Abstract
Introduction
The incidence, characteristics, and prognosis of pulmonary embolism (PE) in Coronavirus disease 2019 (COVID-19) have been poorly investigated.
We aimed to investigate the prevalence and the correlates with the occurrence of PE as well as the association between PE and the risk of mortality in COVID-19.
Methods
Retrospective multicenter study on consecutive COVID-19 patients hospitalized at 7 Italian Hospitals. At admission, all patients underwent medical history, laboratory and echocardiographic evaluation.
Results
The study population consisted of 224 patients (mean age 69 ± 14, male sex 62%); PE was diagnosed in 32 cases (14%). Patients with PE were hospitalized after a longer time since symptoms onset (7 IQR 3–11 days, 3 IQR 1–6 days; p = 0.001) and showed higher D-dimers level (1819 IQR 568–5017 ng/ml vs 555 IQR 13–1530 ng/ml; p < 0.001) and higher prevalence of myocardial injury (47% vs 28%, p = 0.033). At multivariable analysis, tricuspid annular plane systolic excursion (TAPSE; HR = 0.84; 95% CI 0.66–0.98; p = 0.046) and systolic pulmonary arterial pressure (sPAP; HR = 1.12; 95% CI 1.03–1.23; p = 0.008) resulted the only parameters independently associated with PE occurrence. Mortality rates (50% vs 27%; p = 0.010) and cardiogenic shock (37% vs 14%; p = 0.001) were significantly higher in PE as compared with non-PE patients. At multivariate analysis PE was significant associated with mortality.
Conclusion
PE is relatively common complication in COVID-19 and is associated with increased mortality risk. TAPSE and sPAP resulted the only parameters independently associated with PE occurrence in COVID-19 patients.
Keywords: Pulmonary embolism, COVID-19, Echocardiography
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel human coronavirus recently recognized as the cause of the coronavirus disease 2019 (COVID-19). The outbreak sparked in Wuhan, capital city of Hubei province in China, and spread rapidly to other countries, reaching devastating pandemic proportion. Although knowledge on the pathophysiology and on the clinical features of COVID-19 is growing fast [1], the patient management remains largely empirical or based on observations from retrospective studies, small series, and single case reports.
An increasing numbers of studies have showed abnormal serum coagulation parameters in hospitalized patients with severe forms of COVID-19 [2]; moreover, a single-center study conducted by using computed tomography (CT) scan demonstrated the presence of pulmonary thrombi in patients with SARS-CoV-2 related pneumonia [3]. Elevated D-dimer levels were strongly associated with in-hospital mortality [4], and non survivors among infected patients met clinical criteria for disseminated intravascular coagulation (DIC) [2]. In addition, prolonged immobilization in critically ill patients promotes venous stasis and increases the risk of thromboembolic events [5]. Of note, anticoagulant treatment has been associated with lower risk of mortality in patients with severe COVID-19 [6].
The prevalence of pulmonary embolism (PE) in COVID-19 is still poorly investigated. Moreover, the clinical characteristics, predisposing factors and predictors of outcome are largely unknown in this clinical scenario.
2. Methods
2.1. Study design
This is a multi-center observational study including consecutive patients with COVID-19 enrolled at 7 Italian Hospitals (Bergamo, Naples, Sassari and Salerno provinces) contributing to COVID-19 Italian Network (Cov-IT Network) from February 28th to April 20th, 2020. COVID-19 diagnosis was initially based on the World Health Organization criteria and all cases were later confirmed by realtime reverse transcriptase–polymerase chain reaction analysis of throat swab specimens [7].
All patients included in this study were evaluated by the hospital cardiology service and underwent transthoracic echocardiography (TTE) during hospitalization.
This study was conducted according to the Declaration of Helsinki and approved by the institutional ethics committees. The requirement for informed consent from individual patients was waived due to the observational retrospective design of this study.
