Abstract
Background and aims
New data has emerged regarding higher risk of coronavirus disease 2019 (COVID-19), and its severity and complications in patients with type 2 diabetes mellitus (T2DM). However, there is a dearth of evidence regarding type 1 diabetes mellitus (T1DM). This article explores the possibility of COVID 19 induced diabetes and highlights a potential bidirectional link between COVID 19 and T1DM.
Methods
A literature search was performed with Medline (PubMed), Scopus, and Google Scholar electronic databases till October 2020, using relevant keywords (COVID-19 induced diabetes; COVID-19 and type 1 diabetes; COVID-19 induced DKA; new-onset diabetes after SARS-CoV-2 infection) to extract relevant studies describing relationship between COVID-19 and T1DM.
Results
Past lessons and new data teach us that severe acute respiratory syndrome coronaviruses (SARS-CoV and SARS-CoV-2) can enter islet cells via angiotensin converting enzyme-2 (ACE-2) receptors and cause reversible β-cell damage and transient hyperglycemia. There have been postulations regarding the potential new-onset T1DM triggered by COVID-19. This article reviews the available evidence regarding the impact and interlink between COVID-19 and Τ1DM. We also explore the mechanisms behind the viral etiology of Τ1DM.
Conclusions
SARS-CoV-2 can trigger severe diabetic ketoacidosis at presentation in individuals with new-onset diabetes. However, at present, there is no hard evidence that SARS-CoV-2 induces T1DM on it’s own accord. Long term follow-up of children and adults presenting with new-onset diabetes during this pandemic is required to fully understand the type of diabetes induced by COVID-19.
Keywords: SARS-CoV-2, Type 1 diabetes, New-onset diabetes, Hyperglycemia, ACE2 receptors, β-cell damage
Highlights
-
•
Covid-19 related mortality is significantly higher not only in people with type 2 diabetes, but also in type 1 diabetes.
-
•
ACE2 is abundantly expressed in pancreatic islet cells which SARS CoV2 utilises as point of entry into the cells.
-
•
We review all the available evidence regarding among COVID-19 and new-onset diabetes and type 1 diabetes mellitus. .
1. Introduction
Since the onset of COVID 19 pandemic, a great deal of evidence has emerged regarding its relationship with T2DM. However, reports on effects of SARS-CoV-2 infection on people with T1DM have been more recent and relatively sparse. T1DM constitutes about 5% of all diagnosed cases of diabetes and its global incidence is increasing at an alarming rate of about 3% every year [1]. Pre-existing diabetes mellitus is purported to be one of the high-risk factors for developing COVID-19 and related complications [2]. Indeed, there have been reports of COVID-19 induced severe metabolic decompensation of pre-existing or new-onset diabetes such as diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS) [[3], [4], [5], [6], [7]]. More characteristically, SARS-CoV-2 has been suggested as a potential inducer of new-onset T1DM [8]. Coronavirus mediated islet cell damage does not seem to be a novel phenomenon, as evidenced by the experience from previous coronavirus (SARS and MERS) epidemics [9]. However, in this review, we explore the mechanisms of hyperglycemia particularly in relation to COVID 19 illness and also examine the Covid-19 related morbidity and mortality in people with T1DM.
2. Etiology of T1DM – the viral paradigm
T1DM is a genetic autoimmune condition where β-cells are destroyed by the auto-reactive CD4+ and CD8+ T cells. Although >50 candidate genes were identified, poor concordance of T1DM (<50%) in monozygotic twins suggests players beyond genetics. Regional differences in prevalence with incidence in migrants conforming to the incidence of the region of destination, and the North-South gradient with higher figures in northern latitudes indicate non-genetic environmental influences. Well established seasonality of new-onset T1DM led to exploration of viral etiology [10]. The relationship between viral infections and T1DM is complex. Mouse models have demonstrated that while certain viruses could be detrimental to the β-cells and initiate autoimmunity, others can be protective and have preventive effects. However, one needs to exercise caution when extrapolating these findings to human subjects [11].
2.1. Pathogenesis of viral induced β-cell damage: acute vs chronic
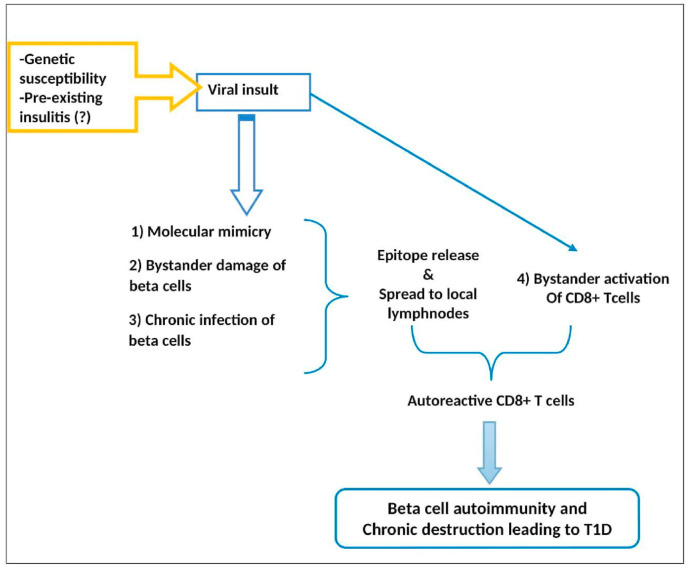
Conceptually, virus induced β-cell damage is due to either 1) direct lytic effects of viral replication and/or, 2) host inflammatory response mediated damage by autoreactive CD + T cells, leading to autoimmunity ( Fig. 1 ). While destruction of >90% of β-cells by direct viral mediated damage leads to non-autoimmune diabetes, limited lysis releases islet cell antigens, which in conjunction with enhanced immune response paves way for autoimmunity. Evidence for the earlier instance is most notable from the cases of fulminant T1DM, reported almost exclusively from Japan and predominantly in adults, preceded by minor upper respiratory or gastrointestinal infections, Mumps, HHV6, Coxsackie B3, B4, HSV, Hepatitis A, Influenza B and parainfluenza. Described as type 1B diabetes, fulminant T1DM is characterized by acute onset of hyperglycemic ketoacidosis, very short (1 week) duration of diabetes symptoms (polyuria, thirst, and body weight loss), absence of islet-related autoantibodies, extremely low C-peptide levels, elevated serum pancreatic enzyme levels, and a HbA1c less than 8.5% on the first visit [[12], [13], [14], [15], [16], [17]].
