Summary
In the United States, more than two-thirds of adolescents and one-third of emerging adults report habitual short sleep duration, which is a known risk factor for psychological distress. The primary aim of this systematic review and meta-analysis was to evaluate the effect of behavioral sleep-promoting interventions on the sleep characteristics (e.g., total sleep time and sleep efficiency) of adolescents and emerging adults (aged 12 to 25 years) who do not have a sleep disorder. The secondary aim was to determine the effect of behavioral sleep-promoting interventions on psychological distress. Multiple electronic databases were searched for relevant randomized controlled trials (RCTs) published in English. Fourteen RCTs were included in the qualitative synthesis (N = 932), seven were included in the meta-analysis (n = 711) to address the primary aim, and three (n = 253) were included to address the secondary aim. The pooled standardized mean difference for sleep-promoting interventions after treatment for total sleep time was 34.92 minutes (95% CI: 8.70, 61.14). Sleep-promoting interventions had no significant effect on sleep efficiency. More RCTs that involve adolescents and emerging adults are needed to determine the effect of sleep-promoting interventions on reducing psychological distress in this high-risk age group.
Keywords: sleep, sleep health, cognitive-behavioral therapy, systematic review, meta-analysis, adolescence, emerging adult, treatment, psychological distress
Habitual short sleep duration is a major public health concern that affects more than two-thirds (68–83%) of adolescents aged 12 to 17 years, and one-third (32.2%) of emerging adults aged 18 to 25 years in the United States [1, 2]. Meeting the recommended sleep duration of 8 to 10 hours per night for adolescents, and 7 to 9 hours per night for emerging adults is difficult due to a combination of biological (e.g., circadian phase delay) and behavioral (e.g., evening use of electronics) [3] interactions, coupled with a need to maintain early wake-up times for work or school [4]. A short sleep duration is associated with psychological distress (symptoms of depression or anxiety) in adolescents and emerging adults, and is an independent risk factor for its persistence a year later [5]. One in four adolescents and emerging adults are affected by psychological distress [6]. However, sleep duration is not typically a direct target for intervention.
Adolescents and emerging adults have the highest prevalence of diagnosed mental illness (25.8%–56.7%) compared with adults aged 26 to 49 years (22.2%), and adults aged 50 years and older (13.8%) [6]. Suicide is the second leading cause of death in adolescents and emerging adults [7]. Further, the transition from adolescence to emerging adulthood is associated with an increased risk of psychological distress and poorer sleep health [8]. The total sleep time measured via actigraphy decreased and variability in total sleep time increased over a two-year period among 343 adolescents transitioning into emerging adulthood [9]. Earlier bedtimes and longer total sleep times are associated with lesser symptoms of depression in adolescents [10]. Several modifiable risks underlie the mechanisms between both sleep disturbance and internalized problems that arise from psychological distress such as stress arousal, emotion processing, and cognitive factors [11]. Therefore, the commonly investigated problems related to psychological distress and sleep health may be amenable to behavioral and cognitive-behavioral interventions aimed at improving sleep [12, 13].
Three previous systematic reviews have addressed behavioral sleep interventions [14, 15, 16]; adolescents were included in one of the reviews [14], whereas adults and adolescents were included in the other two [15, 16]. In the review that included adults, the population evaluated was a broad range of participants without sleep disorders, who were aged 18 to 64 years [15]; the other review included adolescents aged from 12 years through older adults with and without sleep disorders, some with chronic comorbid medical conditions [16]. In the review that included adolescents, an increase in total sleep time was reported [14], whereas improved sleep health (total sleep time was included in the score) was reported in the review that included adults [15], and an improvement in depression symptoms was reported in the other review on adolescents and adults [16]. Self-reported sleep measures were used in all three reviews; however, it should be noted that self-reported total sleep time is often overestimated or subject to self-report bias, since participants mostly report time in bed instead of actual sleep time [17].
The primary aim of this systematic review and meta-analysis was to evaluate the effect of behavioral sleep-promoting interventions on the sleep characteristics of adolescents and emerging adults (aged 12 to 25 years) who do not have a sleep disorder. The secondary aim was to determine the effect of behavioral sleep-promoting interventions on psychological distress (symptoms of depression and anxiety).
This systematic review and meta-analysis fills an important gap in the existing literature as it focuses on adolescents and emerging adults aged 12 to 25 years, a key developmental stage that is associated with a high risk of poor sleep health and psychological distress.
Methods
We conducted this systematic review and meta-analysis in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta analyses Statement [18]. Our protocol was registered with the PROSPERO registry prior to the implementation of the search and can be accessed at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019138021.
The primary aim of this systematic review and meta-analysis was to quantify the effect of behavioral sleep-promoting interventions on sleep characteristics of adolescents and emerging adults who do not have a sleep disorder. The primary outcomes included change in the scores of sleep measures that represent at least one of the following sleep characteristics: total sleep time, sleep efficiency, wake after sleep onset, sleep onset latency measured with actigraphy/polysomnography (PSG), and sleep stages (rapid eye movement sleep, and non-rapid eye movement sleep [stages 1, 2, and 3] as evaluated with PSG only).
The secondary aim of this research was to determine if behavioral sleep-promoting interventions are effective for reducing psychological distress (symptoms of depression and anxiety) or improving mood compared to no intervention (or treatment-as-usual). Secondary outcomes included any change in psychological distress scores after intervention [15].
Eligibility criteria
We included only studies in which the effect of behavioral sleep interventions was assessed using objective sleep measures (e.g., actigraphy or PSG). Additionally, we included studies whose participants were aged 12 to 25 years, a population at a high risk of having poor sleep health and psychological distress. This is because sampling adolescents and adults across a broad range of ages could lead to an underestimation of the effects of these interventions in this high-risk population. Studies that met the following criteria were included in this research: (1) randomized controlled trials (RCTs) of adolescents and emerging adults aged 12 to 25 years that were published in English; and (2) in which sleep characteristics measured using wrist actigraphy or PSG were reported for the intervention and control group(s) in the study. Studies that focused on populations that met the following criteria were excluded: (1) people with sleep disorders (e.g., obstructive sleep apnea, insomnia); (2) people with chronic medical conditions; (3) people with severe comorbid psychiatric illness (e.g., bipolar disorder, schizophrenia); (4) night shift workers; (5) and people with Body Mass Index (BMI) > 35. We also excluded studies in which all intervention groups received pharmacological treatment, or that did not include a non-intervention control group. If the participants of a study were not exclusively adolescents or emerging adults, a sub-analysis was performed when possible.
