Abstract
Introduction
Aflatoxins are secondary metabolites produced mainly by the molds Aspergillus flavus, A. parasiticus and A. nomius, and they contaminate cereals, dry fruits, oilseeds and spices. Aflatoxins have harmful effects in animals and humans, inducing vomiting, diarrhea, hepatitis, cirrhosis, immunosuppression, miscarriages, mutagenic and teratogenic effects, resulting in different cancers. Popcorn (Zea mays everta) is a cereal susceptible to aflatoxin contamination, and there are no reports about the risk of its consumption.
Purpose
A study on the incidence and consumption of aflatoxins in popcorn marketed in the city of Veracruz, Mexico was conducted and evaluated to carry out a risk assessment for human health.
Methods
To obtain popcorn, a random sampling in 30 places was done. Frequency of consumption was obtained with informed consent of participants of 253 surveys that considered gender (56% women and 44% men), age (13 less than 18 years, 218 older than 18 years and 22 older than 60 years) and the average body weight, which was 65.5 kg for women and 72.7 kg for men.
Results
Aflatoxins were found in 47% of the 30 samples. The estimated daily consumption among women was 21 g of popcorn daily with 2.8 ng kg−1 body weight aflatoxin B1 (AFB1) and 18.29 ng kg−1 body weight total aflatoxins, and for men, the values were 3.0 ng kg−1 body weight AFB1, and 16.0 ng kg−1 body weight of total AF; 1 ng kg−1 body weight is recommended as the tolerance limit by the JECFA (2001).
Conclusion
The highest liver cancer risk was detected in men population under 18 years of age, with 0.137 cases in 100,000 persons. The results show that 9.5% of the consumers of AFB1-contaminated popcorn are at risk, and 52.2% are at risk for total aflatoxin exposure. Popcorn is accessible to children with lower weight, increasing the risk.
Keywords: risk assessment, mycotoxins, liver cancer
Plain Language Summary
Aflatoxins are secondary metabolites produced mainly by the molds Aspergillus flavus, A. parasiticus and A. nomius, and they contaminate cereals, as popcorn and other foods. Aflatoxins have harmful mutagenic and teratogenic effects in animals and humans, resulting in different cancers. There are no reports about the risk of consumption of aflatoxin-contaminated popcorn (Zea mays everta). The objective was to obtain the incidence and consumption of aflatoxins in popcorn marketed in the city of Veracruz, Mexico, to carry out a risk assessment for human health based on 253 surveys about the frequency of consumption, gender, age and body weight. The aflatoxin extraction was obtained with AOAC, 2006, method, the purification with immunoaffinity columns and quantitation by high-performance liquid chromatography. Aflatoxins were found in 47% of the 30 samples. Consumption of popcorn by women was 21 g daily with 2.8 ng kg−1 body weight AFB1, and for men were 18.29 ng kg−1 body weight total aflatoxins, the values 1 ng kg−1 body weight is recommended as the tolerance limit by the JECFA, 2001. The conclusion was that the highest risk was detected in the men population under 18 years of age, with 22 g popcorn ingestion. The results show that 9.5% of the consumers of AFB1-contaminated popcorn are at risk, and 52% are at risk for total aflatoxin exposure. Popcorn is accessible to children with lower weight, increasing the risk.
Introduction
Maize (Zea mays L.) has been a staple food in Mexico for more than 7000 years,1 and this country is considered the origin of maize (Zea mays L.).2 Corn is consumed in different ways, one of which is in the form of popcorn, which is produced when the corn, classified as Zea mays Everta, is heated at temperatures of 170°C to 185°C.1,3 The United States of America has the largest production of popcorn (420,000 tons), followed by Argentina (230,000 tons), France and Hungary (40,000 tons), which are the main producers worldwide. Tamaulipas State has the highest production of popcorn in Mexico in 2015, with a total crop yield of 788.54 tons from Reynosa (693.34 tons), Gustavo Díaz Ordaz (63.20 tons) and Camargo (32.00 tons).4 Popcorn can be contaminated with fungi mainly Aspergillus flavus and A. parasiticus,5 which produce toxic secondary metabolites called aflatoxins (AFs), that pass to the corn cob and grains in the field or during storage, although there are few reports about the risk of AFs in regard to popcorn consumption.
