Abstract
Introduction:
Non-steroidal anti-inflammatory drugs (NSAIDs) are associated with adverse cardiovascular outcomes and reportedly overused in American-style football (ASF). However, assessment of ASF NSAID use in the context of cardiovascular risk has not been performed. We sought to characterize NSAID use patterns and the association with cardiovascular risk in a diverse cohort of high school and collegiate ASF athletes.
Methods:
226 ASF athletes, 60 endurance athletes, and 63 non-athletic controls were studied pre- and post-season with echocardiography, vascular applanation tonometry, and clinical data assessment. Qualitative NSAID use throughout the season was recorded at post-season.
Results:
ASF athletes gained weight (Δ0.86±3.9kg, P<0.001), increased systolic blood pressure (SBP, Δ3.1±12mmHg, P<0.001) and pulse wave velocity (PWV, Δ0.2±0.6m/s, P<0.001), and decreased E′ (Δ−1.4±2.8cm/s, P<0.001) across one athletic season. 77% (N=173) of ASF athletes reported sport-specific NSAID use began in middle school. ASF NSAID use was more frequent with ‘weekly’ (N=42,19%) and ‘daily’ (N=32,14%) use compared to endurance athletes (P<0.001) and controls (P=0.02). ASF NSAID use increased in parallel with post-season SBP and weights. ‘Daily’ ASF NSAID users demonstrated the highest post-season SBP (137±13 vs. 128±13mmHg, P=0.002) and weight (109.0±18.6 vs. 95.8±20.5kg, P=0.002) compared to ‘never/rare’ users. Adjusting for player position, SBP, PWV, and E′, increased weight (OR:1.04, 95%CI 1.0–1.08, P=0.037) was associated with more frequent NSAID use.
Conclusions:
Habitual NSAID use commonly begins during adolescence, before full physical maturation, and is associated with cardiovascular risk, particularly increased weight, in ASF athletes. NSAID use frequency should be considered when risk stratifying high-risk ASF athletes.
Keywords: Football, Obesity, Hypertension, Risk factors, Prevention
INTRODUCTION
Through inhibition of cyclooxygenase enzymes,(1) over-the-counter (OTC) nonsteroidal anti-inflammatory drugs (NSAIDs) are considered first-line pharmaceuticals that treat pain and local inflammation associated with common musculoskeletal injuries.(2, 3) However, chronic and overused NSAIDs are also associated with numerous adverse cardiovascular (CV) outcomes including hypertension,(4) coronary artery disease,(5) atrial fibrillation,(6) and congestive heart failure,(7) and therefore contraindicated for regular use in high-risk individuals.(8)
The association between competitive American-style football (ASF) participation and risk of orthopedic injury is well established.(9) ASF athletes are also uniquely at-risk for early acquired CV risk.(10, 11) Specifically, ASF participation is associated with the development of early hypertension(12–14) and acquired pathologic CV phenotypes, all associated with significant weight gain.(11, 15–17) Epidemiologic data also suggest increased CV mortality among retired professional ASF athletes who had the largest playing-time body-mass index (BMI).(11, 18–21) Whether habitual NSAID use, reportedly excessive among ASF athletes,(22–24) also increases CV risk in ASF athletes and impacts long-term outcomes is uncertain.
We sought to provide new insight to these uncertainties by qualitatively detailing NSAID use patterns in young, competitive ASF athletes and by defining the relationship between ASF NSAID use habits with established CV risk factors. We hypothesized that habitual NSAID use among ASF athletes would be increased in prevalence compared to other young and healthy populations and that ASF NSAID use would be associated with the most established markers of ASF CV risk, increased weight and hypertension.(10, 11) To address this hypothesis, we conducted a longitudinal and repeated measures observational cohort study of high school (HS) and collegiate ASF athletes over one competitive training season, capturing changes in clinical and CV phenotypes with cross-sectional, self-reported NSAID usage.
METHODS
Competitively sanctioned HS senior and collegiate freshman ASF and endurance (cross country runners and long-distance swimmers) male athletes were eligible for this study. Only those who successfully completed the season, without injury or leaving the team for any reason, were included in the final analysis. Participants were followed longitudinally across one competitive training season with detailed CV phenotyping including clinical characteristics, complete 2-D trans-thoracic echocardiography, and vascular applanation tonometry. At post-season, subjects were additionally surveyed with detailed written questionnaires outlining qualitative NSAID use habits throughout the season. A non-athletic healthy male undergraduate control comparison group was included and analyzed in parallel fashion with the athletic cohorts. The Emory Institutional Review Board approved all aspects of the study and subjects provided written informed consent, with subject assent and parental consent for participants <18 years old.
Study Population
Subjects were recruited between 2015–2018 and included collegiate freshman ASF and endurance athletes from two National Collegiate Athletic Association (NCAA) Division-I programs [Georgia Institute of Technology (Atlanta, GA) and Furman University (Greenville, SC)], freshman endurance athletes from one NCAA Division-III program [Emory University (Atlanta, GA)], and HS senior ASF and endurance athletes from two local metropolitan high schools [Marist School (Atlanta, GA) and Woodward Academy (Atlanta, GA)]. The HS athletic cohorts were confined to seniors (4th year students) to minimize the impact of pubertal development and to maximize capture of the CV phenotype associated with the respective sport exposure.(17) Non-athletic, healthy undergraduate male controls were recruited from the Emory University student body.