Search strategy
We conducted a controlled vocabulary and keyword search of the following databases: Ovid Medline, Ovid Embase, Ovid PsycINFO, Cochrane Central Register of Controlled Trials, and EBSCO CINAHL Complete. The search was limited to articles published in English language. All searches covered the periods from the date of establishment of each database to May 28, 2019. The Ovid Medline search terms are provided in supplementary table 1. The search strategies were adjusted for syntax as appropriate for each database/platform.
The search was conducted by an expert medical librarian (JB) with input from the investigator (SG) and agreement from a second investigator (MG). The search was then peer-reviewed by a second expert medical librarian. In addition, an ancestry search was conducted for reference lists of identified articles and earlier systematic reviews, together with a forward search (citation tracking) until no additional relevant articles were found (December 31, 2019).
Study selection
The search results were uploaded to EndNote™ (version X8 – Thompson Reuters) and deduplicated. A total of 7,130 references were retrieved, and after removal of duplicates, 5,924 references remained. The final set was uploaded into Covidence™ systematic review software (Veritas Health Information) for screening.
The titles and abstracts of retrieved articles were screened independently by two reviewers (SG and SC) until there was > 95% agreement. The full texts of all articles deemed potentially relevant were obtained and the two reviewers independently assessed them for eligibility against the previously mentioned inclusion/exclusion criteria. All disagreements regarding eligibility were discussed by the two reviewers and if consensus was not reached, it was resolved by a third reviewer (MG). If there were multiple publications that described the same trial, only the study with the largest sample size was included.
Data extraction
Data were extracted and recorded using a customized spreadsheet. Recorded data included study characteristics (authors, title, year, country), intervention type, age, mental health outcome measure used, sleep measure used (PSG or actigraphy), size of the intervention and control groups, and the baseline and outcome data (means [M] and standard deviations [SD]). For studies in which insufficient outcome data were reported, the corresponding authors were contacted to request access to the data.
Risk of bias
The two reviewers independently assessed the risk of bias in the included studies using the Cochrane risk of bias tool [19]. The domains of the Cochrane risk of bias include sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data (< 80%), selective outcome reporting, and ‘other issues.’ We omitted the “blinding of participants and personnel” domain as all interventions were behavioral, and thus participants were not blinded.
Data synthesis
Eligible studies were grouped according to the type of intervention and the results were described narratively. The M and SD or standard error of the mean (SEM) were reported for sleep and other secondary outcomes in each group. The change in M and SD (or SEM) was reported for other secondary outcomes. Meta-analyses were performed using Revman 5.3.5 in cases where the same type of intervention and outcomes were reported in at least three studies. The unstandardized weighted mean difference and the 95% confidence interval (CI) between groups were calculated using a random effects model. This model was selected a priori because independent studies were assumed not to be functionally equivalent as a result of the different protocols and participant groups; therefore, they lacked a common effect size. The I2 statistic was calculated to indicate heterogeneity between studies, with 25%, 50%, and 75% classified as low, medium, and high heterogeneity, respectively [20].
Results
Study selection
The study selection process is illustrated in figure 1. We identified 14 RCTs that met our inclusion criteria. The results presented are based on the data published in these articles, data in supplemental materials, and information obtained through contact with the corresponding author of an article if some pertinent study data were unavailable or unclear. We contacted three corresponding authors, two responded, and one provided additional data.
Figure 1.
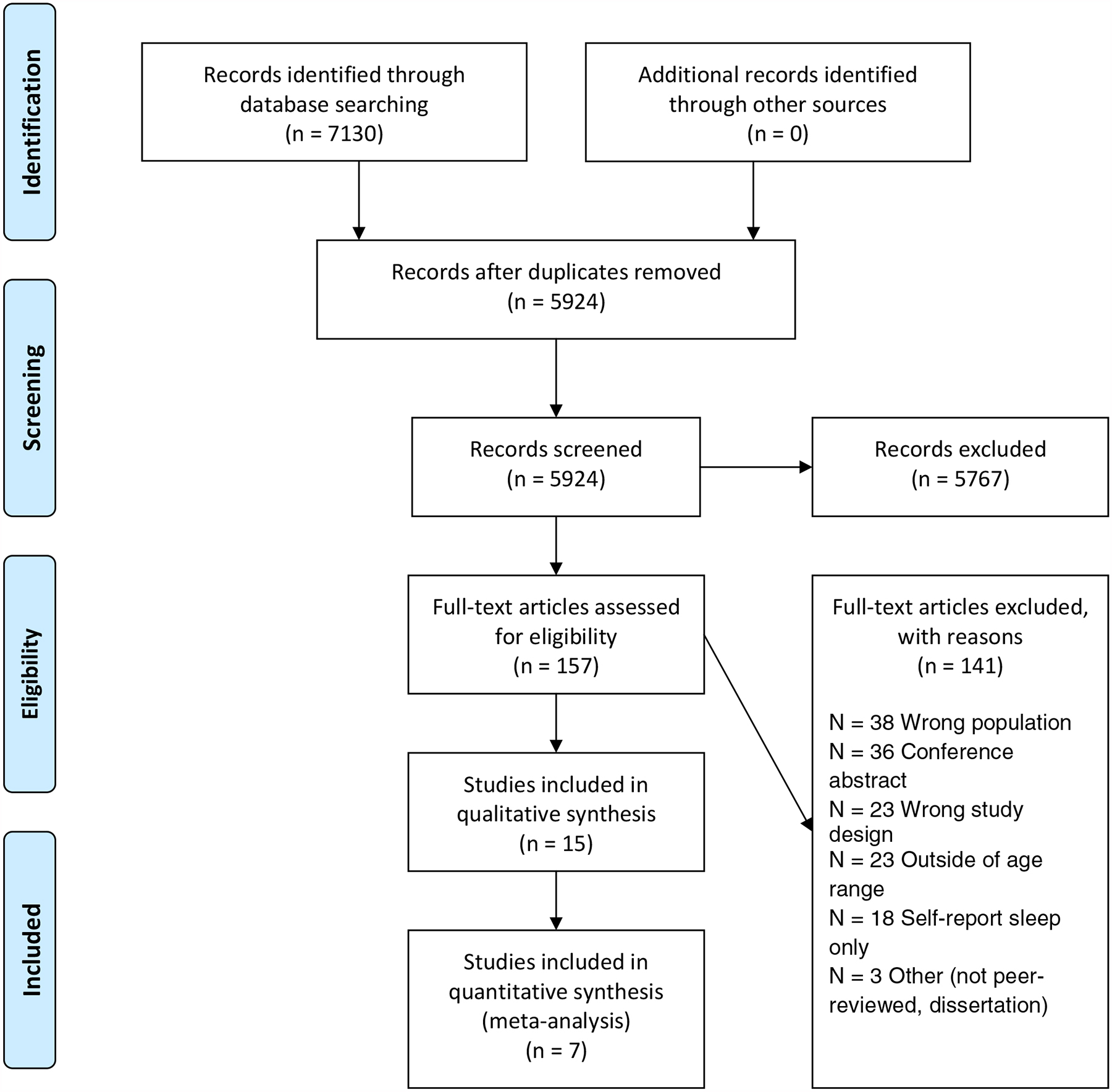
PRISMA Flow Diagram
Characteristics of the included studies
