Abstract
Administrations of intravenous immunoglobulin (IVIG), an immune-modulating blood-derived product, may be beneficial for managing neuropathic pain. Here, we review previous studies to investigate the effectiveness of IVIG in managing neuropathic pain due to various neurological disorders. The electronic databases PubMed, Scopus, Embase, and the Cochrane Library were searched for studies published up to February 2020. Two reviewers independently assessed the studies using strict inclusion criteria. Ten studies were included and qualitatively analyzed. The review included patients with pain due to complex regional pain syndrome (CRPS), diabetic polyneuropathy, and others, such as postherpetic neuralgia and trigeminal neuralgia. We found that IVIG may be one of the beneficial options for managing neuropathic pain from various neurological disorders. In the four articles reviewed, no major adverse effects were reported, and the trend was toward a positive pain-reducing effect in eight articles. However, to confirm the benefits of IVIG on reducing neuropathic pain, more high-quality studies are required.
Keywords: neuropathic pain, intravenous immunoglobulin, complex regional pain syndrome, diabetic polyneuropathy
Introduction
Neuropathic pain is caused by damage of the peripheral or central nervous system and affects 7–10% of the general population.1 Its typical characteristic is burning or electrical sensation and pain induced by non-painful stimuli, such as light touching.1–3 It occurs in various neurological disorders, such as diabetic polyneuropathy, chemotherapy-induced painful neuropathy, complex regional pain syndrome (CRPS), spinal pain, trigeminal neuralgia, postherpetic neuralgia, and other painful neuropathies.1–3 Neuropathic pain is frequently refractory to several treatment methods, such as oral medication (anti-inflammatory drugs, tricyclic antidepressant, and anticonvulsant drugs), physical therapy, and procedures.4–6 Chronic neuropathic pain can also greatly impair patients’ quality of life and cause depression, anxiety, and sleep disturbances.7–9
Neuropathic pain is associated with neuroinflammation.10,11 In patients with neuropathic pain, regardless of the underlying etiology, pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin 1, are overexpressed.10,11 Administration of intravenous immunoglobulin (IVIG), an immune-modulating blood-derived product, may be beneficial for managing neuropathic pain.12,13 IVIG has anti-inflammatory effects, probably induced by the suppression of pro-inflammatory cytokines, blockade of the Fc receptor, and enhancement of antibody catabolism.12–14
To date, several previous studies have evaluated the effectiveness of IVIG for controlling neuropathic pain due to various neurological disorders.10,15–17 However, the effectiveness of IVIG for pain-reducing effect is debatable. In addition, there has been no review about the effectiveness of IVIG for managing neuropathic pain due to various neurological disorders. Here, therefore, we review previous studies to investigate the effectiveness of IVIG for managing neuropathic pain due to various neurological disorders.
Methods
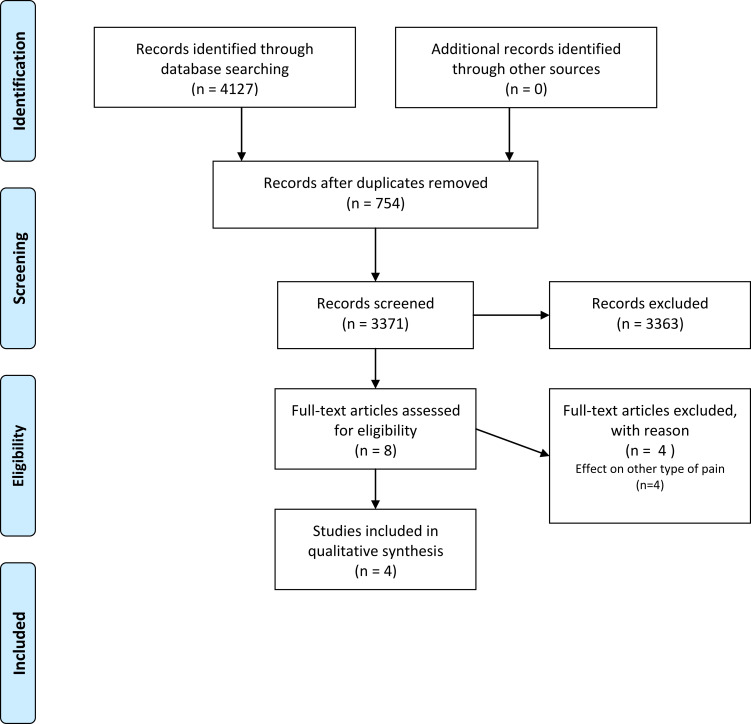