Fig. 1.
Immuno-pathogenesis of beta cell destruction and type 1 diabetes.
Nevertheless, it is the limited β-cell destruction with release of sequestered islet cell antigens and activation of autoreactive T-cells that result in long-term autoimmune damage and subsequent T1DM. In children with recently diagnosed diabetes, hyperexpression of major histocompatibility complex −1 (MHC-1) and interferon-α was observed within the islets that are otherwise completely devoid of these markers [18]. These markers characteristically increase antigen mediated activation of CD8+T Cells, and it is most likely that their enhanced production is viral mediated.
2.2. Mediators of chronic β-cell destruction
The pathological processes mediating chronic β-cell damage are varied. The foremost contender is molecular mimicry, where viral epitope shares a resemblance with host islet protein causing cross-reactivity and autoimmune T cell response against host tissue in susceptible individuals. However, studies aiming to demonstrate molecular mimicry are inconclusive. It is likely that molecular mimicry can accelerate the autoimmune process once it is already started, rather than initiate it on its own [18]. Other proposed mechanisms are bystander T-cell activation, the activation of a T cell independent of an antigen specific T-cell receptor stimulation, and bystander damage, where destruction of β-cells is accelerated by the proinflammatory cytokines released due to infection of adjacent pancreatic cells like alpha, exocrine, endothelial and neuronal cells [[19], [20], [21]]. In addition to the decreased insulin release due to β-cell loss, proinflammatory mediators can result in functional defects like defective glucose mediated insulin release and delay in the conversion of proinsulin to insulin.
Another crucial element is the seeming inability of β-cells to clear viral infections, when compared with alpha cells. Chronicity of β-cell infection was apparent from postmortem studies where expression of viral capsid protein VP1 was detected in the islets of >60% T1DM organ donors while only 8% of non-T1DM samples showed its presence [21]. Chronic β-cell infection results in persistent overexpression of MHC-1 leading to continuous presentation of beta cell epitopes to the immune system, facilitating autoimmunity.
2.3. Putative viruses causing β-cell damage
Though many viruses came to be associated with T1DΜ, namely, enteroviruses (especially Coxsackie B1, B4), mumps, rubella and CMV; so far the most robust evidence for viral induced T1DM is seen with enterovirus, an ssRNA virus of picornavirus family, when enteroviral RNA was detected in the blood of recently diagnosed patients with T1DM (Table 1 ). A systematic review and meta-analysis showed a significant association between enterovirus infection and T1DM-related autoimmunity (OR: 3.7, 95%CI: 2.1–6.8) and clinical T1DM (OR: 9.8, 95%CI: 5.5–17.4) [33]. The Epidemiological Determinants of Diabetes in Young (TEDDY) study that followed 8676 newborn babies with increased genetic risk for T1DM, conferred by a specific HLA genotype, over 15 years, observed that number of respiratory infections occurring in a 9-month period was associated with the subsequent risk of autoimmunity (p < 0.001). For each 1/year increase in infections, the hazard of islet autoimmunity increased by 5.6%. Autoantibodies were more commonly detected in patients with severe respiratory disease, and interestingly, coronaviruses were identified among the different pathogens involved [34]. In the latest update of the TEDDY study, persistent presence of enterovirus B species in a child’s stool appears to predict development of islet autoimmunity, especially antibodies against Insulin [35].
Table 1.
