514–24. doi: 10.2522/ptj.20150668. [DOI] [PubMed] [Google Scholar]
- 69.Koelwyn GJ, Jones LW. Exercise as a candidate antitumor strategy: a window into the future. Clin Cancer Res. 2019;25:5179–81. doi: 10.1158/1078-0432.CCR-19-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]