Data were obtained at 2 time points for each respective cohort. Time point one coincided with the start of pre-season training and time point two was 5–6 months later at a program-specific date corresponding with the immediate conclusion of the season (post-season). Controls were followed starting at the beginning of the academic year and in temporal parallel with the athletic cohorts. Clinical data including age (years), height (cm), self-reported race, and personal or family history of hypertension or coronary artery disease were collected at time point one. Anthropometric data including weight (kg), systolic (SBP) and diastolic blood pressure [DBP (mmHg)] were collected at both time points in all participants. Blood pressure was measured using a manual aneroid sphygmomanometer and an appropriately sized cuff and recorded as the average of 3 measurements with participants in a seated position after at least 10 minutes of rest. As previously proposed, ASF player position was classified as either lineman (LM, tackles, guards, centers, or defensive end positions), or non-lineman (NLM, quarterbacks, running backs, wide receivers, tight ends, linebackers, defensive backs, kickers, or punter positions).(25)
NSAID Use Questionnaires
Detailed multiple choice written surveys were administered to all participants at the post-season time point to qualitatively detail NSAID use throughout the season. Questions posed were similar to a prior survey study assessing NSAID use habits in college ASF.(24) A member of our study team was present to clarify questions for study participants if needed. Participants were asked which oral NSAIDs were used (ibuprofen, naproxen, celecoxib, diclofenac) and the reported NSAID use during the season as a qualitative frequency defined as either ‘never’, ‘rarely’, ‘weekly’, or ‘daily’. For this study, ‘never’ was defined as no NSAID use over the course of the season; ‘rarely’ was defined as NSAID use at least once during the season, but not once per week; ‘weekly’ was defined as NSAID use at least once per week, but not every day; and ‘daily’ was defined as NSAID use at least once per day throughout the season. Finally, participants were asked at which educational level regular sport-specific NSAID use began [middle school (sixth grade to eighth grade), HS (ninth grade to twelfth grade), or college].
2-D Trans-thoracic Echocardiography
Trans-thoracic echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI). 2-dimensional and tissue-Doppler imaging from standard parasternal and apical positions were performed by experienced sonographers. All information was stored digitally and post-study offline data analysis (EchoPAC version 7, GE Healthcare) was performed. Definitions of normality for cardiac structure and function were adopted from the most recent guidelines.(26) Average wall thickness was calculated as the mean of the interventricular septum and posterior wall thickness. Left ventricular (LV) mass was calculated using the area-length method and indexed to body surface area (BSA) and LV ejection fraction was calculated using the modified biplane technique.(26) Comprehensive assessment of cardiac diastolic function using tissue-Doppler imaging was performed. Tissue velocities (E′, A′, and S′) were measured from color-coded images at the lateral and septal mitral annulus. E′ was reported as the average value between the 2 measurements.
Vascular Applanation Tonometry
Arterial stiffness was measured using high-fidelity applanation tonometry (SphygmoCor, Atcor Medical, Australia), which records sequential high-quality pressure waveforms at peripheral pulse sites. The primary measure of arterial function was the carotid-femoral pulse wave velocity (PWV), the gold standard index of arterial stiffness.(27) PWV was measured by acquisition of pressure waveforms within the carotid and femoral arteries and the distance between measurement sites was recorded manually using the “foot-to-foot” method.(28) Pulse wave analysis derivation >80% of the operator index and PWV with <10% standard deviation were required for quality control, with the software programmed to acknowledge internal quality control.
Statistical Analysis
Continuous variables are presented as the mean±standard deviation and categorical variables as percentages. Baseline characteristics for the ASF, endurance, and control groups were compared using the Chi-square or Fisher’s exact test for categorical variables and one-way ANOVA for continuous variables. Pre- to post-season changes in clinical and CV measurements were compared using the paired t-test. NSAID use frequency was compared between groups using the Cochran-Armitage test for trend. Post-season clinical and CV measurements stratified by reported NSAID use frequency were compared using one-way ANOVA with Bonferroni correction for multiple group comparisons (ASF) or a two-sample t-test for comparison between 2 groups (undergraduate controls). A mixed-effects ordinal logistic regression model, incorporating all paired observations made pre- and post-season, was constructed to identify factors associated with increased NSAID use in the ASF cohort. NSAID use was stratified by 3 levels, defined by the self-reported frequency, with ‘never/rarely’ as the reference value. Selected covariates in the model included player field position (LM vs. NLM), weight, SBP, E′, and PWV. Participant-specific random intercepts were incorporated to account for within participant correlation with covariance structure of variance components assuming fixed slope. Analyses were performed with SAS software (version 9.4, Cary, North Carolina). A P-value of ≤0.05 was considered significant.
