Abstract
Background:
Neonatal seizures are associated with death and neurologic morbidity; however, little is known about how neonates with seizures die.
Methods:
This was a prospective, observational cohort study of neonates with seizures treated at seven sites of the Neonatal Seizure Registry. We characterized mode of death, evaluated the association between infant characteristics and mode of death, and evaluated predictors of death or transfer to hospice.
Results:
We enrolled 611 consecutive neonates with seizures, and 90 neonates (15%) died prior to hospital discharge at a median of 11 days of age (range: 1–163 days); 32 (36%) died in the first postnatal week. An additional 19 neonates (3%) were transferred to hospice. The most common mode of in-hospital death was death after extubation amidst concerns for poor neurologic prognosis, in the absence of life-threatening physiologic instability (n=43, 48%). Only one infant died while actively receiving cardiopulmonary resuscitation. In an adjusted analysis, premature birth (OR: 3.06, 95% CI 1.59–5.90) and high seizure burden (OR 4.33, 95% CI 1.88–9.95) were associated with increased odds of death or transfer to hospice.
Conclusion:
In a cohort of neonates with seizures, death occurred predominantly after decisions to withdraw or withhold life-sustaining intervention(s). Future work should characterize how these decisions occur and develop optimized approaches to support families and clinicians caring for newborns with seizures.
Keywords: Neonate, Neurology, Palliative care, Neurocritical Care, Epilepsy, Hospice, Preterm
Introduction:
Seizures are the most common manifestation of neurological dysfunction in newborns and are often the presenting feature of neonatal brain injury.1 Neonates who experience seizures are at high risk for mortality and morbidity; up to one-third of infants with neonatal seizures die, and survivors are at risk for long-term neurodevelopmental impairment and epilepsy.2,3 Neonatal seizures arise from heterogeneous etiologies including brain malformations and hypoxicischemic encephalopathy (HIE), which confer a high risk of death.4
Mortality rates do not reveal the circumstances that surround infant death. Some infants may die in the setting of multiple medical co-morbidities and systemic physiologic instability, while others may die after clinicians and parents decide to withdraw life-sustaining treatment in the face of the infant’s neurologic prognosis. Understanding how infants die – whether death occurred regardless of life-sustaining interventions or instead occurred after parents and clinicians made a decision to limit interventions in the setting of the infant’s neurologic prognosis – is critically important as clinicians consider how to best counsel and support families of newborns with seizure etiologies or comorbidities that confer poor outcomes. Understanding detailed information about death in neonatal seizures is also necessary for the design and interpretation of studies that use mortality as an outcome. Mortality rates alone may obscure important information about how decisions regarding withdrawal of life-sustaining treatment contribute to mortality.
In general, most infants who die during their admission to the neonatal intensive care unit (NICU) do so after life-sustaining treatment is withheld or withdrawn.5 Existing data in a single-center cohort of neonates with HIE similarly suggests that most infant deaths occur in the setting of elective extubation due to concerns for poor future quality of life.6 Infants impacted by neonatal seizures represent a broad range of neonatal neurologic conditions. Despite the high mortality associated with neonatal seizures, little is known about how death occurs in this population.
We aimed to characterize how infants with seizures die, including the timing, location, and mode of death. We hypothesized that infant death would most commonly occur following extubation amidst concerns for poor neurologic prognosis, in the absence of life-threatening physiologic instability.
Methods:
Study design and participants
This was a prospective, consecutive, observational cohort study of all neonates with seizures treated at the seven sites of the Neonatal Seizure Registry (NSR) between 2013–2015. All NSR sites follow the American Clinical Neurophysiology Society guidelines for continuous electroencephalography (cEEG) in neonates and have a level IV neonatal intensive care unit (NICU).7 The institutional review boards at each site approved the study and granted waivers of informed consent. Characteristics of neonates in the NSR cohort have been previously reported, including the clinical characteristics of infants who died among the first 426 infants enrolled.4,8–10
Measurements
Seizure burden was defined as either (1) high [status epilepticus, frequent recurrent seizures without status epilepticus, many (≥7) isolated seizures]; or (2) low [<7 seizures]. Incomplete response to anti-seizure medication was defined as electrographic seizures that recurred > 30 minutes after administration of an adequate first-line anti-seizure medication. Prematurity was defined as gestational age < 37 weeks.