When cereals contaminated with AFs are ingested by animals or humans, they have harmful effects, such as inducing vomiting, diarrhea, hepatitis, cirrhosis, immunosuppression, miscarriages, mutagenic and teratogenic effects, and different cancers. AFs are carcinogenic toxins classified as Grade I, proven to be carcinogenic for humans.6 A. flavus produces only aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2) and generally contaminates maize. A. parasiticus has the ability to produce four basic AFs, AFB1, AFB2, aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2), Supplementary Figure 1, and contaminates different cereals, such as maize,7 rice,8 barley and sorghum; oilseeds, such as nuts,9 almonds, peanuts,10 sunflower seeds, cocoa; spices, such as hot pepper,11 black, green and white peppers,12 and dry fruits.
Regarding AFs in popcorn, there are reports13 that evaluated the risk for health for their ingestion from Brazil,14–16 and Spain17 with a range of AFB1 from 3.72 to 33.5 µg kg−1 and a content of 2.4 µg kg−1 AFB2.
The aim of this work is to determine the risk of AF consumption for human health, from the ingestion of contaminated popped popcorn, studying the exposure.
Methods
Sampling
A grid was drawn on a map of the city of Veracruz to generate a coordinate system that was introduced to the MATLAB statistical software (MathWorks, Natick, Massachusetts, USA) to generate 30 random sampling points, Figure 1. Three hundred grams of popped popcorn were obtained from these 30 sampling points. The popcorn was ground in a mill (Glen, Creston, Stanmore, UK), with a 0.5 mm sieve; each sample was dried at 40°C with an airspeed of 1.5 m/s in a tray dryer (Apex, Construction LTD, Gravesend Kent, UK), until it reached 6% humidity. Samples were vacuum packed in trilaminated bags and stored at −38°C until use.
Figure 1.
Sampling points and survey application of questionnaire in the City of Veracruz, Mexico.
Popcorn Consumption Survey
The consumption of popcorn was assessed through 253 food frequency applied questionnaires, developed with informed consent of participants to obtain information on age, weight, gender, place, frequency and quantity of popcorn consumption, type, and place where popcorn was purchased. The City of Veracruz has 52% women and 48% men18 in 2020. The persons that answer the questionnaire were older than 14 years.
Validation of the AF extraction was performed19 considering the parameters of linearity, limits of detection (LOD) and quantification (LOQ), percentage of recovery and selectivity.20 The B1, B2, G1, and G2 aflatoxin standards (Sigma-Aldrich, St. Louis MO, USA) were dissolved in a mixture of benzene/acetonitrile (98:2 v/v).19 The 425–450 nm absorbance was measured for each AF standard solution with a UV-visible spectrophotometer (Thermo Genesys 10 UV, Madison Wi, USA). A 1000 ng mL−1 AF stock concentration was attained to calculate ten dilutions (0.01, 0.05, 0.1, 0.5, 2.0, 10, 20, 30, 40 and 50 ng mL−1), necessary to obtain the AF calibration curves and the R2 coefficient of determination. Supplementary Figure 2
Aflatoxins in Popcorn
The AFs of 15 g of each homogenized, dry and ground sample were analyzed.19 The homogenate was centrifuged at 2067 x g/15 min, and the supernatant was recovered. The equivalent of 1 g of the sample was taken from the supernatant and diluted (1:4 v/v) in phosphate-buffered solution (PBS) pH of 7.4; the mixture was applied, at a rate of 5 mL per minute, through an Easi-Extract immunoaffinity column for total AFs (R-Biopharm LTD, Glasgow, UK).21 AFs were eluted from the column and dried in an oven (Novatech BTC 9100, Houston, Texas, USA) at 40°C, and AF eluates were derivatized.22,23
AF quantification was performed by high-performance liquid chromatography (HPLC) (Agilent, Series 1200) equipped with an isocratic pump (G1310A Series DE62957044) at a 425–450 nm absorbance range with a fluorescence detector (G1321A Series DE 60456380) and autosampler (G1329A Series DE64761666). The separation was carried out using a C18 E 4.6 x 150 mm VDS Optilab VDSpher 100 column with a particle diameter size of 5 µm, and the ChemStation 32 program was used. The mobile phase was water/acetonitrile/methanol (65:15:20 v/v/v) at 1.2 mL min−1 speed.
Included Variables
Weight Probability Density Function
The population weight density probability function was generated using popcorn consumption frequency survey data.