Fourteen behavioral sleep RCTs were included in this systematic review. A total of 932 participants with ages ranging from 12.2 years to 24 years (M age, 18.1 ± 3.8 years; M BMI, 22.2 ± 1.5; 56.9% female) were included in these trials. The participants were comprised of 693 adolescents (M age, 15.0 ± 1.4 years) and 239 emerging adults (M age 21.2 ± 1.7 years). The characteristics of the included studies are presented in table 1. Sleep was measured via actigraphy in 12 studies, with a combination of actigraphy and PSG/electroencephalography (EEG) in three studies, and with EEG or PSG alone in two studies. For the studies with actigraphy, the procedure was primarily conducted in the homes of the participants, with the exception of one study in which the trial was conducted in a boarding school [21]. For studies with PSG/EEG, the procedure was performed in a laboratory setting. The RCTs were conducted in middle to upper income countries including the United States, England, Brazil, Australia, Canada, the United Kingdom, the Netherlands, Switzerland, Norway, Germany, and Singapore.
Table 1.
Characteristics of studies
| Authors, reference number | Country | Sample | Age in years mean (SD) | Gender N (% female) | Sleep Measure/Setting | Intervention v. Control | Depression measure |
|---|---|---|---|---|---|---|---|
| AL Khatib et al. 2018 [23] | England | N = 42 | 24 (2.2) | 17 (40.5%) | Actigraphy/home | Sleep promotion (sleep extension 11. 5h TIB) v. habitual sleep | CESDa |
| Barber and Cuca1on 2017 [22] | US | N = 78 | 20 (4.8) | 47 (60.3%) | Actigraphy/home | Sleep promotion education (sleep hygiene) v. no education | |
| Baum et al. 2014 [30] | US | N = 40 | 15.5 (0.9) | 20 (50%) | Actigraphy/home | Sleep restriction 6.5 h v. 10 h | |
| Beijamini et al. 2011 [28] | Brazil | N = 21 | 13–14 | 10 (47.6%) | Actigraphy/home | Sleep promotion (sleep hygiene) v. no education | |
| Blake et al. 2016 [24] | Australia | N = 131 | 14.5 (1.0) | 79 (60.3%) | Actigraphy/home | Sleep promotion (CBT/mindfulness-based group) v. active study skills | CESD |
| Cote et al. 2009 [31] | Canada | N = 25 | 20 (3.2) | 19 (76%) | EEG/Lab | Sleep restriction 3h v. 5h. v. 8h | |
| Dewald-Kaufmann et al. 2014 [29] | The Netherlands | N = 55 | 15.4 | 47 (85.5%) | Actigraphy/home | Sleep promotion (gradual sleep extension to 1h and sleep hygiene advice) v. no instruction | CDI |
| Fucito et al. 2017 [27] | US | N = 42 | 20.7 (1.4) | 22 (52.3%) | Actigraphy/home | Sleep promotion (cognitive-behavioral and sleep hygiene) v. education on healthy behaviors | |
| Lo et al. 2016 [21] | Singapore | N = 56 | 16.4 (0.9) | 25 (44.6%) | Actigraphy & PSG/boarding school | Sleep restriction 5h v. 9h | |
| Ødegård et al. 2015 [32] | Norway | N = 33 | 22.7 (3.2) | 17 (51.5%) | Actigraphy/home & PSG/Lab | Sleep restriction 4h v. 9h | |
| Rigney et al. 2015 [25] | Australia | N = 296 | 12.2 (0.6) | 175 (59.1%) | Actigraphy/home | Sleep promotion (sleep hygiene education) v. no education | |
| Saletin et al. 2017 [33] | US | N = 19 | 20 | 10 (52.6%) | PSG/Lab | Sleep restriction 5h with naps v. 5 h no naps | |
| Van Dyk et al. 2017 [26] | US | N = 54 | 15.9 (1.1) | 33 (61.1%) | Actigraphy/home | Sleep promotion (sleep extension 1.5h TIB) v. habitual sleep | POMS |
| Voderholzer et al. 2010 [34] | Germany | N = 27 | 15 (0.9) | 15 (55.5%) | Actigraphy/home & PSG/Lab | Sleep restriction 5h v. 6h v. 7h v. 8h v. 9h |
No post measure results reported for TST, only included in depressive symptoms meta-analysis
Abbreviations:CBT cognitive behavioral therapy; CESD center for the epidemiologic studies depression scale; CDI children’s depression inventory; BDI beck depression inventory; POMS profile of mood states; SCAS spence children’s anxiety scale; TIB time in bed
Eight of the RCTs were focused on sleep promotion, [22–29] whereas six focused on sleep restriction [21, 30–34]. We categorized an intervention as ‘sleep-promoting’ if the participants in the experimental group were encouraged to sleep the recommended hours for their age or if those with habitual short sleep duration were instructed to extend their time in bed by 1 hour to 1.5 hours [23, 26, 29]. Four of these sleep-promotion RCTs included sleep hygiene education [22, 25, 27, 28], two included multiple cognitive-behavioral components [24, 27], one included a mindfulness-based group intervention [24], and three included sleep extension [23, 26, 29] (one with behavioral consultation [23] and one with a sleep hygiene component) [29]. The cognitive-behavioral components included in two of the trials were comprised of sleep education, sleep goals, sleep hygiene and stimulus control, savoring and switching, managing worries, and setback prevention in one of the trials [24]. In another trial, the behavioral components were stimulus control instructions, sleep scheduling, sleep hygiene, and relaxation training, with cognitive strategies to target sleep-disruptive beliefs [27].
