Abstract
Background
Stroke is a leading cause of death and disability worldwide. It remains difficult to treat brain injury and improve functional rehabilitation after cerebral ischemia. Brain-derived neurotrophic factor (BDNF) is involved in ischemic stroke (IS) through interactions in the CREB1-BDNF-NTRk2 pathway. In this study, we aimed to determine the association of NTRK2 gene polymorphisms and the effects of intergenetic interactions in the Chinese population.
Materials and Methods
A total of 400 patients diagnosed with IS and 400 healthy controls were enrolled for genotyping. Detailed sequence-based analysis was predicted through bioinformatical investigation. Polymorphisms associated with miRNA were analyzed by a dual-luciferase reporter assay system.
Results
Analysis of clinical characteristics revealed that IS was highly associated with exposure to cigarette smoking, alcohol intake, as well as metabolic diseases, such as diabetes, hypertension, and higher serum triglyceride concentration. Three polymorphisms in NTRK2 located in the 3ʹ-untranslated region (3ʹ-UTR) were genotyped. Logistic regression analysis showed that IS patients with rs11140793, rs7047042, and rs1221 polymorphisms had a higher risk of stroke and indicated a worse short-term recovery. The mRNA level of NTRK2 was suppressed in a mutant genotype compared with wild genotype. The suppression of NTRK2 was induced by the gain-of-binding ability of certain miRNAs through the direct binding of 3ʹ-UTR.
Conclusion
Our research indicated that, by influencing the expression of NTRK2, the SNPs rs11140793, rs7047042, and rs1221 in the 3′UTR of NTRK2 can be used as risk factors for IS patients.
Keywords: bioinformatics, NTRK2, ischemic stroke, homocysteine, miRNA
Introduction
Approximately 795,000 Americans suffer a stroke each year, and nearly a quarter (185,000) of the strokes are recurrent.1,2 In China, about 1.6 million people die from stroke every year. In other words, about 157 people die from strokes per 100,000 people. Therefore, stroke is the leading cause of disability and death among Chinese adults.3,4 Ischemic stroke (IS) is the most common of all stroke types in China. The most common cause is the de-arterialization of blood in the brain. The detailed pathophysiological causes of IS are not known, but some risk factors such as lifestyle, environment, immunity, inflammation, and genetic factors have been reported.5 Recent data on the inclusion of genetic markers in prediction models of stroke have been varied.6 Genome-wide association studies (GWAS) have been used to investigate the relationship between some genetic variants and IS risk.7 Evidence from studies of twins and family history demonstrates that genetics also influences the pathogenesis of IS and it should be responsible for a large part of IS risk.8 Besides, the data from genome-wide association study (GWAS) have shown that dozens of single nucleotide polymorphism (SNP) are related to IS.9 The most well-studied BDNF single nucleotide polymorphism (SNP) rs6265 has been reported to be related to brain morphology changes and cortical plasticity.10 In the context of stroke, it was also identified that BDNF rs6265 polymorphism was associated with motor recovery of stroke patients.11
A more promising approach has been to intervene with the brain-derived neurotrophic factor (BDNF), for instance, in pathways regulated by BDNF.12 BDNF is the most abundant neurotrophic factor, contributing to nerve cell survival, synaptic plasticity, angiogenesis, and peripheral and central nerve cell growth.13 BDNF is involved in neuro regenerative biological behaviors including synapses in the central nervous system.14 The BDNF genotype is associated with either primary or secondary IS. Reduced serum BDNF levels are associated with IS15 and the BDNF/TrkB signal becomes abnormal. Researcher has proved that in experimental model studies of stroke, both intravenous and intraventricular BDNF injections could reduce infarct size and showed neuroprotective effects.16 Besides, A recent study demonstrated that cerebral ischemia in rats made a difference to the regulation of BDNF isoforms and their associated proteins in a time-dependent manner, and the balance between BDNF and proBDNF in the ischemic cerebral cortex may have an impact on the pathogenesis and recovery from ischemia, such as balance, motor coordination and physical condition.17 Permanent BDNF reduction was found in the core area of cerebral infarction, while long-term upregulation was found in the half area of cerebral infarction, which is considered a neuroprotective mechanism.15,18 The miRNAs can be suppressed by binding to the 3ʹ-untranslated region (3ʹ-UTR), a gene regulation mechanism by transcription. Previous studies have confirmed that functional polymorphisms in 3ʹ-UTR of mRNA played important roles in the occurrence of human diseases.19
Here, we mainly have investigated the single nucleotide polymorphisms (SNPs) in the 3ʹ-UTR of NTRK2. The TrkB polymorphisms (rs11140793AC, rs7047042CG, rs1221CT, rs2277193TC, and rs2277192AG genotypes) were reported to be significantly associated with poststroke depression.20 However, whether these SNPs were associated with the risk of IS lacked thorough investigation. It is not known whether these mutant genotypes will induce the dysregulation of expression. Here we focused on SNPs located in the 3ʹ-UTR of NTRK2, aiming to explore whether these SNPs were associated with the risk of IS. We also predicted all candidate miRNAs that may interact with NTRK2 through bioinformatical analysis.