Aflatoxin Estimated Daily Intake (EDI)
Popcorn consumption data in the city of Veracruz were expressed in terms of consumption per day. The population was classified into groups by gender, age and weight. For each group, the consumption of the population was adjusted to a probability density function (PDF). The Monte Carlo method with @Risk6 software (Palisade, Colorado, USA) was applied. The formula was:
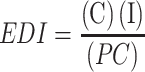 |
where:
EDI = Estimated daily intake
C = PDF of the estimated daily intake of popcorn, g/day.
I = PDF popcorn contamination, ng g−1.
PC = PDF of body weight of the population of the City of Veracruz (kg).
Exposure to AF Due to Popcorn Consumption
The risk of developing liver cancer as a result of popcorn consumption was calculated using the data of the probability functions of weight and consumption, evaluated for each age group and gender. The characterization of the danger by means of the qualitative or quantitative evaluation of the health damages caused by AFs present in the food in humans cannot be carried out with dose-response studies. The ability of AFB1 to cause liver cancer in humans was demonstrated long ago,24,25 with substantial hepatocarcinogenic evidence of the synergistic relationship between the hepatitis B virus and AFB1.26 The World Health Organization (WHO) established that the power of AFB1 to cause cancer in the human population with HBV− and HBV+ was 0.013 and 0.328 per 100,000 inhabitants per year for each ng kg−1 daily exposure, respectively. The exposure units are ng kg−1 body weight/per day (bw/d). In this study, the average AF in the analyzed samples was used. The formula for population exposure to AF through consumption was calculated:27,28
 |
The exposure units are ng kg−1 body weight/per day (bw/d).
Risk of Incidence of Liver Cancer Induced by AFs in Popcorn
Population risk was calculated by multiplying the exposure data and the average potency of AFB1 for the HBV + and HBV− groups for populations in developing countries that have a 25% prevalence of AF-related liver cancer. The equation of AF-related hepatocellular carcinoma risk for populations was estimated:27,28
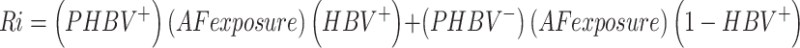 |
where Ri is the cancer risk, with a population fraction of chronic HBV cases (HBV+) and potency estimates, P, for the HBV+ fraction of the population and the fraction of the general population (HBV−).26
Statistical Analysis
The data were subjected to nonparametric statistical analysis, initially applying the Mann–Whitney test and functions of PDF and the Monte Carlo method. Subsequently, to identify significant differences, the Wilcoxon pair test was performed to compare groups by gender.
Results
Validation of the Chemical Method
All the validation parameters (LOD, LOQ, R2, RT, percentage of recovery and sensitivity) were in optimal conditions (Table 1).
Table 1.
Validation Parameters of Basic Aflatoxins and the Hydroxylated Metabolite Aflatoxicol
| Aflatoxin | LOD (ng g−1) | Linearity (Calibration Curves) | Recovery Percentage | |
|---|---|---|---|---|
| Retention Time (Min) | R2 | |||
| AFB1 | 0.01 | 7.085–8.849 | 0.9986 | 97% |
| AFB2 | 0.02 | 17.452–20.228 | 0.9819 | 95% |
| AFG1 | 0.05 | 5.722–5.876 | 0.9897 | 93% |
| AFG2 | 0.05 | 11.215–14.513 | 0.9946 | 96% |
| AFL | 0.01 | 3.032–5.569 | 0.9978 | 98% |
Abbreviations: LOD, limit of detection; R2, coefficient of determination; min, minutes.
Sampling and Survey
Popcorn was found at all points, the results of the consumption of popcorn were based on 300 questionnaires obtained from the conducted survey; however, only 253 people declared themselves as popcorn consumers. The classification of the people surveyed considered gender and age (Table 2). The data obtained in the survey agree with the data reported for the population of Veracruz, of which 53% are women and 47% are men.29 Stratification by age indicates that most of this population is between 15 and 55 years old29 (Table 2).
Table 2.