RESULTS
Baseline characteristics
Of the 244 male ASF and 60 male endurance athletes initially enrolled in this study, 226 ASF athletes and 60 endurance athletes completed the season and were included in the final analysis. 63 male collegiate controls were also enrolled and eligible for final analysis. Among ASF athletes, 92 (41%) were LM and 134 (59%) were NLM. In addition, 59 (26%) ASF athletes were HS seniors and 167 (74%) were college freshmen. Among endurance athletes, 15 (25%) were HS seniors and 45 (75%) were college freshmen. At baseline, ASF athletes were more commonly Black, taller, heavier, and had increased DBP compared to endurance athletes and controls (Table 1).
Table 1.
Baseline Participant Characteristics
| ASF (N=226) | Endurance (N=60) | Controls (N=63) | P-value | |
|---|---|---|---|---|
| Age (years) | 17.8 ± 0.5 | 18 ± 0.9 | 18.4 ± 0.8 | <0.001* |
| Height (cm) | 185 ± 6 | 180 ± 7 | 179 ± 8 | <0.001† |
| Weight (kg) | 97.7 ± 20.0 | 66.4 ± 7.7 | 75.1 ± 11.2 | <0.001* |
| BMI (kg/m2) | 28.3 ± 4.8 | 20.5 ± 1.7 | 23.4 ± 3.2 | <0.001* |
| Systolic Blood Pressure (mm Hg) | 127 ± 12 | 115 ± 14 | 124 ± 13 | <0.001‡ |
| Diastolic Blood Pressure (mm Hg) | 75 ± 9 | 64 ± 9 | 70 ± 10 | <0.001* |
| Black | 93 (41%) | 1 (2%) | 10 (16%) | <0.001* |
| History of Hypertension | 10 (4%) | 0% | 2 (3%) | 0.26 |
| Family History of Hypertension | 81 (36%) | 19 (32%) | 31 (49%) | 0.12 |
| Family History of CAD | 20 (9%) | 10 (17%) | 4 (6%) | 0.10 |
Adjusted P<0.05 between all groups
Adjusted P<0.05 between ASF and other groups
Adjusted P<0.05 between Endurance and other groups
ASF: American-style football; BMI: body mass index CAD: coronary artery disease
Sport-specific changes in weight, BP, and CV function
At the post-season time point, only ASF participants demonstrated significant increases in both weight and SBP (Table 2). Differences in CV function were also present in the ASF athletes compared to endurance athletes (Table 2). While both athlete groups exhibited exercise-induced cardiac hypertrophy, only ASF athletes developed increased PWV and reduced diastolic function. In contrast, endurance athletes demonstrated enhanced diastolic function as defined by increased E′ velocity and no evidence of arterial stiffening. Undergraduate controls did not demonstrate changes in SBP, cardiac structure, or CV function over time.
Table 2.
Longitudinal Changes in Select Clinical and Cardiovascular Measurements
| ASF (N=226) | Endurance (N=60) | Controls (N=63) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-season | Post-season | P-value | Pre-season | Post-season | P-value | Pre-season | Post-season | P-value | |
| Weight (kg) | 97.7 ± 20.0 | 98.5 ± 20.4 | <0.001 | 66.4 ± 7.7 | 67.6 ± 6.7 | 0.07 | 75.1 ± 11.2 | 76.5 ± 11.3 | <0.001 |
| SBP (mm Hg) | 127 ± 12 | 130 ± 13 | <0.001 | 115 ± 14 | 113 ± 12 | 0.33 | 124 ± 13 | 125 ± 12 | 0.45 |
| DBP (mm Hg) | 75 ± 9 | 75 ± 9 | 0.98 | 64 ± 9 | 68 ± 9 | 0.002 | 70 ± 10 | 73 ± 9 | 0.02 |
| LVIDD (mm) | 52.0 ± 4.1 | 52.8 ± 4.3 | <0.001 | 51.0 ± 4.4 | 52.4 ± 4.5 | 0.001 | 50.1 ± 3.9 | 50.0 ± 3.9 | 0.73 |
| Average WT (mm) | 8.9 ± 0.8 | 9.7 ± 1.0 | <0.001 | 8.2 ± 0.8 | 8.5 ± 0.8 | 0.004 | 7.9 ± 0.7 | 8.0 ± 0.7 | 0.71 |
| LV mass/BSA (g/m2) | 91 ± 11 | 100 ± 11 | <0.001 | 100 ± 14 | 110 ±14 | <0.001 | 88 ± 11 | 88 ± 11 | 0.33 |
| Ejection Fraction (%) | 61.0 ± 4.6 | 60.2 ± 5.0 | 0.08 | 59.7 ± 4.6 | 60.0 ± 5.4 | 0.73 | 61.3 ± 4.3 | 61.6 ± 4.5 | 0.59 |
| E′ (cm/sec) | 16.3 ± 2.4 | 15.0 ± 2.9 | <0.001 | 16.8 ± 2.0 | 18.1 ± 2.6 | <0.001 | 16.4 ± 2.4 | 16.7 ± 2.8 | 0.37 |
| PWV (m/sec) | 5.0 ± 0.7 | 5.2 ± 0.8 | <0.001 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.34 | 5.0 ± 0.8 | 4.8 ± 0.7 | 0.08 |
ASF: American-style football; BSA: body surface area; DBP: diastolic blood pressure; E′: tissue Doppler averaged mitral annular early diastolic velocities; LV: left ventricular; LVIDD: left ventricular internal diameter in diastole; PWV: pulse wave velocity; SBP: systolic blood pressure; WT: averaged interventricular septum and posterior wall thickness
Qualitatively reported NSAID use
NSAID use frequency was highest among ASF athletes [N=42 (19%) with ‘weekly’ NSAID use and N=32 (14%) with ‘daily’ use] compared to endurance athletes [N=1 (2%) with ‘weekly’ NSAID use and N=1 (2%) with ‘daily’ use, P<0.001] and non-athletic controls [N=13 (21%) with ‘weekly’ NSAID use and N=1 (2%) with ‘daily’ use, P=0.02] (Table 3). In the ASF cohort, NSAID use frequency was higher among LM [N=18 (20%) with ‘weekly’ NSAID use and N=19 (21%) with ‘daily’ NSAID use] compared to NLM [N=24 (18%) with ‘weekly’ NSAID use and N=13 (10%) with ‘daily’ NSAID use], P=0.02.
