Abstract
Purpose
The forced mid-expiratory flow (FEF25-75%) value is a potentially sensitive marker of obstructive peripheral airflow. We aimed to assess whether FEF25-75% can be an early predictor of chronic obstructive pulmonary disease (COPD).
Patients and Methods
Between July 1, 2007 and June 31, 2009, we identified 3624 patients who underwent a pulmonary function test (PFT) in Gangnam Severance Hospital. We selected 307 patients aged over 40 years without COPD who had normal PFT results at baseline and who had follow-up PFT records more than 1 year later. A FEF25-75% z-score less than −0.8435 was considered low. We defined COPD as a forced expiratory volume in one second/forced vital capacity value of less than 0.7 before July 31, 2019.
Results
Among 307 patients, 91 (29.6%) had low FEF25-75% at baseline. After 10 years, the incidence rate of COPD in the low FEF25-75% group was significantly higher than that in the normal FEF25-75% group (41.8% vs 7.4%; P-value<0.001). The Cox proportional hazard model showed that age (hazard ratio [HR] 1.09; P-value<0.001), smoking status (occasional smoker HR, 4.59; P-value<0.001 and long-term smoker HR, 2.18; P-value=0.023), and low FEF25-75% (HR, 3.31; P-value<0.001) were predictive factors for the development of COPD.
Conclusion
The FEF25-75% value in patients with normal lung function is a useful predictor for the development of COPD. We should carefully monitor patients who present with low FEF25-75% values, even if they have normal lung function.
Keywords: COPD, respiratory function tests, tobacco
Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable chronic airway disease;1 however, the economic burden of terminal COPD is enormous, with COPD being a leading cause of death worldwide. Accurate prediction and early detection of COPD may encourage patients to stop smoking and allow for appropriately timed treatments.2 Ultimately, the ability to predict COPD may prevent airway remodeling, improve prognosis, and diminish its medical and economic burden.3,4 Many researchers have attempted to identify early predictors for COPD development. The pulmonary function test (PFT) is a safe and practical procedure that is widely performed to detect COPD. Recently, a decrease in the forced expiratory volume in one second (FEV1)5 or diffusing capacity of the lungs for carbon monoxide (DLCO)6 in PFT has been suggested as a marker to predict COPD development (FEV1/forced vital capacity [FVC] <0.7). In the clinic, we frequently encounter patients who smoke, have respiratory symptoms, and have normal PFT results. We hypothesized that among some patients with normal lung function, including normal FEV1 and DLCO values, forced mid-expiratory flow (FEF25-75%) may be an early predictive marker for the development of COPD among high-risk individuals.
FEF25-75% is the most sensitive measure of airflow in peripheral airways where primary airflow obstruction originates,7 and it is reduced in early bronchial impairment, which is associated with small airway disease.8–11 Airway remodeling, mucus plugging, and immune cell infiltration induced by cigarette smoking ultimately result in small airway disease, which is a cardinal feature of COPD.12 Some researchers have found that decreased FEF25-75% is frequently observed in patients with COPD.13 We can therefore assume that FEF25-75% might be an earlier marker for COPD than other markers, such as FEV1, DLCO, and FVC. However, no prospective study has examined FEF25-75% as a predictor of COPD.
Thus, in this observational cohort study, we aimed to determine whether the FEF25-75% value measured at baseline can predict COPD development over 10 years.