Materials and Methods
Clinical Information
From July 2010 to October 2016, 400 patients diagnosed with IS were enrolled in the study. The diagnosis of acute IS was based on neurological examination and then validated by CT scan or magnetic resonance imaging (MRI). IS was divided into subtypes according to TOAST criteria. In addition, 400 healthy subjects of the same period were selected as the control group and matched according to age and sex. The control group all went to our hospital for physical examination every year and had no IS. Fasting blood glucose after oral glucose stimulation ≥7.0 mmol/L and/or 11.1 mmol/L at 2 hours after oral glucose or receiving anti-diabetes medication, was defined as diabetes. Smokers were defined as more than ten cigarettes per day with continuous smoking for five years. Drinking exposure was defined as more than 50 mL of alcoholic beverages per day for 5 years. The National Institutes of Health Stroke Scale (NIHSS) score was used to quantify stroke severity at the time of presentation and discharge. This study was approved by the Ethics Committee of Kunshan Rehabilitation Hospital and The Sixth People’s Hospital of Nantong. Written informed consent was obtained from all participants. All experiments on human subjects were conducted in accordance with the Declaration of Helsinki.
Genotyping
Fasting venous blood was drawn from patients and controls. The total DNA of leukocytes was extracted by salt fractionation. The ABI PRISM 7900HT sequence detection system (Applied Biosystems, CA, USA) and the TaqMan allele identification method were used for SNP genotyping. Each 384-well format is allocated five replicate samples and two blank controls for quality control. The allelic discrimination model of the SDS 2.3 software package (Applied Biosystems) was used to calculate the genotyping results.
Bioinformatic Analysis
Bioinformatics software (http://bioinfo.life.hust.edu.cn/miRNASNP/#!/) was used to detect the candidate SNPs that could affect NTRK2 gene regulation via miRNAs.
Cell Line, Culture, and Transfection
Human 293T cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The culture medium included 10% FBS (Invitrogen, Carlsbad, CA, USA) with RMPI-1640 (Gibco, Gaithersburg, MD, USA), and cells were cultured in a humidified 5% CO2 incubator at 37°C. Mimic and control vectors were designed and cloned by Genescript (Nanjing, China). Cells were transfected with Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s protocol.
Construction of Luciferase-Based Reporter Plasmids and Luciferase Reporter Assay
The 3ʹ-UTR fragment containing wild or mutant allele SNPs was amplified by Genscript (Nanjing, Jiangsu). PCR products were cloned into a miR-reporter luciferase system (Ambion, Thermo Fisher Scientific, Waltham, MA, USA). The amplified fragment was verified by Genscript sequencing. PRL-TK containing Renilla luciferase was used for normalization. The 3ʹ-UTR sequence of NTRK2 predicted to interact with miRNAs or the mutant sequence of the predicted target were inserted into the PIR-reporter luciferase HindIII and SacI sites (Genscript, Nanjing, China). HEK-293T cells cultured with 24-well plates were co-transfected with pmIR-reporter vectors containing wild-type or mutant NTRK2-UTR fragments and control vectors, and PRL-TK containing Renilla luciferase was used for normalization. These miRNA mimics and controls were co-transfected into cells.
Statistical Analysis
The statistical analyses were performed using Stata/SE (StataCorp LP, TX, USA). The Student’s t-test and chi-square (χ2) test were used to compare demographic data. Multivariate logistic regression analysis was used to adjust. Odds ratios (OR) and 95% CI were used to estimate the relationship between genotype and IS clinical parameters. P < 0.05 was considered significant.