Table 3.
In-season Reported NSAID Use Frequency
| ASF (N=226) | Endurance (N=60) | Controls (N=63) | |
|---|---|---|---|
| Never/Rarely | 67% (152) | 96% (58) | 77% (49) |
| Weekly | 19% (42) | 2% (1) | 21% (13) |
| Daily | 14% (32) | 2% (1) | 2% (1) |
Never/Rarely: None to not more than once weekly NSAID use
Weekly: At least weekly but not daily NSAID use
Daily: At least daily NSAID use
ASF: American-style football; NSAID: nonsteroidal anti-inflammatory drug
In the ASF cohort, ibuprofen was the most popular NSAID. At the institutions included in this study, IM ketorolac is not permitted for use in the training rooms. Finally, the majority of ASF athletes reported that NSAID use, related to sport participation, began during middle school (N=173, 77%).
Clinical characteristics of ASF athletes stratified by NSAID use
Within the ASF cohort, there were differences in several key post-season clinical measurements stratified by frequency of NSAID use. Specifically, with a higher frequency of in-season NSAIDs, there were parallel increased post-season weights and SBP (Table 4 and Figure 1) with ‘daily’ NSAID users having the largest weight (109.0±18.6 kg) and SBP (137±13 mmHg). While not significant, ‘daily’ NSAID users also demonstrated higher PWV and lower E′ compared to the athletes reporting lower frequencies of NSAID use. Among the non-athletic controls who reported ‘weekly’ NSAID use (only N=1 reporting ‘daily’ NSAID use), there were no significant differences in weight and BP compared to the controls who reported no or rare NSAID use (Table 4).
Table 4.
Select Comparison of Post-season Clinical Measurements Stratified by In-Season NSAID Use
| ASF | Controls | ||||||
|---|---|---|---|---|---|---|---|
| Never/Rarely (N=152) | Weekly (N=42) | Daily (N=32) | P-value | Never/Rarely (N=49) | Weekly (N=13) | P-value | |
| Weight (kg) | 95.8 ± 20.5 | 100.0 ± 18.9 | 109.0 ± 18.6 | 0.003* | 75.6 ± 11.3 | 81.0 ± 10.3 | 0.12 |
| SBP (mm Hg) | 128 ± 13 | 131 ± 10 | 137 ± 13 | 0.002† | 125 ± 12 | 124 ± 14 | 0.66 |
| DBP (mm Hg) | 74 ± 9 | 77 ± 11 | 78 ± 9 | 0.07 | 74 ± 8 | 70 ± 12 | 0.09 |
| PWV (m/sec) | 5.2 ± 0.8 | 5.2 ± 0.7 | 5.4 ± 0.8 | 0.33 | 4.7 ± 0.6 | 5.1 ± 0.8 | 0.08 |
| E′ (cm/sec) | 15.2 ± 2.8 | 14.8 ± 3.5 | 14.1 ± 2.3 | 0.13 | 16.9 ± 2.7 | 15.6 ± 2.9 | 0.14 |
Adjusted (Bonferroni) P=0.002, Daily vs. Never/Rarely
Adjusted (Bonferroni) P=0.002, Daily vs. Never/Rarely
ASF: American-style football; DBP: diastolic blood pressure; E′: tissue-Doppler averaged mitral annular early diastolic velocities; NSAID: nonsteroidal anti-inflammatory drug; PWV: pulse wave velocity; SBP: systolic blood pressure
Figure 1. Comparison of post-season American-style football clinical characteristics (N = 226) stratified by frequency of non-steroidal anti-inflammatory drug (NSAID) use.
