Abstract
Protective effects of quercetin (QUE), polydatin (POL), and folic acid (FA) and their mixtures were tested using zebrafish to model fetal alcohol spectrum disorder in this study. Zebrafish embryos were exposed to 150 mM ethanol for 6 or 22 h and co-treated with QUE, POL, FA, and their mixtures (37.5 – 100.0 μM). Epiboly progression, teratogenic effects, and behavior were evaluated. Ethanol exposure reduced epiboly, and FA and QUE protected against these ethanol-induced defects. POL did not reduce epiboly defects. The mixture QUE+FA showed a possible antagonistic effect. The observed teratogenic effects were similar in all ethanol exposed groups. QUE, FA and QUE+POL reduced the percentage of affected animals, but treatments did not eliminate teratogenic effects. Behavioral measurements were divided into small (between 4 and 8 mm/s) and high swimming activity (> 8 mm/s). All experimental groups displayed a reduction in small swimming activity as compared to control and ethanol groups when exposed to bright light. Additionally, larvae exposed to ethanol were more inhibited than control, not showing a habituation period (after 60 min of experiment) in high swimming activity. Chemical treatments like QUE and POL reduced behavioral defects induced by ethanol exposure. In conclusion, this study presents new evidence that QUE, POL, FA and their mixtures partially protected epiboly, teratogenic, and behavioral defects induced by ethanol exposure. QUE, FA and QUE+POL were more effective in reducing these defects than the other studied compounds and mixtures.
Keywords: Flavonoids, Mixture, Epiboly, Teratogenic effects, Behavior
1. Introduction
A growing number of studies have evaluated protective effects of chemical compounds in the fetal alcohol spectrum disorder (FASD), but the effects of mixtures are unknown. Quercetin (3,3′,4′,5,7-pentahydroxyflavone; QUE) is an important and prevalent natural dietary flavonoid with antioxidant and anti-inflammatory activities found in many foods, which have protective effects of QUE on alcohol-induced liver injuries in a FASD model (Cadena et al., 2013; Zhu et al., 2017; Lee et al., 2019; Ince, 2020). The protective effect of QUE was associated with PI3K/Akt/nuclear factor-κB and STAT3 signaling pathways (Zhu et al., 2017). Polydatin (3, 4, 5-trihydroxystibene-3-b-mono-d-glucoside; POL), a glucoside of resveratrol, was also used to prevent ethanol toxic effects on the liver (Huang et al., 2017; Lai et al., 2018). Furthermore, the therapeutic effect of POL on ethanol-induced learning and memory defects (Zhang et al., 2015) and oxidative stress (Pace et al., 2015) was also evaluated. These protective effects of POL were associated with antioxidant activity through the downregulation of cytochrome P450 2E1 and upregulation of nuclear factor erythroid 2-related factor 2 (Huang et al., 2017). QUE and POL are antioxidants, but the combined effects of these chemicals in a FASD animal model were not studied. Park et al. (2008) studied the combined effects of QUE and resveratrol and showed an additive effect for treatment of obesity. Moreover, Caddeo et al. (2016) showed higher antioxidant activity when QUE and resveratrol were combined than single compounds. Despite the single-use of these flavonoids to treat defects induced by ethanol, possible combined effects have not been reported.
Despite religious and social practices of alcohol use, alcohol consumption results in disease, violence and injuries (WHO, 2018). Alcohol consumption during pregnancy is almost 10% (WHO, 2018), and the prevalence of fetal alcohol syndrome (FAS; a more severe subset of FASD patients) in the general population was reported as 14.6 per 10,000 people in one study (Popova et al., 2017), with FASD prevalence potentially as high as 10% (May et al., 2018). FASD is associated with behavioral defects and several abnormalities in the central nervous system, gastrointestinal and cardiovascular systems in different animal models (Sarmah et al., 2016; Cleal and Parker, 2018; Muralidharan et al., 2018; Collier et al., 2020; Ince, 2020).