Classification of the Population by Gender and Age on Popcorn Consumption
| A. Classification of the Population by Gender, Surveyed on Popcorn Consumption | ||
|---|---|---|
| Number of People | % of the Surveyed Population | |
| Women | 141 | 56 |
| Men | 112 | 44 |
| Total | 253 | 100 |
| B. Stratification by Age of the Surveyed People on the Consumption of Popcorn | ||
| Age (Years) | Number of People | % of the Surveyed Population |
| <18 | 13 | 5 |
| ≥ 18 <60 | 218 | 86 |
| > 60 | 22 | 9 |
| Total | 253 | 100 |
Regarding the values of the probability density function for the age of the popcorn consumers, no significant difference was found in men and women in the same age range (Table 3). On the other hand, Table 4 shows the values generated in the PDF for the weight of popcorn consumers; statistically, the average weight in all age ranges was higher in men than in women, as two Mexican reports support the weight of persons over 14 years old, which was 73.31–73.83 kg in men and 65.52–65.83 kg in women.30,31 However, the values of the PDF for daily popcorn consumption do not show a significant difference between men and women in all age ranges, with values of 20.7 g/day for women and 21.4 g/day for men (Table 5). The values of popcorn consumption obtained in our research are lower than those reported for the USA population,32 in which the population over the age of 14 years consumes, on average, 38.8 g/day, with the highest consumption (45.2 g/day) in the group of over 50 years and the lowest consumption (27.8 g/day) in the population from 4 to 11 years old.
Table 3.
Parameters of the Probability Distribution Function (Normal Log) of the Age of the Consumer Population of the City of Veracruz
| Parameters | Age | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| General | ˂18 | > 18 <60 | >60 | General | ˂18 | > 18 <60 | >60 | |
| Mean | 36a | 14a | 33a | 69a | 33a | 16a | 31a | 72a |
| Median | 30 | 15 | 31 | 69 | 28 | 17 | 28 | 72 |
| Standard deviation | 16 | 3 | 12 | 5 | 16 | 2 | 11 | 8 |
| P95 | 70 | 17 | 55 | 76 | 68 | 17 | 55 | 85 |
| N | 141 | 6 | 121 | 14 | 112 | 7 | 97 | 8 |
Notes: The same letter “a” in the same age range represent that there is no significant difference between men and women, at a level of significance of 5%.
Table 4.
Parameters of the Probability Distribution Function (PDF Log-Normal) Weight (Kg) of the Population
| Parameters | Weight (kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| General | <18 | > 18 <60 | >60 | General | <18 | > 18 <60 | >60 | |
| Mean | 66b | 52b | 66b | 67b | 73ª | 64a | 73ª | 79ª |
| Median | 64 | 53 | 65 | 66 | 72 | 60 | 72 | 78 |
| Standard deviation | 12 | 13 | 12 | 10 | 11 | 8 | 10 | 11 |
| Percentile 95 | 85 | 65 | 90 | 84 | 90 | 76 | 90 | 93 |
| N | 141 | 6 | 121 | 14 | 112 | 7 | 97 | 8 |
Notes: Different letters “a” and “b” are used to differentiate means statistically different at a level of significance of 5%, between the mean weight of men and women, in the same age range.
Table 5.
Parameters of the Probability Distribution Function (Normal PDF Log) of the Daily Intake of Popcorn in the City of Veracruz
| Parameters | Daily Intake of Popcorn (g/Day) | |||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| General | <18 | ˃18 <60 | >60 | General | <18 | ˃18 <60 | >60 | |
| Average (g/day) | 21ª | 28a | 23ª | 5a | 21ª | 28a | 22ª | 4a |
| Median | 8 | 8 | 8 | 4 | 8 | 13 | 8 | 3 |
| Standard deviation | 38 | 5 | 41 | 3 | 30 | 27 | 31 | 5 |
| Percentile 95 | 85 | 14 | 85 | 9 | 73 | 106 | 77.9 | 11 |
Notes: Letter “a” means the average (g/day) represent no significant differences between men and women in the daily intake of popcorn at level of significance of 5%. There is no difference between Average (g/day) women and men for all the age ranges.
Aflatoxins in Popcorn
One type of AF, the AFB1, was detected in at least 47% of the samples, while in 53% of the samples, no AFs were detected. As shown in Table 6, our findings agree with published reports,33 who found AFs in only 48% of the popcorn samples; in other studies,16 only the AFs of 13% of popcorn samples were found. The popcorn consumption of 29 persons was 77 g/month, and the amount of average daily ingested AFs was 0.12 ng kg−1 bw/day. Another study carried out in Spain17 detected AFs in 6% of the popcorn samples analyzed.
Table 6.
