Abstract
Background:
There is no consensus on treatment of irreparable massive rotator cuff tears (MRCT). The goal of this systematic review and meta-analysis was to (1) compare patient-reported outcome (PRO) scores, (2) define failure and reoperation rates, and (3) quantify magnitude of patient response across treatment strategies.
Methods:
MEDLINE, Embase, CENTRAL, and Scopus databases were searched for studies including physical therapy and operative treatment of MRCT. The criteria of the Methodological Index for Nonrandomized Studies were used to assess study quality. Primary outcome measures were PRO scores as well as failure, complication and reoperation rates. To quantify patient response to treatment, we compared changes in the Constant-Murley (CMS) and American Shoulder and Elbow Surgeons (ASES) score to previously reported minimum clinically important difference (MCID) thresholds.
Results:
No level I or II studies were found that met the inclusion / exclusion criteria. Physical therapy was associated with a 30% failure rate and another 30% went on to have surgery. Partial repair was associated with a 45% re-tear rate and 10% reoperation rate. Only graft interposition was associated with a weighted average change that exceeded the MCID for both CMS and ASES score. Latissimus tendon transfer techniques utilizing humeral bone tunnel fixation were associated with a 77% failure rate. Superior capsular reconstruction with fascia lata autograft was associated with a weighted average change that exceeded the MCID for ASES score. Reverse arthroplasty was associated with a 10% prosthesis failure rate and 8% reoperation rate.
Conclusion:
There is a lack of high-quality comparative studies to guide treatment recommendations. Physical therapy compared to surgery is associated with a lower improvement in perceived functional outcome and higher clinical failure rate.
Level of Evidence:
Level IV; Systematic Review
Keywords: Irreparable massive rotator cuff tear, systematic review, meta-analysis, patient-reported outcomes, response to treatment, survival, failure rate, reoperation, complications
As the most common upper extremity condition in people over 50 years old,54 rotator cuff tears represent a significant clinical challenge in our aging population. The overall incidence of rotator cuff tears ranges from 5–40%,52, 53 with approximately 54% of individuals over the age of 60 having a partial or complete rotator cuff tear.63 Massive rotator cuff tears (MRCT), commonly defined as involving a full-thickness tear of at least two tendons10 or measuring greater than five centimeters in the coronal plane,23 are estimated to comprise approximately 20% of all rotator cuff tears and 80% of recurrent tears.5, 43
Increasing rotator cuff tear size is associated with poor outcomes and high structural failure rates following surgical repair.37, 58 A review of 18 studies reporting outcomes after repair of massive tears found a re-tear rate of 78%. Despite the high rate of structural failure, much of the published literature supports an attempt at primary repair.4 However, a number of these MRCT are retracted or lack tendon length so that they cannot be re-attached to their footprint and thus are irreparable. Numerous treatment strategies, such as physical therapy, débridement, partial repair, graft interposition, tendon transfer, superior capsular reconstruction, balloon arthroplasty, and reverse shoulder arthroplasty, have been proposed to treat irreparable MRCT. The comparative efficacy of these treatments remains unclear.
The purpose of this systematic review and meta-analysis was to evaluate the highest quality clinical evidence currently available to recommend either for or against the various treatment options for irreparable MRCT. This was accomplished by (1) comparing patient-reported outcome (PRO) scores across treatment strategies, (2) reporting failure and reoperation rates, and (3) quantitatively evaluating the magnitude of patient response to treatment. We hypothesized that (1) there is a lack of a consistent definition of irreparable MRCT, (2) operative treatment of the irreparable tear leads to greater improvement in PRO scores when compared with nonoperative treatment, and (3) there is no single superior operative treatment strategy due to a lack of high-quality evidence.
Materials and Methods
Search Rationale
This systematic review and meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines. MEDLINE, Embase, CENTRAL (Cochrane Central Register of Controlled Trials), and Scopus databases were searched in November 2019 for studies addressing eight treatment methods for irreparable MRCT: physical therapy, débridement, partial repair, graft interposition, tendon transfer, superior capsular reconstruction, balloon arthroplasty, and reverse shoulder arthroplasty. Separate searches were carried out for each treatment. Search terms included “massive rotator cuff tear” AND terms associated with each treatment: (“physical therapy OR rehabilitation”) / “debridement” / “partial repair” / (“scaffold OR patch OR graft interposition OR platelet rich plasma OR augmentation OR stem cell”) / (“tendon transfer OR latissimus dorsi tendon transfer OR lower trapezius tendon transfer”) / (“superior capsular reconstruction OR superior capsule reconstruction”) / (“(subacromial OR sub-acromial) AND (balloon OR spacer)) OR balloon arthroplasty”) / (“reverse total shoulder arthroplasty OR reverse shoulder arthroplasty OR reverse shoulder prosthesis”). Titles, abstracts, and full texts were screened to identify potentially relevant studies.
Study Eligibility
Eligibility criteria were determined a priori. Inclusion criteria included studies of any level of evidence with a minimum two-year clinical follow-up with criteria defining MRCT and reporting of validated PROs and/or range of motion data. Exclusion criteria included studies that included patients with a repairable rotator cuff tear, rotator cuff tear arthropathy with Hamada stage ≥ 3 (glenohumeral arthritis),28 fractures, rheumatoid arthritis, or instability, as well as case reports, biomechanical studies, reviews, surgical techniques, or studies written in a language other than English. Studies that included patients with or without glenohumeral arthritis were included if data for patients without glenohumeral arthritis were reported separately.
Data Abstraction
Extrapolated data were recorded using a standardized data collection spreadsheet for all sections. This included study design and patient demographics (Supplemental Tables 1a–9a), MRCT diagnosis criteria (Supplemental Tables 1b–9b), clinical outcomes before and after treatment intervention, including VAS pain scores (range 0–10), range of motion, PRO scores, radiographic analysis, failure and revision rates, and complications (Supplemental Tables 1c–9c). All continuous variables were reported as a mean ± standard deviation, unless the standard deviation was unavailable, in which case range was reported, if available.
Assessment of Study Quality
Two reviewers (R.J.S. and D.K.) independently assessed the methodologic quality of all included studies with the Methodological Index for Nonrandomized Studies (MINORS) scoring system.64 Studies with a MINORS score <75% were excluded.
Response to Treatment
To determine variation in magnitude of response to treatment of the Constant-Murley Score (CMS) and the American Shoulder and Elbow Surgeons (ASES) score, we compared pre-to-post-treatment score changes to the minimally clinical important difference (MCID) thresholds determined by previous rotator cuff studies.21, 32 The CMS and ASES were chosen because they were the most frequently reported PROs among included studies. The change in ASES and CMS for each study reporting either PRO as well as the weighted average change in score for each treatment modality were graphically compared to previously reported MCID thresholds. The weighted average change in PRO score was influenced by sample size. For CMS, we used an MCID of 15 for nonoperative treatment and an MCID of 30 for operative management.31 For ASES, we used an MCID of 17 for nonoperative and 39 for operative management.20 All selected MCID threshold values were calculated by prior studies via an anchor-based approach, in which the change in PRO score is anchored to a separate global rating of change questionnaire that determines overall patient improvement with their treatment outcome at final follow-up.
Results
Search Strategy and Data Aggregation
We identified 120 relevant studies with 77 not meeting the inclusion criteria, leaving 43 studies included in this review (Figure 1). All 43 studies were included in the qualitative synthesis and 37 studies were included in the quantitative synthesis. The most common reasons for exclusion were insufficient follow-up, inclusion of rotator cuff tear sizes other than massive (without a subgroup analysis of massive tears), and failure to define the criteria for MRCT. For each treatment strategy, the data were aggregated and pooled where appropriate, such that Table I provides a summary of study design and demographics, Table II includes criteria for defining MRCT, and Table III outlines clinical outcomes, failure rates, and reoperation rates.
Figure 1.
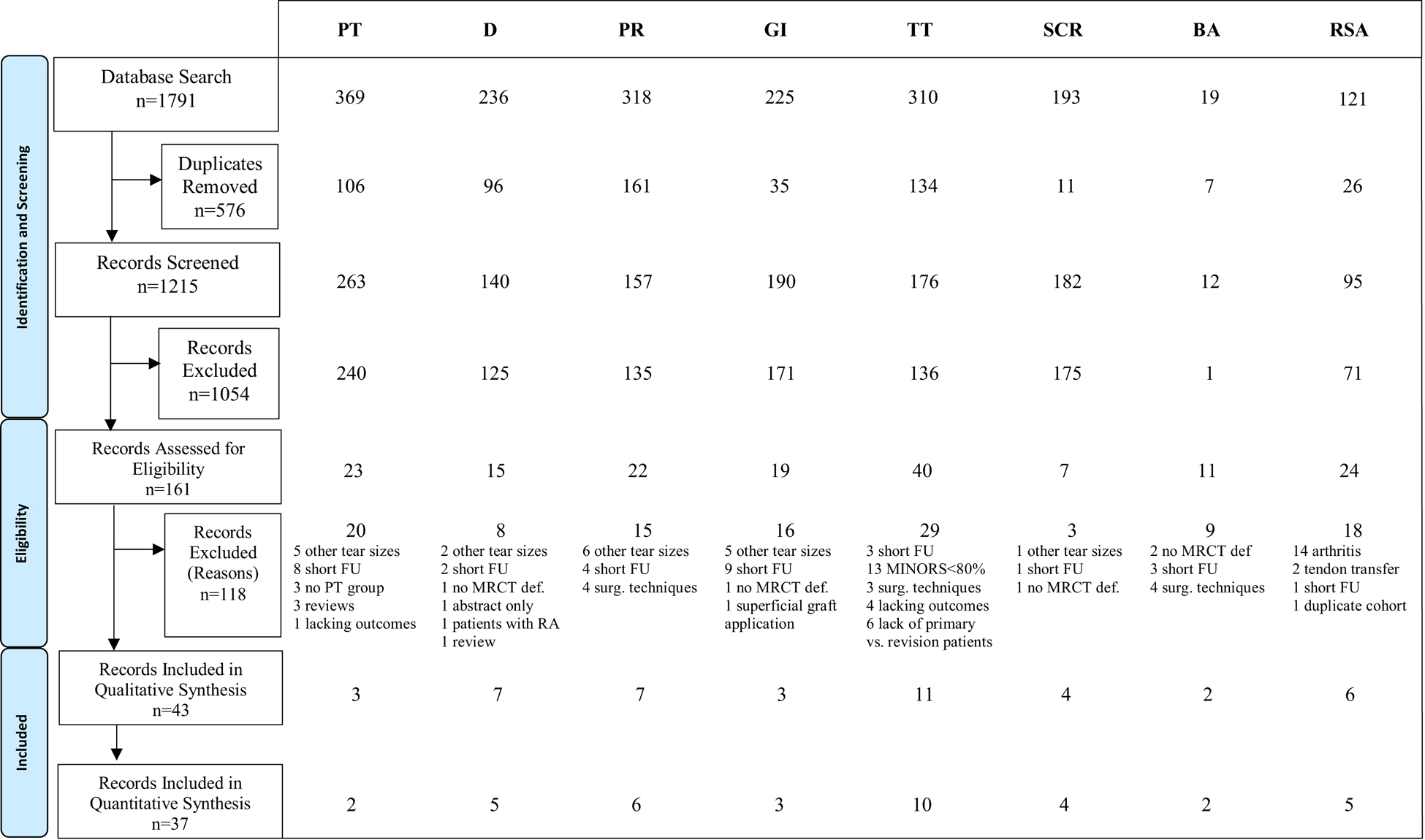
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram representing search and screen process of studies reporting on nonoperative and operative treatment of irreparable massive rotator cuff tears. CENTRAL, Cochrane Central Register of Controlled Trials; PT, Physical therapy; D, Débridement; PR, Partial repair; GI, Graft interposition; TT, Tendon transfer; SCR, Superior capsular reconstruction; BA, Balloon arthroplasty; RSA, Reverse shoulder arthroplasty; FU, Follow-up; MRCT, Massive rotator cuff tear; RA, Rheumatoid arthritis.