Sleep restriction was applied in six trials [21, 30–34]. We categorized interventions as ‘sleep restriction interventions’ if the participants in the experimental group were encouraged to restrict their time in bed to less than the recommended hours for their age. This restriction varied across studies, ranging from 3 hours [31] to 6.5 hours [30]. One of the RCTs that included a sleep restriction intervention of 5 hours of time in bed included daytime naps in their protocol [33].
The depression symptom measures used in the trials included the Center for the Epidemiologic Studies Depression Scale, the Children’s Depression Inventory (CDI), the Beck Depression Inventory, and the Profile of Mood States. There was only one measure of anxiety - the Spence Children’s Anxiety Scale (SCAS).
Risk of bias
The Cochrane Collaboration’s tool for assessing risk of bias was used to evaluate the quality of the included studies; a graph that summarizes the risk of bias of the included studies is presented in supplementary table 2. The majority of the studies were judged to be high-quality studies. Allocation concealment was unclear in four RCTs, blinding of outcome assessments was unclear in 12 RCTs, and sequence generation was unclear in one RCT. Depression symptom measures for adolescents were created or validated, and they had good internal consistency and test-retest reliability.
Effect of behavioral sleep-promoting interventions on total sleep time after treatment
We analyzed seven RCTs (n = 711) that evaluated behavioral sleep promotion. The results of the random effects meta-analysis are illustrated in figure 2. Behavioral sleep-promoting interventions increased total sleep time to a small degree after treatment compared with control conditions not designed to increase total sleep time (standardized mean difference = 34.92 minutes, 95% CI 8.70, 61.14 p = .009). The result had a high heterogeneity (I2 = 100%, p < .00001).
Figure 2.
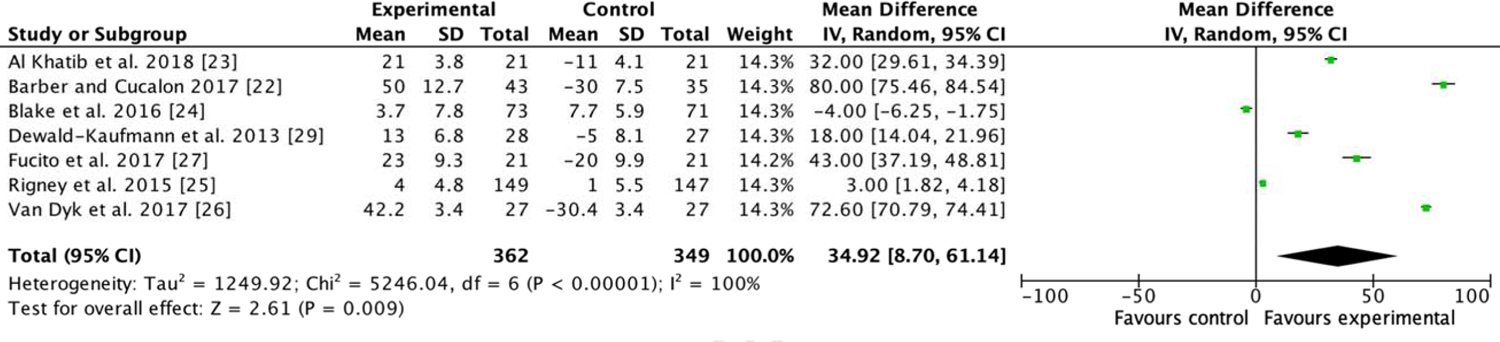
Forest plot for meta-analysis of the effect of behavioral sleep promotion interventions on total sleep time.
Effect of sleep-promoting interventions on sleep efficiency after treatment
Seven RCTs (n = 711) that evaluated behavioral sleep promotion were analyzed to determine the effect of sleep-promoting interventions on sleep efficiency after treatment. The results of the random effects meta-analysis are illustrated in figure 3. Behavioral sleep-promoting interventions improved sleep efficiency after treatment better than control conditions not designed to increase sleep; however, the improvement was not statistically significant (standardized mean difference = −0.01, 95% CI −1.03, 1.02 p = .99) but the result had a high heterogeneity (I2 = 100%, p < .000001).
Figure 3.