Our group proposed a methodology to induce FASD in zebrafish (Danio rerio) as an animal model (Marrs et al., 2010; Muralidharan et al., 2015; Sarmah et al., 2016; Muralidharan et al., 2018). The zebrafish emerged as a model for studying toxic effects of embryonic ethanol exposure, and embryos exposed to ethanol produced effects related to intraoral administration of ethanol in pregnant rodents (Collier et al., 2020). Fernandes et al. (2018) illustrating behavioral defects, including learning and memory, and structural defects in the central nervous system, heart, and craniofacial skeleton. External fertilization in zebrafish helps the study of how ethanol exposure induced defects are related to the exposure time and dosage (Fernandes et al., 2018). Also, the zebrafish is useful for screening of neuroactive drugs (Basnet et al., 2019), and it is possible to evaluate whether these drugs are effective in reducing defects induced by ethanol exposure. In the zebrafish FASD model, the epiboly process, where cells spread over the yolk cell (Kimmel et al., 1995), is affected by embryonic ethanol exposure (Marrs et al., 2010; Sarmah et al., 2013). The study of new therapeutic treatments using the zebrafish epiboly has potential utility for testing in this FASD model. In addition, we previously reproduced morphological defects induced by ethanol exposure in this zebrafish FASD model (Cadena et al., 2020), which also can be used to screen new therapeutic drugs. Finally, behavioral defects induced by ethanol are challenging to measure in zebrafish. However, automated systems to collect behavioral data with standardized time, light, temperature and number of animals can be useful. These methodologies can be suitable for screening the protective effects of chemical compounds like QUE and POL, and their mixtures.
We previously studied the protective effects of 75 μM folic acid (FA) against embryonic exposure to ethanol on behavioral and morphological defects (Cadena et al., 2020). Embryonic ethanol exposure produced FA deficiency (Muralidharan et al., 2013). FA protected against several ethanol-induced teratogenic effects (Muralidharan et al., 2013; Sarmah et al., 2016; Sarmah and Marrs, 2017). We confirmed that FA reduced defects induced by ethanol exposure, but FA does not reduce all defects, particularly behavior defects (Cadena et al., 2020). This study of neuroactive drugs like QUE, POL, FA and their mixtures seeks to overcome these limitations. Using zebrafish as FASD animal model, we investigate whether QUE, POL and FA and their mixtures protect against embryonic ethanol-induced toxic effects in embryonic development and behavior.
2. Material and methods
2.1. Zebrafish maintenance and crossing
The experiments were conducted at Purdue School of Science at IUPUI (Indiana University – Purdue University Indianapolis). Adults zebrafish (Danio rerio; AB strain; 15-months-old) were housed according to OECD 236 (OECD, 2013) and Westerfield (2000) guidelines in an Aquatic Habitats recirculating filtered water system (Pentair Aquatic Eco-Systems, Apopka, FL) with approval from the Indiana University Policy on Animal Care and Use Committee. Adult male/female zebrafish with a 2:1 or 4:2 ratio (Westerfield, 2000) were used for crossing in spawning aquariums (40 males and 16 females). The fertilized eggs were collected 30 min after spawning and then incubated at 28.5 °C in an Echotherm incubator (Torry Pines Scientific, Carlsbad, CA). Only fertilized eggs with normal development (OECD, 2013) were used in the experimental groups.
2.2. Reagents and preparation of stock solutions
Quercetin (QUE) (lot # SLCC9071, CAS number 117-39-5, purity of ≥ 95%, and HPLC grade), polydatin (POL) (lot # WXBC9269V, CAS number 65914-17-2, purity of ≥ 95%, and HPLC grade), folic acid (FA) (lot # 121K01445, CAS number 59-30-3, purity of ≥ 98%, and HPLC grade) and dimethyl sulfoxide (DMSO) (lot # SHBL2891, CAS number 67-68-5, purity of ≥ 99.9%) were purchased from Sigma (St. Louis MO, USA). Ethanol anhydrous (lot # 170421–06, CAS number 64-17-5, purity of ≥ 99.99%) was purchased from Electron Microscopy Sciences (Hatfield, PA, USA). All other reagents utilized were of analytical grade. Stock solutions of QUE, POL, and FA was made fresh before the experiments and prepared by the dissolution in 1% (v/v) of dimethylsulfoxide (DMSO) in embryo medium as aqueous medium obtaining the final nominal concentration of 1,000 μM for each chemical compound.