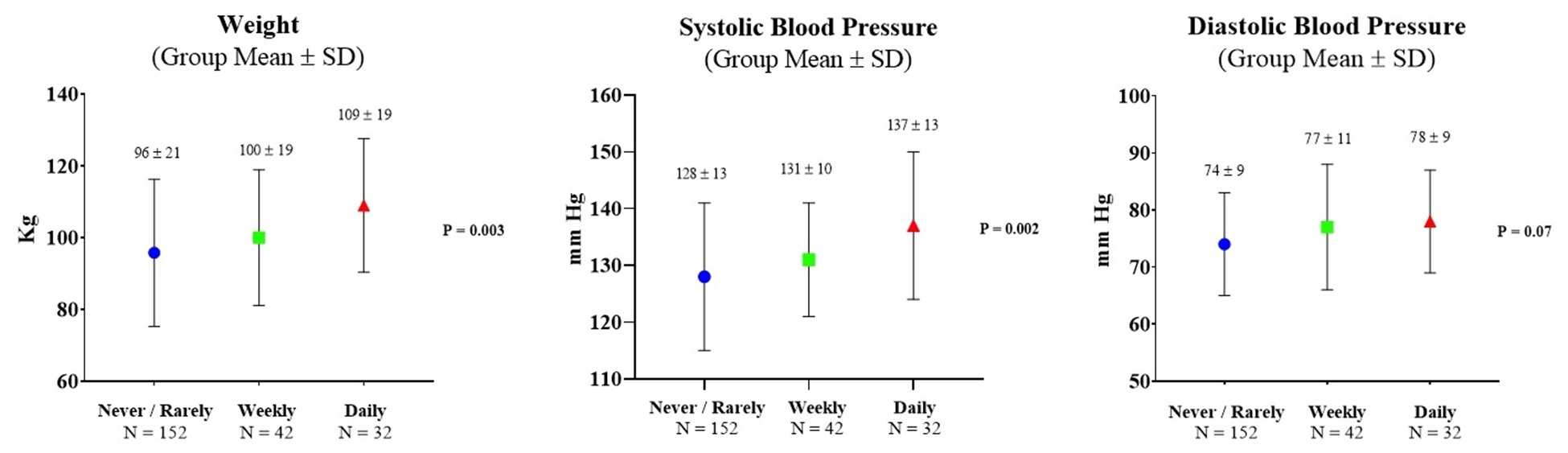
Never/Rarely: None to not more than once weekly NSAID use
Weekly: At least weekly but not daily NSAID use
Daily: At least daily NSAID use
CV risk factors associated with increased ASF NSAID use
A mixed-effects multivariable analysis was performed to determine factors associated with ASF NSAID use (Table 5). After adjusting for player position (LM versus NLM), SBP, E′, and PWV, increased weight emerged as an independent factor associated with a higher frequency of NSAID use (OR 1.04, 95% CI 1.0–1.08, P=0.037). Weight, SBP, E′, and PWV were all incorporated as time-varying pre-and post-season measurements in the model.
Table 5.
Factors Associated with Increased NSAID Use
| Odds Ratio (95% CI) | P-value | |
|---|---|---|
| Player Position (LM vs. NLM) | 0.90 (0.23–3.43) | 0.87 |
| Weight (kg) | 1.04 (1.00–1.08) | 0.037 |
| Systolic Blood Pressure (mm Hg) | 1.02 (0.99–1.06) | 0.16 |
| E′ (cm/sec) | 1.01 (0.89–1.15) | 0.91 |
| Pulse Wave Velocity (m/sec) | 0.48 (0.44–1.46) | 0.48 |
E′: tissue-Doppler averaged mitral annular early diastolic velocities; LM: lineman; NLM: non-lineman; NSAID: nonsteroidal anti-inflammatory drugs
DISCUSSION
This study, designed to determine ASF NSAID use patterns in the context of CV risk, generated the following key findings. First, within a large cohort of combined HS and collegiate ASF athletes, habitual NSAID use was more common among those with established CV risk factors, and importantly, increased weight across the ASF season was associated with an increased frequency of NSAID use. Second, and just as concerning within this diverse ASF cohort, sport-related ASF NSAID use more commonly began early, in middle school, before full physical maturation. Our data suggest that increased weight, a critical pathologic factor linking early ASF-associated CV risk with adverse long term outcomes,(11, 18–21) is also associated with increased NSAID use during competitive ASF training. Taken together, we believe that our findings provide compelling rationale that habitual NSAID use may adversely impact CV risk among competitive ASF athletes and should therefore be considered in the CV risk stratification of high-risk ASF athletes.
Emerging data suggest sub-clinical CV pathology develops in young ASF athletes, particularly among those who gain substantial weight throughout competitive ASF training.(11) Hypertension,(13, 17) relative arterial stiffening,(14, 17) reduced diastolic function,(11, 15, 17) and concentric LV hypertrophy(11, 17) have all previously been observed in separate cohorts of collegiate ASF athletes with weight gain representing a key mechanistic factor associated with most of these maladaptive phenotypes. However, identifying the ASF-specific factors that lead to the development of early CV pathology remain largely speculative. The current study, designed to capture NSAID use frequency in a large and diverse ASF cohort, demonstrates that established CV risk, present among high-risk ASF athletes, is also associated with regular and increased NSAID use. Moreover, our findings suggest an alarmingly high percentage of ASF athletes initiate sport-specific NSAIDs before HS. The discovery that habitual NSAID use is more prevalent among the ASF athletes at highest CV risk, coupled with concerning epidemiologic trends regarding ASF NSAID use, underscores the need to clarify the impact of long-term NSAID use on pathologic CV outcomes in this population.