Table I.
Study design and patient demographics
| Treatment | No. Papers | LOE | MINORS Score | N (M/F) | Age (y) | Follow-up (months) |
|---|---|---|---|---|---|---|
| Physical Therapy | 3 | III (1); IV (2) | 89.6% | 94 (59/35) | 68.3 (54–89) | 32 (24–65) |
| Debridement | 7 | III (1); IV (6) | 88.8% | 256 (160/96) | 65.7 (33–82) | 48 (24–120) |
| Partial Repair | 7 | III (1); IV (6) | 89.0% | 226 (122/93)* | 62.7 (33–81) | 45.2 (24–90) |
| Graft Interposition | 3 | IV (3) | 85.4% | 67 (39/28) | 68.2 (51–85) | 34.3 (24–86) |
| Tendon Transfer | 11 | III (1); IV (10) | 88.1% | 506 (319/187) | 59.2 (53–64.2) | 57.7 (24–147) |
| Arthroscopic-Assisted | 4 | IV (4) | 89.1% | 144 (68/76) | 61.7 (59–64.2) | 70.2 (24–147) |
| Open | 7 | III (1); IV (6) | 87.5% | 362 (251/111) | 57.7 (53–61) | 35.8 (24–77) |
| SCR | 4 | IV (4) | 81.3% | 179 (12/11)* | 64.7 (43–82) | 44.5 (24–60+) |
| HDA | 1 | IV | 87.5% | 38 | 59.4 | 24 |
| TFL | 3 | IV (3) | 79.2% | 141 (12/11)* | 66.4 (65.1–68.0) | 51.4 (24–110) |
| Balloon | 2 | IV (2) | 93.8% | 25 | 68.8 (54–85) | 42 (24–60) |
| RSA | 6 | IV (6) | 85.4% | 247 (48/74)* | 67.5 (34–86) | 39.4 (24–118) |
MINORS score represented as weighted average for each treatment strategy.
N=Total number of patients per treatment group reported; M=male; F=female.
Age and follow-up reported as mean (range) in years and months, respectively.
LOE Level of evidence
SCR Superior Capsular Reconstruction
RSA Reverse Shoulder Arthroplasty
AA Arthroscopic-Assisted
HDA Human Dermal Allograft
TFL Tensor Fascia Lata Autograft
Incomplete reporting of number of patients based on gender
Table II.
Massive rotator cuff tear criteria
| Treatment | No. Papers | Tear Size | Tendon No. | Tendon Retraction (Patte) | Goutallier Fatty Infiltration (SITS) | AHI (mm) or Hamada Classification |
|---|---|---|---|---|---|---|
| Physical Therapy | 3 | NR | ≥2 (3) | Stage 3(1) | Grade 4(1) Grade 3–4 (2) |
AHI Preop 8.2 (1), Postop 5.6 (1) Hamada Stage 1–2 (1) |
| Debridement | 7 | ≥5cm (5) | ≥2 (5) | Stage 2 (1) Stage 3 (1) 4–5cm (1) “Unable to Reattach” (1) |
Grade 3–4 (1) 19 (1) | AHI Preop <5 (1), Preop 5.1 (1) |
| Partial Repair | 7 | ≥5cm (3) | ≥2 (5) ≥3 (2) |
Stage 2(1) | Grade 3–4 (1) Grade 1.9 (1.5–2.2) (2) |
AHI Preop <7 (1) AHI Preop 8.8 (1) Hamada Stage 1 (1) Hamada Stage 1–2 (1) |
| Graft Interposition | 3 | ≥5cm (2) ≥4cm (1) |
≥2 (2) | NR | Grade 3.75 (1) | AHI Preop 7.7 (1), Postop 8.6 (1) |
| Tendon Transfer | 11 | ≥5cm (5) | ≥2 (7) | Stage 2–3 (2) Stage 3 (5) “Excessive” (2) |
Grade 3–4 (11) |
AHI Preop 4.2 (2.3–5.9) (4), Postop 5.1 (4.7–5.7) (3) AHI Preop <5 (1) AHI Preop <7 (1) AHI decreased by 1.5 (1) Hamada Preop 1.7 (0–2) (2), Postop 2.2 (1–5) (2) Hamada Preop Grade 1–2 (1) Hamada Postop Grade 2–3 (1) |
| AA | 4 | ≥5cm (1) | ≥2 (2) | Stage 2–3 (2) Stage 3(1) |
Grade 3–4 (4) |
AHI Preop 3.13 (1), Postop 5.7 (1) AHI Preop <5 (1) Hamada Preop Grade 1–3 (1) |
| Open | 7 | ≥5cm (4) | ≥2 (5) | Stage 3 (4) “Excessive” (2) |
Grade 3–4 (7) |
AHI Preop 4.6 (2.3–5.9) (3), Postop 4.8 (4.7–4.9) (2) AHI Preop <7 (1) AHI decreased by 1.5 (1) Hamada Preop 1.7 (0–2) (3), Postop 2.2 (1–5) (3) Hamada Preop Grade 1–2 (1) Hamada Postop Grade 2–3 (1) |
| SCR | 4 | ≥5cm (1) | ≥2 (3) | ≥5cm (1) | Grade 3–4 (1) Grade 2.6 (2.0–3.7) (3) |
AHI Preop 4.9 (4.6–7.3) (4), Postop 9.1 (8.1–9.9) (4) |
| HDA | 1 | ≥5cm | - | ≥5cm | Grade 3–4 | AHI Preop 7.3, Postop 9.9 |
| TFL | 3 | - | >2 (3) | - | Grade 2.6 (2.0–3.7) (3) | AHI Preop 4.1 (3.4–4.6) (3), Postop 8.8 (8.1–9.7) (3) |
| Balloon | 2 | ≥5cm (2) | ≥2 (1) | NR | Grade 3–4 (1) “Unsuitable for Repair” (1) |
AHI Preop 6.7 (1), Postop 8.0 (1) |
| RSA | 6 | ≥5cm (1) | ≥2 (6) | Stage 2(1) Stage 3(1) |
Grade 3–4 (3) |
AHI Preop <6 (1) AHI Preop <7 (1) Hamada Preop <4 (4) |
SCR Superior Capsular Reconstruction
RSA Reverse Shoulder Arthroplasty
SITS Supraspinatus, Infraspinatus, Teres Minor, Subscapularis
AHI Acromiohumeral Interval
AA Arthroscopic-Assisted
HDA Human Dermal Allograft
TFL Tensor Fascia Lata Autograft
NR Not Recorded
Table III.