Concentration and Types of Aflatoxins in Popcorn
| Samples | Aflatoxin Concentration (µg kg−1) | |||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | AFL | AFt | |
| 1 | 26 | 0 | 46 | 0 | 7 | 79 |
| 2 | 0 | 0 | 0 | 0 | 19 | 19 |
| 3 | 0.2 | 0 | 0 | 2 | 5 | 7 |
| 4 | 0.4 | 38 | 0 | 28 | 54 | 120 |
| 5 | 0 | 0 | 0 | 0 | 9 | 9 |
| 6 | 0 | 0 | 0 | 0 | 14 | 14 |
| 7 | 12 | 15 | 25 | 0 | 4 | 56 |
| 8 | 1 | 0 | 2 | 0 | 3 | 6 |
| 9 | 35 | 0 | 0 | 1 | 0 | 36 |
| 10 | 15 | 0 | 0 | 4 | 0.01 | 19 |
| 11 | 2 | 0 | 0 | 2 | 18 | 22 |
| 12 | 0 | 0 | 0 | 0 | 19 | 19 |
| 13 | 12 | 21 | 0 | 0 | 0 | 33 |
| 14 | 0.3 | 0 | 0 | 2 | 14 | 16 |
| 15 | 0 | 0 | 0 | 1 | 0.4 | 1 |
| 16 | 0 | 0 | 0.1 | 0.4 | 2 | 3 |
| 17 | 0 | 0 | 0 | 0 | 1 | 1 |
| 18 | 0 | 0 | 0 | 1 | 1 | 2 |
| 19 | 0 | 0 | 0 | 0 | 1 | 1 |
| 20 | 0 | 50 | 0 | 1 | 5 | 56 |
| 21 | 0 | 0 | 0 | 1 | 7 | 8 |
| 22 | 0 | 0 | 0.2 | 0 | 20 | 20 |
| 23 | 0 | 0 | 0.2 | 0.3 | 10 | 11 |
| 24 | 0 | 0 | 0.2 | 0 | 14 | 14 |
| 25 | 0.3 | 0 | 0.3 | 0 | 10 | 11 |
| 26 | 0.2 | 0 | 0.3 | 1 | 12 | 14 |
| 27 | 1 | 0 | 0 | 2 | 12 | 15 |
| 28 | 0 | 0 | 0.1 | 0 | 13 | 13 |
| 29 | 3 | 0 | 0 | 0 | 4 | 7 |
| 30 | 0 | 0 | 0 | 0 | 1 | 1 |
| Average | 3.6 | 4.1 | 2.5 | 1.6 | 9.3 | 21.1 |
Note: The fractions < 1 in the AF concentrations are shown depending on the Limit of Detection of each AF.
Abbreviations: AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2; AFL, aflatoxicol; Aft, total aflatoxin.
Discussion
In the present research, the highest incidence of AFB1 was (35 ng g−1) and average concentration (3.6 ng g−1), but AFL, that is also carcinogenic was the highest average concentration of 9.3 ng g−1 followed by the other AFs, Table 6. The average AFt contamination in the popcorn was 21.1 ng g−1, but we must take into account that AFB2 and AFG2, that added 5.7 ng g−1 are not carcinogenic themselves and must be substracted from the carcinogenic AFs (AFB1, AFG1, AFL), giving an intake of carcinogenic AFs of 15.4 ng g−1, higher than the reported in Brazil14–16 and Spain.17
The importance of this study is the analysis of the four basic AFs (AFB1, AFB2, AFG1, AFG2) and the hydroxylated metabolite AFL, that can interconvert with AFB1, increasing the risk in popcorn. No reports of these AFs have been given.
Exposure to AF Through Popcorn Consumption
The population group with the greatest exposure level to AFs as a result of consuming popcorn was men under 18 years of age, probably due to their greater consumption and lower body weight (bw) (Table 7). On the other hand, the population group with the lowest exposure was men over 60 years of age, who have the lowest consumption and the highest bw. On the other hand, women between 18 and 60 years old had the highest exposure for the population in this age range. The average exposure level in women was 3.63 (ng kg−1 bw/per day), while in men, it was 5.018 (ng kg−1 bw/per day). As a result of popcorn consumption, the population has an average exposure of 4.326 (ng kg−1 bw/per day); this value is within the average range of AF exposure (2.9–5.8 ng kg−1 bw/per day) calculated by WHO27 for cluster G05 to which Mexico belongs, and below that reported for the population in Egypt (5.3–20 ng kg−1 bw/per day) due to the consumption of corn-based snacks (Table 7).
Table 7.