Increased body mass, in combination with repetitive blocking and tackling, provides reasoning as to why LM, the largest ASF athletes, may comparatively use more NSAIDs among ASF athletes. Our findings are similar to a prior cross-sectional analysis of 211 collegiate ASF athletes from Holmes and colleagues, in which athletes with BMI >28 kg/m2 reported more in-season NSAID use compared to those with lower BMI (OR=2.06).(24) Our data expand on these important prevalence data by demonstrating a longitudinal association between in-season NSAID use and short-term CV risk in active HS and collegiate ASF athletes. We observed that this relationship is independent of field position (LM vs. NLM), which we believe is not a surprising finding given the high impact, collision nature of the sport.
In the general population, habitual NSAID use is associated with increased risk of hypertension,(4, 29) arterial stiffness,(30) atrial fibrillation,(6) and CV mortality.(8, 31) As such, the long-term implications of our findings should be considered in the context of the compilation of prior data taken from retired professional National Football League (NFL) athletes. In a cross-sectional analysis from Gentry and colleagues, ascending aortic dimensions in 206 retired NFL players were significantly increased compared to population controls (38±5 vs. 34±4 mm, P<0.001), and close to 30% of the retired athletes had aortas >40 mm in maximum dimension. In this study, a history of hypertension and increased body size were associated with increased aortic size.(32) In a second cross-sectional study from Aagaard and colleagues of 460 former NFL players, atrial fibrillation was more common compared to 925 matched population controls (5% vs. 0.5%). In addition to prior ASF participation (OR=5.7), increased body mass (OR=1.9) was also associated with the development of atrial fibrillation.(33) In prior epidemiologic analyses, ASF-associated weight gain predicted later-life CV disease(18) and early CV mortality in former players with high playing-time BMI.(19–21) To what degree habitual NSAID use impacts and leads to these adverse ASF outcomes remains uncertain. Future studies are therefore warranted and will need to be prospective throughout and after cessation of the ASF career. These studies will need to include detailed CV phenotyping as well as careful assessments of renal function. Finally, it will be imperative to control for other potential ASF-specific risk factors, such as sleep apnea,(34–36) in order to differentiate or possibly detect synergy relating to the development of CV pathology.
There are important clinical implications taken from these data. Prior data justify that basic blood pressure and anthropometric measurements can identify ASF athletes at highest CV risk who warrant closer clinical monitoring and potentially medical treatments.(10, 11, 17) Our findings suggest that careful attention to NSAID intake should also be incorporated in the risk stratification process for high-risk ASF athletes encountered in the clinical setting. Practitioners and athletic trainers should also be aware that some asymptomatic ASF athletes may use NSAIDs when seeking legal means for performance enhancement.(37, 38) In addition, we advocate for an emphasis on identifying the behavioral and motivating factors present among adolescent ASF athletes that lead to the early use of NSAIDs as part of ASF participation. Education remains a critical aspect of best clinical practices when caring for ASF athletes. Importantly, routine use of parenteral ketorolac has decreased in university athletic training rooms.(39) While the need for NSAID pharmacotherapy will remain an essential and appropriate component of the clinical care provided to ASF athletes, we concur with the American Heart Association’s recommendation for judicious use(8) and an emphasis on educational efforts regarding safe NSAID use practices for athletes beginning in middle school and throughout HS to the collegiate level.
We acknowledge several noteworthy limitations of this study. First, based on the observational design of this study, we cannot determine causal relationships between NSAID use frequency and increased CV risk. In addition, participants were followed across just one training season and only to the end of the collegiate freshman year. However, the study cohort was multi-center in composition and strengthened by the inclusion of HS senior ASF athletes. Second, NSAID surveys were administered at post-season and subject to recall bias. Given the retrospective recall of NSAID use, frequency was assessed qualitatively and thus the impact of NSAID dose on our CV parameters is unknown. However, the primary goal of this study was to capture ASF NSAID use patterns and the association between NSAID use frequency with known CV risk factors in this population. Even with prospective ascertainment, potential for unreliable NSAID quantification remains. Third, this study was focused on oral NSAID use and did not include institutions that utilize parenteral ketorolac. Fourth, we were unable to assess for potential energy stimulant use in the ASF cohort, which could have impacted changes in BP. Lastly, we acknowledge that there were several baseline differences between our study groups. However, these differences were likely not relevant to the observed differences in NSAID use patterns between groups.
CONCLUSION
Frequent NSAID use is common among competitive ASF athletes and associated with established CV risk factors in this athletic population. ASF NSAID use is strongly associated with increased weight, which has been implicated in the development of CV pathology among active ASF athletes and adverse long-term outcomes in retired professional ASF athletes. Of further concern is the initiation of ASF-specific NSAID use during adolescence that is common among young, physically immature ASF athletes. While further study is warranted to delineate the impact of habitual NSAID use on CV pathology and later-life CV outcomes in this population, careful attention to NSAID use patterns should be strongly considered in the clinical setting and risk stratification of high-risk ASF athletes.