2.2. Transthoracic echocardiography
TTE was performed in accordance with the current guidelines [8]. Left ventricular systolic function was assessed by determining left ventricular ejection fraction (LVEF) through biplane analysis using the modified Simpson's rule. As a parameter of global right ventricular (RV) function, tricuspid annular plane systolic excursion (TAPSE), which reflects the base to apex shortening of the RV in systole, was assessed. RV dysfunction was defined in accordance with the current guidelines [8]. Systolic pulmonary artery pressure (sPAP) was derived from the tricuspid regurgitant jet velocity using systolic trans-tricuspid pressure gradient calculated by the modified Bernoulli equation and the addition of estimated right atrial pressure according to inferior vena cava dimension and inspiratory distensibility [8]. Pulmonary hypertension based on echocardiographic findings is defined according to European Society of Cardiology (ESC) guidelines [9].
2.3. Measures and objectives of the study
Data on patient characteristics and clinical course were retrospectively collected and recorded on an electronic datasheet. In all patients, demographic (age, gender, height, and weight), clinical (comorbidities, pharmacological therapy before and during hospitalization), laboratory (D-dimer, NT-pro-BNP, and high-sensitivity troponin) and echocardiographic data as well as information on patient clinical course (admission in intensive care unit and respiratory support measures) and in-hospital complications [ARDS (acute respiratory distress syndrome), acute cardiac injury, myocardial infarction, acute heart failure] were collected. Chronic kidney disease was defined with eGFR <60 mL/min/1.73 m2, calculated with CKD-EPI. ARDS diagnosis was defined according to the Berlin definition [10]. Acute cardiac injury was defined as elevated cardiac troponin levels with at least one value above the 99th percentile upper reference limit [11]. Cardiogenic shock was defined according to the last position statement from the Heart Failure Association of the ESC [12]. PE was diagnosed according to the last edition of the recommendations by the ESC [13] and all diagnoses were confirmed with computed tomography pulmonary angiography (CTPA). The risk profile (high, intermediate-high, low-intermediate, and low risk) was assessed according to the following clinical and imaging parameters: haemodynamic instability, RV dysfunction, clinical parameters of severity, and elevated cardiac troponin levels [13]. Early PE diagnosis was arbitrary defined when diagnosis was confirmed within 24 h from admission. PE related to major vessel was defined when main pulmonary artery, lobar artery or segmental artery was involved, while minor vessel referred to sub segmental involvement. Late hospitalization was defined after 7 days from symptoms onset, according to ROC analysis.
The primary objective of this study was to describe the prevalence of PE in this multi-center cohort and to identify the clinical, laboratory and echocardiographic parameters correlated to this condition during hospitalization. Secondary objective was to assess the association between PE and the risk of in-hospital mortality.
The number of patients who had died, had been discharged, or were still hospitalized as of May 8th, 2020, date of the analysis, were recorded. The length of hospitalization was also determined.
2.4. Statistical analysis
Distribution of continuous data was assessed with the Kolmogorov–Smirnov test. Normally distributed variables were expressed as mean ± standard deviation, whereas non-normal distributed ones as median and interquartile range. Categorical variables were reported as numbers and percentages. Continuous normally-distributed variables were compared by using the Student t-test; differences between non-normally distributed variables were tested with the Mann-Whitney U test. Categorical variables were compared with chi-squared test, or Fisher exact test, when appropriate. Receiver operating characteristic (ROC) curve analysis was performed to identify the best cutoff values of the D-dimer and high-sensitivity troponin of PE. Optimal threshold value was determined by using the Younden's index and the area under the curve (AUC) was calculated as a measure of their diagnostic accuracy. Survival curves were generated by using the Kaplan–Meier method, and differences among groups were investigated with the Log-Rank test. Univariable and multivariable logistic regression analyses were performed to evaluate the individual and independent association of clinical and echocardiographic variables with the occurrence of PE, and presented as odds ratio (OR) with by their 95% confidence intervals (CI). Multicollinearity was assessed using collinearity diagnostics. The variance inflation factors showed no significant collinearity (<2.5) among the covariates. We used a parsimonious model including variables with p < 0.10 by the univariate test as a candidate for the multivariate analysis.