2.3. Ethanol treatments
Fetal alcohol spectrum disorder (FASD)-like phenotype was induced in zebrafish according to Sarmah et al. (2016). Healthy embryos were kept in embryo medium until 2 hpf (hours postfertilization) and exposed to QUE, POL, FA and their binary and ternary mixtures diluted in embryo medium with or without ethanol (150 mM) according to Table 1 in Petri dishes wrapped with Parafilm®. Also, the final nominal concentration of DMSO was 0.01% (v/v), which does not affect the studied parameters (Christou et al., 2020). Ethanol treatment dishes were placed in chambers with 2% ethanol to minimize ethanol volatilization (Muralidharan et al., 2015). At 24 hpf (22 h of exposure), all solutions were changed for fresh embryo medium. Control and experimental groups were housed in the dark for the first day to prevent flavonoids degradation and then moved into the zebrafish housing facility with a light-dark cycle of 14:10 h and 28.5°C. The developmental stages of embryos were also identified (Kimmel et al., 1995).
Table 1.
Concentrations of the individual compounds and binary and ternary mixtures (A) used for rescue toxic effects induced by ethanol exposition in FASD (Fetal Alcohol Spectrum Disorder) zebrafish model. Simplex lattice experimental design layout (B) for evaluation of interactions between chemical compounds.
| A. Concentrations of the individual compounds and binary and ternary mixtures | ||
|---|---|---|
| Compounds | Concentration | Ethanol exposure |
| Control (Embryo medium) | - | 0 |
| DMSO (Control) | 0.01 % (v/v) | 0 |
| Ethanol | - | 150 mM |
| Folic acid (FA) | 75, 100 μM | 0 |
| Folic acid (FA) | 50, 75 μM | 150 mM |
| Quercetin (QUE) | 75, 100 μM | 0 |
| Quercetin (QUE) | 50, 75 and 100 μM | 150 mM |
| Polydatin (POL) | 75, 100 μM | 0 |
| Polydatin (POL) | 50, 75 and 100 μM | 150 mM |
| QUE+POL | 37.5 μM of each compound = 75 μM | 150 mM |
| QUE+FA | 37.5 μM of each compound = 75 μM | 150 mM |
| POL+FA | 37.5 μM of each compound = 75 μM | 150 mM |
| QUE+POL+FA | 25 μM of each compound = 75 μM | 150 mM |
| B. Simplex lattice experimental design | ||||
|---|---|---|---|---|
| Compounds | Quercetin (μM) | Polydatin (μM) | Folic acid (μM) | Ethanol (mM) |
| QUE | 75 | 0 | 0 | 150 |
| POL | 0 | 75 | 0 | 150 |
| FA | 0 | 0 | 75 | 150 |
| QUE+POL | 37.5 | 37.5 | 0 | 150 |
| QUE+FA | 37.5 | 0 | 37.5 | 150 |
| POL+FA | 0 | 37.5 | 37.5 | 150 |
2.4. Measurement of epiboly
The epiboly of embryos in the experimental groups described in Table 1 was evaluated at 8 hpf, after 6 h of experimental treatment. First, 4% paraformaldehyde in phosphate-buffered saline (PBS) was used to fix the embryos (Marrs et al., 2010) for 24 h. Then, embryos were washed 3 times with PBS. Images were captured using a Leica stereomicroscope with an attached cellphone as a camera. The percentage of epiboly (n ≈ 35 per group with 18 experimental groups ≈ 630 embryos) was determined by the distance between the animal pole to the blastoderm margin divided by the distance between the animal pole and the vegetal pole (Schwend et al., 2011). The measurements were made with Image J software (version 1.52A, 2019, National Institutes of Health, MD).