Clinical outcomes, failure, and reoperation rates
| Treatment | No. Papers | VAS Initial | VAS Final | ROM Initial | ROM Final | PRO Initial | PRO Final | Failure / Survival | Revision/Reoperation |
|---|---|---|---|---|---|---|---|---|---|
| Physical Therapy | 3 | 6.7 (1) | 3.7 (1) |
FF 97 (76–115) (3) Abd 118 (1) ER 44 (1) IR 76 (1) |
FF 133 (129–136) (2) Abd 136 (1) ER 39 (1) IR 66 (1) |
CMS 43 (1) ASES 39 (1) SSV 45 (1) |
CMS 62.4 (56–69) (2) ASES 62 (1) SSV 64 (60–68) (2) |
12/30 (40%) Success 9/30 (30%) Failed PT |
9/30 (30%) Surgery |
| Debridement | 7 | 7.6 (7–7.9) (3) | 3.1 (2–4.3) (3) |
FF 106 (96–115) (2) Abd 74 (57–90) (2) ER 20 (1) |
FF 137 (118–155) (2) Abd 118 (85–150) (2) ER 42 (1) IR T12 (1) Scaption 111 (1) |
CMS 42.7 (31–72.2) (5) ASES 25.5 (24–27) (2) UCLA 11.5 (1) SSV 35.2 (1) qDASH 63.4 (1) |
CMS 63.9 (42.3–89) (6) ASES 62.4 (55–69.8) (2) UCLA 21 (1) SSV 73.2 (1) DASH 41.3 (1) qDASH 24.1 (1) SPADI 38.4 (1) |
NR | 1/23 (4.2%) (1) |
| Partial Repair | 7 | 6.3 (5.6–7) (4) | 1.8 (1.5–2) (4) |
FF 126 (95–168) (3) Abd 90 (1) ER 34 (20–44) (3) IR 84% (1) |
FF 160 (154–172) (4) Abd 150 (120–169) (3) ER 39 (27–54) (5) IR T9 (2) |
CMS 39.6 (36.6–43.1) (3) ASES 44.5 (41–46.6) (3) SSV 34.7 (1) SST 5.6 (1) qDASH 52.5 (1) Oxford 17.8 (1) |
CMS 71.5 (67.5–76.3) (3) ASES 79.3 (78.6–80.1) (3) SSV 74.0 (1) SST 9.1 (1) qDASH 55.8 (1) Oxford 37.1 (1) SANE 96 (1) SPADI 29.5 (1) |
16/111 (14.4%) unsatisfactory outcome (2) 25/55 (45.4%) re-rupture (2) |
16/165 (9.7%) (4) |
| Graft Interposition | 3 | 6.9 (6.8–7) (2) | 1.9 (1–2.8) (2) |
FF 67 (65–69) (2) Abd 64 (60–68) (2) ER 36 (32–39) (2) IR 3.8/10 (3.4–4.2) (2) |
FF 128 (120–136) (2) Abd 127 (120–134) (2) ER 48 (38–57) (2) IR 8.0/10 (7.5–8.4) (2) |
CMS 36.2 (25.7–46.7) (2) ASES 29 (1) UCLA 10.2 (1) SST 2.4 (1) |
CMS 78.3 (72.1–84.5) (2) ASES 74 (1) UCLA 29.4 (1) SST 7.8 (1) |
1/5 (20%) re-rupture (1) | 1/5 (20%) (1) |
| Tendon Transfer | 11 | 7.7 (7.5–7.8) (2) | 2.6 (2.4–2.8) (3) |
FF 95 (58–134) (11) Abd 84 (40–112) (11) ER 18 (12–29) (11) IR L3 (1) |
FF 137 (120–157) (11) Abd 123 (90– 154) (11) ER 33 (23–50) (11) IR L3 (1) |
CMS 35.9 (21–47.3) (10) ASES 39.2 (30.1–48.3) (2) UCLA 6.5 (1) SSV 31.3 (19.3–54) (5) DASH 52 (1) |
CMS 63.5 (58–69.5) (10) ASES 71.7 (66.7–73.2) (3) UCLA 27.5 (1) SSV 66.8 (48.9–78) (5) SST 7 (1) DASH 18 (1) |
29/184 (15.8%) LD re-rupture (4) 8/122 (6.6%) SSC insufficiency (2) 2/122 (1.6%) deltoid avulsion (2) 2/55 (3.6%) stiffness (1) 6/55 (10.9%) LD insufficiency (1) |
24/356 (6.7%) (6) |
| AA | 4 | 7.5±1.0 (1) | 2.7 (2.5–2.8) (2) |
FF 97 (58–134) (4) Abd 64 (51–80) (4) ER 19 (13–29) (4) |
FF 140 (130–157) (4) Abd 115 (93–130) (4) ER 33 (28–42) (4) |
CMS 30.1 (21–37) (4) UCLA 6.5 (1) SSV 22.7 (19.3–26) (2) |
CMS 60.5 (58–65.4) (4) ASES 66.7 (1) UCLA 27.5 (1) SSV 60.0 (48.9–71.1) (2) SST 7 (1) |
28/129 (21.7%) LD re-rupture (3) | 10/115 (8.7%) (2) |
| Open | 7 | 7.8±1.5 (1) | 2.4±1.9 (1) |
FF 93 (70–118) (7) Abd 87 (40–112) (7) ER 19 (14–23) (7) IR L3 (1) |
FF 136 (120–151) (7) Abd 128 (90–154) (7) ER 33 (23–50) (7) IR L3 (1) |
CMS 39.8 (32–47.3) (6) ASES 39.2 (30.1–48.3) (2) SSV 37.0 (28–54) (3) DASH 52 (1) |
CMS 65.4 (60–69.5) (6) ASES 71.7 (70.2–73.2) (2) SSV 71.4 (60–78) (3) DASH 18 (1) |
1/55 (1.8%) LD re-rupture(1) 8/122 (6.6%) SSC insufficiency (2) 2/122 (1.6%) deltoid avulsion (2) 2/55 (3.6%) stiffness (1) 6/55 (10.9%) LD insufficiency (1) |
14/241 (5.8%) (4) |
| SCR | 4 | 5.6 (4.3–6.9) (2) |
1.1 (0.9–1.2) (2) |
FF 97 (84–123) (4) Abd 106 (1) ER 27 (26–27) (3) IR L3 (1) |
FF 154 (148–162) (4) Abd 160 (1) ER 41 (40–43) (3) IR L1 (1) |
ASES 34.3 (23.5–49.5) (4) UCLA 9.9 (1) JOA 50.9 (48.3–53.0) (3) |
ASES 91.2 (85.3–94.3) (4) UCLA 32.4 (1) JOA 92.4 (91.4–93.2) (3) |
11/180 (6.1%) graft failure (4) 3/24 (12.5%) retear of ISP (1) |
6/126 (4.8%) (2) |
| HDA | 1 | 4.3 | 1.2 |
FF 123 Abd 106 |
FF 162 Abd 160 |
ASES 49.5 | ASES 85.3 | 3/38 (7.9%) graft failure | 1/38 (2.6%) |
| TFL | 3 | 6.9 (1) | 0.9 (1) |
FF 89 (84–123) (3) ER 27 (26–27) (3) IR L3 (1) |
FF 152 (148–156) (3) ER 41.4 (40–43) (3) IR L1 (1) |
ASES 29.2 (23.5–35) (3) UCLA 9.9 (1) JOA 50.9 (48.3–52) (3) |
ASES 93.2 (92.3–94.3) (3) UCLA 32.4 (1) JOA 92.4 (91.4–93.2) (3) |
8/142 (5.6%) graft failure (3) 3/24 (12.5%) retear of ISP (1) |
5/88 (5.7%) |
| Balloon | 2 | 6.6 (1) | 2.8 (1) |
FF 71 (1) Abd 65 (1) |
FF 129 (1) Abd 125 (1) |
CMS 38.0 (34.2–41.8) (2) SAS 6.7 (1) |
CMS 67.1 (66.8–67.4) (2) SAS 8.0 (1) |
NR | 1/20 (5%) (1) |
| RSA | 6 | 5.9 (5.5–6.3) (2) | 2.0 (1.9–2.0) (2) |
FF 69 (53–94) (3) ER 29 (21–40) (3) |
FF 133 (122–143) (3) ER 40.2 (29–51) (3) |
CMS 26.5 (23–27.8 (3) ASES 37.5 (33.3–41.6) (2) SST 1.0 (1.6–2.2) (2) |
CMS 59.4 (55–63.4) (3) 74.7 (74–75.4) (2) SST 7.1 (6.5–7.6) (2) |
16/159 (10.1%) prosthesis failure (3) 14/231 (6.1%) fracture (4) 4/206 (1.9%) instability (3) |
19/231 (8.2%) (4) |
AA Arthroscopic-Assisted
SCR Superior Capsular Reconstruction
HDA Human Dermal Allograft
TFL Tensor Fascia Lata Autograft
RSA Reverse Shoulder Arthroplasty
VAS Visual Analogue Scale
PRO Patient-Reported Outcome
CMS Constant-Murley Score
ASES American Shoulder and Elbow Surgeons
UCLA University of California Los Angeles
SSV Subjective Shoulder Value
SST Simple Shoulder Test
SANE Single Assessment Numeric Evaluation
SPADI Shoulder Pain and Disability Index
SAS Shoulder Activity Scale
DASH Disabilities of Arm, Shoulder, and Hand
qDASH Quick Disabilities of Arm, Shoulder, and Hand
JOA Orthopedic Association
ROM Range of Motion
FF Forward Flexion
Abd Abduction
ER External Rotation
IR Internal Rotation
NR Not Reported
PT Physical Therapy
LD Latissimus Dorsi
SSC Subscapularis
ISP Infraspinatus
Physical Therapy
Study Design and Patient Demographics
All three studies were of level III or IV evidence with two prospective and one retrospective study and an average follow-up of 32 months.11, 69, 70 Patients lost to follow-up ranged from 0% to 35%. The number of patients ranged from 19 to 45 (total 94). Nonoperative treatment strategies varied between studies. One study used a home-based three-month anterior deltoid rehabilitation program,69 a second restored passive range of motion and strength without a described duration,70 and a third focused on periscapular and intact rotator cuff muscles and described deltoid muscle coaptation only when the arm was elevated.11
Definition of a MRCT
All three studies defined MRCT as two or more tendon involvement and defined irreparable as fatty muscle infiltration of grade ≥ 3.11, 69, 70
Clinical Outcomes
Complete PRO scores were available for two studies with +13-point and +23-point mean change in CMS and ASES scores, respectively.9;69 Pain scores improved 3 points after physical therapy.69 Complete range of motion data were available from two studies,11, 69, 70 with forward elevation improving by 25°. Meanwhile, Collin et al demonstrated that 53% of patients (24/45) achieved more than 160° forward elevation after treatment.11 Those with subscapularis involvement performed worse than postero-superior rotator cuff tears. Strength improved from 1.1kg to 1.9 kg.69
Survival and Complications
For patients treated with anterior deltoid rehabilitation, 40% (12/30) had a successful outcome, 30% (9/30) chose surgery, and 30% (9/30) did not improve with the rehabilitation program.69 In another study, 18% (7/40) of patients elected to undergo surgery after failing nonoperative treatment.70
Débridement
Study Design and Patient Demographics
All seven articles were of level III or IV evidence with five retrospective22, 30, 35, 38, 41 and two prospective studies.36, 48 All articles had minimal loss to follow-up. The number of patients ranged from 23 to 57 (total 256) with an average age of 65.7 years. Average follow-up was 48 months with two studies reporting follow-up of at least 5 years.22, 38
Definition of a MRCT
There was variability in the criteria used for defining MRCT. The most common were ≥2-tendon involvement or >5 cm anterior-posterior width size. One study referenced either two tendons torn or retraction past the glenoid.41
Clinical Outcomes
Five studies used the CMS with +26-point mean change in scores before and after surgery,22, 30, 35, 36, 41 while two studies used the ASES with +37-point mean change in scores.22, 41 Three studies reported pain scores with an average improvement of 4.5 points.22, 30, 41 Range of motion data were available from five studies,22, 30, 35, 36, 41 but only two measured motion both before and after surgery,22, 30 with forward elevation increasing by 32°.