Mode of death was characterized using an existing paradigm.6,11 Medical records were reviewed for information regarding (1) life-threatening physiologic instability in the 24 hours prior to death, (2) mode of death, and (3) palliative care consultation. Life-threatening physiologic instability was defined as either the presence of extracorporeal membrane oxygenation (ECMO) at the time of death or two or more of the following: (1) persistent desaturation despite 100% oxygen on mechanical ventilation, (2) hypotension despite volume infusion and inotropes, (3) protracted bradycardia in the absence of hypothermia treatment, (4) acidosis, or (5) protracted anuria for > 24 hours. Mode of death was defined as: (1) unstable infants who died while receiving cardio-pulmonary resuscitation (CPR), (2) unstable ventilated infants who died after a decision to withhold CPR, (3) unstable infants who died after a decision to extubate, typically to let a moribund child die in their parents’ arms, (4) stable infants who died after a decision to extubate, typically for quality of life reasons, and (5) stable infants who died after a decision to withhold or withdraw artificial hydration and/or nutrition.
Analysis
We assessed the number of infants with seizures who died and were transferred to hospice. Infant demographic and clinical characteristics were summarized overall and by group (died vs. transferred to hospice) using counts and percentages for categorical variables and medians and ranges for continuous variables. We evaluated the association between infant demographic, clinical characteristics, and mode of death using Fisher’s exact test. We evaluated predictors of death or transfer to hospice using multivariable logistic regression including the following covariates of interest: sex, race, ethnicity, prematurity, seizure etiology (HIE, ischemic stroke, and intracranial hemorrhage), seizure burden, and incomplete response to anti-seizure medication. We performed analyses using Stata version 16.0 (College Station, TX). We considered p-values < 0.05 significant.
Results:
Clinical Characteristics
We enrolled 611 consecutive neonates with seizures. Ninety neonates (15%) died prior to hospital discharge (Table 1). Death occurred at a median of 11 days of age (range 1–163 days, interquartile range (IQR): 4–24 days); 32 (36%) died in the first postnatal week (Figure 1). HIE was the most common seizure etiology among infants who died (n=47, 52%), followed by intracranial hemorrhage (n=11, 12%), brain malformations (n=6, 7%), and central nervous system (CNS) infections (n=6, 7%). Of patients with neonatal seizures due to HIE, 58 (25%) died or were transferred to hospice. Most neonates with intracranial hemorrhage were premature (n=6/11, 55%).
Table 1.
Characteristics of 90 neonates with seizures who died in-hospital during the neonatal admission
| N=90 | |
|---|---|
| Male sex | 43 (48) |
| Gestational age (completed weeks) | |
| < 29 | 7 (8) |
| 29 – 32+6/7 | 6 (7) |
| 33 – 36+6/7 | 16 (18) |
| ≥ 37 | 60 (67) |
| Race | |
| Caucasian | 38 (54) |
| Black | 12 (17) |
| Asian | 6 (8) |
| Other | 15 (21) |
| Hispanic ethnicity | 14 (19) |
| Diagnosis | |
| Hypoxic ischemic encephalopathy | 47 (52) |
| Intracranial hemorrhage | 11 (12) |
| Brain malformation* | 6 (7) |
| CNS infection | 6 (7) |
| Inborn error of metabolism | 4 (4) |
| Ischemic stroke | 3 (3) |
| Neonatal onset epilepsy | 3 (3) |
| Other | 10 (11) |
| Comorbid conditions | |
| Congenital heart disease | 25 (28) |
| Congenital diaphragmatic hernia | 5 (6) |
| Dialysis | 4 (4) |
| ECMO | 17 (19) |
| Age at death (days) | 11 (1–163) |
| Palliative care consultation | 32 (36) |
| Physiologic stability at the time of death** | |
| Life-threatening physiologic instability (total) | 47 (52) |
| Persistent desaturation | 15 (17) |
| Hypotension | 43/88 (49) |
| Bradycardia in the absence of therapeutic hypothermia | 11/87 (13) |
| Protracted anuria | 25/89 (28) |
| Acidosis | 5 (6) |
| ECMO | 17 (19) |
| Mode of Death | |
| Unstable: Died while receiving CPR | 1 (1) |
| Unstable: Died while withholding CPR | 9 (10) |
| Unstable: Died after discontinuation of ECMO | 12 (13) |
| Unstable: Died after extubation to let infant die in parents’ arms | 25 (28) |
| Stable: Elective extubation for QOL considerations | 43 (48) |
| Stable: Died after withdrawal of artificial nutrition and hydration | 0 |
Data are presented as n (%) or median (range). Abbreviations: CNS: central nervous system, ECMO: extracorporeal membrane oxygenation, CPR: cardiopulmonary resuscitation, QOL: quality of life.