Two authors (D.P. and M.C.C) independently performed the literature search using the electronic databases PubMed, Scopus, Embase, and the Cochrane Library. Differences in their search results were resolved through a discussion. In PubMed, a search of ((“Immunoglobulins, Intravenous”[Mesh]) AND (“Neuralgia”[Mesh] OR “Nervous System Diseases”[Mesh] OR “Pain”[Mesh])) was performed. In Scopus, Embase, and the Cochrane library, a search of ((“Intravenous Immunoglobulin” OR “IVIG”) and (“neuralgia” OR “neuropathic pain” OR “nervous system diseases”)) was performed. The search was limited to articles published up to February 29, 2020. Only studies on the effects of IVIG on pain in patients with neuropathic pain were included. Data extraction was performed by two independent reviewers (D.P and M.C.C)(Figure 1).
Figure 1.
Flowchart of this study using PRISMA Flow Diagram.
Notes: Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6(7): e1000097. doi:10.1371/journal.pmed1000097.16 Creative Commons license and disclaimer available from: (http://creativecommons.org/licenses/by/4.0/legalcode).
Results
A total of 4127 potentially relevant articles were found in the primary literature search. After reading the titles and Abstracts and assessing them for eligibility based on the full-text articles, four articles were finally included in this review (Table 1).10,15–17 Among 4127 articles, only four articles were included in this study. Among the included articles, IVIG was used for evaluating the effect of reducing pain due to CRPS in two studies,15,16 diabetic polyneuropathy in one study,17 and neuropathic pain due to various disorders, including CRPS, postherpetic neuralgia, posttraumatic neuropathy, phantom limb pain, and spinal pain in one study.10
Table 1.
Summary of the Included Studies
| # | First Author, Years | Study Design | Number of Patients (E/C) | Treatment Compared with IVIG | IVIG Protocol | Pain Measurement Methods | Outcome Measurement Time, Months | Summary of Outcome | Diagnostic Methods |
|---|---|---|---|---|---|---|---|---|---|
| Complex regional pain syndrome | |||||||||
| 1 | Goebel, 201015 | Randomized crossover study | 13 | – | 0.25 g/kg/day * 2 days | Average 24 hour pain intensity score on an 11-point NRS (0–10) | 6 to 19 days after each infusion session | Decline in VAS score of 1.6 after IVIG treatment was more than placebo. | Diagnosis of Complex Regional Pain Syndrome I or II according to Budapest research criteria. |
| 2 | Goebel, 201716 | RCT | 111 (55/56) | - | Total 0.5/kg | NRS pain value | 6–42 days after the treatment | No significant effect | Diagnosis of Complex Regional Pain Syndrome I or II according to Budapest research criteria. |
| Diabetic polyneuropathy | |||||||||
| 3 | Jann, 202017 | RCT | 23 (11/12) | Placebo | 0.4 g/kg/day for 5 days | VAS, NPSI | 4 weeks after the treatment | ≥50% pain reduction: 63.6% (IVIG) vs 0% (placebo) | Diagnosis confirmed as per the Toronto Diabetic Neuropathy Expert Group criteria17 |
| Various neurological disorders | |||||||||
| 4 | Goebel, 200210 | Single-arm prospective study | 130 | - | Total 9–18 g over 1 week | Average 24 hour pain intensity score on an 11-point NRS (0–10) | Within 2 years after the treatment | 24.1% showed >70% of initial pain | Patients were diagnosed according to IASP (International Association for the Study of Pain) guidelines10 |
Abbreviations: E, experimental group; C, comparison group; IVIG, intravenous immunoglobulin; RCT, randomized controlled trial; VAS, visual analogue scale.