Level of Exposure to Aflatoxins Through the Consumption of Popcorn in the City of Veracruz, Classified by Gender and Age Range of the Consuming Population
| Age Range (Years) | Exposure (ng kg−1 Body Weight/per Say) | |
|---|---|---|
| Women | Men | |
| <18 | 1 | 3 |
| ≥18<60 | 2 | 2 |
| > 60 | 1 | 0.3 |
Risk of Liver Cancer Due to AF Exposure from Popcorn Consumption
The cancer risk values due to AF exposure through the consumption of popcorn are shown in Table 8. A similar behavior was reported for the European population, where population groups under 18 years of age have the highest risk of AF-induced cancer.28,29 On average, the risk of liver cancer in women due to AF exposure from the consumption of popcorn is 0.993 (cancers/year per 100,000 population), while for men, the average risk is 0.137 (cancers/year per 100,000 population). Considering both genders, the average risk of liver cancer due to AF exposure through the consumption of popcorn is 0.1185 (cancers/year per 100,000 population). This result agrees with the range reported27 by the WHO (2018) for food cluster G05 (where Mexico is grouped), which has a risk level of 0.05–1.18 (cancers/year per 100,000 population) as a result of exposure to AF. The average risk calculated in our research is higher than the range (0.01–0.10 cancers/year per 100,000 population) calculated for European countries and other developed countries of the G07 and G08 food security clusters; however, it is lower than the range (0.21–3.94 cancers/year per 100,000 population) reported for sub-Saharan African countries and Haiti in cluster G1328 (Table 8).
Table 8.
Risk of Cancer
| A. Calculation of the Risk of Liver Cancer Due to Aflatoxin Exposure Through the Consumption of Popcorn in the City of Veracruz, Classified by Gender and Age Range of the Consuming Population | ||
|---|---|---|
| Age Range (Years) | Risk (Cancers / Year per 100,000 Population) | |
| Women | Men | |
| <18 | 0.048b | 0.137a |
| ≥18<60 | 0.108a | 0.097a |
| > 60 | 0.024a | 0.016a |
| B. Estimated liver Cancer Cases/100,000 Population/Lifetime Years | ||
| Age Range (Years) | Women Estimated Liver Cancer Cases/100,000 Population/78 Years | Men Estimated Liver Cancer Cases/100,000 Population/73 Years |
| <18 | 4 | 10 |
| ≥18<60 | 8 | 7 |
| > 60 | 2 | 1 |
Notes: Different letters “a” and ”b”, in the same age range represent significant differences between men and women, at a level of significance of 5%.
Conclusion
The present study is accurate because it takes into account four AFs and the prevalent hydroxylated metabolite AFL. The highest risk was detected in the population under 18 years of age, with 22 g popcorn ingestion. The results show that 9.5% of the consumers of AFB1-contaminated popcorn are at risk, and 52% are at risk for total aflatoxin exposure. Popcorn is accessible to children with lower weight, increasing the risk.
The risk of developing liver cancer as a result of exposure to AF through popcorn consumption in Veracruz population is 0.1185 per 100,000 inhabitants; it is recommendable that the population under 18 years of age reduce their popcorn consumption because it contributes to the daily ingestion of carcinogens in food.
Acknowledgments
The authors thank CONACYT for the scholarship given to Tomás Morales-Moo for his Mastership studies. To the Instituto Tecnológico de Veracruz for the popcorn sampling and the Instituto de Biología, Universidad Nacional Autónoma de México (IBUNAM) for the data analysis, funding, methodology and laboratory analysis. The authors also thank IBUNAM´s personnel: Pedro Mercado from the Secretaría Técnica, and Joel Villavicencio, Jorge López, Alfredo Wong, Celina Bernal, provided valuable assistance with, computer analysis and design. Additionally, we thank Georgina Ortega Leite and Gerardo Arévalo for library information.
Funding Statement
Instituto de Biología, Universidad Nacional Autónoma de México, and Instituto Tecnológico de Veracruz.
Ethics and Consent Statements
The Regional Committee for Medical and Health Research Ethics of Veracruz, Mexico approved the use of the data obtained in que questionnaires, for research purposes. We fulfilled all the ethical requirements, and we confirm in the revised manuscript that this study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Byrd JE, Perona MJ, Kinetics of popping of popcorn. Cereal Chem. 2005;82(1):53–59. doi: 10.1094/CC-82-0053 [DOI] [Google Scholar]
- 2.Figueroa JDC, Mauricio RA, Taba S, et al. Kernel characteristics and tortilla making quality of maize accessions from Mexico, the Caribbean, and South and Central America. Latin American maize germplasm conservation: regeneration, in situ conservation, core subsets, and prebreeding. In: Taba S, editor. Proc Workshop CIMMYT; April 7–10, 2003. México DF: CIMMYT; 2003:51–57. [Google Scholar]
- 3.Johnny WP, Schwartzberg HG. Determination of vapor pressure in vapor‐induced puffing. AiChE J. 1994;40(1):160–165. [Google Scholar]
- 4.SIAP, Servicio de información agroalimentaria y pesquera. Anuario estadístico de la producción agrícola por Estado, México. 2015. Datos Abiertos, Estadística de Producción Agrícola. Gobierno de México. Available from: http://infosiap.siap.gob.mx/gobmx/datosAbiertos.php.