The risk of in-hospital death in patients with vs. those without PE was calculated using the Cox proportional hazard regression model and presented as unadjusted and adjusted hazard ratios (HR) with 95% confidence intervals. To account for potential confounders related to the severity of the disease, we performed a multivariable covariates adjustment for age, LVEF, ARDS, and cardiac injury. Model goodness of fit was assessed with Hosmer-Lemeshow test. A p-value <0.05 was considered significant. All tests were two-sided. Analyses were performed with SPSS statistical package, Version 21 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Clinical characteristics of the study population
During the study period, overall 1393 patients for fever and dyspnea were admitted at investigating centers. Of them, 224 consecutive patients (16%) with confirmed diagnosed of COVID-19, who underwent TTE during cardiology consultation were included in the final analysis. The main baseline characteristics of the study population are summarized in Table 1 . The population mean age was 69 ± 14, and male sex rate was 62%. PE was diagnosed in 32 cases (14%). No difference in terms of clinical presentation was reported among groups. Although not statistically significant, PE patients showed lower prevalence of AF (12% vs 22%; p = 0.220) and of anticoagulation therapy before hospital admission (12% vs 20%; p = 0.3280). Use of heparin was reported in 81% of the overall population; no statistical difference in the use of anticoagulant and other pharmacological therapies was showed among groups.
Table 1.
Baseline characteristics of the study population.
| Variables | Total (n = 224) |
Pulmonary embolism (n = 32) |
No pulmonary embolism (n = 192) |
p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 69 ± 14 | 67 ± 15 | 69 ± 13 | 0.469 |
| Male | 127 (62) | 20 (62) | 120 (62) | 0.998 |
| Symptoms | ||||
| Fever | 153 (68) | 25 (78) | 128 (67) | 0.197 |
| Dyspnoea | 158 (70) | 24 (75) | 134 (70) | 0.550 |
| Chest discomfort | 69 (31) | 10 (31) | 59 (31) | 0.958 |
| Cough | 85 (38) | 10 (31) | 75 (39) | 0.399 |
| Syncope | 21 (9) | 4 (12) | 17 (9) | 0.512 |
| Symptoms onset to hospitalization, days Median (IQR) |
6 (2−10) |
7 (3−11) |
3 (1–6) |
0.001 |
| Late presentation | 99 (44) | 22 (69) | 77 (40) | 0.003 |
| Past diagnosis | ||||
| Hypertension | 137 (61) | 19 (59) | 118 (61) | 0.823 |
| Diabetes | 63 (28) | 11 (34) | 52 (27) | 0.524 |
| Dyslipidemia | 60 (30) | 7 (23) | 53 (31) | 0.318 |
| CAD | 35 (16) | 5 (16) | 30 (16) | 0.998 |
| Heart failure | 22 (10) | 5 (16) | 17 (9) | 0.233 |
| History of AF | 46 (21) | 4 (12) | 42 (22) | 0.220 |
| COPD | 45 (20) | 6 (19) | 39 (20) | 0.838 |
| Stroke or TIA | 17 (8) | 1 (3) | 16 (8) | 0.303 |
| CKD | 45 (20) | 6 (19) | 39 (20) | 0.838 |
| Cancer | 27 (12) | 7 (22) | 20 (10) | 0.065 |
| Serum biomarkers | ||||
| Troponin hs, n·99th percentile; peak Median (IQR) |
2.2 (0.2–17.5) |
28 (8–180) |
2 (0.2–9) |
0.025 |
| Acute cardiac injury | 69 (31) | 15 (47) | 54 (28) | 0.033 |
| D-dimer, peak; ng/ml Median (IQR) |
625 (90–2050) |
1819 (568–5017) |
555 (13–1530) |
<0.001 |
| Pro-BNP, peak; pg/ml | 4616 ± 7800 | 4076 ± 3402 | 4765 ± 3654 | 0.782 |
| Cardiovascular drug at hospitalization | ||||
| ACE-I or ARB | 98 (44) | 15 (47) | 83 (43) | 0.700 |
| β-Blocker | 59 (26) | 5 (16) | 54 (28) | 0.137 |
| Ca++ channel blocker | 35 (16) | 5 (16) | 30 (16) | 0.999 |
| Antiplatet agent | 75 (33) | 11 (34) | 64 (33) | 0.908 |
| DAPT | 12 (5) | 2 (6) | 10 (5) | 0.809 |
