Abstract
Elevated rumination, characterized by repetitive, negative self-focused cognition, is common in posttraumatic stress disorder (PTSD) and has been shown to predict the onset and maintenance of the disorder. Neuroimaging research has implicated cortical midline brain structures, including the rostral anterior cingulate cortex (rACC), posterior cingulate cortex (PCC), and isthmus cingulate (IsthCing), in rumination in healthy and depressed populations. While past research has revealed dysfunction in cortical midline regions in PTSD, no studies have yet investigated the structural and functional neural mechanisms underlying rumination in women with PTSD. In the current study, we used structural MRI and resting-state fMRI to examine relationships between rumination and brain volume, as well as resting-state functional connectivity (rsFC) of cortical midline structures in women with PTSD due to interpersonal trauma (N = 71). We performed multiple linear regression analyses to relate brain volume in rACC, PCC, and IsthCing regions to self-reported rumination, after controlling for age and total intracranial volume. We also conducted standard seed-based voxelwise rsFC analyses for significant regions identified in the structural analysis. We found a significant relationship between greater rumination and volume in the left IsthCing (p = .025). Results from the rsFC analyses revealed a significant relationship between greater rumination and diminished rsFC between the left IsthCing and left precuneus (pFWE < .05). These findings provide novel support for alterations in the neural substrates of ruminative thought in women with PTSD. More broadly, we discuss clinical implications for targeted interventions to reduce rumination through psychotherapy or non-invasive brain stimulation.
Keywords: posttraumatic stress disorder, rumination, cortical midline structures, default mode network, isthmus cingulate, precuneus
Introduction
Rumination is characterized by recurring negative thoughts about oneself that are focused on past events or negative emotions (Brinker and Dozois, 2009; Nolen-Hoeksema et al., 2008; Papageorgiou and Wells, 2003). The tendency to ruminate is increasingly recognized as a transdiagnostic vulnerability factor in the development of numerous psychiatric disorders, including depression and posttraumatic stress disorder (PTSD; Aldao et al., 2010; Arditte Hall et al., 2019; Birrer and Michael, 2011; Ehlers et al., 1998; McLaughlin and Nolen-Hoeksema, 2011; Michael et al., 2007). Several studies have demonstrated positive correlations between rumination and PTSD symptoms in trauma-exposed populations (Arditte Hall et al., 2019; Seligowski et al., 2015; Steil and Ehlers, 2000; Szabo et al., 2017). Longitudinal research further suggests that persistent rumination about the trauma predicts PTSD symptom severity and subsequent onset of PTSD (Clohessy and Ehlers, 1999; Ehlers et al., 1998; Michael et al., 2007; Murray et al., 2002). Cognitive theories of PTSD have also emphasized the influence of rumination and negative self-appraisal in maintaining the disorder (Dunmore et al., 1999; Ehlers and Clark, 2000; Foa et al., 1999). Together, this clinical and behavioral research has provided strong support for the role of elevated rumination in the development and maintenance of PTSD. However, the neural mechanisms underlying ruminative thought in PTSD remain unclear.
Several functional and structural neuroimaging studies have characterized the neural systems underlying self-reference and rumination in healthy participants and in depression. Cortical midline brain structures, including the rostral anterior cingulate cortex (rACC), posterior cingulate cortex (PCC), and retrosplenial cortex/isthmus cingulate (IsthCing), have been frequently implicated in self-related cognition (Andrews-Hanna et al., 2010; Qin and Northoff, 2011; Whitfield-Gabrieli et al., 2011) and rumination (Berman et al., 2011; Burkhouse et al., 2017; Cooney et al., 2010; Makovac et al., 2020; Nejad et al., 2013; Zhou et al., 2020). For instance, greater activity in the rACC, PCC, and IsthCing during positive and negative self-reference has been found across a variety of self-related cognition paradigms in healthy participants (Qin and Northoff, 2011). In addition, neuroimaging research has shown that ruminative thought is associated with greater activity and connectivity of the rACC and PCC across both healthy and depressed populations (Berman et al., 2011; Burkhouse et al., 2017; Cooney et al., 2010; Makovac et al., 2020; Nejad et al., 2013; Satyshur et al., 2018; Zhou et al., 2020). While fewer studies have been conducted, existing structural brain imaging research has also revealed correlations between rumination and ACC gray matter volume in healthy individuals (Kühn et al., 2012; Sin et al., 2018).
Previous neuroscientific research in PTSD has provided support for dysfunction in cortical midline regions during social cognition and at rest. For example, task-based neuroimaging studies in individuals with PTSD have reported altered activity within the medial prefrontal cortex and rACC while engaging in self-reference (Bluhm et al., 2012), receiving praise or criticism (Frewen et al., 2010), and thinking about others (Frewen et al., 2017). Resting-state fMRI research has revealed decreased functional connectivity of rACC, PCC/precuneus, and IsthCing regions in PTSD as compared with control participants (Bluhm et al., 2009; Miller et al., 2017; Shang et al., 2014; Sripada et al., 2012; Wang et al., 2016). Yet, to our knowledge, only one study has directly investigated the neural correlates of rumination in PTSD (Buchholz et al., 2016). Buchholz and colleagues (2016) found that trait rumination was correlated with greater activity in the orbitofrontal cortex during an emotional interference task in women with PTSD. Together, neuroimaging research to date suggests that rumination in individuals with PTSD may be associated with alterations in cortical midline structures. Yet, no studies have examined the neural substrates of rumination in women with PTSD using both structural and resting-state brain imaging.
We sought to address these gaps in the literature in the current study by using structural MRI and resting-state fMRI to examine relationships between rumination and brain volume, as well as resting-state functional connectivity (rsFC) of cortical midline structures in women with PTSD due to interpersonal trauma (N = 71). For the behavioral analysis, we hypothesized that rumination would be positively correlated with total PTSD symptom severity and PTSD symptom clusters. For the neuroimaging analyses, we hypothesized that rumination would be correlated with volume and rsFC of cortical midline brain regions in women with PTSD.
Materials and Methods
Participants and Procedure
Participants were women (N = 71) between the ages of 18 and 56, whose target trauma had been an interpersonal traumatic event (physical or sexual assault, molestation, or intimate partner violence) experienced in childhood or adulthood and who had a primary diagnosis of PTSD based on the Diagnostic and Statistical Manual of Mental Disorders (4th ed. text rev.; DSM-IV-TR; APA, 2000). Other comorbid conditions were permitted for this study as long as the primary diagnosis was PTSD. For all participants, their most recent traumatic event had occurred at least 1 month before the time of initial assessment. See Table 1 for participant characteristics.
Table 1.