ACKNOWLEDGEMENTS
This work was entirely supported by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute research grant K23 HL128795 (to Dr. Kim). With this support, Dr. Kim was the principal investigator responsible for the design and conduct of this study: data collection management, analysis, and interpretation of the data; preparation, review, and final approval of the manuscript, and final decision to submit the manuscript for publication. Dr. Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Tso, Dr. Kim, and Ms. Liu (Emory University) are responsible for all final data analysis. We continue to thank the Athletic Departments and the student-athletes at Georgia Institute of Technology, Furman University, Emory University, Marist School, and Woodward Academy for their ongoing support of this research and participation in our sports registry. We also acknowledge Digirad™ and Athletic Heart™ for providing all echocardiographic imaging services.
Footnotes
CONFLICTS OF INTEREST
No conflicts. The results of the present study do not constitute endorsement by ACSM. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
REFERENCES
- 1.Brooks PM, Day RO. Nonsteroidal Antiinflammatory Drugs — Differences and Similarities. N Engl J Med. 1991;324(24):1716–25. doi: 10.1056/nejm199106133242407. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166(7):514. doi: 10.7326/m16-2367. [DOI] [PubMed] [Google Scholar]
- 3.Babatunde OO, Jordan JL, Van Der Windt DA, Hill JC, Foster NE, Protheroe J. Effective treatment options for musculoskeletal pain in primary care: A systematic overview of current evidence. PLOS ONE. 2017;12(6):e0178621. doi: 10.1371/journal.pone.0178621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope JE, Anderson JJ, Felson DT. A Meta-analysis of the Effects of Nonsteroidal Anti-inflammatory Drugs on Blood Pressure. Arch Intern Med. 1993;153(4):477. doi: 10.1001/archinte.1993.00410040045007. [DOI] [PubMed] [Google Scholar]
- 5.Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, et al. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ. 2017:j1909. doi: 10.1136/bmj.j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, Yan Y-P, Zheng X-X, Xu Y-L, Lu J, Hui R-T, et al. Meta-Analysis of Nonsteroidal Anti-Inflammatory Drug Use and Risk of Atrial Fibrillation. Am J Cardiol. 2014;114(10):1523–9. doi: 10.1016/j.amjcard.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbøl EL, et al. Increased Mortality and Cardiovascular Morbidity Associated With Use of Nonsteroidal Anti-inflammatory Drugs in Chronic Heart Failure. Arch Intern Med. 2009;169(2):141. doi: 10.1001/archinternmed.2008.525. [DOI] [PubMed] [Google Scholar]
- 8.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of Nonsteroidal Antiinflammatory Drugs: An Update for Clinicians: A Scientific Statement From the American Heart Association. Circulation. 2007;115(12):1634–42. doi: 10.1161/circulationaha.106.181424. [DOI] [PubMed] [Google Scholar]
- 9.Kerr ZY, Simon JE, Grooms DR, Roos KG, Cohen RP, Dompier TP. Epidemiology of Football Injuries in the National Collegiate Athletic Association, 2004–2005 to 2008–2009. Orthop J Sports Med. 2016;4(9):232596711666450. doi: 10.1177/2325967116664500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Zafonte R, Pascuale‐Leon A, Nadler LM, Weisskopf M, Speizer FE, et al. American‐Style Football and Cardiovascular Health. J Am Heart Assoc. 2018;7(8):e008620. doi: 10.1161/jaha.118.008620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Hollowed C, Liu C, Al-Badri A, Alkhoder A, Dommisse M, et al. Weight Gain, Hypertension, and the Emergence of a Maladaptive Cardiovascular Phenotype Among US Football Players. JAMA Cardiol. 2019;4(12):1221–9. doi: 10.1001/jamacardio.2019.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpinos AR, Roumie CL, Nian H, Diamond AB, Rothman RL. High Prevalence of Hypertension Among Collegiate Football Athletes. Circ Cardiovasc Qual Outcomes. 2013;6(6):716–23. doi: 10.1161/circoutcomes.113.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner RB, Wang F, Isaacs SK, Malhotra R, Berkstresser B, Kim JH, et al. Blood Pressure and Left Ventricular Hypertrophy During American-Style Football Participation. Circulation. 2013;128(5):524–31. doi: 10.1161/circulationaha.113.003522. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Sher S, Wang F, Berkstresser B, Shoop JL, Galante A, et al. Impact of American-Style Football Participation on Vascular Function. Am J Cardiol. 2015;115(2):262–7. doi: 10.1016/j.amjcard.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, et al. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol (1985). 2008;104(4):1121–8. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Wang F, Weiner RB, Deluca JR, Wasfy MM, Berkstresser B, et al. Blood Pressure and LV Remodeling Among American-Style Football Players. JACC: Cardiovasc Imaging. 2016;9(12):1367–76. doi: 10.1016/j.jcmg.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Hollowed C, Patel K, Hosny K, Aida H, Gowani Z, et al. Temporal Changes in Cardiovascular Remodeling Associated with Football Participation. Med Sci Sports Exerc. 2018;50(9):1892–8. doi: 10.1249/mss.0000000000001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churchill TW, Krishnan S, Weisskopf M, Yates BA, Speizer FE, Kim JH, et al. Weight Gain and Health Affliction Among Former National Football League Players. Am J Med. 2018;131(12):1491–8. doi: 10.1016/j.amjmed.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baron S, Rinsky R. Rate and causes of death of National Football League Players. National Institute for Occupational Safety and Health, Services DoHaH; 1994. [Google Scholar]
- 20.Baron SL, Hein MJ, Lehman E, Gersic CM. Body Mass Index, Playing Position, Race, and the Cardiovascular Mortality of Retired Professional Football Players. Am J Cardiol. 2012;109(6):889–96. doi: 10.1016/j.amjcard.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln AE, Vogel RA, Allen TW, Dunn RE, Alexander K, Kaufman ND, et al. Risk and Causes of Death among Former National Football League Players (1986–2012). Med Sci Sports Exerc. 2018;50(3):486–93. Epub 2017/10/28. doi: 10.1249/mss.0000000000001466. [DOI] [PubMed] [Google Scholar]
- 22.Bowen M How NFL players play through pain: ESPN; 2016. [January 9, 2020]. Available from: https://www.espn.com/nfl/story/_/id/14564481/how-nfl-players-play-pain.