2.5. Analysis of the embryonic development
Mortality data are collected and studied at different developmental stages (Kimmel et al., 1995) as embryos (inside the chorion membrane) at 2 days postfertilization (dpf), and as larva at 4–6 dpf. The lethality of embryos was also analyzed (OECD, 2013). Teratogenic effects (OECD, 2013; Silva et al., 2019) were identified in lateral and dorsal views in vivo and after euthanasia. Fish were first euthanized with MS222 after 6 dpf and then fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) pH 7.1 overnight, and then, larvae were washed 3 times with PBS. We consider larvae affected by ethanol exposure when observed under stereomicroscope with, at least, three different teratogenic effects evaluated qualitatively (present or absent) seen as follows: Ocular defects as large inter-eye distance (ED), small eyes (EY); Circulatory defects as pericardial edema (PE), multiple edemas in the body (ME) and yolk sac edema (YSE); Morphological defects as delayed swim bladder inflation (SB), spine deformation (SD), tail deformation (TD) and growth retardation (GR); Locomotor system defects as fish complete paralysis (FP) of muscles (Dubińska-Magiera et al., 2016) and circular swimming (CS) (Kalueff et al., 2013). We also evaluated hatching delay (HD) after 4 dpf (OECD, 2013). These results were presented as a percentage of affected animals (with teratogenic effects) per experimental group using 3 plates for DMSO, QUE, POL and FA (n ≈ 35 per plate with 4 experimental groups ≈ 420 larvae), and 5 plates for groups exposed to alcohol (n ≈ 35 per plate with 8 experimental ≈ 1400 larvae).
2.6. Animal behavior
Only larvae without detectable morphological defects (see item 2.5) were used for the behavioral measurements. The behavior for experimental groups described in Table 1 was analyzed using a Zebrabox (Viewpoint Life Sciences, Lyon, France) tracking system equipped with an infrared camera. The behavioral data were collected in light condition between 11:00 and 15:00, modified from MacPhail et al. (2009). Briefly, larvae (n ≈ 24 per group with 12 experimental groups ≈ 288 larvae) at 6 dpf were placed in 96-well plates and subjected to 10 min of acclimatization in dark and 80-min in 100% bright light. The integration period of 2 minutes was used, and the distance moved (mm/s) was evaluated and divided into small activity (between 4 and 8 mm/s) and high activity (> 8 mm/s).
2.7. Statistical analysis
Statistical planning for the experiments was carried out by simplex lattice mixture design (Table 1) as a basis to determine the number of experimental groups and evaluate the interaction between chemical compounds. FA, QUE and POL concentrations (0 – 100 μM) and their mixtures were used as factors. All data were shown as mean ± SD. ANOVA was used to analyze data, and the means were compared by Tukey’s test (p < 0.05). Statistical analyses were carried out using the Origin Pro Academic 2015 (Origin Lab. Northampton, MA USA) and Statistica 13.5 trial version (TIBCO, USA).
3. Results
3.1. Measurement of epiboly
We exposed embryos to 150 mM ethanol during cleavage until gastrula periods (Kimmel et al., 1995) to evaluate epiboly defects. The results are shown in Figure 1A. As expected, the DMSO (0.01% v/v) did not affect the percent epiboly process, the amount of the yolk cell covered by the growing embryo (Marrs et al., 2010), but ethanol exposure significantly reduced epiboly. FA exposure did not affect epiboly but protected against ethanol-induced defects. QUE exposure also did not affect epiboly and protected against ethanol-induced defects at the same level as FA. However, POL did not prevent epiboly defects induced by ethanol. Despite the protective effect of single compounds, QUE+FA mixture showed a possible antagonistic effect. In addition, 50 μM of QUE, POL or FA individually did not reduce the toxicity induced by ethanol exposure, but mixtures 37.5 μM QUE+POL, POL+FA and 25 μM QUE+POL+FA protected against epiboly defects, showing a possible synergistic effect.