Materials and Methods
Patients and Study Flow
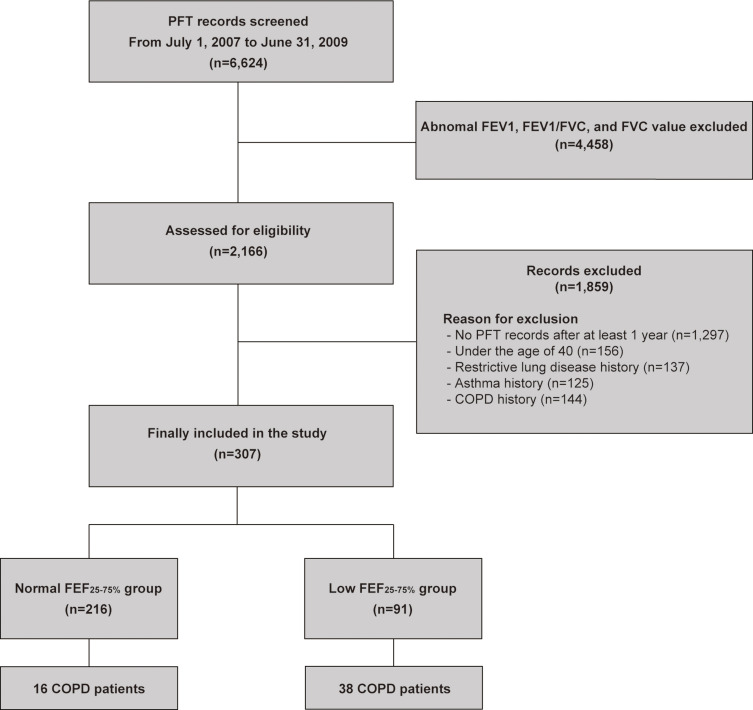
From July 1, 2007 to June 31, 2009, we identified 6624 patients who underwent PFT at Gangnam Severance Hospital. We excluded 4458 patients with abnormal PFT results, defined as at least one of the following three criteria: the FEV1/FVC [<0.7], FVC [<80% of predicted value], and FEV1 [<80% of predicted value]. An additional 1859 patients were excluded for the following reasons: no records of PFT performed more than 1 year after the baseline (n=1297), under 40 years of age (n=156), restrictive lung disease history (n=137), and history of asthma (n=125) and COPD (n=144). Finally, we reviewed the electronic medical records of the remaining 307 patients with normal PFT results at baseline and grouped them according to the FEF25-75% values (Figure 1).
Figure 1.
Flow chart of this study.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEF, forced mid-expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PFT, pulmonary function test.
Parameters
We collected clinical information on sex, age, body mass index (BMI), smoking history, and the following comorbidities: arterial hypertension, diabetes mellitus, coronary heart disease (CHD), old cerebrovascular accident (CVA), alcoholics, reflux esophagitis (RE), peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD), depression, and history of pulmonary tuberculosis. These co-morbidities were checked through electronic medical records. BMI was calculated by dividing the weight in kilograms by the square of height in meters (kg/m2) when the first spirometry was performed. Smoking status was classified as never smoked, occasional smoker (<20 pack-years), or long-term smoker (≥20 pack-years), in accordance with a previous study.14 We calculated pack-years by multiplying the number of packs of cigarettes smoked per day by the number of years the person smoked. This retrospective study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (number: 2019–0556-001). The requirement of informed consent was waived because of the retrospective nature of the study.
PFT
Baseline spirometry tests were performed during the cohort period using Vmax229 and Vmax22 spirometers (SensorMedics Corp., Italia). The procedures were performed following the American Thoracic Society and European Respiratory Society criteria, 2005,15 with all patients seated and wearing nose clips. The procedure was repeated three times, which is an acceptable and reproducible method of effort. When a suitable spirometry test was performed, the FEV1 and FVC values were determined to be the most significant values on the FEV curves, and the two values could be determined on different curves. Baseline FEV1, FEV1/FVC, FVC, and FEF25-75% values were obtained for the study. All of the reference values were based on the Global Lung Function Initiative 2012.16
COPD Development
Patients underwent follow-up PFTs at intervals between 6 months and 1 year. While not on a standard schedule, most patients in this study underwent PFTs frequently. Reasons for follow-up PFTs differed among patients and were as follows: routine follow-up, respiratory symptoms, abnormal lesion in chest images, patient’s request, and doctor’s recommendation. If an obstructive pattern was shown in the PFT, a bronchodilator test was performed to evaluate the reversibility of bronchial obstruction.
We reviewed all PFT results, which were conducted before July 13, 2019. We defined the development of COPD when post-bronchodilation FEV1/FVC was less than 0.7.
Statistical Analysis
All analyses were performed using R Statistical Package (version 4.0.2; Institute for Statistics and Mathematics, Vienna, Austria). The values are expressed as means ± standard deviations for continuous variables and as numbers and percentages for categorical variables. FEF25-75% z-score was treated as both a continuous and categorical dependent variable in the analyses. We used t-tests for continuous variables and chi-square tests for categorical variables to evaluate the relationship between the outcome and independent variables. Univariate and multivariate Cox proportional hazards models were used to analyze factors associated with COPD development. In the univariate analysis, independent variables with a P-value <0.05 were statistically significant and were included in the multivariate model. Cox proportional hazard analysis was used to identify differences in the cumulative development of COPD (%) between the normal FEF25-75% and low FEF25-75% groups.