Brain malformations included holoprosencephaly, lissencephaly, dandy walker malformation, and pachygyria.
Cardiorespiratory instability was defined as either receipt of extracorporeal membrane oxygenation (ECMO) or 2 of the following: persistent desaturation despite 100% oxygen on mechanical ventilation, hypotension despite volume infusion and inotropes, protracted bradycardia in the absence of therapeutic hypothermia, or protracted anuria for 24 hours.
Figure 1.
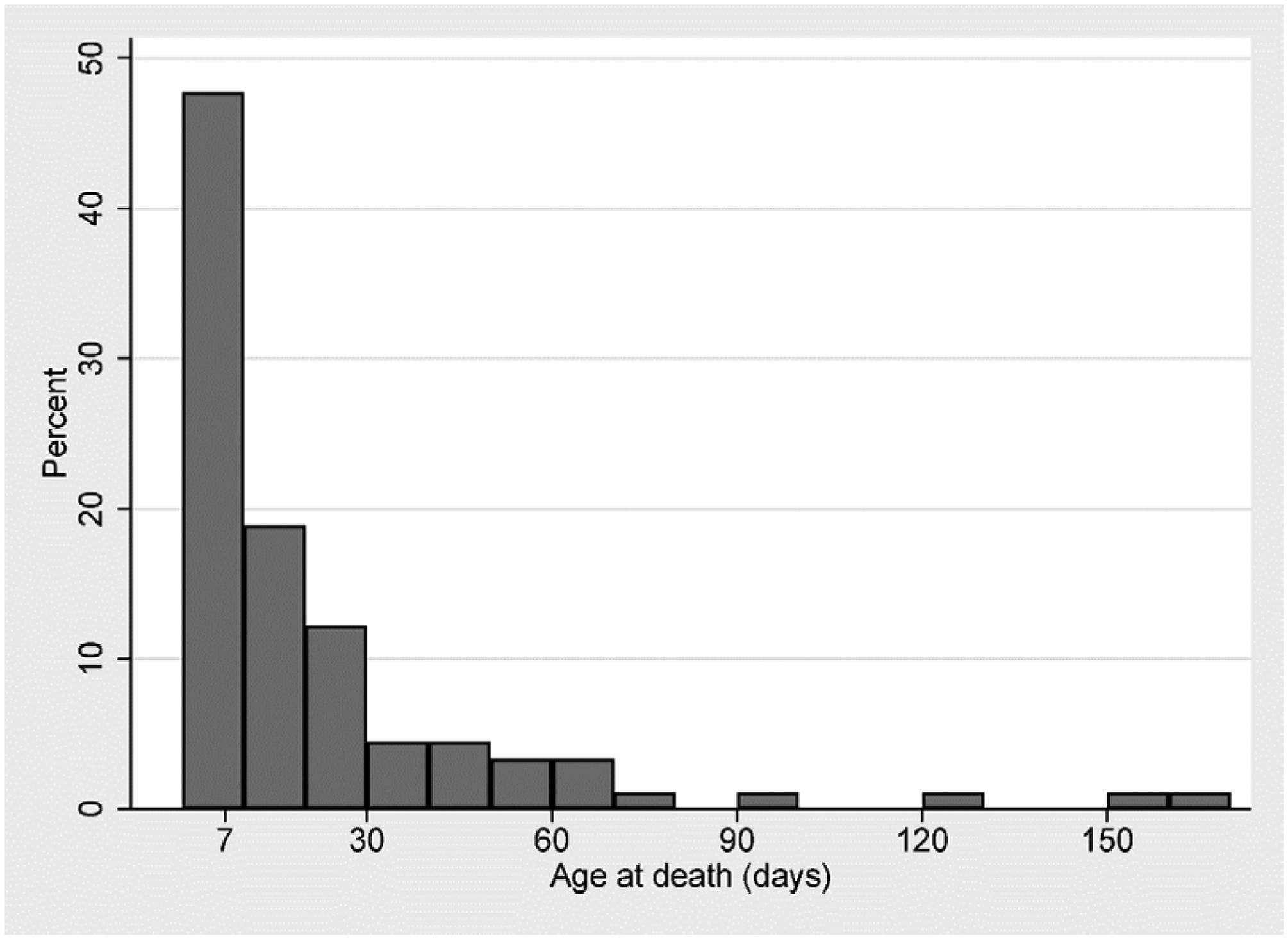
Age at infant death among 90 neonates with seizures.
An additional 19 neonates (3%) from five sites were transferred to hospice (Table 2). Transfer to hospice occurred at a median of 20 days of age (range 6–196 days, IQR 10–31). Death was confirmed in eight of these infants. Seven infants were still living at the time of last documentation, and vital status could not be confirmed in four infants. Most infants (n=11, 58%) transferred to hospice had a diagnosis of HIE.
Table 2.
Characteristics of 19 infants with neonatal seizures who were transferred to hospice during the neonatal admission
| N=19 | |
|---|---|
| Male sex | 12 (63) |
| Gestational age (completed weeks) | |
| < 29 | 1 (5) |
| 29 – 32+6/7 | 1 (5) |
| 33 – 36+6/7 | 1 (5) |
| ≥ 37 | 16 (84) |
| Race | |
| Caucasian | 13 (76) |
| Black | 3 (18) |
| Asian | 1 (6) |
| Other | 0 (0) |
| Hispanic ethnicity | 3 (18) |
| Diagnosis | |
| Hypoxic ischemic encephalopathy | 11 (58) |
| CNS infection | 4 (21) |
| Brain malformation* | 2 (11) |