CRPS
To date, two previous studies evaluated the effect of IVIG for controlling pain due to CRPS.15,16 The first author in these two studies was Goebel. In 2010, Goebel et al16 investigated the effectiveness of IVIG in 13 patients with CRPS. Their study design was a randomized, placebo-controlled, double-blind, crossover trial. The authors gave each infusion to patients for two consecutive days. Changes in the degree of pain were measured 6 to 19 days after the initial treatment and crossover treatment. In the results, a decline in VAS score of 1.6 was higher after IVIG treatment than after placebo. In 2017, Goebel et al15 reported the effectiveness of IVIG in patients with moderate to severe chronic CRPS sustained for 1 to 5 years (IVIG group, n = 55; Placebo group, n = 56). Pain intensity was measured on VAS as a primary outcome, and quality of life was evaluated as a secondary outcome. IVIG was administered on days 1 and 22 after randomization. However, after 6 weeks of IVIG, no statistically significant difference was found between the IVIG and placebo groups in pain intensity or quality of life.
Diabetic Polyneuropathy
Only one study (2020) evaluated the pain-relieving effect of IVIG on diabetic polyneuropathy.17 Jann et al17 recruited 11 patients in the IVIG group and 12 patients in the placebo group and conducted an RCT. One month after IVIG, 7 of 11 patients (63.6%) showed more than 50% reduction in the initial pain, but none of patients in the placebo group (0%) showed more than 50% reduction in the initial pain. They concluded that IVIG is an efficacious treatment for patients with neuropathic pain due to diabetic polyneuropathy.
Others
One prospective observational study evaluated the effectiveness of IVIG for controlling pain from various neurological disorders.10 In 2002, Goebel et al10 (prospective observational study) recruited 11 patients with CRPS, five patients with postherpetic neuralgia, six patients with trigeminal neuralgia, 12 patients with posttraumatic or unknown etiology neuropathic pain, three patients with phantom limb pain, and 21 patients with spinal pain. Of 58 patients, 14 (24.1%) showed more than 70% reduction in the initial pain while 11 (19.0%) showed 25–75% reduction in the initial pain.
Adverse Effects
No major adverse effects were observed after IVIG treatment in any study included in our review. However, a few minor adverse effects were reported. Goebel et al15 reported adverse effects of IVIG as headache, nausea, light-headedness, tiredness, pain elevation, and infusion site reaction. However, of the nine severe adverse events in that study, four occurred after IVIG and five occurred after saline administration. Jann et al18 also reported a few adverse effects of IVIG. In that study, however, one patient developed mild dermatitis psoriasiform in the treatment arm while one patient from the placebo group developed mild “influenza”.17 These studies included in our review suggest that there are no significant side effects of IVIG.
Discussion
Out of four previous studies, except for one study (Goebel et al in 2017: CRPS), IVIG significantly alleviated neuropathic pain. Results of these studies suggest IVIG as a good therapeutic option for alleviating pain due to various neurological disorders.
Although it has not been definitely demonstrated, IVIG may work by binding to perineural pro-inflammatory cytokines or by hindering pro-inflammatory cytokines mRNA expression in neurons, macrophages, and glial cells.10,19,20 In several previous studies, IVIG was demonstrated to have anti-inflammatory effect by suppressing various pro-inflammatory cytokines.21,22
Regarding CRPS, two studies have been reported.15,16 However, the effects of IVIG on CRPS in both studies showed contradictory results, possibly attributed to the following causes. First, the number of patients in the studies was different. In 2017, Goebel et al enrolled 111 patients with CRPS.15 However, in 2010, Goebel et al enrolled only 13 patients with CRPS.16 Second, the duration of CRPS was different in both studies. IVIG treatment exerts a modulation effect on immune activation. Although the mechanism of CRPS is not exactly known, it differs depending on the time course of disease between acute and chronic stages. Considering that steroids, a potent immunomodulator, are more effective in the acute stage of CRPS than in the chronic stage, pathomechanism due to the immune response contributes a little more to the acute stage of CRPS than to the chronic stage.23–28 The disease durations were 2.4 ± 0.9 and 1.6 ± 0.7 years in studies conducted in 2017 and 2010, respectively.15,16 Therefore, the greater effect of IVIG in the study by Goebel et al in 201016 compared to 201715 may be explained by the enrollment of patients in a relatively early phase of CRPS. Moreover, elevated cytokine levels in blood serum seem to be associated with the occurrence of neuropathic pain in patients with CRPS.29 IVIG infusion reduces the levels of some cytokines, and this effect of IVIG seems to alleviate pain. However, in order to find out the exact effect of IVIG on CRPS, additional studies with a larger number of patients and different time periods may be required.