Results
Baseline Clinical Characteristics of Enrolled Patients According to the Groups and the Optimal Cut-off Value for Low FEF25-75%
To classify patients with normal PFT results into low and normal FEF25-75% groups, we initially estimated the optimal cut-off value of FEF25-75% z-score for predicting COPD development using the Youden index method.17 The optimal cut-off value for FEF25-75% z-score was −0.8435 (sensitivity 0.7037 and specificity 0.7905). Among 307 patients, 216 (70.4%) and 91 (29.6%) patients were included in the normal FEF25-75% and low FEF25-75% groups, respectively (Table 1). The baseline clinical characteristics, including BMI, presence of co-morbidity, history of pulmonary tuberculosis, and smoking status were not significantly different between the two groups. However, males were more predominant in the low FEF25-75% group than in the normal FEF25-75% group (73.6% vs 36.1%, P<0.001). In addition, mean patient age was lower in the low FEF25-75% group than in the normal FEF25-75% group (58.9±10.1 vs 62.5±10.5, P=0.006). Regarding co-morbidities, depression was more prevalent in the low FEF25-75% group than in the normal FEF25-75% group (12.1% vs 1.9%, P<0.001) (Table 1).
Table 1.
Baseline Characteristics of Enrolled Patients According to FEF25-75% z-Score
| Variables | Total | Normal FEF25-75% | Low FEF25-75%* | P-value |
|---|---|---|---|---|
| (n=307) | (n=216) | (n=91) | ||
| Male | 145 (47.2%) | 78 (36.1%) | 67 (73.6%) | <0.001 |
| Mean age (years) | 61.4±10.5 | 62.5±10.5 | 58.9±10.1 | 0.006 |
| Height (cm) | 160.7±8.7 | 161.0±9.0 | 160.2±8.0 | 0.486 |
| Weight (kg) | 62.2±9.4 | 62.6±9.7 | 61.2±8.8 | 0.231 |
| BMI (kg/m2) | 24.0±3.0 | 24.1±3.0 | 23.8±3.0 | 0.535 |
| Co-morbidity | ||||
| Hypertension | 126 (41.0%) | 87 (40.3%) | 39 (42.9%) | 0.770 |
| Diabetes | 57 (18.6%) | 39 (18.1%) | 18 (19.8%) | 0.846 |
| CHD | 26 (8.5%) | 19 (8.8%) | 7 (7.7%) | 0.926 |
| Previous CVA | 11 (3.6%) | 8 (3.7%) | 3 (3.3%) | 1.000 |
| RE, PUD or GERD | 18 (5.9%) | 11 (5.1%) | 7 (7.7%) | 0.536 |
| Depression | 15 (4.9%) | 4 (1.9%) | 11 (12.1%) | <0.001 |
| History of tuberculosis | 54 (17.6%) | 33 (15.3%) | 21 (23.1%) | 0.140 |
| Smoking status** | 0.851 | |||
| Never smoker | 197 (66.8%) | 142 (65.7%) | 55 (60.4%) | |
| Occasional smoker | 24 (8.1%) | 16 (7.4%) | 8 (8.8%) | |
| Long-term smoker | 74 (25.1%) | 50 (23.1%) | 24 (26.4%) | |
| Unknown | 12 (3.9%) | 8 (3.7%) | 4 (4.4%) |
Notes: Data are presented as mean ± standard deviation or number of patients (%). *Low FEF25-75%: FEF25-75% z-score below −0.8435 (the optimal cut-off value for predicting COPD development). **Occasional smoker: an adult who has smoked less than 20 pack-years in his or her lifetime; long term smoker: an adult who has smoked over 20 pack-years in his or her lifetime.
Abbreviations: BMI, body mass index; CHD, coronary heart disease; CVA, cerebrovascular accident; FEF, forced mid-expiratory flow; GERD, gastroesophageal reflex disease; PUD, peptic ulcer disease; RE, reflux esophagitis.
Baseline Pulmonary Function According to the Groups
When comparing baseline PFT results between the normal and low groups, FEV1, FEV1/FVC, and FVC values in the low FEF25-75% group were all significantly lower than those in the normal FEF25-75% group (Table 2). However, all PFT results were within the normal range at baseline.
Table 2.