| Anticoagulant | 42 (19) | 4 (12) | 38 (20) | 0.328 |
| Statin | 70 (31) | 7 (22) | 63 (33) | 0.217 |
| Experimental COVID-19 therapies | ||||
| Antiviral | 119 (53) | 16 (50) | 103 (54) | 0.702 |
| Hydroxychloroquine | 178 (79) | 26 (81) | 152 (79) | 0.787 |
| Antibiotics | 165 (74) | 25 (78) | 140 (73) | 0.536 |
| Glucocorticoids | 100 (45) | 16 (50) | 84 (44) | 0.510 |
| UFH or LMWH | 181 (81) | 25 (78) | 156 (82) | 0.591 |
| Echocardiography | ||||
| LVEF (%) | 53 ± 9 | 53 ± 9 | 52 ± 9 | 0.499 |
| TAPSE, mm Median (IQR) |
21 (18–23) |
18 (14–20) |
21 (19–23) |
<0.001 |
| PAPS, mmHg Median (IQR) |
33 (30–40) |
40 (31–50) |
32 (29–40) |
<0.001 |
| Admission | ||||
| ICU | 73 (33) | 11 (34) | 62 (32) | 0.816 |
| Ward | 151 (67) | 21 (66) | 130 (68) | 0.816 |
Categorical data are presented as numbers (%). Continuous data are presented as mean ± SD or median (IQR), as appropriate. CAD, coronary artery disease; AF atrial fibrillation, COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; CKD, chronic kidney disease; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DAPT, dual antiplatelet therapy; UFH, unfractionated heparin; LMWH, low molecular weight heparin; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; sPAP, systolic pulmonary artery pressure; ICU, Intensive care unit.
Patients with PE were hospitalized after a longer time since symptom onset (7 IQR 11–3 days vs 3 IQR 1–6 days in PE and non-PE patients, respectively; p = 0.001) and, therefore, in a later infectious phase (30% vs 13%; p = 0.018). Time between symptom onset and hospitalization was able to discriminate PE (AUC 0.71, CI 95% 0.61–0.78, p < 0.001) with a best cutoff of 7 days (sensitivity = 68%; specificity = 50%).
3.2. Serum biomarker and pulmonary embolism
There was no difference in terms of NT-pro-BNP values among groups (4076 ± 3402 pg/ml vs 4765 ± 3654 pg/ml; p = 0.782). Conversely, serum levels of D-dimer (1819 IQR 568–5017 ng/ml vs 555 IQR 13–1530 ng/ml; p < 0.001) and of high-sensitivity troponin (400 IQR 91–2514 n·99th percentile vs 22 IQR 2–81 n·99th percentile; p = 0.001) were significantly higher in PE as compared to non-PE group. D-dimer level showed a good discriminative ability for PE in hospitalized patients with COVID-19 (AUC 0.73, CI 95% 0.61–0.85, p = 0.002), with a best cutoff of 1743 ng/ml (sensitivity = 63%; specificity = 76%).
3.3. Echocardiography and pulmonary embolism
There was no difference in terms of LVEF between groups (Table 1). PE patients showed lower values of TAPSE (18 IQR 14–20 mm vs 21 IQR 19–23 mm; p < 0.001) and higher sPAP values (40 IQR 31–50 mmHg vs 32 IQR 29–40 mmHg; p < 0.001) compared to those without PE.
3.4. Pulmonary embolism characteristics
Most PE (81%) were diagnosed within the first 24 h from the admission, as reported in Table 2 . Furthermore, in study population PE showed a high pulmonary obstruction grade (bilateral PE in 87% and a major vessel involvement in 91%). Accordingly, among PE patients, 12 (37%) developed a high-risk PE, 3 (9%) intermediate-high risk PE, 7 (22%) intermediate-low risk PE and only 10 (31%) low risk PE.
Table 2.
Pulmonary embolism characteristics.
| PE (n = 32/224) |
|
|---|---|
| Early diagnosis (≤24 h from admission), n (%) | 26 (81) |
| CT scan | |
| Major vessel, n (%) | 29 (91) |
| Minor vessel, n (%) | 3 (9) |
| Bilateral, n (%) | 28 (87) |
| ESC mortality risk | |
| High, n (%) | 12 (37) |
| Intermediate-high, n (%) | 3 (9) |
| Intermediate-low, n (%) | 7 (22) |
| Low, n (%) | 10 (31) |
PE, pulmonary embolism; ESC, European Society of Cardiology.