Recent evidence on role of virus in islet cell autoimmunity and T1D.
| Author & year (reference) | Type of study (case/control) | Test and Sample | Virus | Findings |
|---|---|---|---|---|
|
Schulte BM et al. 2010 [21] |
Case control (10/20) | RT PCR/plama, PBMC, throat, stool | Enterovirus (EV) | All controls are negative. 4/10 PBMC samples, only 1/10 stool samples positive for EV PCR. None of the throat samples are positive, which argues against acute infection, but probably delayed clearance of EV |
|
Laitinen OH et al. 2013 [22] |
Nested case control Study samples from the DIPP cohort (183/366) |
Antibodies | Coxsackie B1 (CB1) | CB1 is associated with increased risk of beta cell autoimmunity, strongest when infection occurred few months before Islet AA appeared (OR: 1.5, 95% CI: 1.0–2.2) CB3, CB6 appear to reduce risk of autoimmunity |
|
Oikarinen S et al. 2013 [23] |
Case control (249/249) | Antibodies | Coxsackie B1 (CB1) | CB1 antibodies are more frequently seen in those with T1D (OR: 1.7, 95% CI: 1.0–2.9) |
|
Stene LC et al. 2010 [24] |
Prospective study in 140 cases seroconverted for IAA from DAISY cohort | RT PCR Blood, rectal swab |
EV | Risk of progression from islet cell autoimmunity to clinical T1D is significantly higher following detection of EV RNA. |
|
Salminen KK et al. 2004 [25] |
Case control From DIPP cohort (12/53) |
Antibodies, RT PCR stool & serum | EV | 83% cases had at least one EV infection before developing Islet AA, while only 42% controls had EV by the same age (p = 0.006) |
|
Dahlquist GG et al. 2004 [26] |
Case control (542/542) | RT PCR of postnatal Day2–4 blood spot samples | EV | Early (fetal, neonatal) EV infection may play a role in T1D pathogenesis (OR: 1.98, 95% CI; 1.04–3.77). No difference seen with CMV, Parvo-B19 |
| Sadeharju K et al. 2003 [27] | Case control (19/84) From TRIGR cohort |
Antibodies and RT PCR | EV | AA-positive children had more enterovirus infections than AA-negative children before the appearance of AA (0·83 versus 0·29 infection per child, P = 0·01) |
| Hiemstra HS et al. 2001 [28] | Clonal CD4+ T cells reactive to GAD65 - from a prediabetic stiff-man syndrome patient. | Synthetic peptide libraries that bind to HLA-DR3, are screened | Cytomegalovirus (CMV) | GAR-65 specific T-cells cross-react with a peptide of hCMV major DNA binding protein, resulting in possible loss of T cell tolerance to GAD65. |
| Honeyman MC et al. 2010 [29] | Comparative | Rotavirus (RV) | Peptides in VP7, immunogenic protein of RV have significant similarity to T cell epitope peptides in IA2 and GAD65. Molecular mimicry with RV could promote autoimmunity to islet antigens. |
|
| Bian X et al. 2016 [30] | Case control Case/control; 42/42 |
Antibodies | Epstein-Barr virus (EBV) | Positive EBV antibody response is associated with significantly higher cases of T1D (OR: 6.6, 95% CI: 2.0–25.7) |
| Nilsson AL et al. 2015 [31] | Case control Case: control: 69/294 |
Antibodies | Parechovirus (PV) | Ljungan virus antibodies correlated with insulin AA, especially in young HLA-DQ8 subjects, suggesting a possible role in T1D. |
| Tapia G et al. 2011 [32] | Nested case control Case/control: 27/53 The MIDIA study cohort |
PCR Fecal samples |
Parechovirus (PV) | Weak association, if PV infection in 3 months prior to development of autoimmunity, warranting further investigation |
DIPP: Diabetes Prediction and Prevention; DAISY: Diabetes and Autoimmunity Study in the Young; TRIGR: Trial to Reduce IDDM in Genetically at Risk; MIDIA: Norwegian acronym for “Environmental Triggers of Type 1 Diabetes” study; AA: Autoantibodies; GAD65: Glutamic acid decarboxylase 65; IA2: Tyrosine phosphatase-like insulinoma Ag 2; PBMC: Peripheral blood mononuclear cells.
Nonetheless, there is substantial epidemiological data contradicting the viral origins of β-cell autoimmunity. Viral data from non-obese diabetic (NOD) mice has shown that coxsackie B3 (CB3) and lymphocytic choriomeningitic virus (LCMV) can offer protection from T1DM by promoting immune tolerance. Early exposure to infections was also deemed to educate the immune system leading to reduced incidence of autoimmune diseases like T1DM as seen in countries with low SES where the incidence of infections is high [33].
2.4. SARS-CoV-1 and diabetes – lessons from the past
Angiotensin converting enzyme (ACE) is the key enzyme in mediating the effects of renin angiotensin aldosterone system (RAAS) by converting angiotensin-I to II. The more recently identified ACE2, a novel homolog of ACE that degrades angiotensin-II to angiotensin-I-VII, was found to be the functional receptor for SARS-CoV-1 and -2 [36]. ACE2 is abundantly present in humans in the epithelia of the lung and small intestine, which might provide possible routes of entry for the SARS-CoV-1, and -2 [37]. Study of 72 human tissues confirmed ACE2 mRNA expression in tissues other than the lung and gastrointestinal system, like testis, cardiovascular, renal, and pancreas [38,39]. Studies from 2003 SARS-CoV-1 epidemic evidenced that even milder SARS pneumonitis cases who did not receive glucocorticoid medications, had higher fasting blood glucose; and hyperglycemia in turn was an independent predictor of higher mortality and morbidity [40]. A follow up study in 2010 of the same cohort, investigating pathogenesis of pancreatic lesions, also found that pancreatic islets are strongly immune-positive for ACE2 while exocrine tissues are only weakly positive. Insulin dependent diabetes occurred during the hospitalization in 20 of the 39 patients (age: 47.2 ± 2.2 years) who received no corticosteroids during the course of SARS disease. Out of these, six had diabetes at discharge. But after 3 years of follow-up, only two had persistent diabetes, suggesting that the damage incurred by islet cells is acute and mostly transient [9].