| Intracranial hemorrhage | 1 (5) |
| Ischemic stroke | 1 (5) |
| Comorbid conditions | |
| Congenital heart disease | 3 (16) |
| Congenital diaphragmatic hernia | 0 (0) |
| Dialysis | 1 (5) |
| ECMO | 0 (0) |
| Palliative care consultation | 10 (59) |
| Outcome | |
| Death confirmed | 8 (42) |
| Still living at time of last documentation | 7 (37) |
| Unknown | 4 (21) |
Data are presented as n (%). Abbreviations: CNS: central nervous system
Brain malformations included holoprosencephaly and dandy walker malformation.
Of infants who died or were transferred to hospice, most had high seizure burden (89/106, 84%) and incomplete response to anti-seizure medication (82/100, 82%). The percent of neonates with seizures and in-hospital death or transfer to hospice at each individual site ranged from 6% to 28%. In unadjusted analyses, infants with HIE were more likely to die or be transferred to hospice than infants whose seizures resulted from all other etiologies. Infants with high seizure burden and incomplete response to anti-seizure medication were also more likely to die or be transferred to hospice (p< 0.001). In the multivariable regression analysis, premature birth (OR: 3.06, 95% CI 1.59–5.90) and high seizure burden (OR 4.33, 95% CI 1.88–9.95) were associated with increased odds of death or transfer to hospice (Table 3). A primary seizure etiology of ischemic stroke was associated with decreased odds of death or transfer to hospice (OR 0.20, 95% CI 0.06–0.72).