- 5.Lawlor PG, Lynch PB. Mycotoxin management. Afric Farm Food Proc. 2005;46:12–13. [Google Scholar]
- 6.IARC (International Agency for Research on Cancer). Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, & International Agency for Research on Cancer. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. World Health Org IARC Monogr. 2002;82:1–592. [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Urueta P, Carvajal M, Méndez I, Meza F, Gálvez A. Survey of aflatoxins in maize tortillas from Mexico City. Food Addit Contam Part B Surveill. 2011;4(1):42–51. [DOI] [PubMed] [Google Scholar]
- 8.Suárez-Bonnet E, Carvajal M, Méndez-Ramírez I, et al. Aflatoxin (B1, B2, G1 and G2) Contamination in rice of Mexico and Spain, from local sources or imported. J Food Sci. 2013;78(11):1822–1829. [DOI] [PubMed] [Google Scholar]
- 9.Adaya-González J, Carvajal-Moreno M, Rojo-Callejas F, Ruiz-Velasco S. Aflatoxins in walnut (Juglans regia L.), pecan (Carya illinoinensis (Wangenh.) K. Koch) and cashew (Anacardium occidentale L.) nuts of Mexico. Pharm Anal Acta. 2015;6(2):338–350. doi: 10.4172/2153-2435.1000338 [DOI] [Google Scholar]
- 10.Alvarado-Hernández JR, Carvajal-Moreno M, Rojo-Callejas F, Ruiz-Velasco S. Aflatoxins in natural peanut (Arachis hypogaea L.) of Mexico City. Validation of the immunoaffinity column extraction and HPLC quantification method. J Plant Biochem Physiol. 2016;4(2):168–178. doi: 10.4172/2329-9029.1000168 [DOI] [Google Scholar]
- 11.Rosas-Contreras C, Carvajal-Moreno M, Rojo-Callejas F, Ruiz-Velasco S. Identification and HPLC quantification of AFs in dried hot peppers (Capsicum annum L.), in Mexico and other countries. J Drug Metab Toxicol. 2016;7(1):198–208. doi: 10.4172/2157-7609.1000198 [DOI] [Google Scholar]
- 12.Garduño-García JI, Carvajal-Moreno M, Rojo-Callejas F, Ruiz-Velasco S. Detection of Aflatoxins, mutagens and carcinogens, in black, white and green peppers (Piper nigrum L.). J Microb Biochem Technol. 2017;9(3):95–104. doi: 10.4172/1948-5948.1000350 [DOI] [Google Scholar]
- 13.Caldas ED, Silva SC, Oliveira JN. Aflatoxins and ochratoxin A in food and the risks to human health. Rev Saude Publica. 2002;36(3):319–323. doi: 10.1590/s0034-89102002000300010 [DOI] [PubMed] [Google Scholar]
- 14.Leiko-Sekiyama B, Braga-Ribeiro A, Machinski MJ. Aflatoxins, ochratoxin A and zearalenone in maize-based food products. Braz J Microbiol. 2005;36(3):289–294. [Google Scholar]
- 15.Amaral KASD, Nascimiento GB, Sekiyama BL, Janeiro V, Machinski JM. Aflatoxins in products based in maize, commercialized in Brazil and risks for human health . Food Sci Technol. 2006:26(2), 336-342. doi: 10.1590/S0101-20612006000200016 [DOI] [Google Scholar]
- 16.Jager AV, Tedesco MP, Souto PCMDC, Oliveira CAFD. Assessment of aflatoxin intake in São Paulo, Brazil. Food Control. 2013;33(1):87–92. [Google Scholar]
- 17.Alborch L, Bragulat MR, Castellá G, Abarca ML, Cabañes FJ, Mycobiota and mycotoxin contamination of maize flours and popcorn kernels for human consumption commercialized in Spain. Food Microbiol. 2012;32(1):97–103. doi: 10.1016/j.fm.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 18.INEGI, Instituto Nacional de Estadística y Geografía. Panorama Demográfico; 2020. Available from: http://ceieg.veracruz.gob.mx/wp-content/uploads/sites/21/2019/10/2.-Censo-2020_CEIEG_INEGI.pdf. Accessed July20, 2020
- 19.AOAC, Association of Official Analytical Chemists. Natural toxins Horwitz W, Latimer GW, Trucksess MW, editors. In: Official Methods of Analysis of AOAC International. 18th ed. Gaithersburg (MD): AOAC International; 2006:1–51. Available from: http://documents.mx/documents/aoac-2005.html. Accessed October31, 2020. [Google Scholar]
- 20.Cruz-Rueda V. Validation Course of Analytical Methods. Capacitation Center R.H. World SA CV, 2016. Available from: www.rhworld.com.mx.