Results
Subject Characteristics
As shown in Table 1, there was no significant difference in age and sex distribution between IS patients and healthy controls. Smoking and alcohol exposure, diabetes, hypertension, and other common risk factors were found to have an increased risk of IS compared with the control group (P<0.05). In addition, traditional cardiovascular and cerebrovascular disease biomarkers such as total cholesterol, HDL-C, and LDL-C are also related to the occurrence of IS.
Table 1.
Clinical Characteristics of Ischemic Stroke Patients and Healthy Controls
| Characteristics | Cases (n=400) | Control (n=400) | p valuea |
|---|---|---|---|
| Age | >0.05 | ||
| >60 | 349 | 351 | |
| <60 | 51 | 49 | |
| Gender | >0.05 | ||
| Male | 276 | 244 | |
| Female | 124 | 156 | |
| Smoking exposure | <0.001 | ||
| Yes | 301 | 181 | |
| No | 99 | 219 | |
| Drinking exposure | <0.001 | ||
| Yes | 279 | 199 | |
| No | 121 | 201 | |
| Diabetes | 0.007 | ||
| Yes | 241 | 203 | |
| No | 159 | 197 | |
| Hypertension | <0.001 | ||
| Yes | 311 | 129 | |
| No | 89 | 271 | |
| Total cholesterol(mmol/L) | 4.88(4.11–5.14) | 4.79(4.12–5.13) | >0.05 |
| HDL-C(mmol/L) | 0.87(0.44–1.71) | 1.52(1.08–1.87) | <0.001 |
| LDL-C(mmol/L) | 4.99 (4.11–6.21) | 2.83(1.77–3.31) | <0.001 |
Note: aStudent t-test for continuous variable, Chi-squared test for categorical variable
Genotype and Allele Analysis
Three polymorphisms including rs11140793, rs7047042, and rs1221 were found to locate in the 3ʹ-UTR region of NTRK2. We explored the effects of the three candidate SNPs on IS susceptibility in our case-control participants. The results of chi-square statistical analysis showed that the genotypes of the three SNPs in the healthy control group all conformed to the Hardy-Weinberg equilibrium distribution pattern. As presented in Table 2, logistic regression analysis showed that the sensitivity of the AC genotype and CC genotype to IS was significantly higher than that of the AA genotype (OR=1.68, 95% CI=0.83–1.87; OR=1.79, 95% CI=0.91–1.99). We also found that polymorphisms in rs7047042 indicated a different distribution compared with the control group. Logistic regression analysis showed that NTRK2 rs7047042 CG and GG genotypes were significantly correlated with the increased risk of IS compared with CC genotypes (OR: 1.12; CG genotype, 95% CI: 1.01–1.65, OR: 1.31; GG genotype, 95% CI: 1.08–1.78). For rs1221 polymorphisms, we also found that patients with the CT or TT genotype have a higher IS risk than patients with the CC genotype (OR: 1.33; CT genotype, 95% CI: 1.19–2.33, OR: 1.41; TT genotype, 95% CI: 1.21–2.45).
Table 2.
Genotype Frequencies of the NTRK2 Polymorphisms in is Patients and Healthy Controls
| Genotype | Cases (n =400) | Controls (n = 400) | OR (95% CI) a |
P Valuea | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| rs11140793 | ||||||
| AA | 322 | 80.5 | 331 | 82.7 | 1.00 | |
| AC | 53 | 13.3 | 51 | 12.7 | 1.68 (0.83–1.87) | 0.03 |
| CC | 25 | 6.2 | 18 | 4.6 | 1.79 (0.91–1.99) | 0.01 |
| rs7047042 | ||||||
| CC | 371 | 92.7 | 382 | 95.5 | 1.00 | |
| CG | 23 | 5.8 | 16 | 4 | 1.12 (1.01–1.65) | 0.02 |
| GG | 6 | 1.5 | 2 | 0.5 | 1.31 (1.08–1.78) | 0.02 |
| rs1221 | ||||||
| CC | 344 | 86.0 | 351 | 87.7 | 1.00 | |
| CT | 38 | 9.5 | 37 | 9.3 | 1.33 (1.19–2.33) | 0.01 |
| TT | 18 | 4.5 | 12 | 3 | 1.41 (1.21–2.45) | 0.005 |
Note: aThe ORs, 95% CIs and P value were calculated after adjusting for smoking, drinking and other characteristics listed in Table 1.