Results of Lung Function Test According to the FEF25-75% z-Score
| Variables | Total | Normal FEF25-75% | Low FEF25-75%* | P-value |
|---|---|---|---|---|
| (n=307) | (n=216) | (n=91) | ||
| FEV1 (L) | 2.5±0.6 | 2.6±0.6 | 2.2±0.6 | <0.001 |
| FEV1 (% predicted) | 105.0±16.6 | 109.8±16.2 | 93.5±11.2 | <0.001 |
| FVC (L) | 3.2±0.8 | 3.3±0.8 | 3.0±0.8 | 0.007 |
| FVC (% predicted) | 97.6±13.5 | 99.7±14.3 | 92.7±10.0 | <0.001 |
| FEF25-75% (L/sec) | 2.2±0.8 | 2.4±0.8 | 1.6±0.5 | <0.001 |
| FEF25-75% (% predicted) | 82.9±25.7 | 93.0±23.4 | 58.7±10.0 | <0.001 |
| FEV1/FVC | 77.1±4.8 | 78.6±4.7 | 73.6±2.9 | <0.001 |
Notes: *Low FEF25-75%: FEF25-75% z-score below −0.8435 (the optimal cut-off value for predicting COPD development)
Abbreviations: FEF, forced mid-expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Significant Predictive Factors for the Development of COPD
Over the 10-year follow-up, 54 patients (17.6%) developed COPD among 307 patients. The incidence of COPD in the low FEF25-75% group was significantly higher than that in the normal FEF25-75% group (38 patients, 41.8% vs 16 patients, 7.4%; P<0.001). We identified significant factors for the development of COPD using Cox proportional hazard analysis. In the univariate analysis, age, depression, smoking status, FEV1, FEV1/FVC, and FEF25-75% z-scores were significant factors in COPD development (Table 3). We selected significant factors in the univariate analysis and included them in the multivariate analysis. However, the variance inflation factor of FEV1 z-scores was 6.390 in the multivariate analysis model, and it was not analyzed owing to multi-collinearity with other variables. The multivariate analysis revealed that the significant factors for COPD development were age (HR, 1.088; 95% confidence interval [CI], 1.050–1.128), smoking status (occasional smoker HR, 4.586; 95% CI, 1.913–10.993 and long-term smoker HR, 2.179; 95% CI, 1.115–4.258), FEV1/FVC z-score (HR, 0.452; 95% CI, 0.219–0.936), and FEF25-75% z-score (HR, 0.453; 95% CI, 0.267–0.766). However, depression was not a significant factor for the development of COPD in the multivariate analysis (HR, 1.550; 95% CI, 0.674–3.566) (Table 3).
Table 3.
Significant Factors for Development of COPD
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | VIF | |
| Low FEF25-75%* † | 5.665 | 3.156–10.166 | <0.001 | 3.308 | 1.650–6.632 | <0.001 | |
| Sex (male) | 1.616 | 0.941–2.777 | 0.082 | ||||
| Age (years) | 1.038 | 1.009–1.068 | 0.009 | 1.088 | 1.050–1.128 | <0.001 | 1.254 |
| BMI (kg/m2) | 0.927 | 0.844–1.017 | 0.108 | ||||
| Co-morbidity | |||||||
| Hypertension | 1.668 | 0.975–2.856 | 0.062 | ||||
| Diabetes | 1.504 | 0.817–2.770 | 0.190 | ||||
| CHD | 1.077 | 0.387–2.993 | 0.887 | ||||
| Previous CVA | 1.088 | 0.264–4.489 | 0.907 | ||||
| RE, PUD, or GERD | 1.074 | 0.385–2.993 | 0.892 | ||||
| Depression | 2.979 | 1.337–6.639 | 0.008 | 1.550 | 0.674–3.566 | 0.302 | 1.057 |
| History of tuberculosis | 1.063 | 0.564–2.001 | 0.850 | ||||
| Smoking status** | |||||||
| (reference: never smoked) | |||||||
| Occasional smoker | 2.242 | 0.968–5.195 | 0.060 | 4.586 | 1.913–10.993 | <0.001 | 1.166 |
| Long-term smoker | 2.003 | 1.118–3.586 | 0.020 | 2.179 | 1.115–4.258 | 0.023 | 1.379 |
| PFT | |||||||
| FEV1 z-score | 0.743 | 0.620–0.890 | 0.001 | ||||
| FVC z-score | 0.875 | 0.743–1.030 | 0.109 | ||||
| FEV1/FVC z-score | 0.284 | 0.182–0.444 | <0.001 | 0.452 | 0.219–0.936 | 0.033 | 2.034 |
| FEF25-75% z-score | 0.366 | 0.256–0.523 | <0.001 | 0.453 | 0.267–0.766 | 0.003 | 1.882 |
Notes: †Adjusted for age, depression, smoking status, and FEV1/FVC z-score. *Low FEF25-75%: FEF25-75% z-score below −0.8435 (the optimal cut-off value for predicting COPD development). **Occasional smoker: an adult who has smoked less than 20 pack-years in his or her lifetime; long-term smoker: an adult who has smoked over 20 pack-years in his or her lifetime.