- 23.Tokish JM, Powell ET, Schlegel TF, Hawkins RJ. Ketorolac Use in the National Football League. Phys Sportsmed. 2002;30(9):19–24. doi: 10.3810/psm.2002.09.428. [DOI] [PubMed] [Google Scholar]
- 24.Holmes N, Cronholm PF, Duffy AJ, Webner D. Nonsteroidal Anti-Inflammatory Drug Use in Collegiate Football Players. Clin J Sport Med. 2013;23(4):283–6. doi: 10.1097/jsm.0b013e318286d0fa. [DOI] [PubMed] [Google Scholar]
- 25.Croft LB, Belanger A, Miller MA, Roberts A, Goldman ME. Comparison of National Football League Linemen Versus Nonlinemen of Left Ventricular Mass and Left Atrial Size. Am J Cardiol. 2008;102(3):343–7. doi: 10.1016/j.amjcard.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 28.Yasmin Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92(10):595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 29.Johnson AG, Nguyen TV, Day RO. Do Nonsteroidal Anti-inflammatory Drugs Affect Blood Pressure? A Meta-Analysis. Ann Intern Med. 1994;121(4):289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Claridge M, Hobbs S, Quick C, Day N, Bradbury A, Wilmink T. Nonsteroidal antiinflammatory drugs are associated with increased aortic stiffness. Vasc Health Risk Manag. 2005;1(2):149–53. doi: 10.2147/vhrm.1.2.149.64082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt M, Lamberts M, Olsen A-MS, Fosbøll E, Niessner A, Tamargo J, et al. Cardiovascular safety of non-aspirin non-steroidal anti-inflammatory drugs: review and position paper by the working group for Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J. 2016;37(13):1015–23. doi: 10.1093/eurheartj/ehv505. [DOI] [PubMed] [Google Scholar]
- 32.Gentry JL III, Carruthers D, Joshi PH, Maroules CD, Ayers CR, de Lemos JA, et al. Ascending Aortic Dimensions in Former National Football League Athletes. Circ Cardiovasc Imaging. 2017;10(11). Epub 2017/11/11. doi: 10.1161/circimaging.117.006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aagaard P, Sharma S, McNamara DA, Joshi P, Ayers CR, De Lemos JA, et al. Arrhythmias and Adaptations of the Cardiac Conduction System in Former National Football League Players. J Am Heart Assoc. 2019;8(15). doi: 10.1161/jaha.118.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Hollowed C, Irwin-Weyant M, Patel K, Hosny K, Aida H, et al. Sleep-Disordered Breathing and Cardiovascular Correlates in College Football Players. Am J Cardiol. 2017;120(8):1410–5. doi: 10.1016/j.amjcard.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George CF, Kab V, Levy AM. Increased prevalence of sleep-disordered breathing among professional football players. N Engl J Med. 2003;348(4):367–8. Epub 2003/01/24. doi: 10.1056/nejm200301233480422. [DOI] [PubMed] [Google Scholar]
- 36.Rice TB, Dunn RE, Lincoln AE, Tucker AM, Vogel RA, Heyer RA, et al. Sleep-disordered breathing in the National Football League. Sleep. 2010;33(6):819–24. Epub 2010/06/17. doi: 10.1093/sleep/33.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warner DC, Schnepf G, Barrett MS, Dian D, Swigonski NL. Prevalence, attitudes, and behaviors related to the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in student athletes. J Adolesc Health. 2002;30(3):150–3. doi: 10.1016/s1054-139x(01)00325-1. [DOI] [PubMed] [Google Scholar]
- 38.Matava M, Brater DC, Gritter N, Heyer R, Rollins D, Schlegel T, et al. Recommendations of the National Football League Physician Society Task Force on the Use of Toradol(R) Ketorolac in the National Football League. Sports Health. 2012;4(5):377–83. doi: 10.1177/1941738112457154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carver TJ, Schrock JB, Kraeutler MJ, McCarty EC. The Evolving Treatment Patterns of NCAA Division I Football Players by Orthopaedic Team Physicians Over the Past Decade, 2008–2016. Sports Health. 2018;10(3):234–43. doi: 10.1177/1941738117745488. [DOI] [PMC free article] [PubMed] [Google Scholar]