Table 3.
Clinical characteristics associated with death or transfer to hospice on multivariable logistic regression analysis.
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Male sex | 0.71 (0.42–1.20) | 0.20 |
| Race and ethnicity | ||
| Caucasian | [Reference] | |
| Hispanic | 0.64 (0.31–1.33) | 0.23 |
| Black | 0.86 (0.41–1.82) | 0.70 |
| Asian | 0.64 (0.20–1.98) | 0.24 |
| Prematurity | 3.06 (1.59 – 5.90) | 0.001 |
| Etiology | ||
| Hypoxic ischemic encephalopathy | 1.41 (0.77 – 2.57) | 0.27 |
| Ischemic stroke | 0.20 (0.06–0.72) | 0.01 |
| Intracranial hemorrhage | 0.81 (0.35–1.87) | 0.62 |
| Seizure severity | ||
| High seizure burden | 4.33 (1.88–9.95) | 0.001 |
| Incomplete response to anti-seizure medication | 1.34 (0.59–3.02) | 0.49 |
Mode of Death
Approximately half (n=43/90, 48%) of infants who died in-hospital were physiologically stable at the time of death. Most in-hospital deaths occurred after a decision to extubate (n=68/90, 76%). The most common mode of in-hospital death was death in stable infants who died after a decision to extubate (n=43/90, 48%). Twelve children (13%) died following a decision to discontinue ECMO. Of these 12 infants, most were born at term (n=8, 67%), died at > 7 days of age (n=8, 67%), and had a primary diagnosis of HIE (n=10, 83%). Only one infant died while actively receiving CPR. While detailed information regarding mode of death was not available for all infants transferred to hospice, three infants who were transferred to hospice ultimately died after a decision to withhold or withdraw artificial hydration and nutrition. All of these infants had a diagnosis of HIE and were treated at the same institution.
In HIE, death typically occurred in stable infants, who died after a decision to extubate (p=0.026). Other neonatal seizure etiologies were not associated with a particular mode of death. High seizure burden was associated with physiologic instability (p=0.012). Most infants with low seizure burden who died were stable infants who died after a decision to extubate (p=0.045). Infant sex, gestational age, race, and ethnicity were not associated with mode of death.
Palliative Care
Palliative care service utilization was higher among infants who were transferred to hospice (n=10/17, 59%), compared to those who died in the hospital (n=32/90, 36%), although the difference was not statistically significant (p=0.1). The involvement of palliative care team support was associated with mode of death and was most common for unstable infants who died after a decision to withhold CPR, followed by stable infants who died after a decision to extubate (p<0.001). No infants who died after a decision to discontinue ECMO received a palliative care consult. All sites had a dedicated palliative care service. The frequency of palliative care consultation varied by site; at the lowest utilization site, 6% of infants who died or transferred to hospice received a palliative care consult, as compared to 100% at the highest utilization site.
Discussion:
Characterizing how infants die is critical to interpreting the impact of illness or interventions in neonatal care.12 In this cohort, the overwhelming majority of neonates with seizures died after decisions to withhold or withdraw life-sustaining treatment. The most common mode of death, representing nearly half of the infant deaths in this cohort, was death in a stable infant, who died after a decision to extubate. These results highlight that mortality rates in neonates with seizures are likely driven by treatment decisions about the initiation or withdrawal of life-sustaining therapies. Clinicians must be prepared to help parents caring for infants with seizures understand and process information about neurologic prognosis, future quality of life, and complex decision making.