Survival and Complications
Complications were seldom reported. One study reported 4.9% of patients (2/41) developing complex regional pain syndrome type 135 while another study reported 6.1% (2/33) and 3.0% (1/33) developed seromas and infections, respectively.22
Partial Repair
Study Design and Patient Demographics
All seven articles were of level III55 or IV evidence.30, 48, 55,9, 12, 16, 21 Study sample size ranged from 11 to 90 (total 226) with an average age of 62.7 years. Two studies had greater than 5% loss to follow-up.12, 21 Average follow-up was 45.2 months with two studies reporting follow-up of at least 5 years.12, 21
Definition of a MRCT
All studies defined MRCT by number of tendons torn (two torn tendons in five studies12, 16, 21, 48, 55 and three torn tendons in two studies.9, 30 Three studies included tear size (≥ 5 cm) as an additional criterion.16, 48, 55 Three studies reported preoperative fatty infiltration,9, 30, 55 three the acromiohumeral interval,9, 16, 21 and two the Hamada classification,9, 12 demonstrating variability in criteria used for defining MRCT.
Clinical Outcomes
Six studies reported PROs both before and after surgery. The mean change in scores with CMS30;21;55 and ASES16;48;9 was +32 points and +35 points, respectively. Pain scores improved by about 4.5 points.16; 9;12;30 Three studies reported motion both before and after surgery, with forward elevation and external rotation improving on average by 30° and 11°, respectively.12, 16, 30
Survival and Complications
Pooled rates for re-tear defined as re-rupture on postoperative MRI or ultrasound,9, 30 unsatisfactory outcomes,12, 21 and revision surgery16;48;30;21 were 45% (25/55), 14% (16/111), and 9.7% (16/165), respectively. Reasons for revision surgery included re-tear (12/16), infection (2/16), anchor loosening (1/16), or AC joint cyst (1/16).
Graft Interposition
Study Design and Patient Demographics
The three articles were of level IV evidence with one prospective study and two retrospective studies.2, 44, 51 (Supplemental Table 5d) The number of patients ranged from five to 41 (total 67) with an average age of 68.2 years and an average follow-up of 34.3 months.
Definition of MRCT
There was variability in the criteria used for defining MRCT with ≥ two tendon involvement reported in two articles.2, 44, 51 All three articles quantified tear size with two reporting > 5cm44, 51 and one > 4cm2 tear size for the diagnosis of MRCT.
Clinical Outcomes
Two of three studies used the CMS with +42-point mean change in scores before and after surgery,2, 51 while one study used the ASES with +45-point mean change in scores.44 Two studies reported pain scores with an average improvement of 5 points.44, 51 Range of motion before and after surgery was reported for two studies,2, 51 with forward elevation and external rotation improving on average by 61° and 12°, respectively.2, 51
Survival and Complications
Pooled rates for re-tear2, 51 and revision surgery2, 51 were 20% (1/5) and 20% (1/5), respectively.
Tendon Transfer
Study Design and Patient Demographics
All eleven articles were of level III49 or IV evidence.8, 14, 17, 18, 24, 25, 27, 32–34, 49 Study sample size ranged from 14 to 86 (total 506) with an average of 59 years. Average follow-up was 57.7 months with two studies reporting follow-up of at least 9 years.17, 25 It is worth noting that there was overlap in the cohorts of patients reported by two separate pairs of studies,25, 27, 34;24 (Supplemental Table 6a) which slightly skews conclusions drawn from analysis of all patients between the eleven studies.
All studies utilized the latissimus dorsi tendon8, 14, 17, 24, 25, 27, 32–34, 49 except for Elhassan et al who transferred the lower trapezius.18 The latissimus transfer surgeries were performed either using the open two-incision technique popularized by Gerber26 or an arthroscopic-assisted approach.14, 27, 33, 34 Operative technique details are outlined in Supplemental Table 6d.
Definition of MRCT
All studies defined MRCT as either involving ≥ 2 tendons (i.e., supraspinatus and infraspinatus),17, 18, 24, 25, 33, 34, 49 or measuring ≥ 5 cm,8, 24, 32, 34, 49 with two studies24, 49 requiring both criteria. Nine of eleven articles reported tendon retraction to at least the level of the glenoid or medial to it5; 11;17, 18, 27, 33, 34; 44 and all studies observed fatty infiltration of Goutallier grade ≥ 3.8, 14, 17, 18, 24, 25, 27, 32–34, 49
Clinical Outcomes
All but one study18 reported CMS, with a mean change of +28 points (+30 points for arthroscopic treatment and +26 points for open treatment). The mean change in ASES was +33 points.17, 49 Pain scores improved by about 5.1 points.17, 33 All studies reported motion both before and after surgery, with forward elevation and external rotation improving on average by 43° and 15°, respectively.
Survival and Complications
Pooled rates for tendon transfer re-tear,14, 17, 18, 25, 27, 34 rotator cuff tear,24, 25, 34 deltoid deficiency,17, 24, 25 and revision surgery17, 18, 24, 25, 27, 34 were 14.6% (35/239), 6.6% (8/122), 1.6% (2/122), 6.7% (24/356), respectively. Twenty-seven of the 35 tendon transfer failures (77%) occurred secondary to humeral bone tunnel fixation with tendon tubularization compared to eight failures with greater tuberosity footprint fixation (23%).27, 34 Postoperative complications included hematoma (8%; 23/286),17, 18, 27, 32, 34 greater tuberosity fracture (7.3%; 4/55),27 deep infection (3.3%; 7/214),14, 18, 27, 32, 34 stiffness (3.1%; 6/193),17, 25, 32 and nerve dysesthesias (2.1%; 9/431).14, 17, 24, 25, 27, 32, 34, 49
Superior Capsular Reconstruction
Study Design and Patient Demographics
All four retrospective articles were of level IV evidence including 177 patients with an average age of 64.7 years and an average follow-up of 44.5 months.45–47, 57 There was overlap in the cohorts reported by three studies,45–47 which slightly skews conclusions drawn from analysis of patients receiving fascia lata autograft. There was variability noted concerning graft characteristics (i.e., type, size, thickness) and glenoid fixation. (Supplemental Table 7d).
Definition of MRCT
Pennington et al defined MRCT using tear size ≥ 5 cm and further characterized muscle quality with the Goutallier grading classification,57 while Mihata et al defined MRCT by two or more tendon invlovement.45–47 All studies reported preoperative fatty infiltration and average changes in the acromiohumeral interval (AHI) ranging from 3.4 mm preoperatively to 9.6 mm postoperatively.45–47, 57
Clinical Outcomes
All studies used the ASES score with +57-point mean change in scores before and after surgery (+36 points for human dermal allograft and +64 points for tensor fascia lata autograft). Two studies reported pain scores with an average improvement of 3.9 points.45, 57 Range of motion before and after surgery was reported for all studies, with forward elevation improving on average by 57°.
Survival and Complications
Pooled rates for structural failure and revision surgery were 6.1% (11/180) and 4.8% (6/126), respectively. Rates of graft tear and revision surgery were 7.9% (3/38) and 2.6% (1/38) with use of human dermal allograft, respectively.57. Rates of infraspinatus re-tear, graft tear, and revision surgery were 12.5% (3/24), 5.6% (8/142), and 5.7% (5/88) with use of tensor fascia lata autograft, respectively.45–47
Balloon Arthroplasty
Study Design and Patient Demographics
Two articles, one retrospective case series and one prospective case series, were included and both were of level IV evidence including 25 patients with an average age of 68.8 years and an average follow-up of 42 months.59, 62
Definition of MRCT
Both studies defined MRCT using tear size ≥ 5 cm. Ricci et al59 also required ≥ two tendons to be torn and Goutallier grade ≥ 3, while Senekovic noted the presence of substantial fatty infiltration deemed unsuitable for repair in all patients without qualitatively assessing its severity.62
Clinical Outcomes
Both studies used the CMS with +29-point mean change in scores before and after surgery. One study reported pain scores with an average improvement of 3.8 points.59 Range of motion before and after surgery was reported for one study, with forward elevation improving on average by 58°.62
Survival and Complications
No complications were noted and one patient (5%; 1/20) needed eventual conversion to RSA within the five-year follow-up period.62
Reverse Shoulder Arthroplasty
Study Design and Patient Demographics
All six articles were retrospective case series of level IV evidence.3, 19, 29, 50, 65, 67 The number of patients included ranged from 17 to 64 (total 247) with an average age of 67.5 years and an average follow-up of 39.4 months. Age data were available from only three studies.19, 29, 50
Definition of MRCT
Tendon number was the most commonly referenced criterion, with a minimum two tendon tear.29, 50, 67;3, 65;15 All studies referenced either the acromiohumeral interval3, 19 or the Hamada classification,27, 29, 50, 67; 60 but the Hamada classification was primarily used to exclude arthritis and not to diagnose MRCT. Of the three studies that assessed tendon retraction,3, 19, 65 a common value for the degree of retraction was not identified. Three studies used the Goutallier classification and considered grade ≥ 3 to be consistent with MRCT.3, 19, 67
Clinical Outcomes
Three studies reported the CMS with +32-point mean change in scores before and after surgery,3;67;19 while two studies reported the ASES score with +37-point mean change in scores.29;50 Two studies reported pain scores with an average improvement of 3.9 points.29;50 Range of motion before and after surgery was reported for three studies, with forward elevation improving on average by 64°.27, 29, 50, 67
Survival and Complications
Pooled rates for prosthesis failure,3, 19, 29, 50, 65, 67 fracture,3, 19, 29, 50, 65, 67 instability,3, 19, 29, 50, 65, 67 and revision surgery3, 19, 29, 50, 65, 67 were 10.1% (16/159), 6.1% (14/231), 1.9% (4/206), and 8.2% (19/231), respectively. One study provided an estimated 90.7% survival at 52 months, with the end-point defined as component revision, removal, loosening, or a worsening ASES score.50
Response to Treatment
The magnitude of change in CMS and ASES score for each treatment strategy compared to the MCID threshold for nonoperative and operative treatment are provided in Figures 2 and 3. Twenty-six studies reported sufficient data for CMS score comparison to MCID. The weighted average change in CMS was greater than MCID for partial repair, graft interposition, balloon arthroplasty, and reverse shoulder arthroplasty. Fifteen studies reported sufficient data for ASES score comparison to MCID. The weighted average change in ASES score was greater than MCID for physical therapy, graft interposition, and superior capsular reconstruction with tensor fascia lata autograft.
Figure 2.