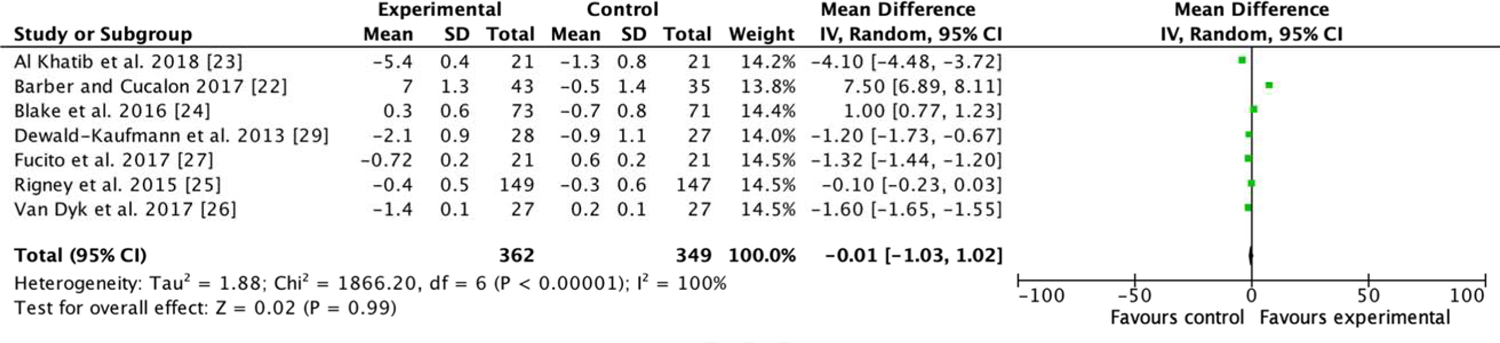
Forest plot for meta-analysis of the effect of behavioral sleep promotion interventions on sleep efficiency.
Effect of behavioral sleep-promoting interventions on symptoms of depression
The effect of behavioral sleep-promoting interventions on depression symptoms in adolescents was tested in three RCTs (n = 253) [24, 26, 35]. The effect of the interventions was not significant in two of the trials [24, 26, 35]. However, in Dewald-Kaufmann’s trial of 55 adolescents with chronic sleep reduction, the depression symptoms of the participants in the sleep improvement group (gradual sleep extension of up to an additional one hour of time in bed with sleep hygiene advice) decreased significantly (a small effect size was noted) (β = −0.41, SE = 0.16, p = .01) [35].
Effect of sleep-promoting interventions on anxiety symptoms
The effect of sleep promotion on anxiety symptoms was tested in only two trials [24, 26], and an effect on anxiety symptoms was only reported in one [24]. There was an improvement in the anxiety symptoms (measured with the SCAS) of adolescents (M age, 14.5 ±0.95 years) in the sleep improvement group, a multicomponent cognitive-behavioral, mindfulness-based intervention group with a small effect size (β = 3.49, SE = 1.67, CI = 0.23, 6.75) [24]. In the other RCT, the sleep-promoting intervention did not have a significant effect on the anxiety symptoms of the adolescents in the sleep improvement group (sleep extension of 1.5 hours) compared to those in the habitual sleep group [26].
Sleep restriction, mood, and cognition
Sleep restriction was described in six trials included in the present review [21, 30–34]. However, we were unable to analyze the findings of the trials due to the varied application of sleep restriction interventions on the treatment group versus the control group (e.g., 6.5 hours versus 10 hours; 3 hours versus 5 hours versus 8 hours; 9 hours versus 4 hours, etc.). One of the trials included naps in the protocol for the sleep restriction group [33], and inconsistency or omission in the reports of recovery sleep after intervention. Overall, it was feasible for both adolescents and emerging adults to follow the sleep restriction protocol (>80%) [21, 30–34]. The sleep restriction interventions had a negative effect on positive and negative mood [31], preservation of slow wave sleep, and memory consolidation in recovery sleep [34]; they also led to a poorer working memory, executive function, and sustained attention [21], and more subjective sleepiness despite naps [33].
Sleep restriction was reported to have a negative effect on mood in two trials, [30, 31] and a negative effect on the ability to regulate emotions in one of the trials [30]. In Baum et al.’s trial, 40 adolescents (M age, 15.5 ± 0.9 years) were randomized 1:1 into a short sleep restriction (6.5 hours in bed) group or a healthy total sleep time (10 hours in bed) control group for five nights [30]. The participants in the sleep restriction group reported more anxiety symptoms and a worsened ability to regulate emotions during the experiment (p = .001). However, there were no differences between depression symptoms of both groups (p > .05) [30]. In Cote et al.’s trial of 25 emerging adults (M age, 20 ± 3.2 years), the participants were randomized into an 8-hour (n = 12), a 5-hour (n = 13), or a 3-hour (n = 13) sleep restriction group. Sleep restriction had a negative effect on positive mood for both the 5-hour and 3-hour groups (p < .01 and p < .001 respectively), but the positive mood was slightly better in the 8-hour group (p < .05) [31]. Comparatively, negative mood was not a sensitive measure of the dose-dependent effects of sleep restriction for any of the groups (8 hours, 5 hours, or 3 hours) [31].
In Voderholzer et al.’s trial of 27 adolescents (M age, 15 ± 0.9 years), a higher preservation of slow wave sleep measured via PSG, and more resilient memory consolidation was recorded in the 5-hour sleep restriction group (n = 15) than in the control group (8 hours) (n = 12) [34]. On the other hand, memory recovery was not recorded in another sleep restriction trial with adolescents of a similar age (M age, 16.4 ± 0.9 years) [21]. In Lo et al.’s trial of 56 adolescents (M age, 16.4 ± 0.9 y), the 5-hour sleep restriction group demonstrated incremental deterioration of sustained attention, working memory, executive function, as well as an increase in subjective sleepiness that did not fully improve even after two nights of recovery sleep [21].