Abbreviations: BMI, body mass index; CHD, coronary heart disease; CI, confidence interval; CVA, cerebrovascular accident; FEF, forced mid-expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GERD, gastroesophageal reflex disease; HR, hazard ratio; PFT, pulmonary function test; PUD, peptic ulcer disease; RE, reflux esophagitis; VIF, variance inflation factor.
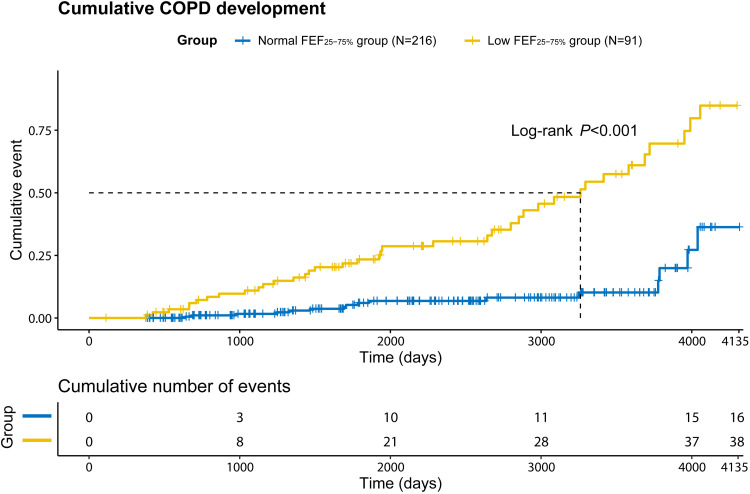
The low FEF25-75% group was also a significant risk factor for the development of COPD in the multivariate analysis (HR, 3.308; 95% CI, 1.650–6.632). In the Kaplan-Meier curve, the cumulative development of COPD was significantly higher in the low FEF25-75% group than in the normal FEF25-75% group (Figure 2; P<0.001).
Figure 2.
Differences in cumulative development of COPD (%) between normal FEF25-75% and low FEF25-75% groups.
Abbreviations: COPD, chronic obstructive pulmonary disease; FEF, forced mid-expiratory flow.
Comparison of PFT Parameters for Predicting COPD Development
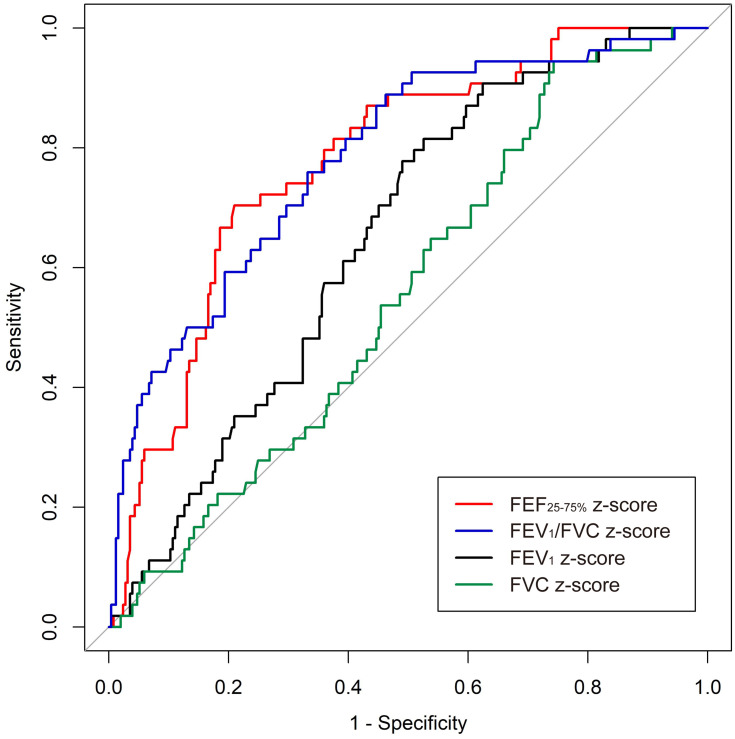
We obtained and compared the receiver operating characteristic (ROC) curve of each PFT parameter for predicting COPD development (Figure 3). The area under the ROC curve was the highest for FEF25-75% z-score (0.779; 95% CI, 0.715–0.843) and FEV1/FVC z-score (0.783; 95% CI, 0.716–0.851), followed by FEV1 z-score (0.649; 95% CI, 0.578–0.720) and FVC z-score (0.557; 95% CI, 0.480–0.634).