The miR-Binding Was Attenuated by SNPs
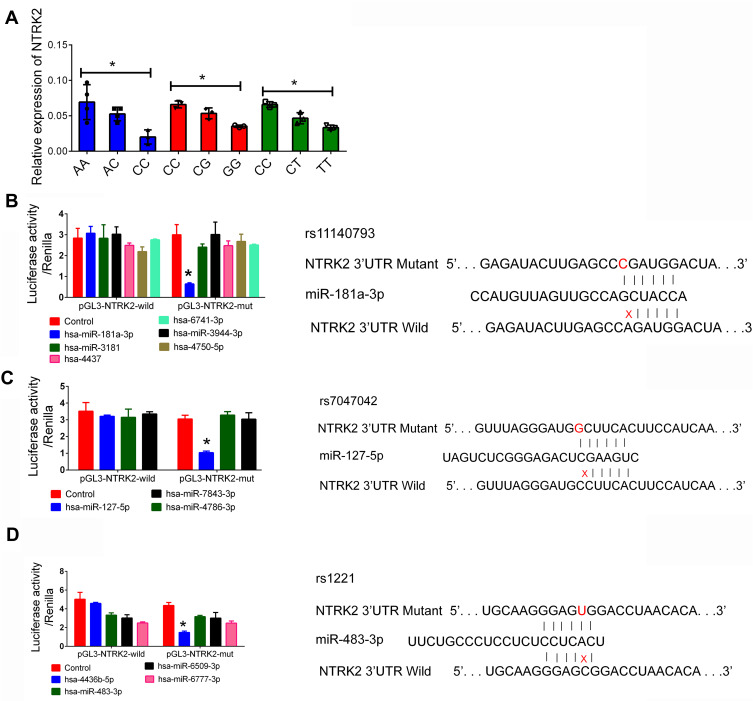
Because NTRK2 acted in a beneficial role during the progression of IS, increasing levels of NTRK2 were associated with a lower risk of IS. Here we found that all the three SNPs were highly associated with the increased risk of IS, indicating that all the SNPs could be associated with a lower level of NTRK2. Next, we investigated the mRNA level of NTRK2 in plasma samples. We found that patients harboring mutant NTRK2 DNA presented a decreased level of NTRK2 mRNA (Figure 1A). Whether the suppression of NTRK2 was associated with miRNA-associated interactions are unknown. As predicted by bioinformatics analysis, the miRNAs which have the potential to bind with the 3ʹ-UTR of NTRK2 are presented in Table 3. A total of six candidate miRNAs that may interact with rs11140793 through binding were listed according to the expression correlation value by prediction software. Three miRNAs were predicted for rs7047042 whereas four miRNAs were predicted for rs1221. We then cloned the 13 candidate miRNA mimics and transfected cells with either wild-type or mutant type NTRK2. A luciferase assay confirmed that miR-181a-3p could directly bound with the NTRK2 mutant (CC genotype of rs11140793) causing the suppression of NTRK2 luciferase (Figure 1B), miR-127-5p could interact with the NTRK2 mutant (GG genotype of rs7047042, Figure 1C), and miR-483-3p could interact with the TT genotype of rs1221 (Figure 1D).
Figure 1.
The function miRNAs associated with NTRK2 polymorphisms. (A): Relative expression of NTRK2 mRNA in patients with different genotypes of IS. (B): Luciferase of rs11140793 was detected by miRNA. (C): Luciferase of rs7047042 was detected by miRNA. (D): Luciferase of rs1221 was detected by miRNA. 293T cells were co-transfected with miRNAs simulator or control Renilla luciferase vector PRL-SV40 for 48 h. Luciferase activity in fireflies and renal cells was determined using the luciferase assay kit. The luciferase firefly signal was normalized to the renal luciferase signal. Data are expressed as mean ± standard deviation. *P < 0.05.
Table 3.
Candidate miRNAs Associated with NTRK2 Polymorphisms Predicted by Bioinformatics Analysis
| Gene | SNP | miRNA-ID | ΔG Binding (kCal/mol) |
|---|---|---|---|
| NTRK2 | rs11140793 | hsa-miR-181a-3p | −15.32 |
| hsa-miR-3181 | −14.60 | ||
| hsa-miR-3944-3p | −17.90 | ||
| hsa-4437 | −20.35 | ||
| hsa-4750-5p | −18.49 | ||
| hsa-6741-3p | −14.67 | ||
| rs7047042 | hsa-miR-127-5p | −9.01 | |
| hsa-miR-4786-3p | −14.83 | ||
| hsa-miR-7843-3p | −19.25 | ||
| rs1221 | hsa-4436b-5p | −18.36 | |
| hsa-miR-483-3p | −15.99 | ||
| hsa-miR-6509-3p | −13.95 | ||
| hsa-miR-6777-3p | −12.41 |
Note: ΔG binding (kCal/mol): binding energy based on ensemble free energy.