Participant Demographics and Study Variables
| Variable | Mean | SD | Range |
|---|---|---|---|
| Age | 31.93 | 9.39 | 18-56 |
| Educationa | 14.83 | 2.49 | 6.5-20 |
| Total PTSD severity | 66.51 | 16.83 | 35-104 |
| Re-experiencing | 17.65 | 6.44 | 3-34 |
| Avoidance/numbing | 26.56 | 7.89 | 11-48 |
| Hyperarousal | 22.20 | 6.54 | 6-35 |
| Time since first traumaa | 14.20 | 10.73 | 0-39 |
| BDI-II | 24.82 | 10.34 | 5-46 |
| Rumination | 99.97 | 23.72 | 41-136 |
Notes. Posttraumatic Stress Disorder = PTSD; Beck Depression Inventory- Version II = BDI-II; PTSD severity corresponds to the total score within the Clinician-Administered PTSD scale (CAPS). Re-experiencing, Avoidance/numbing, & Hyperarousal correspond to clusters within the CAPS. Rumination scores correspond to the total score on the ruminative thought style questionnaire (Brinker & Dozois, 2009).
Education and time since first trauma are reported in years.
All participants were recruited through a multi-disciplinary center at a large Midwestern university that primarily treats adult women who have experienced interpersonal trauma. All participants gave informed consent according to a protocol reviewed and approved by the institutional review board at the university. At the time of study recruitment and participation, all women met the following inclusion criteria: no current use of psychotropic prescription or nonprescription medications; no previous trauma-focused therapy; no current drug/alcohol dependence or alcohol/substance use disorder; no Axis II disorders; no history of any psychotic disorder or bipolar disorder; no history of head trauma, no active suicidal or homicidal ideation, no MRI contraindications (e.g., metallic implants, implanted medical devices). Participants were also excluded from the sample if they were involved in a currently abusive relationship or were being stalked.
Clinical and Behavioral Assessments
PTSD Symptoms.
Symptoms of PTSD were assessed using the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995), which is a semi-structured 30-item clinically administered interview that measures the validity, severity, and improvement of DSM-IV-TR PTSD symptoms over a time period of interest (e.g., past month). The CAPS also consists of separate ratings for each PTSD symptom based on both frequency and intensity of symptoms based on the DSM-IV-TR (APA, 2000). The CAPS has demonstrated high internal consistency (Cronbach’s αs=.92–.99) and is an accepted valid measure of PTSD symptoms and diagnosis (Blake et al., 1995). The internal consistency of the CAPS in this sample was high (Cronbach’s α=.95). We computed the severity of PTSD symptoms over the past month for all PTSD symptoms (total PTSD severity) and for the following symptom clusters: re-experiencing, avoidance/numbing, and hyperarousal.
Time Since First Trauma.
Time since first trauma (in years) was calculated for each participant by subtracting the age of the participant’s first traumatic event from their current age. We did not report this variable for participants without exact age of first trauma information (n = 6).
Depression Symptoms.
Depression severity was assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996). The BDI-II is 21-item self-report inventory used to measure depression-related symptom severity during the past two weeks. The BDI-II has good psychometric properties with a Cronbach’s α=.91 and test-retest reliability of .93 (Beck, Steer, Ball, & Ranieri, 1996; Beck, Steer, & Brown, 1996). The internal consistency of the BDI-II in the current sample was high (Cronbach’s α=.90).
Rumination.
Trait levels of rumination were measured using the Ruminative Thought Style Questionnaire (RTS; Brinker and Dozois, 2009), a 20-item questionnaire which assesses the tendency toward global rumination or repetitive, intrusive, and uncontrollable thoughts, accounting for temporal focus (e.g. past-oriented thoughts) and affective valence (e.g. negative thoughts). Example items include: “I find that some thoughts come to mind over and over throughout the day” or “I have never been able to distract myself from unwanted thoughts.” The RTS has high internal consistency (Cronbach’s αs=.87-.94) and good test-retest reliability (Brinker and Dozois, 2009; Tanner et al., 2013). In the present study, internal consistency for RTS was high (Cronbach’s α=.94). Total RTS scores were used for all analyses.
Structural and Functional MRI Data Acquisition
All structural and functional MRI data were acquired using a Siemens 3T TrioTrim MRI scanner (Siemens, Erlangen, Germany). High-resolution T1-weighted structural images and two resting-state fMRI scans were acquired. See Supplementary Methods for acquisition parameters. During the resting-state scans (~8min), the participants were asked to keep their eyes open and fixate on a cross. In the current study, we analyzed the first resting-state scan.
Processing of Structural Data and Regions of Interest for Volumetric Analysis
The averaged T1-weighted images were processed using FreeSurfer, version 5.1 (www.nmr.mgh.harvard.edu/freesurfer), as previously described (Fischl and Dale, 2000). Briefly, the automated procedure includes skull-stripping, registration, intensity normalization, Talairach transformation, tissue segmentation, and surface tessellation. The default analysis pipeline in FreeSurfer provides an automatic parcellation of the cortical surface in anatomical regions using surface-based anatomical atlases. We used the volumetric values for brain regions of interest (ROIs) described below, which were derived from cortical thickness and surface area measurements.
Based on our hypothesized interest in brain areas consistently implicated in self-focused thought and rumination (Bluhm et al., 2012; Cooney et al., 2010; Murray et al., 2015; Qin and Northoff, 2011), we selected the following ROIs for both right and left hemispheres: rACC, PCC, and IsthCing from the Desikan-Killany atlas (Desikan et al., 2006). Using these 3 ROIs (6 including both hemispheres), we examined the relationship between volume in these regions and rumination in women with PTSD.
Processing of Functional Data for Resting-State Analysis
Preprocessing.
The resting-state functional data were processed using AFNI, FSL, and ANTs (Cox, 1996; FMRIB Software Library; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/; http://stnava.github.io/ANTs/). Data were preprocessed using the following initial steps: images deobliqued (3dWarp), first three volumes removed (3dcalc), motion corrected by rigid body alignment to the first EPI acquisition (3dvolreg), despiked the 3D+time series to remove time series outliers (3dDespike), temporally filtered (band-pass: 0.009 Hz < f < 0.08 Hz; 3dTproject) and spatially smoothed with a 3D 4-mm full-width half-maximum (FWHM) Gaussian kernel (3dmerge). Next, the skull-stripped anatomical scan was rigidly coregistered with the T1, then with the EPI, and diffeomorphically aligned to Montreal Neurological Institute (MNI)-152 template space using a symmetric normalization algorithm in ANTs (Avants and Gee, 2004). Normalized T1 anatomical images were also segmented into gray matter, white matter, and CSF using FAST in FSL (Zhang et al., 2001). White matter and CSF segments were used as masks to extract a representative time series from each tissue type.
In the final preprocessing steps we conducted a GLM (3dDeconvolve) to account for motion and other typical nuisance variables (Ciric et al., 2017): six motion parameters (three translations, three rotations) obtained from the rigid body alignment of EPI volumes and their six derivatives, the white matter time series and its derivative, and the ventricular (CSF) time series and its derivative. To further control for individual motion within the GLM, volumes were censored for excessive motion. These final preprocessed resting-state files were used in the functional connectivity analysis detailed below.
Motion Analysis.