Figure 3.
Comparison of AUC of ROC curves.
Abbreviations: AUC, area under the curve; FEF, forced mid-expiratory flow; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ROC, receiver operating characteristic.
Discussion
In this 10-year follow-up observational cohort study, we showed that patients with decreased FEF25-75% were susceptible to develop COPD, despite the fact that they showed normal lung function, including normal FEV1 and FVC values. FEF25-75% was an independent risk factor for COPD even after adjustment for age, smoking history, and FEV1/FVC at baseline. As far as we know, this is the first study to reveal a significant association between FEF25-75% and the development of COPD. Small airway obstruction is a fundamental feature of COPD, and FEF25-75% reflects small airway disease.18 However, earlier research studies have described that FEF25-75% has high variability, and thus, its normal range is large.19 Therefore, FEF25-75% has not been studied for medical use. Although FEF25-75% might have poor repeatability based on earlier studies, the predictive power of FEF25-75% for COPD development can cover a large variable range of FEF25-75%. In this study, to reduce the variability of FEF25-75%, we analyzed the z-score by calibrating the age, sex, height, and race of each patient. The predictability and HR of FEF25-75% for COPD development were equivalent to those of FEV1/FVC, the parameter used in the diagnostic definition of COPD.
FEF25-75% measures airway flow rates on an FVC segment, which describes the flow from medium-to-small airways. The impairment of FEF25-75% indicates the impairment of medium-sized and small airways.20 Compromised FEF25-75% is frequently observed in patients with bronchial hyper-responsiveness in asthma or allergic rhinitis.21 Children with allergic rhinitis/asthma and decreased FEF25-75% have elevated values of fractional exhaled nitric oxide.22 Impairment of FEF25-75% is a marker of early bronchial impairment in allergic rhinitis and bronchiolitis obliterans.8,9 However, the superiority of FEF25-75% over FEV1/FVC has not been demonstrated in regard to sensitivity.23 In COPD, FEF25-75% is decreased in smokers compared to healthy individuals.24 Moreover, FEF25-75% is decreased in individuals exposed to second-hand smoke11 or those with air trapping observed on chest computed tomography.25 However, the role of FEF25-75% as a predictor for the development of COPD has not been well studied. Instead, several studies have investigated the parameters associated with FEF25-75% and obstructive disease.20 Although objective criteria for analysis are lacking, the concavity of the flow-volume curve is often used for classifying the obstructive pattern of patients.20 Recently, the global concavity index (100*[reference FEF50%–measured FEF50%]/reference FEF50%) and peripheral concavity index ([reference FEF75%-measured FEF75%]/reference FEF75%) were reported to be related to obstructive disease.26 However, further research is required.
COPD is a chronic airway disease induced by extended exposure to toxic particles, including components of cigarettes. The small airways that are exposed to toxic materials chronically demonstrate marked remodeling with thickened airway wall.27 This remodeling is caused by wound healing in response to injury by toxic materials, including cigarette smoking, viruses, and bacteria. Airway remodeling includes wall thickness of airway, increased density of inflammatory cells, fibrosis, and smooth muscle hyperplasia. Mucus plugs, which are a significant feature of COPD, also lead to small airway dysfunction.12 Immune cell infiltration induced by smoking precedes fibrosis of small airways and loss of lung tissue.28 These above mechanisms might identify subclinical COPD patients with small airway dysfunction before the impairment of lung function and may reveal impairment of FEF25-75% in advance.
We found that old age and smoking status were significant predictors for the development of COPD in this study. Age and smoking history are well-known critical factors for COPD.1 We demonstrated that well-known factors for the development of COPD are statistically significant in our sample. FEV1 is a well-known marker for airway obstruction and is used to measure severity of airway obstruction and can predict mortality.29 In our univariate analysis, FEV1 was significant. However, it showed multi-collinearity with FEF25-75% and FEV1/FVC and was therefore not included in the multivariate analysis model. We speculated that compared to FEV1, FEF25-75% is a better predictor for the development of COPD in the comparison of ROC in patients with normal lung function. We can therefore assume that FEF25-75% can be an earlier marker for COPD than FEV1.