3. COVID-19 and T1DM: bidirectional link
Earlier reports from Italy and China noticed a curious lack of people with T1DM in hospitalized cohorts of SARS-CoV-2, that made the authors wonder if the immunological attributes of T1DM are in some way protective [41,42]. However, it is more plausible that lock-down measures with special directives of caution to people with pre-existing conditions like diabetes, fear of infection, more parental supervision while staying at home encouraged young people to avoid crowded places as well as take better care of their diabetes. Nonetheless, as we try to comprehend this unfolding pandemic, new evidence is emerging that COVID-19 not only increases the risk of DKA and mortality in those with T1DM & T2DM, but also could potentially induce new-onset T1DM.
3.1. Does COVID-19 increase mortality/morbidity in T1DM?
Diabetes has long been associated with increased susceptibility to and severity of infections. Hyperglycemia, by altering immune response and causing cytokine dysregulation, is an inherently proinflammatory and procoagulant state [[43], [44], [45], [46], [47]]. In an observational study on 59 hospitalized adult COVID-19 patients, patients with hyperglycemia had higher IL-6 and D-dimer levels, which reduced significantly with optimal glucose control, supporting the permissive role hyperglycemia plays in enhancing inflammation and creating procoagulant state independent of viral mediation [4]. Hence T1DM and T2DM, especially with poor glycemic control become high-risk pre-existing conditions for many bacterial and viral infections including SARS-CoV-2.
Multiple centers reported that COVID-19 induces DKA and increases the length of hospital stay in those with diabetes [5]. Even as evidence mounts on the increased COVID-19 related mortality and morbidity in those with pre-existing diabetes, most of these observations were in relation to people with T2DM, who typically have a range of co-morbidities like hypertension, obesity, cardiovascular disease etc., unlike the relatively younger and otherwise fit T1DM community [6,48].
In a preliminary report from a multicenter USA study, out of 64 adults with T1DM who have confirmed or suspected COVID-19, more than 50% reported hyperglycemia, and nearly one-third had DKA [49]. In a nationwide analysis in England, adjusted for age, sex, deprivation, ethnicity, and geographical region, compared with people without diabetes, the odds ratios for in-hospital COVID-19-related death were 3·51 (95% CI 3·16–3·90) in people with T1DM and 2·03 (1·97–2·09) in people with T2DM. These effects were attenuated to ORs of 2·86 for T1DM and 1·80 for T2DM when also adjusted for underlying cardio/cerebrovascular disease, though other potential confounders like BMI, hypertension, kidney disease, and tobacco smoking were not adjusted for [50]. Moreover, people younger than 40 years with either type of diabetes were at very low absolute risk of in-hospital death with COVID-19, further indicating that comorbidities might have contributed significantly to the increased mortality. Moreover, evidence hitherto points towards a milder covid-19 in children with better prognosis when compared with adults [51].
In a population-based cohort study of all the people with T1DM and T2DM who were registered to general practice in England, COVID-19 related mortality is higher (Hazard ratio 2.23 in T1DM, 1.61 in T2DM) in those with prior higher HbA1c of 10% (86 mmol/mol) versus in those with a HbA1c of 6.5–7.0% (48–53 mmol/mol). In addition, older age (>70 years), non-white ethnicity, co-morbidities like previous stroke or cardiac failure or renal compromise and socio-economic deprivation are associated with increased mortality, in both T1DM and T2DM [52].
These studies indicate that COVID-19 increases mortality even in people with T1DM, especially in older age groups with co-existing renal or cardiac disease. In patients with diabetes, it is important to maintain optimal glycemic control by frequent blood glucose and ketone measurements, and adjusting insulin regime accordingly.
3.2. Challenges in the management of individuals with T1DM
As it is becoming more evident that the length of hospital-stay, risk of complications and overall mortality from COVID-19 are higher with poor glycemic control, this could be partly due to the adverse effects of certain therapies currently under trials to treat severe cOVID-19 [52,53].
Hydroxychloroquine, an immunomodulatory agent that was extensively used during the initial phases of the pandemic, can decrease insulin degradation at the cellular level and stimulate insulin-mediated glucose transport, resulting in potential hypoglycemia [54,55]. On the other hand, antiviral drugs such as lopinavir and ritonavir could lead to hyperglycemia and worsen glycemic control [56]. Glucocorticoids, which were seen to improve outcomes in COVID-19 related severe acute respiratory distress syndrome and hence became an integral part of treatment regime for hospitalized patients, can lead to marked hyperglycemia by reducing insulin sensitivity as well as by interfering with the actions of glucagon like peptide-1 and stimulating production of glucagon [57]. Some of the challenges in managing individuals with T1DM during COVID-19 pandemic are given in Table 2 .
Table 2.