Excessive motion was assessed using the following criteria: maximum framewise motion displacement > 4 mm, and/or total scan time < 3 min after censoring all time points with framewise motion displacement > .2 mm and extreme timeseries displacement. Ten participants in the present study were excluded based on these criteria. We selected these motion thresholds based on recommendations from previous research (Power et al., 2012; Yan et al., 2013). Average root-mean-squared (RMS) displacement was used as a summary measure of individual participant motion (Ciric et al., 2017). Average RMS was not correlated with rumination (r = −.05, p = .69), thus it was not included as a covariate in the rsFC analyses.
rsFC Analysis.
We performed seed-based voxelwise rsFC analyses (Biswal et al., 1995) using only ROIs demonstrating a significant relationship in the volumetric analysis. To create the seed ROIs for the rsFC analysis, the transformation matrix from the registration procedure was used to align the seed ROIs masks provided in FreeSurfer to MNI-152 template space. For each participant, the mean resting-state BOLD time series from each seed ROI was included in a GLM (3dDeconvolve) to compute the correlation between each seed ROI’s time series and all other voxels in the brain. To create the correlation maps for each seed ROI, we performed the following steps (as in Philippi et al., 2015): used GLM output to convert R2 values to correlation coefficients (r) and used Fisher’s r-to-z transform to convert r to z-scores. Resulting z-score maps were then entered into the second-level statistical analyses.
Statistical Analysis
Behavioral Data Analysis.
Linear regression analyses were performed to examine the relationship between PTSD symptom severity and rumination (version 25; SPSS/IBM, Chicago, IL). Four separate regression analyses were conducted for each of the PTSD symptom severity scores for the past month as the predictor/independent variable (total PTSD severity, re-experiencing, avoidance/numbing, and hyperarousal) and rumination as the dependent variable.
Volumetric Analysis.
To investigate the association between self-related brain regions and rumination in PTSD, we conducted six separate linear regression analyses with each ROI volume (right/left rACC, right/left PCC, right/left IsthCing) as the predictor/independent variable and rumination as the dependent variable (version 25; SPSS/IBM, Chicago, IL). Age and total intracranial volume were also included as covariates in all volumetric regression models. Age was included to account for associations between age and gray matter volume (Giorgio et al., 2010; Walhovd et al., 2005); intracranial volume was included to control for differences in overall brain size across participants (Good et al., 2001; Murphy et al., 1992).
Resting-State Analysis.
For ROIs that were significant in the volumetric analysis, we performed multivariate regression analyses (3dttest++ in AFNI) to further determine whether rumination was also related to rsFC of self-related brain regions. This rsFC analysis was conducted in 61 of the participants, after excluding those with excessive motion (n = 10). To correct for multiple comparisons, we implemented a family-wise error (FWE) correction approach at the cluster level using a whole-brain mask (3dClustSim in AFNI version updated August 2018; Carp, 2012; Forman et al., 1995) and applied cluster-extent thresholding. To address the non-Gaussian nature of fMRI data (Eklund et al., 2016), the autocorrelation function (−acf) was used to calculate the FWHM for each subject (3dFWHMx in AFNI). Our results were FWE cluster-corrected at the whole brain level, with a predefined voxelwise threshold of p < 0.001 (uncorrected) with a cluster-corrected voxel size of ≥ 40 voxels significant at pFWE < .05. Regression results were overlaid on the normalized mean anatomical image.
Results
Behavioral Data Analysis
Regression analyses (Table 2) revealed a significant relationship between rumination and total PTSD symptom severity in individuals with PTSD (t(67) = 4.59, p < .001, f2 = .22). Additionally, rumination was significantly associated with all PTSD symptom clusters: re-experiencing (t(67) = 2.99, p < .01, f2 = .13), avoidance/numbing (t(67) = 2.74, p < 0.01, f2 = .11), and hyperarousal (t(67) = 3.27, p < .01, f2 = .16). When controlling for depression symptoms, results for total PTSD severity (t(64) = 2.32, p = .024, f2 = .47) and re-experiencing (t(64) = 2.60, p = .011, f2 = .18) remained significant (Table S1). In addition, findings for total PTSD severity and PTSD symptom clusters remained significant when including time since first trauma as a covariate in the analyses (Table S2).
Table 2.
Multiple Linear Regression for Posttraumatic Stress Disorder (PTSD) Symptom Clusters and Rumination
| Total PTSD severity | Re-experiencing | Avoidance/Numbing | Hyperarousal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β |
| (Constant) | 36.64 | 7.99 | 8.42 | 3.17 | 16.10 | 3.92 | 12.05 | 3.19 | ||||
| Rumination | 0.30 | 0.08 | .42** | 0.09 | 0.03 | .34* | 0.10 | 0.04 | .31* | 0.10 | 0.03 | .37* |
Notes.
p < .01;
p < .001
Volumetric Analysis
We found relationships between rumination and greater volume of self-related brain regions (Tables S3-S5). Rumination was significantly associated with increased left IsthCing volume (t(66) = 2.29, p = .025, f2 = .54; Figure 1). There were no significant relationships between ruminative thought and volume of ACC, PCC, or right IsthCing (ps = .061-.876, Supplementary Methods). After controlling for depression symptoms, the significant relationship for left IsthCing volume was trend-level (t(62) = 1.94, p = .057). Further, the left IsthCing finding did not survive Bonferroni correction (p < .008).
Figure 1. Rumination associated with greater left isthmus cingulate volume.
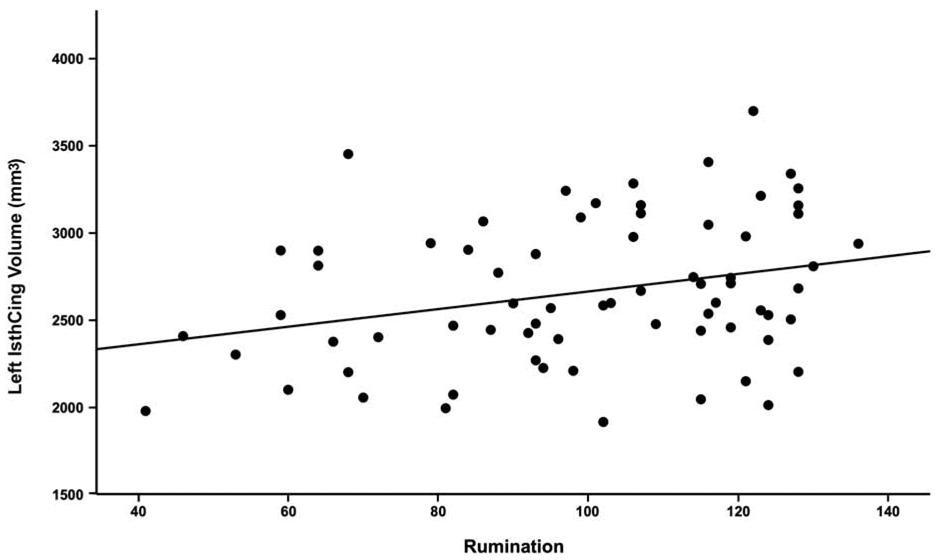
Zero-order correlation plot shows significant positive relationship between left isthmus cingulate volume (mm3) and rumination. *Plot line does not depict other covariates included in full regression model (age, intracranial volume).