Early diagnosis and prediction of COPD are essential for improving the prognosis of COPD patients.1 We can identify patients susceptible to the development of COPD and provide interventions to prevent COPD. Furthermore, we should recommend patients with decreased FEF25-75% to quit smoking and educate them about lifestyle issues, including avoiding dust, appropriate vaccinations, regular exercise, and nutritional support, even when they do not have symptoms or show reduced lung function.27 Early intervention, management, and close monitoring will help prevent the progression of COPD. Regular follow-up of patients can detect COPD in the early stage, leading to improved treatment timing, prevention of airway remodeling, and improvement of severity and prognosis.
This study had some limitations. First, this study was conducted at a single institute with a limited number of patients. In this respect, the optimal cut-off value of FEF25-75% used in this study cannot be generalized. Therefore, further large-scale studies are needed. However, we had sufficient patients to obtain significant results of well-known risk factors, including age and smoking history. Second, the FEF25-75% value is known to have considerable variability, and the normal range was shown to be quite broad in earlier studies. However, previous studies also reported that FEF25-75% is relatively reliable when FVC and FEV1 are normal.29 We included patients with normal FVC and FEV1, specifically to evaluate FEF25-75%. In addition, because all values were calibrated as the z-score, we could reduce the variability of FEF25-75%. Third, we included patients that were assessed with two different types of spirometers, resulting in potential systematic differences between lung function measurements.30 Finally, the interval between PFTs varied. In Korea, the national health insurance covers almost all Koreans, allowing patients to use medical services frequently and quickly. Thus, frequent PFTs are usually conducted. Most of our study patients underwent PFT at a 6-month to 1-year interval. We think this varied interval of PFT is not a major problem that would weaken the power of the results of the present study.
Conclusions
This retrospective, observational cohort study revealed that the FEF25-75% value in patients with normal lung function can be useful in predicting the development of COPD. We should thus carefully monitor patients who present with low FEF25-75% values who are susceptible to COPD, even though they may have normal lung function. Early interventions in these patients, including smoking cessation, timed vaccinations, sufficient exercise, and environmental care, may help improve their prognosis.
Funding Statement
There is no funding to report.
Abbreviations
BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DLCO, diffusing capacity of the lungs for carbon monoxide; FEF25-75%, forced mid-expiratory flow25-75%; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; HR, hazard ratio; PFT, pulmonary function test; PUD, peptic ulcer disease; RE, reflux esophagitis.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
Institutional Review Board (IRB) of Gangnam Severance Hospital (number: 2019-0556-001).
Consent for Publication
The requirement of informed consent was waived because of the retrospective nature of the study, and all data were collected in accordance with the amended Declaration of Helsinki.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Park YB, Rhee CK, Yoon HK, et al. Revised (2018) COPD clinical practice guideline of the korean academy of tuberculosis and respiratory disease: a summary. Tuberc Respir Dis (Seoul). 2018;81(4):261–273. doi: 10.4046/trd.2018.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devine JF. Chronic obstructive pulmonary disease: an overview. Am Health Drug Benefits. 2008;1(7):34–42. [PMC free article] [PubMed] [Google Scholar]
- 3.Csikesz NG, Gartman EJ. New developments in the assessment of COPD: early diagnosis is key. Int J Chron Obstruct Pulmon Dis. 2014;9:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336–349. doi: 10.1111/ijcp.12522 [DOI] [PubMed] [Google Scholar]
- 5.Park HJ, Byun MK, Rhee CK, Kim K, Kim HJ, Yoo KH. Significant predictors of medically diagnosed chronic obstructive pulmonary disease in patients with preserved ratio impaired spirometry: a 3-year cohort study. Respir Res. 2018;19(1):185. doi: 10.1186/s12931-018-0896-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey BG, Strulovici-Barel Y, Kaner RJ, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J. 2015;46(6):1589–1597. doi: 10.1183/13993003.02377-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 8.Ciprandi G, Cirillo I, Klersy C, et al. Role of FEF25-75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am J Rhinol. 2006;20(6):641–647. doi: 10.2500/ajr.2006.20.2914 [DOI] [PubMed] [Google Scholar]