Challenges in managing individuals with TIDM.
| COVID-19 induced Challenges | Effects on individuals with T1DM |
|---|---|
| Use of drugs, such as chloroquine and hydroxychloroquine | Higher risk of glycemic fluctuations and hypoglycemia |
| Effect of ‘lock down’ | Lack of physical interaction with peers Reduced physical activity Increased screen time Intake of less healthy food Psychological stress Irregular sleep pattern |
| Increased risk of DKA | Fear of contracting COVID-19 in a hospital and delay in seeking medical attention in case of an illness Difficulties accessing medical supplies |
3.3. COVID-19, pancreatitis, and new onset diabetes
Recent virologic data from Germany (Hoffmann et al.) and China (Zhou et al.) reveal important commonalities between SARS-CoV-2 and SARS-CoV-1 infections, and demonstrate that SARS-CoV-2 uses the same ACE2 receptor as SARS-CoV-1 for host cell entry [58,59]. As the substantially high transmissibility of SARS-CoV-2 relative to SARS-CoV-1 is becoming evident, one may speculate that the new virus might exploit cellular attachment factors with higher efficiency than SARS-CoV-1, causing more robust infection of ACE2+ cells.
3.3.1. COVID-19 and pancreatitis
Despite the findings of islet cell infection, new onset hyperglycemia and diabetes, there have been no reports of acute pancreatitis with the SARS-CoV-1 epidemic of 2003. However, the effects of SARS-CoV-2 seem to differ in this perspective, with many cases of COVID-19 related acute pancreatitis being reported during the last few months [[60], [61], [62], [63], [64]]. In a case series of 52 patients with acute COVID-19, eight patients experienced pancreatic injury in the form of abnormal elevation in lipase or amylase [65]. Whether this pancreatic injury is due to the direct cytopathic effect of the virus, or the indirect result of severe systemic inflammatory response and multiorgan dysfunction in the context of severe COVID-19 illness is yet to be established.
A distinct subset of moderate pancreatitis with a benign course was described by researchers at Liverpool, UK. Out of 35 patients presenting with acute pancreatitis over a period of 6 weeks during March/April 2020, 10 were positive for SARS-CoV-2, and 5 of these were excluded as they had a clearly defined etiology. The remaining five patients were male, overweight or obese, had abdominal pain, mildly elevated amylase, and pancreatico-duodenal inflammation with hepatic steatosis on CT scan. Though all had persistenty elevated inflammatory markers, none had either transient or persistent multiorgan failure. Three of them had new onset diabetes requiring Insulin, with two going home on Insulin [66]. Though none of these reports prove causality, the role of direct viral cytopathic damage of pancreas can not be discounted, and it seems that endocrine islet cells are particularly more vulnerable to the viral insult.
3.3.2. COVID-19 and new onset diabetes
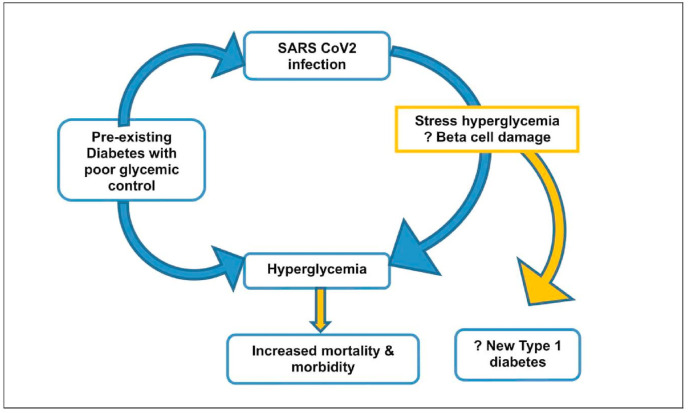
New-onset hyperglycemia is being increasingly described with COVID-19 in adults without a previous history of diabetes, albeit with significant mortality and morbidity. While infection induced inflammation and cytokine activation and resultant insulin resistance could lead to stress hyperglycemia, it is uncertain as to what extent the direct viral destruction of islet cells with decreased insulin production and release might be contributing [67] (Fig. 2 ). COVID-19 can also act as an infectious trigger that could decompensate and precipitate DKA in patients with new-onset T1DM and T2DM. During early months of pandemic in Italy, 23% fewer annual cases of new childhood diabetes were reported, though the ones presenting had more severe DKA in 2020 than in 2019 (44.3% vs. 36%, respectively) [68]. A two-fold increase in DKA and severe ketoacidosis at diabetes diagnosis in children and adolescents during the COVID-19 pandemic was reported from Germany, while an increase in referral of children with DKA was reported from the UK when compared with previous years. However, the underlying reasons for this phenomenon may be multifactorial and reflect reduced access to primary care services, parental fear of approaching the health care system during the pandemic period resulting in delayed diagnosis of new cases of T1DM [[69], [70], [71]]. A more recent multicenter study from the UK describes an apparent increase in new-onset T1DM in children, with evidence of SARS-CoV-2 infection or exposure in some of these. Seventy per cent (21/30) children presented with DKA and 52% (11/21) had severe DKA (pH 6.82–7.05). Of the five children with positive results (2 of 21 tested were SARS-CoV-2 PCR positive and 3 of 16 tested were SARS-CoV-2 IgG positive), three presented with severe DKA and refractory hypokalemia, and one PCR positive child suffered a hypokalemia-related cardiac arrest but recovered fully. Interestingly, majority had only a short duration of preceding symptoms of diabetes, refuting the previous notion of delayed presentation as the reason for increase in incidence of DKA at disease onset [8]. SARS-CoV-2 reduces ACE2 expression, leading to decreased degradation of angiotensin II, which can cause increased secretion of aldosterone and renal potassium loss. Whether this phenomenon was the basis for severe hypokalemia seen in the PCR positive child, needs further evidence. There are a few case reports of COVID-19 inducing acute onset diabetes and DKA in several individuals, mimicking T1DM. However, on follow-up there was reduced need for insulin and ultimately insulin could be discontinued in all the three patients. At last follow-up, these patients had normoglycemia on oral antihyperglycemic medication [7,72].