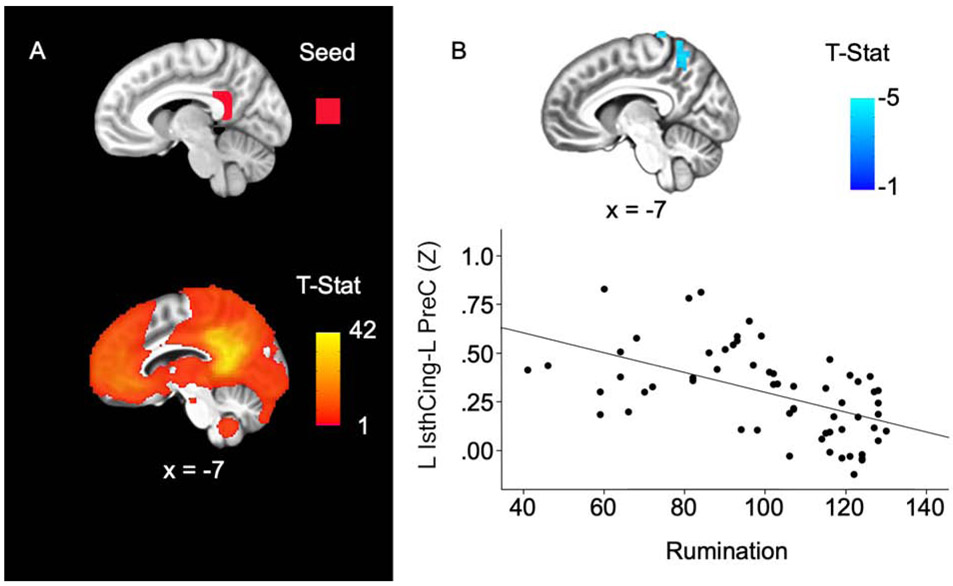
Resting-State Analysis
To follow-up the volumetric finding, we also performed a rsFC analysis to determine whether rumination was associated with rsFC of the left IsthCing. Across all participants, activity of the left IsthCing was positively correlated with other cortical midline regions including the PCC, precuneus, and medial prefrontal cortex (Figure 2A). Regression analyses revealed that greater levels of ruminative thought were associated with diminished rsFC between the left IsthCing and the left precuneus, extending to lateral parietal cortex (pFWE < .05; Figure 2B; Table 3). These rsFC results remained significant after controlling for depression symptoms (Table S6).
Figure 2. Rumination associated with reduced left isthmus cingulate-left precuneus connectivity.
A. Top: Left isthmus cingulate seed ROI (red); Bottom: Left isthmus cingulate connectivity across the entire sample (p < .001). B. Top: Higher rumination scores were associated with reduced connectivity between left isthmus cingulate and left precuneus extending to lateral parietal cortex. Bottom: Scatterplot shows the relationship between rumination and left isthmus cingulate connectivity values (z-scores). Results survived whole-brain cluster correction (pFWE < .05, p = .001 uncorrected). All results are displayed on the group average structural MRI in MNI-space. L = left, IsthCing = isthmus cingulate cortex, PreC = precuneus.
Table 3.
Resting-State Regression Results for Left Isthmus Cingulate Connectivity Related to Rumination
| Seed ROI | Cluster location | MNI coordinates (x, y, z) |
Cluster size (voxels) |
t-value | Average raw connectivity (z-score) |
|---|---|---|---|---|---|
| Left IsthCing |
Left precuneus extending to lateral parietal cortex | −7, −54, 54 | 108 | −4.73 | 0.29 |
Notes. ROI = region of interest; IsthCing = isthmus cingulate cortex. Regression results from resting-state analysis were significant at pFWE = 0.05, p = 0.001 uncorrected.
Discussion
This is the first study, to our knowledge, to use both structural and resting-state imaging to investigate the neural correlates of rumination in women with PTSD. In the behavioral analysis we found significant positive correlations between rumination and total PTSD symptom severity and PTSD symptom clusters. The volumetric and resting-state analyses revealed significant relationships between rumination and gray matter volume and rsFC of the left IsthCing. We focus our discussion on the major findings as well as potential clinical implications.
Consistent with our hypothesis, we found a significant relationship between elevated rumination and total PTSD symptom severity, even after controlling for depressive symptoms. These results replicate prior research reporting reliable associations between frequent rumination and more severe PTSD symptoms (Arditte Hall et al., 2019; Seligowski et al., 2015; Steil and Ehlers, 2000; Szabo et al., 2017). Given that we assessed global aspects of ruminative thought, our findings also align with previous meta-analyses indicating that trait rumination may yield larger effect sizes than trauma-focused rumination in PTSD (Szabo et al., 2017). However, we did not examine trauma-focused rumination in the present study. Thus, subsequent research will be needed to determine whether there are differences between trait rumination and trauma-focused rumination in relation to gray matter volume and rsFC in PTSD.
Our results showing associations between rumination and all three PTSD symptom clusters recapitulate the findings of a recent meta-analysis on the topic (Szabo et al., 2017). We also found that only re-experiencing symptoms remained significant after controlling for depressive symptoms. Although avoidance and hyperarousal have also been associated with rumination, there is some evidence that intrusive re-experiencing symptoms may be more strongly related to ruminative thought in PTSD (Szabo et al., 2017). From a clinical perspective, it has been suggested that rumination may serve as a maladaptive or avoidant coping strategy to respond to intrusive memories (Clohessy and Ehlers, 1999; Ehlers and Clark, 2000; Steil and Ehlers, 2000). Other experimental research in nonclinical populations indicates that engaging in ruminative cognition can contribute directly to enhancing intrusive memories and distress. For example, studies in healthy participants have demonstrated that rumination induction following a traumatic film was associated with increased intrusive memories (Ball and Brewin, 2012; Zetsche et al., 2009) and intrusion-related distress (Kubota and Nixon, 2017). However, other research has reported no significant increases in intrusive memories following rumination induction (Ehring et al., 2009; Marks et al., 2018; Stavropoulos and Berle, 2020), suggesting that other factors (e.g., gender, time after trauma) may also contribute to the relationship between rumination and intrusive memories in PTSD. For example, given that re-experiencing symptoms can be more severe in women with PTSD (Charak et al., 2014; Olff, 2017), sex differences could partially explain the relationships rumination and re-experiencing in our study as well as prior research. Though, additional research in samples of men and women with PTSD will be required to directly address this question. In terms of other factors, in the present study, time since first trauma did not appear to have a significant effect on the association between trait rumination and re-experiencing symptoms.