Polymorphisms Predicted a Worse Outcome in is Patients
Because the increase of NTRK2 expression can promote tHcy, it may lead to a better prognosis in IS patients. Next, we evaluated the relationship between polymorphism and also the outcome of short-term IS. The evaluation used the NIHSS. According to the NIHSS measurement, different genotypes had significant differences in the initial severity of stroke in the acute phase. A negative value indicated a clinical improvement in IS outcome. We found that all three polymorphisms predicted a worse prognosis in IS patients. NTRK2 mRNA expression and circulating tHcy concentration were also examined. Our data showed that NTRK2 expression was low and tHcy level was increased in patients with mutant NTRK2 (Table 4).
Table 4.
Clinical Outcome Correlation of NTRK2 Polymorphisms with is Patients
| Feature | Genotype (rs11140793) | AC vs AA* P value |
CC vs AA* P value |
||
|---|---|---|---|---|---|
| AA | AC | CC | |||
| NIHSS ac (Mean/SD) | 3.1/1.1 | 4.2/1.5 | 4.8/1.1 | 0.02 | 0.005 |
| NIHSS 3m (Mean/SD) | 0.6/0.1 | 0.8/0.2 | 1.1/0.2 | 0.03 | 0.002 |
| tHcy (mmol/L) | 12.3/1.8 | 13.5/1.5 | 15.5/1.4 | 0.01 | 0.001 |
| NTRK2 (Mean/SDb) | 0.08/0.022 | 0.06/0.018 | 0.04/0.11 | 0.02 | 0.003 |
| Feature | Genotype (rs7047042) |
CG vs CC* P value |
GG vs CC* P value |
||
| CC | CG | GG | |||
| NIHSS ac (Mean/SD) | 3.2/1.1 | 3.5/1.3 | 4.1/2.1 | 0.04 | 0.02 |
| NIHSS 3m (Mean/SD) | 0.7/0.1 | 0.9/0.2 | 1.0/0.2 | 0.03 | 0.01 |
| tHcy (mmol/L) | 11.8/1.9 | 12.1/1.6 | 14.2/1.8 | 0.01 | 0.04 |
| NTRK2 (Mean/SDb) | 0.09/0.012 | 0.06/0.021 | 0.04/0.19 | 0.02 | 0.03 |
| Feature | Genotype (rs1221) |
CT vs CC* P value |
TT vs CC* P value |
||
| CC | CT | TT | |||
| NIHSS ac (Mean/SD) | 3.5/1.1 | 3.6/1.7 | 4.2/2.1 | 0.12 | 0.03 |
| NIHSS 3m (Mean/SD) | 1.1/0.4 | 1.1/0.6 | 1.3/0.8 | 0.23 | 0.02 |
| tHcy (mmol/L) | 10.8/1.9 | 13.2/0.2 | 13.1/0.3 | 0.001 | 0.002 |
| NTRK2 (Mean/SDb) | 0.06/0.032 | 0.03/0.021 | 0.01/0.011 | 0.01 | 0.03 |
Notes: *Student t-test for either genotype distributions or allele frequencies in IS patients. bRelative expression of NTRK2 normalized with GAPDH. NIHSS ac, initial stroke severity during the acute phase; NIHSS 3m, stroke severity after 3 months; MTHFR: Relative expression of MTHGR mRNA in IS patients.