As for diabetic polyneuropathy, only one RCT was conducted despite its result being positive.17 Furthermore that study had a small number of patients. Accordingly, it is hard to draw a conclusion on the use of IVIG for managing pain due to diabetic polyneuropathy. Moreover, evidence is lacking on various neurological disorders other than CRPS, and diabetic neuropathy, despite some positive pain-reducing results reported in one study. That study was limited in that they did not evaluate the effect of IVIG in each neurological disorder separately.10
Conclusions
This narrative review shows that IVIG may manage neuropathic pain due to various neurological disorders. In the four articles reviewed, no major adverse effects were reported, and the trend was toward a positive pain-reducing effect. To confirm the benefits of IVIG on reducing neuropathic pain, more high-quality studies are required, because only four studies are included. Moreover, the protocols for IVIG infusion used in each study were heterogeneous; therefore, the most effective protocol for IVIG infusion for controlling neuropathic pain should be evaluated in the future. Also, pain-reducing effect is known to be initiated at 1 or 2 days after IVIG infusion, but the time when the maximal pain relief occurs and the duration of its pain-reducing effect have not been clearly known.12 Studies on changes in the effectiveness of IVIG over time would be helpful to elucidate the most appropriate protocol for IVIG infusion.
Funding Statement
The present study was supported by a National Research Foundation of Korea grant funded by the Korean government (grant no. NRF-2019M3E5D1A02068106).
Abbreviations
IVIG; intravenous immunoglobulin (IVIG), PPS; postpolio syndrome, CRPS; complex regional pain syndrome, TNF- α; tumor necrosis factor-α, VAS; visual analogue scale, RCTs; randomized controlled trials.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Author Contributions
Min Cheol Chang: Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, acquisition of data, revision of manuscript and critical revision of manuscript for intellectual content. Donghwi Park: Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, acquisition of data, revision of manuscript and critical revision of manuscript for intellectual content.
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors drafted or wrote, or substantially revised or critically reviewed the article. All authors have agreed on the journal to which the article will be submitted, reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage, and agree to take responsibility and be accountable for the contents of the article.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang MC, Boudier-Reveret M, Choo YJ, Hsiao MY. An unusual presentation of neuropathic pain following cervical spinal cord injury: a case report. BMC Neurol. 2020;20:61. doi: 10.1186/s12883-020-01644-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nascimento OJ, Pessoa BL, Orsini M, et al. Neuropathic pain treatment: still a challenge. Neurol Int. 2016;8:6322. doi: 10.4081/ni.2016.6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther. 2017;6:25–33. doi: 10.1007/s40122-017-0091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang S, Chang MC. Effect of bipolar pulsed radiofrequency on chronic cervical radicular pain refractory to monopolar pulsed radiofrequency. Ann Palliat Med. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Yang S, Chang MC. Effect of repetitive transcranial magnetic stimulation on pain management: a systematic narrative review. Front Neurol. 2020;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang MC. The effects of ultrasound-guided corticosteroid injection for the treatment of hemiplegic shoulder pain on depression and anxiety in patients with chronic stroke. Int J Neurosci. 2017;127:958–964. doi: 10.1080/00207454.2017.1281274 [DOI] [PubMed] [Google Scholar]
- 8.Duenas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457–467. doi: 10.2147/JPR.S105892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goebel A, Netal S, Schedel R, Sprotte G. Human pooled immunoglobulin in the treatment of chronic pain syndromes. Pain Med. 2002;3:119–127. doi: 10.1046/j.1526-4637.2002.02018.x [DOI] [PubMed] [Google Scholar]