The results from our volumetric analysis revealed a positive correlation between rumination and gray matter volume in the left IsthCing, in line with our hypothesis. These findings agree with previous neuroimaging research on self-reference and rumination in healthy and depressed participants (Andrews-Hanna et al., 2010; Berman et al., 2011; Burkhouse et al., 2017; Cooney et al., 2010; Kelley et al., 2002; Makovac et al., 2020; Nejad et al., 2013; Qin and Northoff, 2011; Whitfield-Gabrieli et al., 2011; Zhou et al., 2020). Furthermore, the IsthCing/retrosplenial cortex has been implicated in conditioned fear memories in animals (Cowansage et al., 2014; Wang et al., 2019), and autobiographical memory and unintentional mind-wandering in healthy participants (Christoff et al., 2016; Golchert et al., 2017; Vann et al., 2009), which may be relevant to individuals with PTSD. For example, a meta-analysis of symptom provocation neuroimaging studies found consistently greater activity in the retrosplenial cortex in response to trauma-related stimuli in PTSD patients as compared with trauma-exposed controls (Sartory et al., 2013). In another study, individuals with PTSD exhibited increased activity in the retrosplenial cortex while retrieving emotionally intense autobiographical memories (St. Jacques et al., 2011). Together with our results, we speculate that the IsthCing/retrosplenial cortex may contribute to both intrusive memories and ruminative thought in PTSD. However, it is also possible that alterations in the IsthCing/retrosplenial cortex and other DMN regions are associated with a ruminative style of thinking or ruminative subtype across both healthy and clinical populations (Zhou et al., 2020). Future research in individuals with PTSD as well as healthy populations will be necessary to establish common patterns of brain activity during rumination and intrusive re-experiencing.
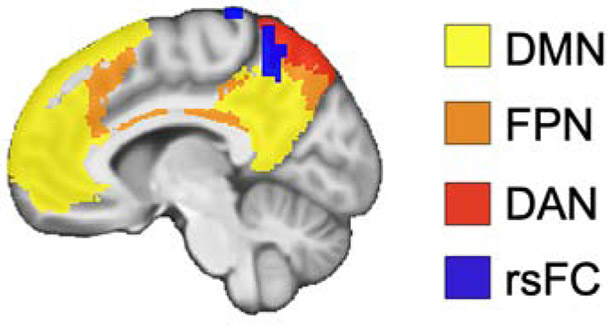
In our resting-state analyses, we found that greater levels of ruminative thought were associated with diminished rsFC between the left IsthCing and the left precuneus, extending to lateral parietal cortex. Tracing studies in monkeys indicate that there are bilateral structural connections between the isthmus cingulate/retrosplenial cortex and the precuneus (Cavanna and Trimble, 2006; Kobayashi and Amaral, 2007, 2003; Morris et al., 1999; Parvizi et al., 2006; Van Hoesen et al., 1993). Functionally, in addition to cortical midline structures, the IsthCing/retrosplenial cortex and the precuneus are also part of the default mode network (DMN), a network consistently implicated in self-referential cognition (Andreasen et al., 1995; Andrews-Hanna et al., 2010; Whitfield-Gabrieli et al., 2011). Consistent with our resting-state results, previous studies have found reduced DMN activity and connectivity in PTSD (Akiki et al., 2017; Bluhm et al., 2009; Koch et al., 2016; Miller et al., 2017). Besides the DMN, the cluster we identified in the left precuneus also overlaps with other cortical networks involved in executive functions, including the frontoparietal network, dorsal attention network, and salience network (Figure 3; Yeo et al., 2011). Relatedly, the cluster intersects with sensorimotor and cognitive subdivisions of the precuneus, with the central precuneus anatomically and functionally connected with regions in the cognitive/associative network (Margulies et al., 2009). Taken together, these results indicate that rumination is associated with reduced rsFC between the DMN and executive control networks in PTSD. We speculate that such aberrant connectivity in PTSD could disrupt the ability to shift attention away from repetitive negative self-focused cognition to other thoughts and external information. Consistent with this hypothesis, previous research has reported altered rsFC between DMN and executive control network regions in relation to negative self-focus (Philippi et al., 2018) and rumination (Jacob et al., 2020; Misaki et al., 2020; Satyshur et al., 2018) in depression. For example, a recent study reported that higher rumination scores were associated with lower connectivity strength in the precuneus in patients with major depressive disorder (Jacob et al., 2020). In sum, our findings suggest that there may be common neurobiological substrates underlying rumination in PTSD and depression.
Figure 3. Resting-state connectivity results overlap with default mode network and executive control networks.
Significant resting-state functional connectivity results from left isthmus cingulate-precuneus (rsFC; blue) are overlaid on masks of DMN (yellow), FPN (orange), and DAN (red) from a resting-state fMRI study of 1000 participants (Yeo et al., 2011). Results and network masks are displayed on the group average structural MRI in MNI-space. Default mode network = DMN, frontoparietal network = FPN, dorsal attention network = DAN.
The results from the current study could also have important clinical implications for the development of targeted treatments aimed at decreasing rumination in PTSD. For instance, clinical research indicates that rumination-focused cognitive behavioral therapy is associated with positive outcomes for patients with depression (Watkins, 2015). Although no randomized control trials have been conducted in PTSD, a preliminary study in adolescent Rwandan survivors of genocide further suggests that rumination-focused therapy may be effective for treating PTSD symptoms (Sezibera et al., 2009). Moreover, research has shown that trauma-focused treatments that target maladaptive cognitions, including rumination, are associated with the best treatment outcomes (LoSavio et al., 2017). Additional research is warranted to evaluate whether treatments that target ruminative thought can further improve symptoms and/or treatment response in PTSD.
Besides psychotherapy, non-invasive brain stimulation could possibly be applied to the precuneus to reduce rumination by normalizing connectivity within and between the DMN and executive control networks in individuals with PTSD. For example, transcranial direct current stimulation and transcranial magnetic stimulation approaches targeting the dorsolateral prefrontal cortex have been shown to decrease PTSD symptoms (Ahmadizadeh and Rezaei, 2018; Berlim and Van den Eynde, 2014), depressive symptoms (Berlim et al., 2013; Gaynes et al., 2014; Liston et al., 2014), and rumination in healthy participants (Baeken et al., 2017; De Raedt et al., 2017). Further, transcranial magnetic stimulation to the precuneus has been found to alter early autobiographical memory retrieval in healthy individuals (Hebscher et al., 2020) and improve episodic memory in early Alzheimer’s disease (Koch et al., 2018). Future work will be necessary to investigate the effects of precuneus stimulation on rumination in individuals with PTSD.
There were several limitations to the present study. First, our study examined the neural correlates of a self-report measure of rumination using structural MRI and resting-state fMRI. Subsequent research will be necessary to determine whether trauma-focused rumination and/or rumination induction paradigms in task-based fMRI are also associated with cortical midline structures and connectivity with executive control networks in PTSD. Second, the study included women with PTSD due to interpersonal trauma. Considering the potential sex differences in PTSD prevalence and symptom severity (Charak et al., 2014; Olff, 2017), these results will require replication in male and female participants with PTSD and participants exposed to different trauma types. Third, although the effect size was large for the left IsthCing in the volumetric analysis (f2 = .54), this finding did not survive Bonferroni correction. Therefore, future studies with larger sample sizes will be needed to replicate these results.