Discussion
It is important to identify the risk of stroke recurrence in patients because about a quarter of strokes recur and have a poor response to therapy.21 Cerebral or systemic ischemic events may highly increase morbidity and mortality.22 Therefore, secondary prevention in patients with recent cerebrovascular events is of critical importance.23 IS endangers the health and quality of life of patients, and at the same time, it also brings a heavy burden to patients, their families, and society. At present, it is worth noting that it may be possible to enhance survival pathways by binding to TrkB receptors, such as the pathways found to be regulated by BDNF. In addition, studies have shown that BDNF/TrkB has a beneficial effect after acute stroke and can stimulate later nerve repair.24
NTRK2 consists of 24 exons and is located on chromosome 9 (q22.1), with a length of about 24 KB.25 It encodes a subtype of the BDNF receptor, TrkB, which is bound to mBDNF with a high affinity.26 The BDNF/TrkB signaling pathway enhances neuroplasticity including neurogenesis, neurite dendrite, and synapse. BDNF promotes neuronal cell survival through dimerization, increased tyrosine kinase activity, and full-length TrkB phosphorylation of the high-affinity receptor.27 NTRK2 can activate and trigger three related cascade signaling pathways, including MAPK/ERK, PI3K/Akt, and PLCγ28
There is also increasing evidence that association analysis based on genes and pathways is more effective than association studies based on SNPs because they increase the ability to identify true associations. However, it should be pointed out that analysis based on gene-gene interactions is rarely applied to IS.29,30 Functional SNPs located at miRNA binding sites can affect the binding of miRNA to target genes, by reverse inhibition or activating the expression of target genes, and altering susceptibility to human diseases.
There were some limitations to consider in this study. Our study only followed up the short-term prognosis of patients. The influence of SNPs on the long-term rehabilitation and functional prognosis of patients needs further research. Therefore, our results need to be confirmed in independent samples or other populations.
Conclusion
In summary, our current research showed that NTRK2 SNP gene variants were associated with more severe initial stroke severity and worse short-term recovery after IS. In addition, miRNAs could regulate these three SNPs, resulting in decreased expression of NTRK2. This may be a favorable factor in the prognosis of patients with IS.
Funding Statement
This work was supported by grant from the Science and Technology Development Plan of Suzhou (Grant Number: SYS2019010).
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Li F, Chen QX, Peng B, Chen Y, Yao T, Wang G. Microalbuminuria in patients with acute ischemic stroke. Neurol Res. 2019;41(6):498–503. [DOI] [PubMed] [Google Scholar]
- 2.Numis AL, Fox CK. Arterial ischemic stroke in children: risk factors and etiologies. Curr Neurol Neurosci Rep. 2014;14(1):422. doi: 10.1007/s11910-013-0422-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gairolla J, Kler R, Modi M, Khurana D. Leptin and adiponectin: pathophysiological role and possible therapeutic target of inflammation in ischemic stroke. Rev Neurosci. 2017;28(3):295–306. doi: 10.1515/revneuro-2016-0055 [DOI] [PubMed] [Google Scholar]
- 4.Okada Y, Numata T, Sato-Numata K, et al. Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction. Curr Top Membr. 2019;83:205–283. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in china: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi: 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 6.Li F, Yang L, Yang R, et al. Ischemic stroke in young adults of northern china: characteristics and risk factors for recurrence. Eur Neurol. 2017;77(34):115–122. doi: 10.1159/000455093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng JH, Zhang Z, Ye Q, Ye ZS, Xia NG. Characteristics of the ischemic stroke patients whose seizures occur at stroke presentation at a single institution in Eastern China. J Neurol Sci. 2018;387:46–50. doi: 10.1016/j.jns.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 8.Traylor M, Farrall M, Holliday EG, et al. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11(11):951–962. doi: 10.1016/S1474-4422(12)70234-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matarin M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol. 2007;6(5):414–420. doi: 10.1016/S1474-4422(07)70081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cerebral Cortex. 2010;20(5):1254–1262. doi: 10.1093/cercor/bhp189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Vliet R, Ribbers GM, Vandermeeren Y, Frens MA, Selles RW. BDNF Val66Met but not transcranial direct current stimulation affects motor learning after stroke. Brain Stimul. 