- 11.Sommer C, Schafers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x [DOI] [PubMed] [Google Scholar]
- 12.Goebel A. Immunoglobulin responsive chronic pain. J Clin Immunol. 2010;30(Suppl 1):S103–108. [DOI] [PubMed] [Google Scholar]
- 13.Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255(Suppl S3):3–6. doi: 10.1007/s00415-008-3002-0 [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Park JS, Park D. Successful IVIG treatment without discontinuation of TNF-alpha blocker in Guillain-Barre syndrome induced by adalimumab in patient with Crohn’s disease. Neurol Sci. 2018;39:595–598. doi: 10.1007/s10072-017-3179-z [DOI] [PubMed] [Google Scholar]
- 15.Goebel A, Baranowski A, Maurer K, Ghiai A, McCabe C, Ambler G. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152:152–158. doi: 10.7326/0003-4819-152-3-201002020-00006 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med; 2009;6(7):e1000097. doi: 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goebel A, Bisla J, Carganillo R, et al. Low-dose intravenous immunoglobulin treatment for long-standing complex regional pain syndrome: a randomized trial. Ann Intern Med. 2017;167:476–483. doi: 10.7326/M17-0509 [DOI] [PubMed] [Google Scholar]
- 18.Jann S, Fazio R, Cocito D, et al. High-dose intravenous immunoglobulin is effective in painful diabetic polyneuropathy resistant to conventional treatments. Results of a double-blind, randomized, placebo-controlled, multicenter trial. Pain Med. 2020;21:576–585. doi: 10.1093/pm/pnz331 [DOI] [PubMed] [Google Scholar]
- 19.Jann S, Beretta S, Moggio M, Adobbati L, Pellegrini G. High-dose intravenous human immunoglobulin in polymyositis resistant to treatment. J Neurol Neurosurg Psychiatry. 1992;55:60–62. doi: 10.1136/jnnp.55.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung L, Cahill CM. TNF-alpha and neuropathic pain–a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goncalves Dos Santos G, Delay L, Yaksh TL, Corr M. Neuraxial cytokines in pain states. Front Immunol. 2019;10:3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das M, Karnam A, Stephen-Victor E, et al. Intravenous immunoglobulin mediates anti-inflammatory effects in peripheral blood mononuclear cells by inducing autophagy. Cell Death Dis. 2020;11:50. doi: 10.1038/s41419-020-2249-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci. 1997;825:179–193. [DOI] [PubMed] [Google Scholar]
- 24.Lee BJ, Kim JY, Cho HJ, Park D. Sphingosine 1-phosphate receptor modulation attenuate mechanical allodynia in mouse model of chronic complex regional pain syndrome by suppressing pathogenic astrocyte activation. Reg Anesth Pain Med. 2020;45:230–238. doi: 10.1136/rapm-2019-100801 [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Park JS, Park D. Anti-allodynic effect of interleukin 10 in a mouse model of complex regional pain syndrome through reduction of NK1 receptor expression of microglia in the spinal cord. J Pain Res. 2018;11:1729–1741. doi: 10.2147/JPR.S166624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park D. Pramipexole-induced limb dystonia and its associated complex regional pain syndrome in idiopathic Parkinson’s disease: a case report. Medicine (Baltimore). 2017;96:e7530. doi: 10.1097/MD.0000000000007530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henson P, Bruehl S. Complex regional pain syndrome: state of the art update. Curr Treat Options Cardiovasc Med. 2010;12:156–167. doi: 10.1007/s11936-010-0063-z [DOI] [PubMed] [Google Scholar]
- 28.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113:713–725. doi: 10.1097/ALN.0b013e3181e3db38 [DOI] [PubMed] [Google Scholar]
- 29.Park D. Recurrent complex regional pain syndrome type I in a patient with amyotrophic lateral sclerosis: a case report. Neurol Sci. 2018;39:1487–1488. doi: 10.1007/s10072-018-3305-6 [DOI] [PubMed] [Google Scholar]
- 30.Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain. 2012;13:10–20. doi: 10.1016/j.jpain.2011.10.003 [DOI] [PubMed] [Google Scholar]