Figure 1.
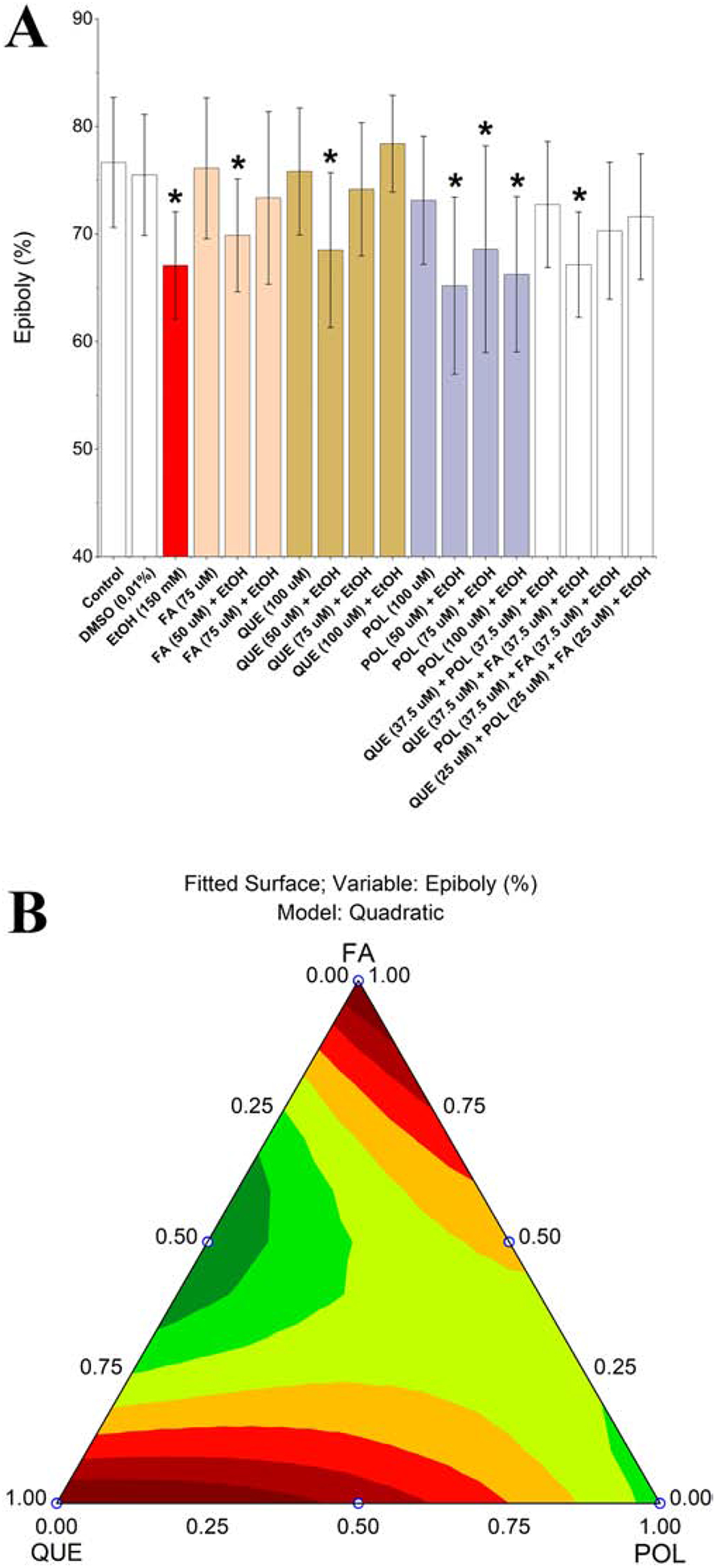
Zebrafish ethanol exposure during the embryogenesis after 8 hpf (A). The epiboly process was considered affected when * = p < 0.05. Legend: Con – Control; DMSO – 0.01% (v/v) Dimethyl sulfoxide; EtOH – Ethanol; FA – Folic acid; QUE – Quercetin; POL - Polydatin. Each experimental group was compared with the control group by one-way ANOVA (F(16,735) = 16.38 p < 0.05) followed by Tukey’s test (DMSO p = 1.00; EtOH p < 0.001, FA p = 1.00, FA (50 μM) + EtOH p < 0.001, FA (75 μM) + EtOH p = 0.97, QUE p = 1.00, QUE (50 μM) + EtOH p < 0.001, QUE (75 μM) + EtOH p = 0.98, QUE (100 μM) + EtOH p = 0.99, POL p = 0.98, POL (50 μM) + EtOH p < 0.001, POL (75 μM) + EtOH p < 0.001, POL (100 μM) + EtOH p = p < 0.001, QUE+POL + EtOH p = 0.70, QUE+FA + EtOH p < 0.001, POL+FA + EtOH p = 0.03, QUE+POL+FA + EtOH p = 0.26. Ternary contour plot (B) of variables QUE, POL, and FA for a quadratic model (F(3,253) = 7.84 p < 0.05) evaluating the single and interaction effects between these compounds and their mixtures. QUE and FA were more suitable to reduce epiboly defects induced by ethanol exposure (red areas).
Ternary contour plot (Figure 1B) showed that single QUE and FA were more effective in the reduction the epiboly defects than their mixtures. In addition, Figure 1B is useful to show the synergistic effect between QUE+POL and POL+FA to protect against epiboly defects. Concentrations of single compounds that did not rescue epiboly defects, namely QUE (50 μM), FA (50 μM) and POL (50 μM), were excluded from the subsequent experiments, and we maintained all binary mixtures and POL (75 μM) in subsequent experiments.
3.2. Analysis of the embryonic development
Next, we determined whether ethanol-induced defects on epiboly correlates with developmental defects after 22 h of exposure. Results are shown in Figure 2A, revealing several teratogenic effects in the groups exposed to ethanol. Mortality was less than 10% after 2 dpf for all groups, and the indicator of lethality was coagulation (OECD, 2013). After 4 dpf, only the ethanol group had mortality higher than 10% (12%). We considered animals dead when they did not have a heartbeat and did not respond to mechanical stimuli. This mortality was associated with teratogenic effects in ethanol exposed groups. The typically teratogenic effects (see section 2.5) observed in all ethanol exposed groups were measured and shown in Figure 3. These teratogenic effects (Figure 2A) were similar in all ethanol exposed groups. However, the percentage of affected animals had a significant variation between the experimental groups. QUE, FA, QUE+POL reduced the percentage of affected animals but did not completely protect against ethanol-induced defects. Ternary contour plot (Figure 2B) showed that mixtures of QUE+POL were more effective in reducing the percentage of affected animals indicating a synergistic effect. Single QUE and FA treatments were also effective at reducing ethanol-induced defects.
Figure 2.