Discussion
We reviewed the results of trials on behavioral interventions designed to improve total sleep time and sleep efficiency. The meta-analysis showed that behavioral sleep interventions improve total sleep time but have no significant effect on sleep efficiency. Due to inconsistent reporting in the included studies, we were unable to determine the effect of behavioral sleep interventions on other important sleep outcomes such as sleep onset latency, wake after sleep onset, sleep fragmentation, or sleep stages. Only one of the studies with adolescents recorded a small effect of behavioral sleep interventions on depression symptoms. This may partially be because the exclusion criteria of the present review did not permit the inclusion of trials that included adolescents with clinical-level mental health problems. Depression or anxiety symptoms were measured in only five studies on adolescents but not in trials on emerging adults. It appears that increasing total sleep time improves symptoms of psychological distress, whereas restricting sleep worsens psychological distress in adolescents. The potential effects of sleep-promoting interventions on psychological distress, specifically depression and anxiety symptoms, in adolescents and emerging adults warrants further investigation.
Clinical implications
Since psychological distress is a major risk factor for suicide, there is great interest in the possibility that sleep is a modifiable risk factor for psychological distress in both adolescents and emerging adults who are at high risk of suicide [36]. Despite this interest, there has been limited emphasis and work in this area, particularly among this high-risk population. The negative effect of sleep restriction on psychological distress warrants further investigation. In one of the trials included in the present review, after only a few days of restricting sleep to a level that is experienced regularly by millions of adolescents on school nights (e.g., 6.5 hours), adolescents had worsened anxiety symptoms and a decreased ability to regulate negative emotions [30]; notably, sleep restriction did not have an effect on depression symptoms in this RCT [30]. Although the causality between short sleep duration and psychological distress remains unclear, it is likely that short sleep duration plays a role in psychological distress based on the linear association noted in previous studies [5], and the direct effect that was noted when sleep was restricted in adolescents [30, 31]. Based on these findings, we recommend that researchers should plan carefully and develop a safety monitoring system when designing interventions with a sleep restriction component for adolescents and emerging adults, considering the high prevalence of psychological distress in this age group. We also suggest including measures of both anxiety and depression symptoms considering the high prevalence of both anxiety and depression symptoms in this age group [30]. Excluding patients with severe anxiety, severe depression, or other severe psychiatric illnesses (e.g., bipolar disorder, schizophrenia) is another important consideration for protocols with a sleep restriction component (e.g., Cognitive behavioral therapy for insomnia [CBT-I]).
On another note, interventions designed to improve sleep knowledge, extend sleep, or interventions with behavioral, cognitive-behavioral, and/or mindfulness components had either a neutral or positive effect on the depression or anxiety symptoms of adolescents. These programs were well tolerated and were feasible for both adolescents and emerging adults; participants in the experimental groups of these studies extended their total sleep time. Two behavioral interventions had the dual benefit of improving total sleep time and psychological distress; one had a small effect on depression symptoms, [35] and the other had a small effect on anxiety symptoms of adolescents [24]. The intervention that had a statistically significant effect on the depression symptoms of adolescents was gradual sleep extension with sleep hygiene advice [35]. It may be that the CDI was more sensitive at identifying the effects of the intervention on depression symptoms. Another possibility is that the intervention itself, by gradually extending sleep combined with sleep hygiene advice, improves both total sleep time and depression symptoms. In a literature review of 12 studies that included adolescents (aged 14 to 19 years) who received sleep education, sleep knowledge increased in most groups; however, these interventions did not have a significant effect on self-reported sleep behavior, and did not increase sleep duration or improve sleep hygiene [37]. Since this previous review was based on self-reported sleep behavior, it is likely that sleep education without behavioral components does not produce changes in sleep behavior. The multicomponent cognitive-behavioral, mindfulness-based sleep improvement intervention improved total sleep time and had a small effect on anxiety symptoms [24]. Larger RCTs are needed to determine whether sleep improvement interventions have a large-scale effect on psychological distress in this age group. However, based on our systematic review, these sleep-promoting interventions have the potential to improve total sleep time in adolescents and emerging adults who generally have short sleep duration.
Limitations
Due to inconsistent reporting, we were only able to analyze total sleep time and sleep efficiency. Reports of baseline and post-intervention values for other sleep outcomes (e.g., sleep onset latency, wake after sleep onset, sleep fragmentation, sleep stages) were unavailable as well. Thus, due to the limited number of studies performed with simultaneous PSG, we cannot determine whether lengthening or promoting healthy sleep has a negative effect on other sleep parameters that indicate difficulty initiating (e.g., sleep onset latency) or maintaining sleep overnight (e.g., wake after sleep onset, sleep fragmentation), or whether it reduces sleep quality (e.g., sleep stages).
In terms of psychological distress, we could not analyze the effect of behavioral sleep-promoting interventions on depression or anxiety symptoms in adolescents, and we were unable to determine their effect on either depression or anxiety symptoms in emerging adults. We only included studies that used objective sleep measures; if studies that used self-report sleep measures had been included, it may have been possible to analyze the effect of sleep improvement interventions on depression symptoms in emerging adults, and anxiety symptoms in both adolescents and emerging adults. In their meta-analysis, Gee et al. [16] determined that non-pharmacologic sleep interventions were effective at reducing the depression symptoms of participants of a broad range of ages, who had sleep problems (e.g., sleep disturbance, sleep apnea, parasomnias) and a wide range of comorbid mental health diagnoses and some chronic comorbid medical diagnoses. This result leaves a gap in our understanding of the effects of sleep improvement interventions on psychological distress in adolescents and emerging adults who are at a high risk of both poor sleep and poor mental health, and do not have a sleep disorder or severe mental illness or comorbid medical diagnosis.
We were also unable to analyze the effect of sleep restriction on total recovery total sleep time and sleep efficiency or other sleep outcomes. It would be beneficial if there were larger samples in which the same or similar sleep restriction protocols were studied to determine the effect of sleep restriction (a component of CBT-I) on recovery sleep outcomes in participants without insomnia disorder. A goal of CBT-I is to limit sleep opportunities to increase the drive for sleep and ultimately improve homeostatic regulation of sleep [38]. This approach would help determine if CBT-I could be a consideration for those without clinically significant insomnia.