- 9.Patterson GM, Wilson S, Whang JL, et al. Physiologic definitions of obliterative bronchiolitis in heart-lung and double lung transplantation: a comparison of the forced expiratory flow between 25% and 75% of the forced vital capacity and forced expiratory volume in one second. J Heart Lung Transplant. 1996;15(2):175–181. [PubMed] [Google Scholar]
- 10.Malerba M, Radaeli A, Olivini A, et al. Association of fef25-75% impairment with bronchial hyperresponsiveness and airway inflammation in subjects with asthma-like symptoms. Respiration. 2016;91(3):206–214. doi: 10.1159/000443797 [DOI] [PubMed] [Google Scholar]
- 11.Bird Y, Staines-Orozco H. Pulmonary effects of active smoking and secondhand smoke exposure among adolescent students in Juarez, Mexico. Int J Chron Obstruct Pulmon Dis. 2016;11:1459–1467. doi: 10.2147/COPD.S102999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higham A, Quinn AM, Cancado JED, Singh D. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res. 2019;20(1):49. doi: 10.1186/s12931-019-1017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson PA, Clearie K, Menzies D, Vaidyanathan S, Lipworth BJ. Assessment of small-airways disease using alveolar nitric oxide and impulse oscillometry in asthma and COPD. Lung. 2011;189(2):121–129. doi: 10.1007/s00408-010-9275-y [DOI] [PubMed] [Google Scholar]
- 14.Ohar JA, Sadeghnejad A, Meyers DA, Donohue JF, Bleecker ER. Do symptoms predict COPD in smokers? Chest. 2010;137(6):1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. European Respiratory J. 2005;26(2):319. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: [DOI] [PubMed] [Google Scholar]
- 18.Deepak D, Prasad A, Atwal SS, Agarwal K. Recognition of small airways obstruction in asthma and COPD the road less travelled. J Clin Diagnostic Res. 2017;11(3):TE01–TE05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lung function testing: selection of reference values and interpretative strategies. American thoracic society. Am Rev Respir Dis. 1991;144(5):1202–1218. [DOI] [PubMed] [Google Scholar]
- 20.Hoesterey D, Das N, Janssens W, et al. Spirometric indices of early airflow impairment in individuals at risk of developing COPD: spirometry beyond FEV1/FVC. Respir Med. 2019;156:58–68. doi: 10.1016/j.rmed.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciprandi G, Cirillo I. Forced expiratory flow between 25% and 75% of vital capacity may be a marker of bronchial impairment in allergic rhinitis. J Allergy Clin Immunol. 2011;127(2):549; discussion 550–541. doi: 10.1016/j.jaci.2010.10.053 [DOI] [PubMed] [Google Scholar]
- 22.Ciprandi G, Tosca MA, Cirillo I, et al. Impaired FEF25-75 may predict high exhaled nitric oxide values in children with allergic rhinitis and/or asthma. J Biol Regul Homeost Agents. 2012;26(1 Suppl):S27–33. [PubMed] [Google Scholar]
- 23.Lebecque P, Kiakulanda P, Coates AL. Spirometry in the asthmatic child: is FEF25-75 a more sensitive test than FEV1/FVC? Pediatr Pulmonol. 1993;16(1):19–22. doi: 10.1002/ppul.1950160105 [DOI] [PubMed] [Google Scholar]
- 24.Kornmann O, Beeh KM, Beier J, et al. Newly diagnosed chronic obstructive pulmonary disease. Clinical features and distribution of the novel stages of the global initiative for obstructive lung disease. Respiration. 2003;70(1):67–75. doi: 10.1159/000068417 [DOI] [PubMed] [Google Scholar]
- 25.Lee SM, Seo JB, Lee SM, Kim N, Oh SY, Oh YM. Optimal threshold of subtraction method for quantification of air-trapping on coregistered CT in COPD patients. Eur Radiol. 2016;26(7):2184–2192. doi: 10.1007/s00330-015-4070-z [DOI] [PubMed] [Google Scholar]
- 26.Johns DP, Walters JAE, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis. 2014;6(11):1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 28.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974;291(15):755–758. doi: 10.1056/NEJM197410102911503 [DOI] [PubMed] [Google Scholar]
- 29.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952 [DOI] [PubMed] [Google Scholar]
- 30.Milanzi EB, Koppelman GH, Oldenwening M, et al. Considerations in the use of different spirometers in epidemiological studies. Environmental Health. 2019;18(1):39. doi: 10.1186/s12940-019-0478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]