Conclusion
Our results provide novel support for alterations in the structural and functional neural substrates of ruminative thought in women with PTSD. The volumetric and resting-state analyses revealed significant relationships between rumination and gray matter volume of the left IsthCing and rsFC between the IsthCing and precuneus. Together, these findings suggest that aberrant connectivity between cortical midline structures and executive control networks may underlie ruminative cognition in PTSD and other psychiatric disorders, such as depression.
Supplementary Material
We investigated the neural correlates of ruminative thought in women with PTSD
Greater PTSD symptom severity and symptom clusters were correlated with rumination
Rumination was associated with increased gray matter volume in the IsthCing
Rumination was also related to reduced connectivity between IsthCing and precuneus
Findings suggest shared neural substrates of rumination in PTSD and depression
Acknowledgments
This work was supported by NIH grants K23 MH090366 (PI: Steven E. Bruce) and RC1 MH089704 (PI: Yvette Sheline). We also thank all of the participants for their contributions in making this research possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Declarations: The authors declare no competing financial interests. The authors have not pre-registered this research with an independent, institutional registry and the authors do not currently have data, analytic methods, and study materials available to other researchers. However, the authors are willing to provide this information to other researchers upon request.
The authors report no potential conflicts of interest.
References
- Ahmadizadeh M-J, Rezaei M, 2018. Unilateral right and bilateral dorsolateral prefrontal cortex transcranial magnetic stimulation in treatment post-traumatic stress disorder: A randomized controlled study. Brain Research Bulletin 140, 334–340. 10.1016/j.brainresbull.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, Abdallah CG, 2017. A network-based neurobiological model of PTSD: Evidence from structural and functional neuroimaging studies. Current Psychiatry Reports 19, 81 10.1007/s11920-017-0840-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Schweizer S, 2010. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review 30, 217–237. 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and statistical manual of mental disorder, text revision (DSM-IV-TR). American Psychiatric Association, Washington, D.C. [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD, 1995. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152, 1576–1585. 10.1176/ajp.152.11.1576 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL, 2010. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 65, 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arditte Hall KA, Davison EH, Galovski TE, Vasterling JJ, Pineles SL, 2019. Associations Between Trauma-Related Rumination and Symptoms of Posttraumatic Stress and Depression in Treatment-Seeking Female Veterans. Journal of Traumatic Stress 32, 260–268. 10.1002/jts.22385 [DOI] [PubMed] [Google Scholar]
- Avants B, Gee JC, 2004. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage 23, S139–S150. 10.1016/j.neuroimage.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Baeken C, Remue J, Vanderhasselt M-A, Brunoni AR, De Witte S, Duprat R, Koster EHW, De Raedt R, Wu G-R, 2017. Increased left prefrontal brain perfusion after MRI compatible tDCS attenuates momentary ruminative self-referential thoughts. Brain Stimulation 10, 1088–1095. 10.1016/j.brs.2017.09.005 [DOI] [PubMed] [Google Scholar]
- Ball SC, Brewin CR, 2012. The Effect of Rumination on Intrusive Images and Mood: An Experimental Investigation Using the Trauma Film Paradigm. Journal of Experimental Psychopathology 3, 297–309. 10.5127/jep.019511 [DOI] [Google Scholar]
- Berlim MT, Van den Eynde F, 2014. Repetitive Transcranial Magnetic Stimulation over the Dorsolateral Prefrontal Cortex for Treating Posttraumatic Stress Disorder: An Exploratory Meta-Analysis of Randomized, Double-Blind and Sham-Controlled Trials. Can J Psychiatry 59, 487–496. 10.1177/070674371405900905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim MT, Van den Eynde F, Daskalakis ZJ, 2013. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychological Medicine 43, 2245–2254. 10.1017/S0033291712002802 [DOI] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J, 2011. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience 6, 548–555. 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrer E, Michael T, 2011. Rumination in PTSD as well as in Traumatized and Non- Traumatized Depressed Patients: A Cross-Sectional Clinical Study. Behavioural and Cognitive Psychotherapy 39, 381–397. 10.1017/S1352465811000087 [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med 34, 537–541. 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress 8, 75–90. 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Frewen PA, Coupland NC, Densmore M, Schore AN, Lanius RA, 2012. Neural correlates of self-reflection in post-traumatic stress disorder. Acta Psychiatrica Scandinavica 125, 238–246. 10.1111/j.1600-0447.2011.01773.x [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RW, Théberge J, Lanius RA, 2009. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry & Neuroscience : JPN 34, 187–194. [PMC free article] [PubMed] [Google Scholar]
- Brinker JK, Dozois DJA, 2009. Ruminative thought style and depressed mood. Journal of Clinical Psychology 65, 1–19. 10.1002/jclp.20542 [DOI] [PubMed] [Google Scholar]
- Buchholz KR, Bruce SE, Koucky EM, Artime TM, Wojtalik JA, Brown WJ, Sheline YI, 2016. Neural correlates of trait rumination during an emotion interference task in women with PTSD. Journal of Traumatic Stress 29, 317–324. 10.1002/jts.22112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Jacobs RH, Peters AT, Ajilore O, Watkins ER, Langenecker SA, 2017. Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cognitive, Affective, & Behavioral Neuroscience 17, 394–405. 10.3758/s13415-016-0486-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J, 2012. The secret lives of experiments: Methods reporting in the fMRI literature. NeuroImage 63, 289–300. 10.1016/j.neuroimage.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR, 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Charak R, Armour C, Elklit A, Angmo D, Elhai JD, Koot HM, 2014. Factor structure of PTSD, and relation with gender in trauma survivors from India. European Journal of Psychotraumatology 5, 25547 10.3402/ejpt.v5.25547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KCR, Spreng RN, Andrews-Hanna JR, 2016. Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews Neuroscience 17, 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C, Gur RC, Gur RE, Bassett DS, Satterthwaite TD, 2017. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage 154, 174–187. 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clohessy S, Ehlers A, 1999. PTSD symptoms, response to intrusive memories and coping in ambulance service workers. British Journal of Clinical Psychology 38, 251–265. 10.1348/014466599162836 [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH, 2010. Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience 10, 470–478. 10.3758/CABN.10.4.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M, 2014. Direct Reactivation of a Coherent Neocortical Memory of Context. Neuron 84, 432–441. 10.1016/j.neuron.2014.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research 29, 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- De Raedt R, Remue J, Loeys T, Hooley JM, Baeken C, 2017. The effect of transcranial direct current stimulation of the prefrontal cortex on implicit self-esteem is mediated by rumination after criticism. Behaviour Research and Therapy 99, 138–146. 10.1016/j.brat.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dunmore E, Clark DM, Ehlers A, 1999. Cognitive factors involved in the onset and maintenance of posttraumatic stress disorder (PTSD) after physical or sexual assault. Behaviour Research and Therapy 37, 809–829. 10.1016/S0005-7967(98)00181-8 [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM, 2000. A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy 38, 319–345. 10.1016/S0005-7967(99)00123-0 [DOI] [PubMed] [Google Scholar]