Fig. 2.
The bidirectional dynamic of SARS CoV2 and diabetes.
4. Future directions
At this point, it would be mostly conjectural to say that SARS-CoV-2 exposure contributed to the rise in DKA by precipitating or accelerating onset of T1DM. Our understanding so far is uncertain if this new-onset diabetes is classic T1DM or some new form of diabetes. Whether the severe COVID-19 induced hyperglycemia noticed in some individuals would remit on a long run as seen with SARS-CoV-1 induced diabetes is also unclear. How COVID-19 changes the natural history of disease in those with pre-existing diabetes is difficult to surmise.
To address some of these issues, an international group of diabetes researchers have established a global registry of patients with COVID-19–related diabetes (covidien.e-dendrite.com), as part of CoviDIAB Project. The goal of the registry is to establish the extent and phenotype of new-onset diabetes that is defined by hyperglycemia, confirmed COVID-19, a negative history of diabetes, and a history of a normal glycated hemoglobin level [73].
5. Conclusion
COVID-19 is an indiscriminate disease with unequal vulnerability. While hyperglycemia is seen to increase mortality and morbidity related to COVID-19, the virus itself can induce/worsen hyperglycemia, culminating in a vicious cycle. While we comprehend the intriguing mechanism of COVID-19 inducing diabetes or worsening the existing disease, we are still left with some unanswered questions. Is COVID-19 induced β-cell damage transient or permanent? Can COVID-19 linger on in the beta β-cells, causing chronic infection and new-onset T1DM? As this pandemic evolves, coordinated global efforts might throw some light upon these important concerns. Until that time, it is prudent to keep a diligent and close long term follow up of children and adults presenting with new-onset diabetes during this pandemic and also those with hyperglycemia induced by severe COVID-19.
Funding
None.
Declaration of competing interest
The authors declare that there are no conflicting interests relevant to this article.
Acknowledgments
Nil.
References
- 1.DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 2.Docherty A.B., Harrison E.M., Green C.A. medRxiv; 2020. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sardu C., D’Onofrio N., Balestrieri M.L. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020 doi: 10.1111/dom.14057. 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apicella M., Campopiano M.C., Mantuano M. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. The lancet. Diabetes & Endocrinology. 2020;8(9):782–792. doi: 10.1016/s2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy P.K., Kuchay M.S., Mehta Y., Mishra S.K. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020;14(5):1459–1462. doi: 10.1016/j.dsx.2020.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unsworth R., Wallace S., Oliver N.S., Yeung S., Kshirsagar A., Naidu H., Kwong R.M.W., Kumar P., Logan K.M. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020 doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 9.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippi C.M., von Herrath M.G. Viral trigger for type 1 diabetes: pros and cons. Diabetes. 2008;57(11):2863–2871. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppieters K.T., Boettler T., von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1) doi: 10.1101/cshperspect.a007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imagawa A., Hanafusa T., Miyagawa J., Matsuzawa Y. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. Osaka IDDM Study Group. N Engl J Med. 2000;342(5):301–307. doi: 10.1056/NEJM200002033420501. [DOI] [PubMed] [Google Scholar]
- 13.Imagawa A., Hanafusa T., Uchigata Y., Kanatsuka A., Kawasaki E., Kobayashi T., Shimada A., Shimizu I., Toyoda T., Maruyama T., Makino H. Fulminant type 1 diabetes: a nationwide survey in Japan. Diabetes Care. 2003;26(8):2345–2352. doi: 10.2337/diacare.26.8.2345. [DOI] [PubMed] [Google Scholar]
- 14.Sano H., Terasaki J., Tsutsumi C., Imagawa A., Hanafusa T. A case of fulminant type 1 diabetes mellitus after influenza B infection. Diabetes Res Clin Pract. 2008;79(3):e8–e9. doi: 10.1016/j.diabres.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Goto A., Takahashi Y., Kishimoto M. A case of fulminant type 1 diabetes associated with significant elevation of mumps titers. Endocr J. 2008;55(3):561–564. doi: 10.1507/endocrj.k07e-126. [DOI] [PubMed] [Google Scholar]
- 16.Akatsuka H., Yano Y., Gabazza E.C. A case of fulminant type 1 diabetes with coxsackie B4 virus infection diagnosed by elevated serum levels of neutralizing antibody. Diabetes Res Clin Pract. 2009;84(3):e50–e52. doi: 10.1016/j.diabres.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Hwang Y.C., Jeong I.K., Chon S., Oh S., Ahn K.J., Chung H.Y., Woo J.T., Kim S.W., Kim J.W., Kim Y.S. Fulminant Type 1 diabetes mellitus associated with acute hepatitis A. Diabet Med. 2010 Mar;27(3):366–367. doi: 10.1111/j.1464-5491.2010.02930.x. [DOI] [PubMed] [Google Scholar]