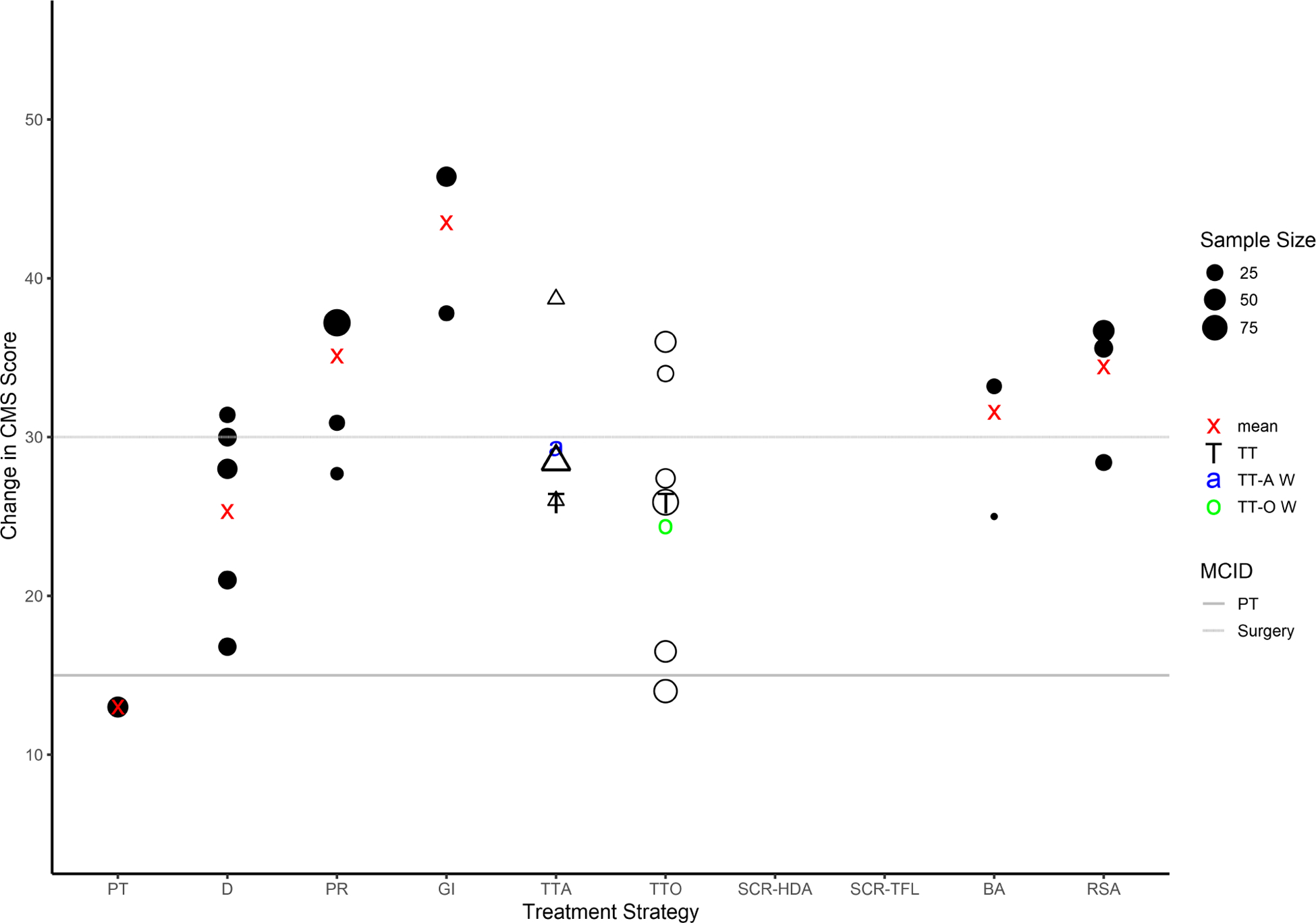
Change in CMS for each treatment strategy compared to MCID threshold for either nonoperative or operative intervention. Sample size is directly proportional to the size of the circle (or triangle for arthroscopic tendon transfer). Influenced by the sample size, the red “x” denotes the weighted average change in CMS for all treatment strategies except tendon transfer. The “T” denotes the weighted average change in CMS for all tendon transfers. The blue “a” represents the weighted average change in CMS for arthroscopic tendon transfer. The green “o” represents the weighted average change in CMS for open tendon transfer. PT, Physical therapy; D, Débridement; PR, Partial repair; GI, Graft interposition; TTA, Arthroscopic tendon transfer; TTO, Open tendon transfer; SCR-HDA, Superior capsular reconstruction—human dermal allograft; SCR-TFL, Superior capsular reconstruction—tensor fascia lata autograft; BA, Balloon arthroplasty; RSA, Reverse shoulder arthroplasty; MCID, Minimum clinically important difference.
Figure 3.
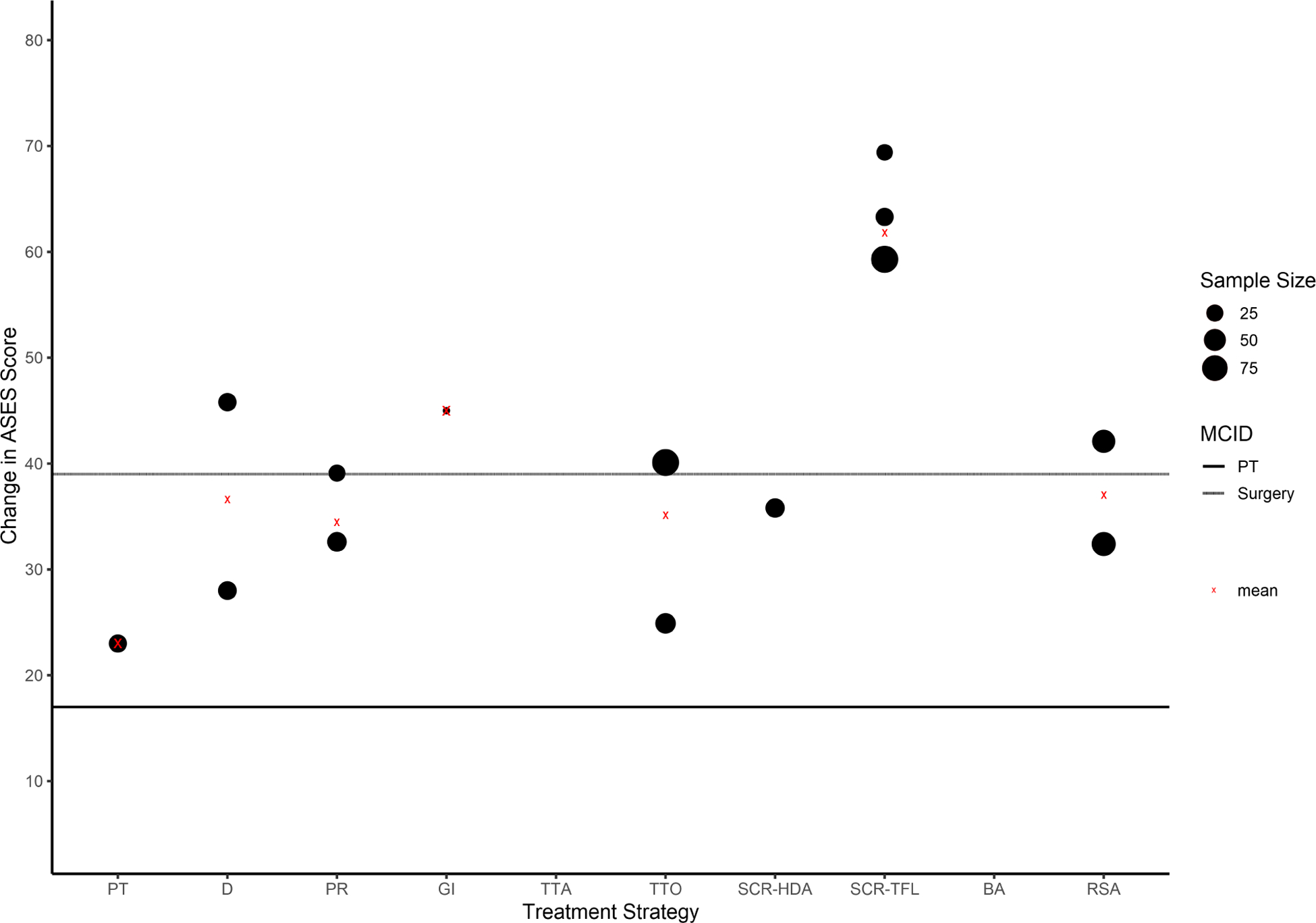
Change in ASES score for each treatment strategy compared to MCID threshold for either nonoperative or operative intervention. Sample size is directly proportional to the size of the circle. The red “x” denotes the weighted average change in CMS for all treatment strategies, which is influenced by sample size. PT, Physical therapy; D, Débridement; PR, Partial repair; GI, Graft interposition; TTA, Arthroscopic tendon transfer; TTO, Open tendon transfer; SCR-HDA, Superior capsular reconstruction—human dermal allograft; SCR-TFL, Superior capsular reconstruction—tensor fascia lata autograft; BA, Balloon arthroplasty; RSA, Reverse shoulder arthroplasty; MCID, Minimum clinically important difference.
Discussion
Our study findings clearly show the absence of high-quality literature on irreparable MRCT. Of all 43 studies, only 9.3% (4/43) were of level III evidence with the remaining of level IV evidence. As such, it is difficult to definitively recommend either for or against one treatment strategy over another for the management of irreparable MRCT. These findings agree with the recommendations provided by the American Academy of Orthopaedic Surgeons 2019 clinical practice guideline on management of rotator cuff injuries. The authors found insufficient evidence to support the efficacy of physical therapy, partial repair, tendon transfer, superior capsular reconstruction (SCR), débridement, allograft augmentation, or RSA in the treatment of irreparable tears, instead concluding based on consensus clinical opinion alone that these treatments may improve patient reported outcomes.1
Treatment decisions when the rotator cuff cannot be repaired will have to be made with professional judgement, surgeon experience, patient expectations, ability to complete postoperative rehabilitation, and a shared decision making process between the surgeon and the patient. In such scenarios, appropriate use criteria (AUC) can provide guidance by considering clinical experience, patient factors (smoking status, worker’s compensation, etc.), and disease type (tear size and fatty infiltration) to indicate the appropriateness of a given intervention for a specific clinical scenario.56, 60
Our current study suggests that physical therapy compared to surgery may lead to high failure rates and inferior clinical outcomes for irreparable MRCT. Physical therapy is promoted to be the first line of treatment when a patient is medically unfit, does not wish to proceed with surgery, or demonstrates a positive response to non-operative care.60 However, with the numbers available, 60% of the patients (18/30) in this review did not respond to physical therapy or went on to have surgery.