- Ehlers A, Mayou RA, Bryant B, 1998. Psychological predictors of chronic posttraumatic stress disorder after motor vehicle accidents. Journal of Abnormal Psychology 107, 508–519. 10.1037/0021-843X.107.3.508 [DOI] [PubMed] [Google Scholar]
- Ehring T, Szeimies A-K, Schaffrick C, 2009. An experimental analogue study into the role of abstract thinking in trauma-related rumination. Behaviour Research and Therapy 47, 285–293. 10.1016/j.brat.2008.12.011 [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113, 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97, 11050 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Ehlers A, Clark DM, Tolin DF, Orsillo SM, 1999. The Posttraumatic Cognitions Inventory (PTCI): Development and Validation. Psychological Assessment 11, 303–314. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC, 1995. Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magn. Reson. Med 33, 636–647. 10.1002/mrm.1910330508 [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Densmore M, Stevens TK, Lanius RA, 2010. Social emotions and emotional valence during imagery in women with PTSD: Affective and neural correlates. Psychological Trauma: Theory, Research, Practice, and Policy 2, 145–157. 10.1037/a0019154 [DOI] [Google Scholar]
- Frewen PA, Thornley E, Rabellino D, Lanius R, 2017. Neuroimaging the traumatized self: fMRI reveals altered response in cortical midline structures and occipital cortex during visual and verbal self- and other-referential processing in women with PTSD. European Journal of Psychotraumatology 8, 1314164 10.1080/20008198.2017.1314164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, Jonas DE, Evans TS, Viswanathan M, Lohr KN, 2014. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. The Journal of Clinical Psychiatry 75, 477–489. 10.4088/JCP.13r08815 [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H, 2010. Age-related changes in grey and white matter structure throughout adulthood. NeuroImage 51, 943–951. 10.1016/j.neuroimage.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchert J, Smallwood J, Jefferies E, Seli P, Huntenburg JM, Liem F, Lauckner ME, Oligschläger S, Bernhardt BC, Villringer A, Margulies DS, 2017. Individual variation in intentionality in the mind-wandering state is reflected in the integration of the default-mode, fronto-parietal, and limbic networks. NeuroImage 146, 226–235. 10.1016/j.neuroimage.2016.11.025 [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ, 2001. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage 14, 21–36. 10.1006/nimg.2001.0786 [DOI] [PubMed] [Google Scholar]
- Hebscher M, Ibrahim C, Gilboa A, 2020. Precuneus stimulation alters the neural dynamics of autobiographical memory retrieval. NeuroImage 210, 116575 10.1016/j.neuroimage.2020.116575 [DOI] [PubMed] [Google Scholar]
- Jacob Y, Morris LS, Huang K-H, Schneider M, Rutter S, Verma G, Murrough JW, Balchandani P, 2020. Neural correlates of rumination in major depressive disorder: A brain network analysis. NeuroImage: Clinical 25, 102142 10.1016/j.nicl.2019.102142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF, 2002. Finding the Self? An Event-Related fMRI Study. Journal of Cognitive Neuroscience 14, 785–794. 10.1162/08989290260138672 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG, 2007. Macaque monkey retrosplenial cortex: III. Cortical efferents. Journal of Comparative Neurology 502, 810–833. 10.1002/cne.21346 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG, 2003. Macaque monkey retrosplenial cortex: II. Cortical afferents. Journal of Comparative Neurology 466, 48–79. 10.1002/cne.10883 [DOI] [PubMed] [Google Scholar]
- Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, Motta C, Maiella M, Marra C, Cercignani M, Martorana A, Caltagirone C, Bozzali M, 2018. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. NeuroImage 169, 302–311. 10.1016/j.neuroimage.2017.12.048 [DOI] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2016. Aberrant resting-state brain activity in PTSD: a meta-analysis and systematic review. Depression and Anxiety 33, 592–605. 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Kubota R, Nixon RDV, 2017. An Analogue Investigation into the Effect of Trauma-related Rumination on Trauma Intrusions and the Moderating Role of Trait Rumination and Depression. Journal of Experimental Psychopathology 8, 413–442. 10.5127/jep.058516 [DOI] [Google Scholar]
- Kühn S, Vanderhasselt M-A, De Raedt R, Gallinat J, 2012. Why ruminators won’t stop: The structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorders 141, 352–360. 10.1016/j.jad.2012.03.024 [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ, 2014. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biological Psychiatry 76, 517–526. 10.1016/j.biopsych.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoSavio ST, Dillon KH, Resick PA, 2017. Cognitive factors in the development, maintenance, and treatment of post-traumatic stress disorder. Current Opinion in Psychology 14, 18–22. 10.1016/j.copsyc.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Makovac E, Fagioli S, Rae CL, Critchley HD, Ottaviani C, 2020. Can’t get it off my brain: Meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Research: Neuroimaging 295, 111020 10.1016/j.pscychresns.2019.111020 [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M, 2009. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106, 20069 10.1073/pnas.0905314106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks EH, Franklin AR, Zoellner LA, 2018. Can’t get it out of my mind: A systematic review of predictors of intrusive memories of distressing events. Psychological Bulletin 144, 584–640. 10.1037/bul0000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Nolen-Hoeksema S, 2011. Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy 49, 186–193. 10.1016/j.brat.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael T, Halligan SL, Clark DM, Ehlers A, 2007. Rumination in posttraumatic stress disorder. Depress. Anxiety 24, 307–317. 10.1002/da.20228 [DOI] [PubMed] [Google Scholar]
- Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M, 2017. Default Mode Network Subsystems Are Differentially Disrupted in Posttraumatic Stress Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2, 363–371. 10.1016/j.bpsc.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Tsuchiyagaito A, Al Zoubi O, Paulus M, Bodurka J, 2020. Connectome-wide search for functional connectivity locus associated with pathological rumination as a target for real-time fMRI neurofeedback intervention. NeuroImage: Clinical 26, 102244 10.1016/j.nicl.2020.102244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN, 1999. Architecture and connections of retrosplenial area 30 in the rhesus monkey (macaca mulatta). European Journal of Neuroscience 11, 2506–2518. 10.1046/j.1460-9568.1999.00672.x [DOI] [PubMed] [Google Scholar]