- 18.Christen U., Edelmann K.H., McGavern D.B., Wolfe T., Coon B., Teague M.K., Miller S.D., Oldstone M.B., von Herrath M.G. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114(9):1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz M.S., Bradley L.M., Harbertson J., Krahl T., Lee J., Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 20.Op de Beeck A., Eizirik D.L. Viral infections in type 1 diabetes mellitus--why the β cells? Nat Rev Endocrinol. 2016;12(5):263–273. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulte B.M., Bakkers J., Lanke K.H., Melchers W.J., Westerlaken C., Allebes W., Aanstoot H.J., Bruining G.J., Adema G.J., Van Kuppeveld F.J., Galama J.M. Detection of enterovirus RNA in peripheral blood mononuclear cells of type 1 diabetic patients beyond the stage of acute infection. Viral Immunol. 2010;23(1):99–104. doi: 10.1089/vim.2009.0072. [DOI] [PubMed] [Google Scholar]
- 22.Laitinen O.H., Honkanen H., Pakkanen O., Oikarinen S., Hankaniemi M.M., Huhtala H., Ruokoranta T., Lecouturier V., André P., Harju R., Virtanen S.M., Lehtonen J., Almond J.W., Simell T., Simell O., Ilonen J., Veijola R., Knip M., Hyöty H. Coxsackievirus B1 is associated with induction of β-cell autoimmunity that portends type 1 diabetes. Diabetes. 2014 Feb;63(2):446–455. doi: 10.2337/db13-0619. [DOI] [PubMed] [Google Scholar]
- 23.Oikarinen S., Tauriainen S., Hober D., Lucas B., Vazeou A., Sioofy-Khojine A., Bozas E., Muir P., Honkanen H., Ilonen J., Knip M., Keskinen P., Saha M.T., Huhtala H., Stanway G., Bartsocas C., Ludvigsson J., Taylor K., Hyöty H., VirDiab Study Group Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014 Feb;63(2):655–662. doi: 10.2337/db13-0620. [DOI] [PubMed] [Google Scholar]
- 24.Stene L.C., Oikarinen S., Hyöty H., Barriga K.J., Norris J.M., Klingensmith G., Hutton J.C., Erlich H.A., Eisenbarth G.S., Rewers M. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010 Dec;59(12):3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salminen K.K., Vuorinen T., Oikarinen S., Helminen M., Simell S., Knip M., Ilonen J., Simell O., Hyöty H. Isolation of enterovirus strains from children with preclinical Type 1 diabetes. Diabet Med. 2004 Feb;21(2):156–164. doi: 10.1111/j.1464-5491.2004.01097.x. [DOI] [PubMed] [Google Scholar]
- 26.Dahlquist G., Forsberg J., Hagenfeldt L., Boman J., Juto P. Increased prevalence of enteroviral RNA in blood spots from newborn children who later developed type 1 diabetes: a population-based casecontrol study. Diabetes Care. 2004;27:285–286. doi: 10.2337/diacare.27.1.285. [DOI] [PubMed] [Google Scholar]
- 27.Sadeharju K., Hämäläinen A.M., Knip M., Lönnrot M., Koskela P., Virtanen S.M., Ilonen J., Akerblom H.K., Hyöty H., Finnish Trigr Study Group Enterovirus infections as a risk factor for type I diabetes: virus analyses in a dietary intervention trial. Clin Exp Immunol. 2003 May;132(2):271–277. doi: 10.1046/j.1365-2249.2003.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiemstra H.S., Schloot N.C., van Veelen P.A. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 2001;98(7):3988–3991. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honeyman M.C., Stone N.L., Falk B.A., Nepom G., Harrison L.C. Evidence for molecular 549 mimicry between human T cell epitopes in rotavirus and pancreatic islet 550 autoantigens. J Immunol. 2010;184(4):2204–2210. doi: 10.4049/jimmunol.0900709. [DOI] [PubMed] [Google Scholar]
- 30.Bian X., Wallstrom G., Davis A. Immunoproteomic profiling of antiviral antibodies in new-onset type 1 diabetes using protein arrays. Diabetes. 2016;65(1):285–296. doi: 10.2337/db15-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson A.L., Vaziri-Sani F., Broberg P., Elfaitouri A., Pipkorn R., Blomberg J., Ivarsson S.A., Elding Larsson H., Lernmark Å. Serological evaluation of possible exposure to Ljungan virus and related parechovirus in autoimmune (type 1) diabetes in children. J Med Virol. 2015 Jul;87(7):1130–1140. doi: 10.1002/jmv.24127. [DOI] [PubMed] [Google Scholar]
- 32.Tapia G., Cinek O., Rasmussen T., Grinde B., Stene L.C., Ronningen K.S. Longitudinal study 533 of parechovirus infection in infancy and risk of repeated positivity for multiple islet 534 autoantibodies: the MIDIA study. Pediatr Diabetes. 2011;12(1):58–62. doi: 10.1111/j.1399-5448.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- 33.Yeung W.C., Rawlinson W.D., Craig M.E. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ. 2011;342:d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lönnrot M., Lynch K.F., Elding Larsson H. Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia. 2017;60(10):1931–1940. doi: 10.1007/s00125-017-4365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.https://www.diabetes.org/newsroom/press-releases/2020/latest-TEDDY-report-outlines-research-on-t1d-and-celiac-disease Accessed 1st October,2020.