Débridement and partial repair showed improvements in VAS pain scores, functional range of motion and PRO scores with lower reoperation rates compared to physical therapy. The majority of débridement studies did not meet the MCID threshold, and as such, débridement may not be a successful treatment strategy. However, Walch et al66 investigated débridement with concomitant biceps tenotomy in 307 patients with full-thickness rotator cuff tears, finding that this combination of procedures led to significant clinical improvement. While this study did not exclusively investigate MRCT, this treatment strategy may be considered in the appropriate patient. A drawback to partial repair was the high re-tear rate (45%; 25/55) and the majority of studies did not meet the MCID threshold.
Surgical reconstruction (graft interposition / tendon transfer) compared to physical therapy showed superior improvements in pain scores, forward elevation, and mean change in CMS and ASES scores. All three graft interposition studies exceeded the MCID threshold, and as such, graft interposition should be investigated further. Arthroscopic-assisted tendon transfer utilizing greater tuberosity fixation techniques are favored over humeral bone tunnel fixation techniques as the latter are associated with a high failure rate (77%; 27/35). Based on the available evidence, open tendon transfer may not be a successful treatment strategy as the majority of studies did not meet the MCID for either ASES or CMS.
SCR and balloon arthroplasty are relatively new procedures with a paucity of data reporting clinical outcomes and rates of failure, revision surgery, and complications. With the numbers available, both SCR and balloon arthroplasty led to an improvement in pain scores, forward elevation, and PRO scores. However, of concern is the high structural failure rate of SCR using human dermal allograft, which has been reported to range from 15–75%6, 7, 15, 39, 57, 68, compared to SCR using tensor fascia lata autograft, with failure rates reported to range from 5–36%13, 40, 42, 45–47 (Table IV). Based on the available evidence, SCR may be considered using fascia lata autograft, and further studies are needed to determine success of SCR with human dermal allograft and efficacy of balloon arthroplasty.
Table IV.
Superior capsular reconstruction graft failure rates
| Author (Year) | Graft Type | N | n with Post-Op MRI | Timing of Imaging (years) | Graft Failure Rate |
|---|---|---|---|---|---|
| Hirihara (2017) | HDA | 8 | 5 | 2 | 1/5 (20%) |
| Denard (2018) | HDA | 59 | 20 | 1 | 11/20 (55%) |
| Pennington (2018) | HDA | 88 | 4 | 2 | 3/4 (75%) |
| Burkhart (2019) | HDA | 10 | 10 | 1 | 3/10 (30%) |
| Woodmass (2019) | HDA | 34 | Not Reported | Not Reported | 22/34 (65%)* |
| Burkhart (2020) | HDA | 41 | 26 | 1 | 4/26 (15%) |
| Lacheta (2020) | HDA | 22 | 21 | 0.2 | 9/21 (43%) |
| HDA Total | 262 | 86 | 32/86 (37%) | ||
| Mihata (2013) | TFL | 24 | 24 | 3 | 4/24 (17%) |
| De Campos Azevedo (2018) | TFL | 22 | 22 | 0.5 | 2/22 (9%) |
| Lee (2018) | TFL | 36 | 36 | 1 | 13/36 (36%) |
| Lim (2018) | TFL | 31 | 31 | 1 | 9/31 (29%) |
| Mihata (2018) | TFL | 88 | 88 | 5 | 4/88 (5%) |
| Mihata (2019) | TFL | 30 | 30 | 2.5 | 3/30 (10%) |
| TFL Total | 231 | 231 | 35/231 (15%) |
SCR: Superior Capsule Reconstruction
HDA: Human Dermal Allograft
TFL: Tensor Fascia Lata Autograft
N=Total number of patients
Graft failure rate determined by clinical examination rather than advanced imaging
Reverse shoulder arthroplasty was found to improve pain scores, functional motion and PRO scores compared to physical therapy. However, this treatment strategy has an 8.2% (19/231) reoperation rate and a 10.1% (16/159) prosthesis failure rate. In light of this, we agree with the AUC that reverse arthroplasty should be considered only in a healthy elderly patient with pseudoparalysis from a chronic irreparable massive tear.56, 60
We found considerable variability in the definition of MRCT. Thirty-two studies required a minimum tear size for diagnosis (i.e., ≥ 5cm), and twenty-three studies required a minimum number of involved tendons (i.e., two). Meanwhile, thirteen studies required both a minimum tear size and a minimum number of involved tendons, and two studies required either a minimum tendon retraction length or a minimum amount of fatty infiltration. Clearly, there is inconsistent reporting on what defines MRCT. How to define MRCT may depend on treatment strategy and patient expectations (i.e., pain relief, restore motion, limit progression of radiographic changes). A recent study using the Delphi method determined with 90% agreement that MRCT should be defined as either axial or coronal tendon retraction to the glenoid rim and/or a tear with ≥67% of the tuberosity exposed in the sagittal plane.61
The major limitation of this review is the lack of high-quality evidence available on the treatment of irreparable MRCT. There were only three comparative studies, all of which compared débridement to partial repair, while the majority were case series (72%; 31/43). Without better quality studies, it is difficult to make evidence-based recommendations for clinical care. Second, we observed an inconsistent reporting of PROs, pain scores, range of motion, strength, failure rates, revision surgery, and complication rates across all treatment strategies. There were twelve different PROs used, with CMS (27 studies) and ASES (17 studies) scores most commonly reported. Similarly, six of 43 studies (14%) reported motion data in four planes (forward flexion, internal rotation, external rotation, and abduction) before and after surgery. Third, we were unable to perform a comprehensive quantitative synthesis due to inconsistent outcome instrument selection. Standardized data collection and reporting are keys to data transparency, and instituting a minimum data set requirement could improve the quality of future studies. Fourth, the results of our quantitative analysis are highly dependent on MCID values selected from prior studies. While separate MCIDs were chosen for operative and nonoperative treatments, the operative MCID available was calculated using data from patients undergoing complete rotator cuff repair only. It is highly likely that each treatment strategy will have a unique MCID threshold if separately determined by anchor-based methodology.
Further limitations of our quantitative analysis are those inherent to anchor-based MCID methods. First, MCID values are highly impacted by the patient population being studied, with less healthy cohorts having lower baseline scores and more opportunity for score improvement. This is particularly relevant when considering the functional impairment seen in patients with irreparable MRCT. Second, anchor-based approaches are subject to recall bias. Results of the global rating of change questionnaire administered to patients at final follow-up are likely influenced by recent developments in each patient’s health status and therefore may reflect a single time-point snapshot of health status rather than magnitude of change from baseline. Third, the timing of MCID determination influences the magnitude of recall bias, with a longer follow-up duration introducing more susceptibility to bias. Lastly, many studies determining MCID are limited by small subject numbers and wide confidence intervals. Gagnier et al evaluated 222 patients with full-thickness rotator cuff tears, but only 22 patients had a minimal clinical improvement. This small subset was further evaluated to determine the ASES MCID for surgical treatment, which was found to have a fragile confidence interval of −7.57 to 85.57.20 Robust MCID values for each treatment strategy matched for age, gender, and racial differences need to be determined through studies with larger sample sizes utilizing a combination of anchor- and distribution-based approaches.
Conclusions
Due to the paucity of high-quality clinical studies available for guiding management of irreparable MRCT, it is currently not possible to recommend for or against any specific treatment strategy. Rather, clinical experience, patient factors, patient expectations, and rotator cuff tear characteristics should guide clinical decision-making. Physical therapy compared to surgical treatments may have inferior outcomes. Standardized data collection, reporting, and terminology are key to enhancing the quality of evidence-based medicine. There is a need to unequivocally define the MCID for various MRCT treatment strategies that will lead to improved interpretation of outcomes. Significant opportunities exist for multi-center research groups to embark on high-quality comparative clinical studies to improve our understanding and management of MRCT.
Supplementary Material
Sources of Support / Funding:
Research reported in this publication was partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K08AR072092 (D.K.). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Study Group Name: American Shoulder & Elbow Surgeons Massive cuff Evaluation and Research IniTiative Investigators (ASES MERIT Investigators)
Ethical Committee approval: The study was exempt from local institutional review board approval because of study type (i.e., systematic review and meta-analysis).
Conflicts of interest/Disclosures:
David Kovacevic, MD: This author is a committee member of American Shoulder and Elbow Surgeons and Orthopaedic Research Society, and serves on the editorial or governing board for Journal of Bone and Joint Surgery.
Robert J. Suriani Jr, BA: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Brian M. Grawe, MD: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Edward H. Yian, MD: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Mohit N. Gilotra, MD: This author is a paid presenter / speaker for Arthrex Inc.
S. Ashfaq Hasan, MD: This author is a board / committee member of AAOS.
Umasuthan Srikumaran, MD MBA: This author receives financial or material support from Arthrex, DePuy, Smith & Nephew, Stryker, Thieme, and Wright Medical Technology; is a paid consultant for Conventus and Fx Shoulder; holds stock or stock options with Quantum OPS and Tigon Medical; receives research support from Stryker; has a family member who is an employee for Abbott; is a paid presenter / speaker for Fx Shoulder; and receives publishing royalties from Thieme.
Samer S. Hasan, MD PhD: This author receives financial or material support from Arthrex, DePuy, and DJ Orthopaedics; is a board / committee member for AAOS, American Shoulder and Elbow Surgeons; serves on the editorial / governing board for Arthroscopy and Orthopedics Today; receives research support from DJ Orthopaedics and OrthoSpace; is a paid presenter / speaker for Arthrex; is a paid consultant for DJ Orthopaedics and OrthoSpace; receives IP royalties from DJ Orthopaedics; holds stock of stock options with ROM3.
Frances Cuomo, MD: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Robert T. Burks, MD: This author is a board or committee member of American Orthopaedic Society for Sports Medicine; paid consultant for DePuy Mitek; receives IP royalties and is an unpaid consultant for Arthrex; is a paid presenter / speaker for DePuy Mitek; and holds stock or stock options with KATOR.