- Murphy DGM, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B, 1992. Age-Related Differences in Volumes of Subcortical Nuclei, Brain Matter, and Cerebrospinal Fluid in Healthy Men as Measured With Magnetic Resonance Imaging. JAMA Neurology 49, 839–845. 10.1001/archneur.1992.00530320063013 [DOI] [PubMed] [Google Scholar]
- Murray J, Ehlers A, Mayou RA, 2002. Dissociation and post-traumatic stress disorder: two prospective studies of road traffic accident survivors. British Journal of Psychiatry 180, 363–368. 10.1192/bjp.180.4.363 [DOI] [PubMed] [Google Scholar]
- Murray RJ, Debbané M, Fox PT, Bzdok D, Eickhoff SB, 2015. Functional connectivity mapping of regions associated with self- and other-processing. Hum. Brain Mapp 36, 1304–1324. 10.1002/hbm.22703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C, 2013. Self-Referential Processing, Rumination, and Cortical Midline Structures in Major Depression. Frontiers in Human Neuroscience 7, 666 10.3389/fnhum.2013.00666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S, 2008. Rethinking rumination. Perspectives on Psychological Science 3, 400–424. 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Olff M, 2017. Sex and gender differences in post-traumatic stress disorder: an update. European Journal of Psychotraumatology 8, 1351204 10.1080/20008198.2017.1351204 [DOI] [Google Scholar]
- Papageorgiou C, Wells A, 2003. An Empirical Test of a Clinical Metacognitive Model of Rumination and Depression. Cognitive Therapy and Research 27, 261–273. 10.1023/A:1023962332399 [DOI] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A, 2006. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA 103, 1563 10.1073/pnas.0507729103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Cornejo MD, Frost CP, Walsh EC, Hoks RM, Birn R, Abercrombie HC, 2018. Neural and behavioral correlates of negative self-focused thought associated with depression. Hum. Brain Mapp 39, 2246–2257. 10.1002/hbm.24003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, Koenigs M, 2015. Altered resting-state functional connectivity in cortical networks in psychopathy. J. Neurosci. 35, 6068 10.1523/JNEUROSCI.5010-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G, 2011. How is our self related to midline regions and the default-mode network? NeuroImage 57, 1221–1233. 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schürholt B, Lebens M, Seitz RJ, Schulze R, 2013. In Search of the Trauma Memory: A Meta-Analysis of Functional Neuroimaging Studies of Symptom Provocation in Posttraumatic Stress Disorder (PTSD). PLOS ONE 8, e58150 10.1371/journal.pone.0058150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyshur MD, Layden EA, Gowins JR, Buchanan A, Gollan JK, 2018. Functional connectivity of reflective and brooding rumination in depressed and healthy women. Cognitive, Affective, & Behavioral Neuroscience 18, 884–901. 10.3758/s13415-018-0611-7 [DOI] [PubMed] [Google Scholar]
- Seligowski AV, Lee DJ, Bardeen JR, Orcutt HK, 2015. Emotion Regulation and Posttraumatic Stress Symptoms: A Meta-Analysis. Cognitive Behaviour Therapy 44, 87–102. 10.1080/16506073.2014.980753 [DOI] [PubMed] [Google Scholar]
- Sezibera V, Van Broeck N, Philippot P, 2009. Intervening on Persistent Posttraumatic Stress Disorder: Rumination-Focused Cognitive and Behavioral Therapy in a Population of Young Survivors of the 1994 Genocide in Rwanda. J Cogn Psychother 107–113. 10.1891/0889-8391.23.2.107 [DOI] [Google Scholar]
- Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, Liao W, Zhang W, 2014. Alterations in Low-Level Perceptual Networks Related to Clinical Severity in PTSD after an Earthquake: A Resting-State fMRI Study. PLOS ONE 9, e96834 10.1371/journal.pone.0096834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin ELL, Shao R, Geng X, Cho V, Lee TMC, 2018. The Neuroanatomical Basis of Two Subcomponents of Rumination: A VBM Study. Frontiers in Human Neuroscience 12, 324 10.3389/fnhum.2018.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I, 2012. Neural Dysregulation in Posttraumatic Stress Disorder: Evidence for Disrupted Equilibrium between Salience and Default Mode Brain Networks. Psychosomatic medicine 74, 904–911. 10.1097/PSY.0b013e318273bf33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Botzung A, Miles A, Rubin DC, 2011. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research 45, 630–637. 10.1016/j.jpsychires.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos A, Berle D, 2020. The influence of ruminative processing mode on the trajectory of intrusive memories following a negative mood induction. Journal of Behavior Therapy and Experimental Psychiatry 68, 101528 10.1016/j.jbtep.2019.101528 [DOI] [PubMed] [Google Scholar]
- Steil R, Ehlers A, 2000. Dysfunctional meaning of posttraumatic intrusions in chronic PTSD. Behaviour Research and Therapy 38, 537–558. 10.1016/S0005-7967(99)00069-8 [DOI] [PubMed] [Google Scholar]
- Szabo YZ, Warnecke AJ, Newton TL, Valentine JC, 2017. Rumination and posttraumatic stress symptoms in trauma-exposed adults: a systematic review and meta-analysis. Anxiety, Stress, & Coping 30, 396–414. 10.1080/10615806.2017.1313835 [DOI] [PubMed] [Google Scholar]
- Tanner A, Voon D, Hasking P, Martin G, 2013. Underlying Structure of Ruminative Thinking: Factor Analysis of the Ruminative Thought Style Questionnaire. Cognitive Therapy and Research 37, 633–646. 10.1007/s10608-012-9492-1 [DOI] [Google Scholar]
- Van Hoesen GW, Morecraft RJ, Vogt BA, 1993. Connections of the Monkey Cingulate Cortex, in: Vogt BA, Gabriel M (Eds.), Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhäuser Boston, Boston, MA, pp. 249–284. 10.1007/978-1-4899-6704-6_9 [DOI] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA, 2009. What does the retrosplenial cortex do? Nature Reviews Neuroscience 10, 792–802. 10.1038/nrn2733 [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B, 2005. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging 26, 1261–1270. 10.1016/j.neurobiolaging.2005.05.020 [DOI] [PubMed] [Google Scholar]
- Wang G, Xie H, Wang L, Luo W, Wang Y, Jiang J, Xiao C, Xing F, Guan J-S, 2019. Switching From Fear to No Fear by Different Neural Ensembles in Mouse Retrosplenial Cortex. Cerebral Cortex 29, 5085–5097. 10.1093/cercor/bhz050 [DOI] [PubMed] [Google Scholar]
- Wang T, Liu J, Zhang J, Zhan W, Li L, Wu M, Huang H, Zhu H, Kemp GJ, Gong Q, 2016. Altered resting-state functional activity in posttraumatic stress disorder: A quantitative meta-analysis. Scientific Reports 6, 27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, 2015. Psychological treatment of depressive rumination. Current Opinion in Psychology 4, 32–36. 10.1016/j.copsyc.2015.01.020 [DOI] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castañón A, Triantafyllou C, Saxe R, Gabrieli JDE, 2011. Associations and dissociations between default and self-reference networks in the human brain. NeuroImage 55, 225–232. 10.1016/j.neuroimage.2010.11.048 [DOI] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP, 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76, 183–201. 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche U, Ehring T, Ehlers A, 2009. The effects of rumination on mood and intrusive memories after exposure to traumatic material: An experimental study. Journal of Behavior Therapy and Experimental Psychiatry 40, 499–514. 10.1016/j.jbtep.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S, 2001. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging 20, 45–57. 10.1109/42.906424 [DOI] [PubMed] [Google Scholar]
- Zhou H-X, Chen X, Shen Y-Q, Li L, Chen N-X, Zhu Z-C, Castellanos FX, Yan C-G, 2020. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage 206, 116287 10.1016/j.neuroimage.2019.116287 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.