- 36.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 39.Fignani D., Licata G., Brusco N. SARS-CoV-2 receptor Angiotensin I-Converting Enzyme type 2 is expressed in human pancreatic islet β-cells and is upregulated by inflammatory stress. bioRxiv. 2020 doi: 10.1101/2020.07.23.208041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J.K., Feng Y., Yuan M.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 41.Pitocco D., Tartaglione L., Viti L. Lack of type 1 diabetes involvement in SARS-COV-2 population: only a particular coincidence? Diabetes Res Clin Pract. 2020;164:108220. doi: 10.1016/j.diabres.2020.108220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi N., Caputo G.M., Weitekamp M.R., Karchmer A.W. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341(25):1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 44.Tiwari S., Pratyush D.D., Gahlot A., Singh S.K. Sepsis in diabetes: a bad duo. Diabetes Metab Syndr. 2011;5(4):222–227. doi: 10.1016/j.dsx.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Carey I.M., Critchley J.A., DeWilde S., Harris T., Hosking F.J., Cook D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- 46.Magliano D.J., Harding J.L., Cohen K., Huxley R.R., Davis W.A., Shaw J.E. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care. 2015;38(7):1274–1280. doi: 10.2337/dc14-2820. [DOI] [PubMed] [Google Scholar]
- 47.Wang W., Chen H., Li Q. Fasting plasma glucose is an independent predictor for severity of H1N1 pneumonia. BMC Infect Dis. 2011;11:104. doi: 10.1186/1471-2334-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J., Huang J., Zhu G. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: a retrospective cohort study. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebekozien O.A., Noor N., Gallagher M.P., Alonso G.T. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care. 2020;43(8):e83–e85. doi: 10.2337/dc20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barron E., Bakhai C., Kar P. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The lancet Diabetes & endocrinology. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holman N., Knighton P., Kar P. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. The lancet. Diabetes & Endocrinology. 2020;8(10):823–833. doi: 10.1016/s2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., Klonoff D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salman P.M., Quevedo I., Arias M. Hypoglycemia due to hydroxychloroquine, an uncommon association but to keep in mind, case report and review of literature. J Diabetes Metab Disord Control. 2020;7(1):6–7. doi: 10.15406/jdmdc.2020.07.00193. [DOI] [Google Scholar]
- 55.Unübol M., Ayhan M., Guney E. Hypoglycemia induced by hydroxychloroquine in a patient treated for rheumatoid arthritis. J Clin Rheumatol. 2011;17(1):46–47. doi: 10.1097/RHU.0b013e3182098e1f. [DOI] [PubMed] [Google Scholar]
- 56.Paengsai N., Jourdain G., Salvadori N. Recommended first-line antiretroviral therapy regimens and risk of diabetes mellitus in HIV-infected adults in resource-limited settings. Open Forum Infect Dis. 2019;6(10):ofz298. doi: 10.1093/ofid/ofz298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deng F., Gao D., Ma X. Corticosteroids in diabetes patients infected with COVID-19. Ir J Med Sci. 2020:1–3. doi: 10.1007/s11845-020-02287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan Z., Chen L., Li J. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hadi A., Werge M., Kristiansen K.T. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members. Pancreatology. 2020;20(4):665–667. doi: 10.1016/j.pan.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anand E.R., Major C., Pickering O., Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107(7) doi: 10.1002/bjs.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghosh A., Gupta V., Misra A. COVID19 induced acute pancreatitis and pancreatic necrosis in a patient with type 2 diabetes, Diabetes & Metabolic Syndrome. Clin Res Rev. 2020 doi: 10.1016/j.dsx.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159(1):367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szatmary P, Arora A, Raraty MGT, Dunne DFJ, Baron RD, Halloran CM. Emerging phenotype of SARS-CoV2 associated pancreatitis. Gastroenterology. doi: 10.1053/j.gastro.2020.05.069. [DOI] [PMC free article] [PubMed]
- 67.Ceriello A., De Nigris V., Prattichizzo F. Why is hyperglycemia worsening COVID-19 and its prognosis? Diabetes Obes Metabol. 2020 doi: 10.1111/dom.14098. 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rabbone I., Schiaffini R., Cherubini V. Has COVID-19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020 doi: 10.2337/dc20-1321. [DOI] [PubMed] [Google Scholar]
- 69.Basatemur E., Jones A., Peters M., Ramnarayan P. Paediatric critical care referrals of children with diabetic ketoacidosis during the COVID-19 pandemic. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-320471. [DOI] [PubMed] [Google Scholar]
- 70.Kamrath C., Mönkemöller K., Biester T. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324(8):801–804. doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Potier L., Julla J.B., Roussel R. COVID-19 symptoms masking inaugural ketoacidosis of type 1 diabetes. Diabetes Metab. 2020;S1262–3636(20) doi: 10.1016/j.diabet.2020.05.004. 30081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuchay M.S., Reddy P.K., Gagneja S., Mathew A., Mishra S.K. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr. 2020;14(6):2039–2041. doi: 10.1016/j.dsx.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubino F., Amiel S.A., Zimmet P. New-onset diabetes in covid-19. N Engl J Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]