Andrew G. Green, MD: This author receives financial / material support from Arthrex, JBJS, and Smith & Nephew; is a board / committee member of AAOS and American Shoulder and Elbow Surgeons; is a paid consultant for DJ Orthopaedics; holds stock or stock options with IlluminOss Medical and Pfizer; serves on the editorial / governing board for JBJS and Techniques in Shoulder and Elbow Surgery; is a paid presenter / speaker and receives research support from DJ Orthopaedics; receives publishing royalties from JBJS, and receives IP royalties from Wright Medical Technology.
Wesley M. Nottage, MD: This author is a board / committee member for the American Orthopaedic Society for Sports Medicine, the American Shoulder and Elbow Surgeons, and Arthroscopy Association of North America; holds stock or stock options with Johnson & Johnson.
Sai Theja, MSc: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Hafiz F. Kassam, MD: This author serves on the editorial / governing board for the Journal of Shoulder and Elbow Arthroplasty.
Maarouf A. Saad, BS: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Miguel A. Ramirez, MD: This author serves on the editorial / governing board for the Journal of Shoulder and Elbow Surgery, is a paid consultant and paid presenter / speaker for Stryker.
Rodney J. Stanley, MD: This author is a board / committee member for the American Shoulder and Elbow Surgeons.
Matthew D. Williams, MD: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Vidushan Nadarajah, BA: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Alexis C. Konja, MPH: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Jason L. Koh, MD: This author is a board / committee member for American Academy of Orthopaedic Surgeons, ACL Study Group, American Orthopaedic Society for Sports Medicine, American Shoulder and Elbow Surgeons, Arthroscopy Association of North America, Herodicus Society, Illinois Association of Orthopaedic Surgeons, International Patellofemoral Study Group, International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine, and the Patellofemoral Foundation; is a paid consultant for Flexion; holds stock or stock options with Acuitive and Marrow Access Technologies; is an employee of Marrow Access Technologies; and serves on the editorial or governing board for Orthopaedic Journal of Sports Medicine.
Andrew S. Rokito, MD: This author, their immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article.
Charles M. Jobin, MD: This author is a paid consultant for Acumed LLC, Consortium of Focused Orthopedists LLC, DePuy, Wright Medical Technology, and Zimmer Biomet; is a paid presenter / speaker for Acumed LLC, Wright Medical Technology, and Zimmer Biomet; is a board / committee member for the American Shoulder and Elbow Surgeons and American Board of Orthopaedic Surgery; receives research support from Acumed LLC; and serves on the editorial / governing board for the Journal of the American Academy of Orthopaedic Surgeons.
William N. Levine, MD: This author is a board / committee member of the American Shoulder and Elbow Surgeons; serves on the editorial / governing board for the Journal of the American Academy of Orthopaedic Surgeons; is an unpaid design team member for Zimmer.
Christopher C. Schmidt: This author is a board / committee member of the American Shoulder and Elbow Surgeons; is a paid consultant for Arthrex.
References
- 1.American Academy of Orthopaedic Surgeons. Management of rotator cuff injuries clinical practice guideline. In. https://www.aaos.org/globalassets/quality-and-practice-resources/rotator-cuff/rotator-cuff-cpg-final-12-20-19.pdf
- 2.Audenaert E, Van Nuffel J, Schepens A, Verhelst M, Verdonk R. Reconstruction of massive rotator cuff lesions with a synthetic interposition graft: a prospective study of 41 patients. Knee Surg Sports Traumatol Arthrosc 2006;14:360–364. 10.1007/s00167-005-0689-7 [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Gonzalez J-F, Chuinard C, Bicknell R, Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg 2009;18:600–606. 10.1016/j.jse.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 4.Burkhart SS, Barth JR, Richards DP, Zlatkin MB, Larsen M. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthroscopy 2007;23:347–354. 10.1016/j.arthro.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 5.Burkhart SS, Danaceau SM, Pearce CE Jr. Arthroscopic rotator cuff repair: analysis of results by tear size and by repair technique—margin convergence versus direct tendon-to-bone repair. Arthroscopy 2001;17:905–912. [DOI] [PubMed] [Google Scholar]
- 6.Burkhart SS, Hartzler RU. Superior capsular reconstruction reverses profound pseudoparalysis in patients with irreparable rotator cuff tears and minimal or no glenohumeral arthritis. Arthroscopy 2019;35:22–28. 10.1016/j.arthro.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 7.Burkhart SS, Pranckun JJ, Hartzler RU. Superior capsular reconstruction for the operatively irreparable rotator cuff tear: Clinical outcomes are maintained 2 years after surgery. Arthroscopy 2020;36:373–380. 10.1016/j.arthro.2019.08.035 [DOI] [PubMed] [Google Scholar]
- 8.Castricini R, De Benedetto M, Familiari F, De Gori M, De Nardo P, Orlando N et al. Functional status and failed rotator cuff repair predict outcomes after arthroscopic-assisted latissimus dorsi transfer for irreparable massive rotator cuff tears. J Shoulder Elbow Surg 2016;25:658–665. 10.1016/j.jse.2015.08.043 [DOI] [PubMed] [Google Scholar]
- 9.Chen K-H, Chiang E-R, Wang H-Y, Ma H-L. Arthroscopic partial repair of irreparable rotator cuff tears: factors related to greater degree of clinical improvement at 2 years of follow-up. Arthroscopy 2017;33:1949–1955. 10.1016/j.arthro.2017.06.047 [DOI] [PubMed] [Google Scholar]
- 10.Cofield R Subscapular muscle transposition for repair of chronic rotator cuff tears. Surg Gynecol Obstet 1982;154:667–672. [PubMed] [Google Scholar]
- 11.Collin P, Gain S, Huu FN, Lädermann A. Is rehabilitation effective in massive rotator cuff tears? Orthop Traumatol Surg Res 2015;101:S203–S205. 10.1016/j.otsr.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Cuff DJ, Pupello DR, Santoni BG. Partial rotator cuff repair and biceps tenotomy for the treatment of patients with massive cuff tears and retained overhead elevation: midterm outcomes with a minimum 5 years of follow-up. J Shoulder Elbow Surg 2016;25:1803–1809. 10.1016/j.jse.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 13.de Campos Azevedo CI, Ângelo ACLPG, Vinga S. Arthroscopic superior capsular reconstruction with a minimally invasive harvested fascia lata autograft produces good clinical results. Orthop J Sports Med 2018;6:2325967118808242 10.1177/2325967118808242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Casas R, Lois M, Cidoncha M, Valadron M. Clinic and electromyographic results of latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Orthop Surg Res 2014;9:83 10.1186/s13018-014-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy 2018;34:93–99. 10.1016/j.arthro.2017.08.265 [DOI] [PubMed] [Google Scholar]
- 16.Duralde XA, Bair B. Massive rotator cuff tears: the result of partial rotator cuff repair. J Shoulder Elbow Surg 2005;14:121–127. 10.1016/j.jse.2004.06.015 [DOI] [PubMed] [Google Scholar]
- 17.El-Azab HM, Rott O, Irlenbusch U. Long-term follow-up after latissimus dorsi transfer for irreparable posterosuperior rotator cuff tears. J Bone Joint Surg Am 2015;97:462–469. 10.2106/JBJS.M.00235 [DOI] [PubMed] [Google Scholar]
- 18.Elhassan BT, Wagner ER, Werthel J-D. Outcome of lower trapezius transfer to reconstruct massive irreparable posterior-superior rotator cuff tear. J Shoulder Elbow Surg 2016;25:1346–1353. 10.1016/j.jse.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 19.Favard L, Berhouet J, Colmar M, Boukobza E, Richou J, Sonnard A et al. Massive rotator cuff tears in patients younger than 65 years. What treatment options are available? Orthop Traumatol Surg Res 2009;95:19–26. 10.1016/j.otsr.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 20.Gagnier JJ, Robbins C, Bedi A, Carpenter JE, Miller BS. Establishing minimally important differences for the American Shoulder and Elbow Surgeons score and the Western Ontario Rotator Cuff Index in patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg 2018;27:e160–e166. 10.1016/j.jse.2017.10.042 [DOI] [PubMed] [Google Scholar]
- 21.Galasso O, Riccelli DA, De Gori M, De Benedetto M, Orlando N, Gasparini G et al. Quality of life and functional results of arthroscopic partial repair of irreparable rotator cuff tears. Arthroscopy 2017;33:261–268. 10.1016/j.arthro.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 22.Gartsman GM. Massive, irreparable tears of the rotator cuff. Results of operative débridement and subacromial decompression. J Bone Joint Surg Am 1997;79:715–721. [DOI] [PubMed] [Google Scholar]
- 23.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2000;82:505–515. [DOI] [PubMed] [Google Scholar]
- 24.Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am 2006;88:113–120. 10.2106/JBJS.E.00282 [DOI] [PubMed] [Google Scholar]
- 25.Gerber C, Rahm SA, Catanzaro S, Farshad M, Moor BK. Latissimus dorsi tendon transfer for treatment of irreparable posterosuperior rotator cuff tears: long-term results at a minimum follow-up of ten years. J Bone Joint Surg Am 2013;95:1920–1926. 10.2106/JBJS.M.00122 [DOI] [PubMed] [Google Scholar]
- 26.Gerber C, Vinh TS, Hertel R, Hess CW. Latissimus dorsi transfer for the treatment of massive tears of the rotator cuff. A preliminary report. Clin Orthop Relat Res 1988:51–61. [PubMed] [Google Scholar]
- 27.Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy 2015;31:599–607. e1. 10.1016/j.arthro.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 28.Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res 2011;469:2452–2460. 10.1007/s11999-011-1896-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartzler RU, Steen BM, Hussey MM, Cusick MC, Cottrell BJ, Clark RE et al. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg 2015;24:1698–1706. 10.1016/j.jse.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 30.Heuberer PR, Kölblinger R, Buchleitner S, Pauzenberger L, Laky B, Auffarth A et al. Arthroscopic management of massive rotator cuff tears: an evaluation of débridement, complete, and partial repair with and without force couple restoration. Knee Surg Sports Traumatol Arthrosc 2016;24:3828–3837. 10.1007/s00167-015-3739-9 [DOI] [PubMed] [Google Scholar]