3.5. Predictors of pulmonary embolism
The results of the logistic regression for the occurrence of PE during hospitalization are summarized in Table 3 . At univariable analyses, D-dimer, acute cardiac injury, time between hospitalization and symptom onset, TAPSE and sPAP were significantly associated with PE. At multiple logistic regression only TAPSE (HR = 0.84; 95% CI 0.66–0.98; p = 0.046) and sPAP (HR = 1.12; 95% CI 1.03–1.23; p = 0.008) resulted independently associated with PE. The goodness of fit of the model was confirmed by the Hosmer-Lemeshow test (p = 0.870).
Table 3.
Univariable and multivariable regression analyses for the occurrence of pulmonary embolism.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR (95% CI) |
p-Value | OR (95% CI) |
p-Value | |
| Pulmonary embolism | ||||
| TAPSE, mm | 0.78 (0.73–0.88) |
<0.001 | 0.84 (0.66–0.98) |
0.046 |
| sPAP, mmHg | 1.08 (1.04–1.12) |
<0.001 | 1.12 (1.03–1.23) |
0.008 |
| Time between hospitalization and symptoms onset, days | 1.07 (1.03–1.11) |
0.001 | – | – |
| D-dimer, valuea | 1.02 (1.006–1.045) |
0.010 | – | – |
| Acute cardiac injury | 2.25 (1.05–4.83) |
0.037 | – | – |
CI, confidence interval; OR, odds ratio; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion.
Odds ratio reflects risk with increases of 0.01.
3.6. Pulmonary embolism and outcome
Follow up rate was >99%, with only 1 patient lost at follow-up. The median follow-up length was 19 days (IQR 5–27). As of May 8, 2020, date of the analysis, 24 patients (10%) were still hospitalized (4% of them in intensive care unit). The proportions of adverse events during hospitalization are summarized in Table 4 .
Table 4.
In-hospital adverse events in the enrolled patients.
| Total (n = 224) |
Pulmonary embolism (n = 32) |
No pulmonary embolism (n = 192) |
p-Value | |
|---|---|---|---|---|
| Mortality | 68 (30) | 16 (50) | 52 (27) | 0.010 |
| Cardiogenic shock | 39 (17) | 12 (37) | 27 (14) | 0.001 |
| ARDS | 107 (48) | 20 (62) | 87 (45) | 0.072 |
| IMV | 68 (30) | 13 (41) | 55 (29) | 0.172 |
| NIV | 100 (45) | 16 (50) | 84 (44) | 0.510 |
ARDS, acute respiratory distress syndrome; IMV, invasive mechanical ventilation; NIV, non-invasive ventilation.
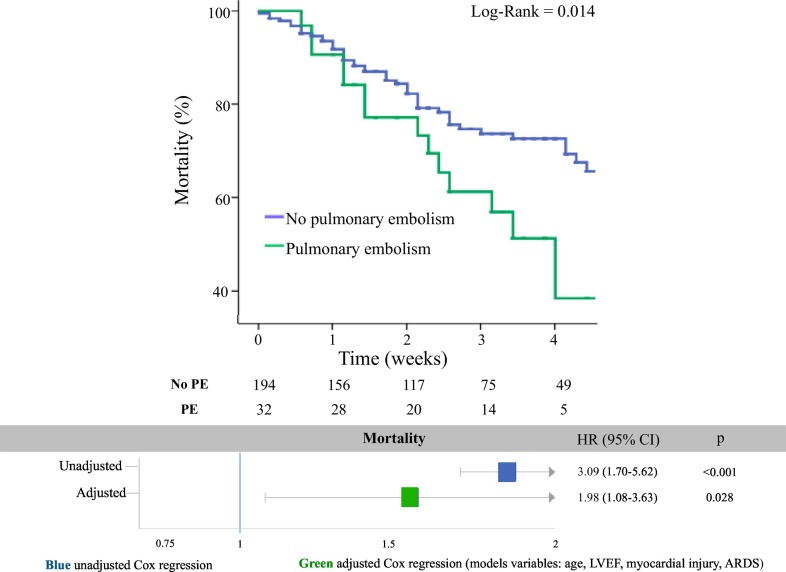
The percentages of death for any cause (50% vs 27%; p = 0.010), and cardiogenic shock (37% vs 14%; p = 0.001) were significantly higher in PE patients than in non-PE patients (Table 3). Kaplan-Meier curves showed a significantly lower survival in PE than in non-PE patients (Log-Rank = 0.014; Fig. 1 ). The risk of mortality was significantly higher in PE as compared with non-PE group at both unadjusted (HR 3.09, 95% CI 1.70–5.71; p < 0.001) and adjusted regression model (HR 1.97, 95% CI 1.08–3.63; p = 0.028). At the multivariable regression analysis, the PE emerged as a strong predictor for mortality (Fig. 1). The goodness of fit of the model was confirmed by the Hosmer-Lemeshow test (p = 0.840).