Death occurred in a variety of circumstances. Most neonates who did not survive died in-hospital and approximately one-third of infants died during their first postnatal week. A minority of infants were transferred to hospice; some of these children ultimately survived. Unexpected survival after neonatal brain injury has been described previously13 and underscores the importance of ensuring families have longitudinal follow-up with health care team members beyond the neonatal period. Pediatric neurologists are well-equipped to help families process an unexpected outcome and to manage ongoing neurologic symptoms.
High seizure burden (defined as status epilepticus, frequent recurrent seizures without status epilepticus, or many (≥7) isolated seizures was associated with four-fold increased odds of death or transfer to hospice. A seizure etiology of ischemic stroke was associated with decreased odds of death or transfer to hospice. We hypothesize that these relationships are related to the severity of the underlying brain injury. These findings highlight that neurologists are highly likely to be involved in these complex cases and should be prepared to support families and other clinicians as they make decisions about the provision or discontinuation of life-sustaining treatment.
Families and clinicians received the support of a palliative care team in a minority of cases, despite the availability of such services at all participating study centers. Palliative care consultation was more common for infants who died after a decision to either withhold CPR or to extubate in the setting of physiologic stability. This finding highlights the complexity associated with these clinical scenarios, which can require additional decision making and communication support.14 Utilization of palliative care consultation and hospice varied by site, likely reflecting varied availability of services and local culture.15
A small number of infants died after a decision to withhold or withdrawal artificial hydration and/or nutrition; all three of these infants were from a single institution and were transferred to hospice. Withholding or withdrawing artificial nutrition and hydration in the newborn period is controversial.16–19 Professional guidelines suggest that this practice is acceptable in select circumstances, including cases in which the child “permanently lacks awareness and the ability to interact with the environment” and when the provision of fluids and nutrition serve solely to “prolong and add morbidity to the process of dying.”20 The complexity of prognostication required to make these determinations underscores the importance of close coordination between multi-disciplinary clinicians, including pediatric neurologists, neonatologists, and palliative care specialists.
This study is strengthened by the large, multi-center, consecutive cohort design, but it is not without limitations. We lack information regarding brain injury severity, for example, neuroimaging, EEG background patterns, seizure semiology, autopsy data, and degree of intracranial hemorrhage, which may influence parent and clinician decision making. We also do not have access to comprehensive socioeconomic and demographic variables, including insurance status and income, which have been shown to be related to mode of death in the HIE population.21 Maternal, placental, and intrapartum factors may influence infant mortality and are not fully assessed in this dataset.22,23 This study uses information regarding circumstances around the time of death as a proxy for real-time decision making. The definition of physiologic stability, also used in previous studies,6,11 does not include indicators of neurologic stability, which may inform immediate mortality risk. Future studies should prospectively evaluate the complex decisions that occur between parents and clinicians and the conversations that precede them.
Decisions to withhold or withdraw life-sustaining treatment are common for neonates with seizures and occur throughout the hospital course in varied circumstances. Neurologists caring for infants with seizures must be prepared to partner with families as they make these challenging decisions. Rates of infant mortality in neonatal seizures are influenced by parent-clinician decision making; how these decisions occur may vary between settings. Future work should characterize how end of life decisions occur and how to best support families and clinicians caring for newborns with seizures.