- 31.Holmgren T, Öberg B, Adolfsson L, Hallgren HB, Johansson K. Minimal important changes in the Constant-Murley score in patients with subacromial pain. J Shoulder Elbow Surg 2014;23:1083–1090. 10.1016/j.jse.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Irlenbusch U, Bracht M, Gansen H-K, Lorenz U, Thiel J. Latissimus dorsi transfer for irreparable rotator cuff tears: a longitudinal study. J Shoulder Elbow Surg 2008;17:527–534. 10.1016/j.jse.2007.11.022 [DOI] [PubMed] [Google Scholar]
- 33.Kanatlı U, Özer M, Ataoğlu MB, Öztürk BY, Gül O, Çetinkaya M et al. Arthroscopic-assisted latissimus dorsi tendon transfer for massive, irreparable rotator cuff tears: technique and short-term follow-up of patients with pseudoparalysis. Arthroscopy 2017;33:929–937. 10.1016/j.arthro.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 34.Kany J, Grimberg J, Amaravathi RS, Sekaran P, Scorpie D, Werthel JD. Arthroscopically-assisted latissimus dorsi transfer for irreparable rotator cuff insufficiency: modes of failure and clinical correlation. Arthroscopy 2018;34:1139–1150. 10.1016/j.arthro.2017.10.052 [DOI] [PubMed] [Google Scholar]
- 35.Klinger H-M, Spahn G, Baums M, Stecket H. Arthroscopic débridement of irreparable massive rotator cuff tears—a comparison of débridement alone and combined procedure with biceps tenotomy. Acta Chir Belg 2005;105:297–301. 10.1080/00015458.2005.11679720 [DOI] [PubMed] [Google Scholar]
- 36.Klinger H-M, Steckel H, Ernstberger T, Baums MH. Arthroscopic débridement of massive rotator cuff tears: negative prognostic factors. Arch Orthop Trauma Surg 2005;125:261–266. 10.1007/s00402-004-0738-6 [DOI] [PubMed] [Google Scholar]
- 37.Kluger R, Bock P, Mittlböck M, Krampla W, Engel A. Long-term survivorship of rotator cuff repairs using ultrasound and magnetic resonance imaging analysis. Am J Sports Med 2011;39:2071–2081. 10.1177/0363546511406395 [DOI] [PubMed] [Google Scholar]
- 38.König MA, Braunstein VA. Tendon repair leads to better long-term clinical outcome than débridement in massive rotator cuff tears. Open Orthop J 2017;11:546–553. 10.2174/1874325001611010546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacheta L, Horan MP, Schairer WW, Goldenberg BT, Dornan GJ, Pogorzelski J et al. Clinical and imaging outcomes after arthroscopic superior capsule reconstruction with human dermal allograft for irreparable posterosuperior rotator cuff tears: a minimum two year follow up. Arthroscopy 2020. 10.1016/j.arthro.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 40.Lee S-J, Min Y-K. Can inadequate acromiohumeral distance improvement and poor posterior remnant tissue be the predictive factors of re-tear? preliminary outcomes of arthroscopic superior capsular reconstruction. Knee Surg Sports Traumatol Arthrosc 2018;26:2205–2213. 10.1007/s00167-018-4912-8 [DOI] [PubMed] [Google Scholar]
- 41.Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic débridement of massive irreparable rotator cuff tears. Arthroscopy 2008;24:743–748. 10.1016/j.arthro.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 42.Lim S, AlRamadhan H, Kwak J-M, Hong H, Jeon I-H. Graft tears after arthroscopic superior capsule reconstruction (ASCR): pattern of failure and its correlation with clinical outcome. Arch Orthop Trauma Surg 2019;139:231–239. 10.1007/s00402-018-3025-7 [DOI] [PubMed] [Google Scholar]
- 43.Lo IK, Burkhart SS. Arthroscopic revision of failed rotator cuff repairs: technique and results. Arthroscopy 2004;20:250–267. 10.1016/j.arthro.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 44.Mihara S, Fujita T, Ono T, Inoue H, Kisimoto T. Rotator cuff repair using an original iliotibial ligament with a bone block patch: preliminary results with a 24-month follow-up period. J Shoulder Elbow Surg 2016;25:1155–1162. 10.1016/j.jse.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 45.Mihata T, Lee TQ, Hasegawa A, Fukunishi K, Kawakami T, Fujisawa Y et al. Five-year follow-up of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. J Bone Joint Surg Am 2019;101:1921–1930. 10.2106/JBJS.19.00135 [DOI] [PubMed] [Google Scholar]
- 46.Mihata T, Lee TQ, Hasegawa A, Kawakami T, Fukunishi K, Fujisawa Y et al. Arthroscopic superior capsule reconstruction can eliminate pseudoparalysis in patients with irreparable rotator cuff tears. Am J Sports Med 2018;46:2707–2716. 10.1177/0363546518786489 [DOI] [PubMed] [Google Scholar]
- 47.Mihata T, Lee TQ, Watanabe C, Fukunishi K, Ohue M, Tsujimura T et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy 2013;29:459–470. 10.1016/j.arthro.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 48.Moser M, Jablonski MV, Horodyski M, Wright TW. Functional outcome of surgically treated massive rotator cuff tears: a comparison of complete repair, partial repair, and débridement. Orthopedics 2007;30:479–482. 10.3928/01477447-20070601-05 [DOI] [PubMed] [Google Scholar]
- 49.Moursy M, Forstner R, Koller H, Resch H, Tauber M. Latissimus dorsi tendon transfer for irreparable rotator cuff tears: a modified technique to improve tendon transfer integrity. J Bone Joint Surg Am 2009;91:1924–1931. 10.2106/JBJS.H.00515 [DOI] [PubMed] [Google Scholar]
- 50.Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am 2010;92:2544–2556. 10.2106/JBJS.I.00912 [DOI] [PubMed] [Google Scholar]
- 51.Nada A, Debnath U, Robinson D, Jordan C. Treatment of massive rotator-cuff tears with a polyester ligament (Dacron) augmentation: clinical outcome. J Bone Joint Surg Br 2010;92:1397–1402. 10.1302/0301-620X.92B10.24299 [DOI] [PubMed] [Google Scholar]
- 52.Neer CS 2nd. Impingement lesions. Clin Orthop Relat Res 1983;173:70–77. [PubMed] [Google Scholar]
- 53.Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg 2014;23:445–455. 10.1016/j.jse.2013.07.054 [DOI] [PubMed] [Google Scholar]
- 54.Oliva F, Osti L, Padulo J, Maffulli N. Epidemiology of the rotator cuff tears: a new incidence related to thyroid disease. Muscles Ligaments Tendons J 2014;4:309–314. 10.11138/mltj/2014.4.3.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey R, Tafazal S, Shyamsundar S, Modi A, Singh HP. Outcome of partial repair of massive rotator cuff tears with and without human tissue allograft bridging repair. Shoulder Elbow 2017;9:23–30. 10.1177/1758573216665114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pappou IP, Schmidt CC, Jarrett CD, Steen BM, Frankle MA. AAOS appropriate use criteria: optimizing the management of full-thickness rotator cuff tears. J Am Acad Orthop Surg 2013;21:772–775. 10.5435/JAAOS-21-12-772 [DOI] [PubMed] [Google Scholar]
- 57.Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic superior capsular reconstruction with acellular dermal allograft for the treatment of massive irreparable rotator cuff tears: short-term clinical outcomes and the radiographic parameter of superior capsular distance. Arthroscopy 2018;34:1764–1773. 10.1016/j.arthro.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 58.Rashid MS, Cooper C, Cook J, Cooper D, Dakin SG, Snelling S et al. Increasing age and tear size reduce rotator cuff repair healing rate at 1 year: data from a large randomized controlled trial. Acta Orthop 2017;88:606–611. 10.1080/17453674.2017.1370844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricci M, Vecchini E, Bonfante E, Micheloni GM, Berti M, Schenal G et al. A clinical and radiological study of biodegradable subacromial spacer in the treatment of massive irreparable rotator cuff tears. Acta Biomed 2017;88:75–80. 10.23750/abm.v88i4-S.6797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt CC, Morrey BF. Management of full-thickness rotator cuff tears: appropriate use criteria. J Shoulder Elbow Surg 2015;24:1860–1867. 10.1016/j.jse.2015.05.042 [DOI] [PubMed] [Google Scholar]
- 61.Schumaier A, Kovacevic D, Schmidt C, Green A, Rokito A, Jobin C et al. Defining massive rotator cuff tears: a Delphi consensus study. J Shoulder Elbow Surg 2020;29:674–680. 10.1016/j.jse.2019.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senekovic V, Poberaj B, Kovacic L, Mikek M, Adar E, Markovitz E et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg 2017;137:95–103. 10.1007/s00402-016-2603-9 [DOI] [PubMed] [Google Scholar]
- 63.Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 1995;77:10–15. [DOI] [PubMed] [Google Scholar]
- 64.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003;73:712–716. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 65.Valenti P, Sauzieres P, Katz D, Kalouche I, Kilinc AS. Do less medialized reverse shoulder prostheses increase motion and reduce notching? Clin Orthop Relat Res 2011;469:2550–2557. 10.1007/s11999-011-1844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walch G, Edwards TB, Boulahia A, Nové-Josserand L, Neyton L, Szabo I. Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. J Shoulder Elbow Surg 2005;14:238–246. 10.1016/j.jse.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 67.Wall B, O’connor DP, Edwards TB, Nové-Josserand L, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476–1485. 10.2106/JBJS.F.00666 [DOI] [PubMed] [Google Scholar]
- 68.Woodmass JM, Wagner ER, Borque KA, Chang MJ, Welp KM, Warner JJ. Superior capsule reconstruction using dermal allograft: early outcomes and survival. J Shoulder Elbow Surg 2019;28:S100–S109. 10.1016/j.jse.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 69.Yian EH, Sodl JF, Dionysian E, Schneeberger AG. Anterior deltoid reeducation for irreparable rotator cuff tears revisited. J Shoulder Elbow Surg 2017;26:1562–1565. 10.1016/j.jse.2017.03.007 [DOI] [PubMed] [Google Scholar]
- 70.Zingg P, Jost B, Sukthankar A, Buhler M, Pfirrmann C, Gerber C. Clinical and structural outcomes of nonoperative management of massive rotator cuff tears. J Bone Joint Surg Am 2007;89:1928–1934. 10.2106/JBJS.F.01073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