Fig. 1.
Kaplan-Meier curves for survival free from all-cause death in PE (green line) vs. Non-PE (blue line) group (panel A). Unadjusted and adjusted risk of mortality in PE- vs. Non-PE group (panel B). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
The main findings of the present study can be summarized as follows:
-
1.
PE is a relatively common complication in hospitalized patients with COVID-19;
-
2.
TAPSE and sPAP, as echocardiographic parameters of RV dysfunction and of pulmonary hypertension, are independently associated with the occurrence of PE;
-
3.
Patients with PE showed a higher risk of death independently from other parameters of disease severity in COVID-19.
In our cohort of hospitalized patients with COVID-19, pulmonary embolism was a common complication (about 14% of cases), with a prevalence largely higher than reported in the last edition of PE ESC guidelines [13]. COVID-19 may promote thromboembolic events through several potential mechanisms. First, a severe inflammatory response and disseminated intravascular coagulation may occur in COVID-19 patients predisposing to micro- and macrovascular pulmonary thrombosis [14]; second, virus-induced local inflammatory reactions [15] may affect endothelial cell function leading to vessel wall damage; third, adverse drug-drug interaction between anticoagulant agents and experimental COVID-19 therapies may oblige clinicians to switch oral anticoagulant drugs to heparin/LMWH with associated out of therapeutic range periods of time and increase in thromboembolic events [5]; fourth, limited mobility of bedridden patients, particularly in peculiar setting such as intensive care unit; fifth, difficulties in patients care related to the risk of infection, including the mobilization of patients intubated or with critical ill disease; sixth, the misperception that antithrombotic agents might confer increased risk for contracting COVID-19, which may have led to untoward interruption of anticoagulation in some patients [14].
In the present analysis, we tested several variables potentially associated with PE, including well-known risk factors such as age or malignancy, and we found that only TAPSE and sPAP were independently associated with the occurrence of this condition. Our study, by including patients systematically assessed by cardiologists, is the first real-world pandemic study reporting echocardiographic data in all COVID-19 patient enrolled. Echocardiography has been poorly performed in previous reports on COVID-19, which focused on the importance of clinical, computed tomography or laboratory values for PE diagnosis and prognostic stratification [16]. Of note, TTE acquisition is complex to be performed in patients infected by SARS-CoV-2 and it needs specific precautions [17]. However, TTE is the first-line imaging modality for early detection of PE, and may be diriment in patients with a well-founded suspicion by clinical and laboratory data [13].
D-dimer, cardiac injury, late hospitalization after symptoms onset, all showed a significant association with PE at univariable analyses. However, they did not confirm a significant association after adjustment for TAPSE and sPAP at the multivariable model. These results emphasize the central role of TTE for PE diagnosis, also in the COVID-19 clinical setting.