2017;10(5):882–892. doi: 10.1016/j.brs.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 12.Harward SC, Hedrick NG, Hall CE, et al. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538(7623):99–103. doi: 10.1038/nature19766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii T, Warabi E, Mann GE. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic Biol Med. 2019;133:169–178. doi: 10.1016/j.freeradbiomed.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Holt LM, Hernandez RD, Pacheco NL, Torres Ceja B, Hossain M, Olsen ML. Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. eLife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correia CT, Coutinho AM, Sequeira AF, et al. Increased BDNF levels and NTRK2 gene association suggest a disruption of BDNF/TrkB signaling in autism. Genes Brain Behav. 2010;9(7):841–848. doi: 10.1111/j.1601-183X.2010.00627.x [DOI] [PubMed] [Google Scholar]
- 16.Schabitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31(9):2212–2217. doi: 10.1161/01.STR.31.9.2212 [DOI] [PubMed] [Google Scholar]
- 17.Rahman M, Luo H, Sims NR, Bobrovskaya L, Zhou XF. Investigation of Mature BDNF and proBDNF Signaling in a Rat Photothrombotic Ischemic Model. Neurochem Res. 2018;43(3):637–649. [DOI] [PubMed] [Google Scholar]
- 18.Wessels JM, Wu L, Leyland NA, Wang H, Foster WG. The brain-uterus connection: brain derived neurotrophic factor (BDNF) and its receptor (Ntrk2) are conserved in the mammalian uterus. PLoS One. 2014;9(4):e94036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue YH, Bai XD, Zhang HJ, et al. Gene polymorphisms affect the effectiveness of atorvastatin in treating ischemic stroke patients. Cellular Physiology Biochemistry. 2016;39(2):630–638. doi: 10.1159/000445654 [DOI] [PubMed] [Google Scholar]
- 20.Liang J, Yue Y, Jiang H, et al. Genetic variations in the p11/tPA/BDNF pathway are associated with post stroke depression. J Affect Disord. 2018;226:313–325. doi: 10.1016/j.jad.2017.09.055 [DOI] [PubMed] [Google Scholar]
- 21.Wang GL, Zhang R, Zhou YT, et al. Combined effects of a body shape index and serum c-reactive protein on ischemic stroke incidence among mongolians in China. Biomedical Environmental Sciences. 2019;32(3):169–176. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Chen L, Xu C, et al. Expression profile and bioinformatics analysis of circular RNAs in acute ischemic stroke in a South Chinese Han population. Sci Rep. 2020;10(1):10138. doi: 10.1038/s41598-020-66990-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Yoshimura S, Delcourt C, et al. Thrombolysis outcomes in acute ischemic stroke by fluid-attenuated inversion recovery hyperintense arteries. Stroke. 2020;51(7):2240–2243. doi: 10.1161/STROKEAHA.119.028550 [DOI] [PubMed] [Google Scholar]
- 24.Tejeda GS, Esteban-Ortega GM, San Antonio E, Vidaurre OG, Diaz-Guerra M. Prevention of excitotoxicity-induced processing of BDNF receptor TrkB-FL leads to stroke neuroprotection. EMBO Mol Med. 2019;11(7):e9950. doi: 10.15252/emmm.201809950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong ZY, Yu SS, Wang ZJ, Zhu YZ. SCM-198 ameliorates cognitive deficits, promotes neuronal survival and enhances CREB/BDNF/TrkB signaling without affecting abeta burden in abetapp/PS1 mice. Int J Mol Sci. 2015;16(8):18544–18563. doi: 10.3390/ijms160818544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tejeda GS, Ayuso-Dolado S, Arbeteta R, Esteban-Ortega GM, Vidaurre OG, Diaz-Guerra M. Brain ischaemia induces shedding of a BDNF-scavenger ectodomain from TrkB receptors by excitotoxicity activation of metalloproteinases and gamma-secretases. J Pathol. 2016;238(5):627–640. doi: 10.1002/path.4684 [DOI] [PubMed] [Google Scholar]
- 27.Chow R, Wessels JM, Foster WG. Brain-derived neurotrophic factor (BDNF) expression and function in the mammalian reproductive Tract. Hum Reprod Update. 2020;26(4):545–564. doi: 10.1093/humupd/dmaa008 [DOI] [PubMed] [Google Scholar]
- 28.An JJ, Kinney CE, Tan JW, Liao GY, Kremer EJ, Xu B. TrkB-expressing paraventricular hypothalamic neurons suppress appetite through multiple neurocircuits. Nat Commun. 2020;11(1):1729. doi: 10.1038/s41467-020-15537-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Z, Ding X, Yang Q, et al. Association between single-nucleotide polymorphisms of the tyrosine kinase receptor b (trkb) and post-stroke depression in China. PLoS One. 2015;10(12):e0144301. doi: 10.1371/journal.pone.0144301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ropret S, Zupanc T, Komel R, Videtic Paska A. Investigating the associations between polymorphisms in the NTRK2 and NGFR genes and completed suicide in the Slovenian sample. Psychiatr Genet. 2015;25(6):241–248. doi: 10.1097/YPG.0000000000000109 [DOI] [PubMed] [Google Scholar]