Conclusions
We determined that the total sleep time of adolescents and emerging adults can be improved with behavioral sleep-promoting interventions. In addition, these interventions were well tolerated, feasible, and acceptable for the participating adolescents and emerging adults. Sleep-promoting interventions with gradual sleep extension and sleep hygiene advice components appear to have the dual benefit of improving sleep and reducing depression symptoms in adolescents without a clinical sleep disorder. Larger samples of adolescents, as well as a sampling of emerging adults, are needed to determine the feasibility and effects of behavioral sleep-promoting interventions in this high-risk age group. Researchers should also consider including moderators (e.g., age, sex, type of intervention) when these larger studies are available for meta-analysis. This approach would be highly beneficial considering the side effects and adverse effects associated with pharmacologic sleep treatments.
Supplementary Material
Practice points.
The focus of previous systematic reviews and meta-analyses of sleep interventions was on individuals across a broad range of ages with clinical sleep disorders and/or mental disorders.
The large number of adolescents and emerging adults that report poor sleep health, but are not diagnosed with a sleep disorder, calls for more interventions that are effective for people in this subgroup.
Behavioral sleep-promoting interventions with gradual sleep extension and sleep hygiene advice components appear to have the dual benefit of improving sleep and reducing depression symptoms in adolescents without a clinical sleep disorder.
Behavioral sleep promotion interventions are feasible and well tolerated in both adolescents and emerging adults.
Restricting sleep in adolescents should be done with caution and careful monitoring, given the limited available research on this age group and the negative finding in one of the studies.
Research agenda.
Larger, more diverse samples of adolescents and emerging adults are needed to determine the feasibility and effect of behavioral sleep-promoting interventions in this high-risk age group.
Larger samples that apply the same or similar sleep restriction protocols are needed to determine the effect of sleep restriction (a component of CBT-I) on recovery sleep outcomes in people without insomnia disorder.
Funding:
National Institute for Nursing Research (NINR), T32 NR0008346
Abbreviations
- BMI
Body mass index
- CBT-I
Cognitive behavioral therapy for insomnia
- CDI
Children’s depression inventory
- CI
Confidence interval
- EEG
Electroencephalography
- M
Mean
- PSG
Polysomnography
- RCT
Randomized controlled trial
- SCAS
Spence children’s anxiety scale
- SD
Standard deviation
- SEM
Glossary of terms
- Forest plot
A graphical representation of the results of a meta-analysis.
- Heterogeneity
A variability in an observed effect size that is greater than would be expected by chance alone.
- Total sleep time
The total length of time spent asleep over a period of 24 hours.
- Sleep onset latency
The length of time it takes to transition from full wakefulness to sleep after “lights out”. A shorter transition time is considered ideal.
- Sleep efficiency
The percentage of time in bed spent asleep. The ratio of the total time spent asleep (total sleep time) compared to the total amount of time spent in bed (total sleep time/time in bed X 100). A higher percentage is considered ideal.
- Sleep hygiene
Psychoeducation on healthy sleep behaviors and sleep-promoting environmental conditions.
- Sleep extension
Behavioral instruction aimed at increasing time in bed as a way of increasing total sleep time. Typically, time in bed is increased by 1 hour to 1.5 hours from baseline. The recommended sleep duration is 8 to 10 hours for adolescents aged 12 to 17 years, and 7 to 9 hours for emerging adults.
- Sleep restriction
Behavioral instructions aimed at restricting time in bed as a way of increasing homeostatic sleep drive. Time allowed in bed is initially restricted to the average time perceived as sleep time per night and then adjusted to ensure that sleep efficiency remains above 85%.
- Cognitive behavioral therapy for insomnia
A multicomponent psychological intervention comprising a range of strategies designed to target the behavioral and cognitive underpinnings of insomnia. It may include stimulus control therapy, sleep restriction, sleep hygiene, relaxation training, sleep environment improvement, and biofeedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest.
Contributor Information
Stephanie Griggs, Case Western Reserve University, Frances Payne Bolton School of Nursing, Cleveland, Ohio, 44106.
Margaret Grey, Yale University, School of Nursing and School of Medicine, West Haven, Connecticut 06477.
References
- [1].Peltzer K, Pengpid S. Sleep duration and health correlates among university students in 26 countries. Psychol Health Med 2016; 21: 208–20. [DOI] [PubMed] [Google Scholar]
- [2].Wheaton AG, Jones SE, Cooper AC, Croft JB. Short Sleep Duration Among Middle School and High School Students - United States, 2015. MMWR Morb Mortal Wkly Rep 2018; 67: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mazzer K, Bauducco S, Linton SJ, Boersma K. Longitudinal associations between time spent using technology and sleep duration among adolescents. J Adolesc 2018; 66: 112–9. [DOI] [PubMed] [Google Scholar]
- [4].Owens J Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics 2014; 134: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Glozier N, Martiniuk A, Patton G, Ivers R, Li Q, Hickie I, et al. Short sleep duration in prevalent and persistent psychological distress in young adults: the DRIVE study. Sleep 2010; 33: 1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kessler RC, Avenevoli S, Green J, Gruber MJ, Guyer M, He Y, et al. National comorbidity survey replication adolescent supplement (NCS-A): III. Concordance of DSM-IV/CIDI diagnoses with clinical reassessments. J Am Acad Child Adolesc Psychiatry 2009; 48: 386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention. WISQARS leading causes of death reports. 1999–2017.