Consistently with previous data, we found a D-dimer cutoff of 1743 ng/ml, which is three- to four-fold higher than the normal threshold value (500 ng/ml) routinely adopted for PE diagnosis in the general population [13,18]. D-dimer is usually abnormal in COVID-19, probably due to the inflammatory response induced by SARS-CoV-2 infection and to the hypoxia-inducible transcription factor-dependent signaling pathway [19]. In this context, the discriminative ability of D-dimer is substantially reduced as compared to the general population, and the evidence of high D-dimer serum level by itself cannot be considered for diagnostic purpose. The multivariable analysis did not confirm the association between D-dimer and PE, pointing out the intrinsic limitation of this parameter for PE diagnosis in the context of COVID-19. We also found an association between cardiac injury and PE. RV pressure overload due to acute PE may precipitate RV myocardium ischemia and serum release of cardiac biomarkers of necrosis. Indeed, high-sensitivity troponin is a prognostic risk parameter in PE reflecting the severity of RV dysfunction and of hemodynamic derangement during the acute phase. However, cardiac injury was not significantly associated to PE at multivariable analysis, probably due to the poor specificity of high-sensitivity troponin, which it may be increased in a large proportion of patients with COVID-19 (ranging from 18.8% to 27.8%) as a consequence of multiple potential mechanisms [20]. To our knowledge, this is the first study showing an association between late hospitalization after symptom onset and PE in COVID-19. Although delay to hospitalization did not emerged as an independent correlate with PE, this finding can be a clinical parameter of interest to suspect PE in patients with COVID-19. We hypothesize that the relationship between late admission and PE can be explained by the longer bed rest, later anticoagulant prophylaxis administration as well as by the pathophysiological mechanism involved in the later phases of COVID-19 clinical course, characterized by the interplay between systemic hyper-inflammation state [21], immuno-mediated phenomenon and clotting system activation [22,23].
Accordingly, most PE diagnosis were confirmed within 24 h after admission, suggesting that VTE was not related to hospitalization; probably PE in COVID-19 is a progressive pathological process, that begins in the early infection stage, when the patient is still at home, and becomes clinically manifest only in late infectious phase becoming an important cause of hospitalization.
Previous reports showed that COVID-19 patients with established cardiovascular disease and/or cardiovascular risk factors have worse prognosis and they are more likely to be admitted in intensive care unit and to need ventilatory support [24]. In the present study, we demonstrated that patients complicated by PE experienced a more severe prognosis, independently from the coexistence of other clinical conditions of severity such as older age, left ventricular systolic dysfunction, ARDS, and cardiac injury. By obstructing pulmonary arterial flow with thrombi, PE interferes with both circulation and gas exchange, with dramatic consequence in COVID-19, which is characterized by a diffuse pulmonary infiltrates and fibrosis. Moreover, in about two-third of our cohort of COVID-19 patients complicated by PE, we found signs of cardiovascular involvement represented by elevated cardiac troponin levels and/or RV dysfunction (accounting for the high proportion of patients with high or intermediate risk PE).
These results emphasize the importance of early recognition of PE, and the need for expanding TTE indications in hospitalized patients with COVID-19.
These findings may have relevant implications for clinicians: first, pulmonary thromboembolism is a highly probable clinical entity in severe COVID-19, and clinicians should consider all COVID-19 patients at risk of venous thromboembolism, especially in the presence of late hospitalization after symptom onset, high risk serum biomarker profile and echocardiographic evidence of RV dysfunction and pulmonary hypertension. The early recognition of PE risk factors can help physicians to start prompt, full-dose, anticoagulation therapy. Second, due to the high risk of mortality, COVID-19 patients complicated by PE should be closely monitored during hospitalization and, whenever possible, admitted in intensive/subintensive care unit.
Our study must be evaluated in the light of some limitations. First, this is an observational retrospective study. Moreover, we included only patients evaluated by cardiologists and for whom TTE data were available, which may have influenced the generalizability of our results. Larger prospective studies on unselected patients are needed to confirm our preliminary findings in terms of prevalence, correlates, and clinical outcome of COVID-19 patients complicated by PE. Second, because of the limited sample size of PE group, our study was not properly powered for hard clinical outcome measures, who should be judged just as explorative. Third, the registry did not collect data on type (UFH, LMWH) and dose of anticoagulation therapy, however all patients with confirmed PE received full anticoagulation dose. Lastly, we were not able to analyze the influence of the different experimental COVID-19 therapies on clinical outcome.
5. Conclusions
Pulmonary embolism is a relatively common complication during COVID-19 pandemic and it is associated with poor prognoses and increased risk of mortality during hospitalization. Several clinical and laboratory parameters are associated with PE, but only echocardiographic parameters of RV dysfunction and of pulmonary hypertension, such as TAPSE and sPAP, are independently associated with PE occurrence.
Declaration of competing interest
Prof. Parodi reported receiving consulting or lecture fees from AstraZeneca, Bayer, Chiesi, Daiichi Sankyo/Eli Lilly, and Merck Sharp Dohme. The remaining Authors have no conflict of interest to disclose.