Study funding:
Supported by a grant from the Pediatric Epilepsy Research Foundation. MEL is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number K12NS098482 and the Derfner Foundation. CJC is supported by NIH K23 NS092923. CJW is supported by K02NS102598.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glass HC, Grinspan ZM, Shellhaas RA. Outcomes after acute symptomatic seizures in neonates. Semin Fetal Neonatal Med. 2018;23(3):218–222. [DOI] [PubMed] [Google Scholar]
- 2.Watkins A, Szymonowicz W, Jin X, Yu VV. Significance of seizures in very low-birthweight infants. Dev Med Child Neurol. 1988;30(2):162–169. [DOI] [PubMed] [Google Scholar]
- 3.Heljic S, Uzicanin S, Catibusic F, Zubcevic S. Predictors of Mortality in Neonates with Seizures; a Prospective Cohort Study. Med Arch. 2016;70(3):182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary Profile of Seizures in Neonates: A Prospective Cohort Study. J Pediatr. 2016;174:98–103 e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner J, Sharma J, Lantos J, Kilbride H. How infants die in the neonatal intensive care unit: trends from 1999 through 2008. Arch Pediatr Adolesc Med. 2011;165(7):630–634. [DOI] [PubMed] [Google Scholar]
- 6.Lemmon ME, Boss RD, Bonifacio SL, Foster-Barber A, Barkovich AJ, Glass HC. Characterization of Death in Neonatal Encephalopathy in the Hypothermia Era. J Child Neurol. 2017;32(4):360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28(6):611–617. [DOI] [PubMed] [Google Scholar]
- 8.Shellhaas RA, Chang T, Wusthoff CJ, et al. Treatment Duration After Acute Symptomatic Seizures in Neonates: A Multicenter Cohort Study. J Pediatr. 2017;181:298–301 e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass HC, Shellhaas RA, Tsuchida TN, et al. Seizures in Preterm Neonates: A Multicenter Observational Cohort Study. Pediatr Neurol. 2017;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shellhaas RA, Wusthoff CJ, Tsuchida TN, et al. Profile of neonatal epilepsies: Characteristics of a prospective US cohort. Neurology. 2017;89(9):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhagen AA, Janvier A, Leuthner SR, et al. Categorizing neonatal deaths: a cross-cultural study in the United States, Canada, and The Netherlands. J Pediatr. 2010;156(1):33–37. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen AA, Janvier A. The continuing importance of how neonates die. JAMA Pediatr. 2013;167(11):987–988. [DOI] [PubMed] [Google Scholar]
- 13.Pal S, Jones J, Job S, Maynard L, Curley A, Clarke P. Characteristics of babies who unexpectedly survive long term after withdrawal of intensive care. Acta Paediatr. 2016;105(5):468–474. [DOI] [PubMed] [Google Scholar]
- 14.Lemmon ME, Bidegain M, Boss RD. Palliative care in neonatal neurology: robust support for infants, families and clinicians. J Perinatol. 2016;36(5):331–337. [DOI] [PubMed] [Google Scholar]
- 15.Feudtner C, Womer J, Augustin R, et al. Pediatric palliative care programs in children’s hospitals: a cross-sectional national survey. Pediatrics. 2013;132(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 16.Carter BS, Leuthner SR. The ethics of withholding/withdrawing nutrition in the newborn. Semin Perinatol. 2003;27(6):480–487. [DOI] [PubMed] [Google Scholar]
- 17.Porta N, Frader J. Withholding hydration and nutrition in newborns. Theor Med Bioeth. 2007;28(5):443–451. [DOI] [PubMed] [Google Scholar]
- 18.Beranger A, Boize P, Viallard ML. [The practices of withdrawing artificial nutrition and hydration in the neonatal intensive care unit: a preliminary study]. Arch Pediatr. 2014;21(2):170–176. [DOI] [PubMed] [Google Scholar]
- 19.Levi BH. Withdrawing nutrition and hydration from children: legal, ethical, and professional issues. Clin Pediatr (Phila). 2003;42(2):139–145. [DOI] [PubMed] [Google Scholar]
- 20.Diekema DS, Botkin JR, Committee on B. Clinical report--Forgoing medically provided nutrition and hydration in children. Pediatrics. 2009;124(2):813–822. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan G, Mathur A, Zaniletti I, et al. Withdrawal of Life-Support in Neonatal Hypoxic-Ischemic Encephalopathy. Pediatric Neurology. 2019;91:20–26. [DOI] [PubMed] [Google Scholar]
- 22.Turner JM, Mitchell MD, Kumar SS. The physiology of intrapartum fetal compromise at term. Am J Obstet Gynecol. 2020;222(1):17–26. [DOI] [PubMed] [Google Scholar]
- 23.Barros FC, Papageorghiou AT, Victora CG, et al. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr. 2015;169(3):220–229. [DOI] [PubMed] [Google Scholar]