- [8].Sacker A, Cable N. Transitions to adulthood and psychological distress in young adults born 12 years apart: constraints on and resources for development. Psychol Med 2010; 40: 301–13. [DOI] [PubMed] [Google Scholar]
- [9].Park H, Chiang JJ, Irwin MR, Bower JE, McCreath H, Fuligni AJ. Developmental trends in sleep during adolescents’ transition to young adulthood. Sleep Med 2019; 60: 202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gangwisch JE, Babiss LA, Malaspina D, Turner JB, Zammit GK, Posner K. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep 2010; 33: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cowie J, Alfano CA, Patriquin MA, Reynolds KC, Talavera D, Clementi MA. Addressing sleep in children with anxiety disorders. Sleep Med Clinics 2014; 9: 137–48. [Google Scholar]
- [12].Baglioni C, Spiegelhalder K, Feige B, Nissen C, Berger M, Riemann D. Sleep, depression and insomnia–a vicious circle? Current Psychiatry Rev 2014; 10: 202–13. [Google Scholar]
- [13].Dahl RE, Harvey AG. Sleep in children and adolescents with behavioral and emotional disorders. Sleep Med Clinics 2007; 2: 501–11. [Google Scholar]
- [14].Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, Allen NB. Systematic review and meta-analysis of adolescent cognitive–behavioral sleep interventions. Clin Child Fam Psychol Rev 2017; 20: 227–49. [DOI] [PubMed] [Google Scholar]
- [15].Murawski B, Wade L, Plotnikoff RC, Lubans DR, Duncan MJ. A systematic review and meta-analysis of cognitive and behavioral interventions to improve sleep health in adults without sleep disorders. Sleep Med Rev 2018; 40: 160–9. [DOI] [PubMed] [Google Scholar]
- [16].Gee B, Orchard F, Clarke E, Joy A, Clarke T, Reynolds S. The effect of nonpharmacological sleep interventions on depression symptoms: A meta-analysis of randomised controlled trials. Sleep Med Rev 2018; 43: 118–28 [DOI] [PubMed] [Google Scholar]
- [17].Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Sleep duration: how well do self-reports reflect objective measures? The CARDIA Sleep Study. Epidemiology 2008; 19: 838–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nagendrababu V, Duncan H, Tsesis I, Sathorn C, Pulikkotil S, Dharmarajan L, et al. Preferred reporting items for systematic reviews and meta analyses for abstracts: best practice for reporting abstracts of systematic reviews in Endodontology. Int Endod J 2019; 52: 1096–107 [DOI] [PubMed] [Google Scholar]
- [19].Jørgensen L, Paludan-Müller AS, Laursen DR, Savović J, Boutron I, Sterne JA, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev 2016; 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lo JC, Ong JL, Leong RLF, Gooley JJ, Chee MWL. Cognitive Performance, Sleepiness, and Mood in Partially Sleep Deprived Adolescents: The Need for Sleep Study. Sleep 2016; 39:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barber LK, Cucalon MS. Modifying the Sleep Treatment Education Program for Students to include technology use (STEPS-TECH): intervention effects on objective and subjective sleep outcomes. Stress Health 2017; 33: 684–90. [DOI] [PubMed] [Google Scholar]
- [23].Al Khatib HK, Hall WL, Creedon A, Ooi E, Masri T, Harding SV, et al. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. Am J Clin Nutr 2018; 76: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Blake M, Waloszek JM, Schwartz O, Raniti M, Simmons JG, Blake L, et al. The SENSE study: Post intervention effects of a randomized controlled trial of a cognitive-behavioral and mindfulness-based group sleep improvement intervention among at-risk adolescents. J Consult Clin Psychol 2016; 84: 1039–51. [DOI] [PubMed] [Google Scholar]
- [25].Rigney G, Blunden S, Maher C, Dollman J, Parvazian S, Matricciani L, et al. Can a school-based sleep education programme improve sleep knowledge, hygiene and behaviours using a randomised controlled trial. Sleep Med 2015; 16: 736–45. [DOI] [PubMed] [Google Scholar]
- [26].Van Dyk TR, Zhang N, Catlin PA, Cornist K, McAlister S, Whitacre C, et al. Feasibility and Emotional Impact of Experimentally Extending Sleep in Short-Sleeping Adolescents. Sleep 2017; 40: 1. [DOI] [PubMed] [Google Scholar]
- [27].Fucito LM, DeMartini KS, Hanrahan TH, Yaggi HK, Heffern C, Redeker NS. Using sleep interventions to engage and treat heavy-drinking college students: A randomized pilot study. Alcohol Clin Exp Res 2017; 41: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Beijamini F, Louzada FM. Are educational interventions able to prevent excessive daytime sleepiness in adolescents? Biol Rhythm Res 2012; 43: 603–13. [Google Scholar]
- [29].Dewald-Kaufmann JF, Oort F, Meijer A. The effects of sleep extension on sleep and cognitive performance in adolescents with chronic sleep reduction: an experimental study. Sleep Med 2013; 14: 510–7. [DOI] [PubMed] [Google Scholar]
- [30].Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry 2014; 55: 180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cote KA, Milner CE, Smith BA, Aubin AJ, Greason TA, Cuthbert BP, et al. CNS arousal and neurobehavioral performance in a short-term sleep restriction paradigm. J Sleep Res 2009; 18: 291–303. [DOI] [PubMed] [Google Scholar]
- [32].Odegard SS, Omland PM, Nilsen KB, Stjern M, Gravdahl GB, Sand T. The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clin Neurophysiol 2015; 126: 1979–87. [DOI] [PubMed] [Google Scholar]
- [33].Saletin JM, Hilditch CJ, Dement WC, Carskadon MA. Short daytime naps briefly attenuate objectively measured sleepiness under chronic sleep restriction. Sleep 2017; 40: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Voderholzer U, Piosczyk H, Holz J, Landmann N, Feige B, Loessl B, et al. Sleep restriction over several days does not affect long-term recall of declarative and procedural memories in adolescents. Sleep Med 2011; 12: 170–8. [DOI] [PubMed] [Google Scholar]
- [35].Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry 2014; 55: 273–83. [DOI] [PubMed] [Google Scholar]
- [36].Hirsch JK, Visser PL, Chang EC, Jeglic EL. Race and ethnic differences in hope and hopelessness as moderators of the association between depressive symptoms and suicidal behavior. J Am Coll Health 2012; 60: 115–25. [DOI] [PubMed] [Google Scholar]
- [37].Blunden SL, Chapman J, Rigney GA. Are sleep education programs successful? The case for improved and consistent research efforts. Sleep Med Rev 2012; 16: 355–70. [DOI] [PubMed] [Google Scholar]
- [38].Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev 2015; 19: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