References
- 1.Russo V., Di Maio M., Attena E., Silverio A., Scudiero F., Celentani D., Lodigiani C., Di Micco P. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacol. Res. 29 May 2020 doi: 10.1016/j.phrs.2020.104965. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caruso D., Zerunian M., Polici M., Pucciarelli F., Polidori T., Rucci C., Guido G., Bracci B., de Dominicis C., Laghi A. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;3:201237. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canonico M.E., Siciliano R., Scudiero F., Sanna G.D., Parodi G. The tug-of-war between coagulopathy and anticoagulant agents in patients with COVID-19. Eur. Heart J. Cardiovasc. Pharmacother. 2020 May 8 doi: 10.1093/ehjcvp/pvaa048. pii: pvaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paranjpe I., Fuster V., Lala V., Russak A., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. (S0735-1097(20)35218-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. Jan 28, 2020. https://www.who.int/docs/defaultsource/coronaviruse/clinical-management-of-novel-cov.pdf
- 8.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., Lancellotti P., Muraru D., Picard M.H., Rietzschel E.R., Rudski L., Spencer K.T., Tsang W., Voigt J.U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J CVI. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 9.Galie N., Humbert M., Vachieryc J.L., Gibbs S., Lang I., Torbicki A., Simonneau G., Peacock A., Vonk Noordegraaf A., Beghetti M., Ghofrani A., Gomez Sanchez M.A., Hansmann G., Klepetko W., Lancellotti P., Matucci M., McDonagh T., Pierard L.A., Trindade P.T., Zompatori M. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2015 doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 10.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Mickley H., Crea F., Van de Werf F., Bucciarelli-Ducci C., Katus H.A., Pinto F.J., Antman E.M., Hamm C.W., De Caterina R., Januzzi J.L., Jr., Apple F.S., Alonso Garcia M.A., Underwood S.R. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 12.Chioncel O., Parissis J., Mebazaa A., Thiele H., Desch S., Bauersachs J., Harjola V.P., Antohi E.L., Arrigo M., Gal T.B., Celutkiene J., Collins S.P., DeBacker D., Iliescu V.A., Jankowska E., Jaarsma T., Keramida K., Lainscak M., Lund L., Lyon A.R. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur. J. Heart Fail. 2020 May 29 doi: 10.1002/ejhf.1922. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., Huisman M.V., Humbert M., Jennings C.S., Jiménez D., Kucher N., Lang I.M., Lankeit M., Lorusso R., Mazzolai L., Meneveau N., Ní Áinle F., Prandoni P., Pruszczyk P., Righini M. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur. Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 14.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. JACC. 2020;S0735-1097(20) doi: 10.1016/j.jacc.2020.04.031. (35008-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scudiero F., Parodi G. Dual antiplatelet therapy in patients with acute coronary syndrome during COVID-19 pandemia: the right choice at right time. J. Cardiovasc. Med. (Hagerstown) 2020;21(8):535–537. doi: 10.2459/JCM.0000000000001028. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Olivé I., Sintes H., Radua J., Capa J.A., Rosella A. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir. Med. 2020 May;13:106023. doi: 10.1016/j.rmed.2020.106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skulstad H., Cosyns B., Popescu B.A., Galderisi M., Di Salvo G., Donal E., Donal E., Petersen S., Gimelli A., Haugaa K.H., Muraru D., Almeida A.G., Schulz-Menger J., Dweck M.R., Pontone G., Sade L.E., Gerber B., Maurovich-Horvat P., Bharucha T. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur. Heart J. Cardiovasc. Imaging. 2020;0:1–7. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Xi R., Hu J., Chen Q., Shen W., Zhang R., Yan X. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020 Apr 19 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta N., Zhao Y.Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jose RJ and Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. DOI: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed]
- 23.Di Micco P., Russo V., Carannante N., Imparato M., Rodolfi S., Cardillo G., Lodigiani C. Clotting factors in COVID-19: epidemiological association and prognostic values in different clinical presentations in an Italian cohort. J. Clin. Med. 2020 May 7;9(5) doi: 10.3390/jcm9051371. pii: E1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]