- 21.R-Biopharm Rhône LTD. Easi-extract, aflatoxin (RP71/RP70); 2016. Available from: https://food.r-biopharm.com/products/easi-extract-aflatoxin-2/. Accessed October31, 2020.
- 22.Kok WT. Derivatization reactions for the determination of aflatoxins by liquid chromatography with fluorescence detection. J Chromatogr. 1994;659(1–2):127–137. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama H, Goda Y, Tanaka T, Toyoda M, Determination of aflatoxins B1, B2, G1 and G2 in spices using a multifunctional column clean-up. J Chromatogr A. 2001;932(1–2):153–157. doi: 10.1016/S0021-9673(01)01211-0 [DOI] [PubMed] [Google Scholar]
- 24.WHO (World Health Organization). Evaluation of certain food additives and contaminants. Forty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Tech Rep Series No. 952. Geneva; 2008. Available from: https://apps.who.int/iris/bitstream/handle/10665/44062/WHO_TRS_952_eng.pdf. Accessed October31, 2020. [PubMed] [Google Scholar]
- 25.Codex Alimentarius. International Food Standards. Guidelines for the simple evaluation of dietary exposure to food additives CAC/GL 3-1989. Adopted 1989. Revision 2014. CAG/GL 3-1989. FAO, WHO; September 15, 2014. Available from: file:///C:/Users/IB/Downloads/cxg_003e%20(5).pdf.
- 26.Wogan GN, Kensler TW, Groopman JD. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma: a review. Food Addit Contam. 2012;29(2):249–257. doi: 10.1080/19440049.2011.563370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO (World Health Organization). Safety Evaluation of Certain Contaminants in Food. WHO Food Addit Series: 74, FAO JECFA Monogr 19 bis; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/276868/9789241660747-eng-pdf. Accessed October31, 2020.
- 28.Schrenk D, Bignami M, Bodin L, et al. Scientific opinion–risk assessment of aflatoxins in food. EFSA, European Food Safety Authority, EFSA panel on contaminants in the food chain. EFSA J. 2020;18(3):6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SEFIPLAN, Ministry of Financial and Planning from the State of Veracruz government . System of Municipal Information. Cuadernillos Municipales (Municipal Booklets). Veracruz, 2019. Available from: https://ceieg.veracruz.gob.mx/2019/05/09/cuadernillos-municipales-2019/. AccessedMarch12, 2020.
- 30.Wall-Martínez HA, Ramírez-Martínez A, Wesolek N, et al. Statistical analysis of corn improved mycotoxin exposure estimates for the population of Veracruz City, Mexico. Food Addit Contam Part A. 2017;34(5):864–879. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Camarillo E, Ramírez-Martínez A, Vargas-Ortíz M, et al., Consumption data for the main cheeses (Mexican style fresh and Oaxaca) for dietary exposure assessment among the population of Veracruz city, Mexico. Adv Dairy Res J. 2016;4:164–172. [Google Scholar]
- 32.Grandjean AC, Fulgoni III VL, Reimers KJ, Agarwal S. Popcorn consumption and dietary and physiological parameters of US children and adults: analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2002 dietary survey data. J Amer Dietetic Assoc. 2008;108(5):853–856. [DOI] [PubMed] [Google Scholar]
- 33.Andrade GCRM, Pimpinato RF, Francisco JG, Monteiro SH, Calori-Domingues MA, Tornisielo VL. Evaluation of mycotoxins and their estimated daily intake in popcorn and cornflakes using LC-MS techniques. LWT. 2018;95:240–246. doi: 10.1016/j.lwt.2018.04.073 [DOI] [Google Scholar]