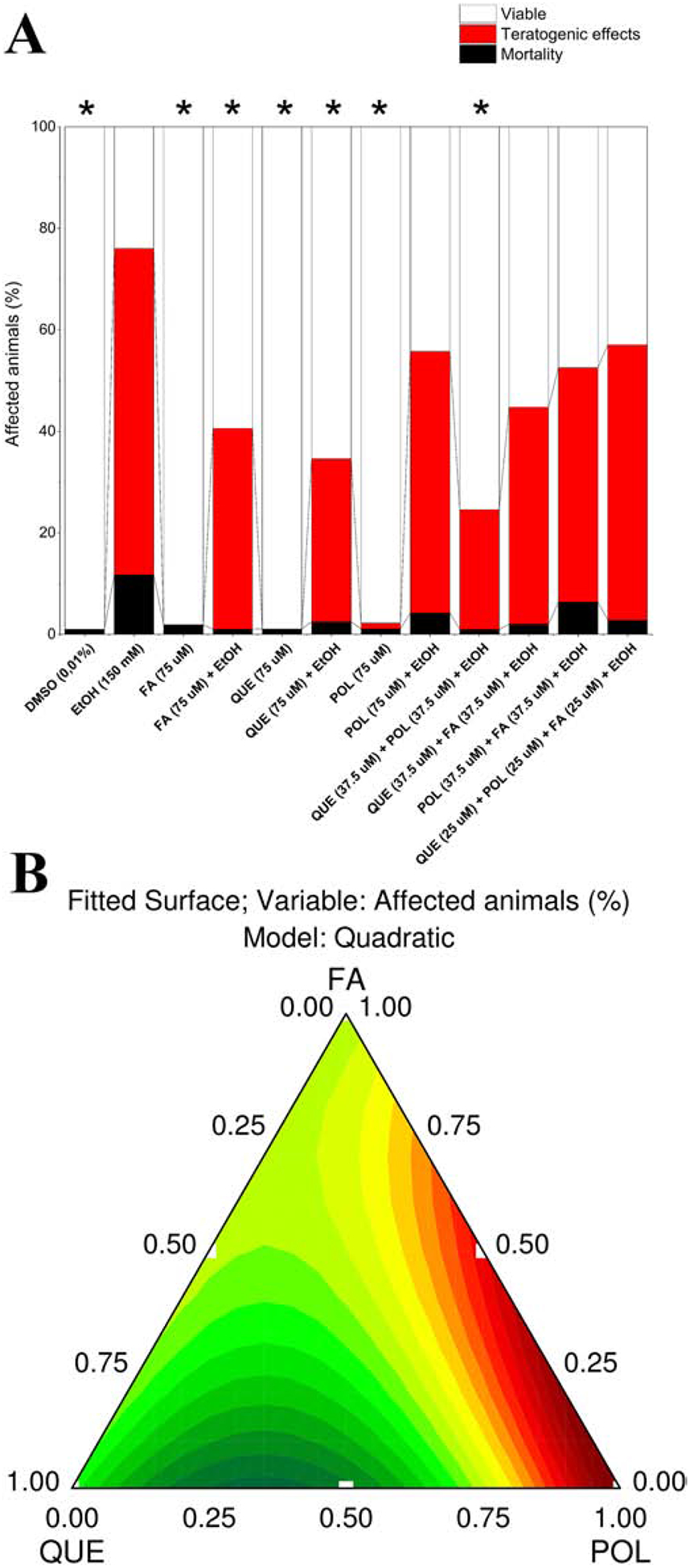
Percentage of affected animals (A) at 4 dpf after 150 mM ethanol embryonic exposure. The reduction in the percentage of affected animals was considered significant when * = p < 0.05. Legend: DMSO – 0.01% (v/v) Dimethyl sulfoxide; EtOH – Ethanol; FA – Folic acid; QUE – Quercetin; POL - Polydatin. Each experimental group was compared with the EtOH group by one-way ANOVA (F(11,54) = 10.76 p < 0.05) followed by Tukey’s test (DMSO p < 0.001, FA p < 0.001, FA (75 μM) + EtOH p = 0.02, QUE p < 0.001, QUE (75 μM) + EtOH p = 0.004, POL p < 0.001, POL (75 μM) + EtOH p = 0.61, QUE+POL + EtOH p < 0.001, QUE+FA + EtOH p = 0.08, POL+FA + EtOH p = 0.39, QUE+POL + FA + EtOH p = 0.69). Ternary contour plot (B) of variables QUE, POL, and FA for a quadratic model (F(3,23) = 4.95 p < 0.05) evaluating the single and interaction effects between these compounds and their mixtures. QUE, QUE+POL and FA were more suitable to reduce the percentage of affected animals (green and yellow areas).
Figure 3.
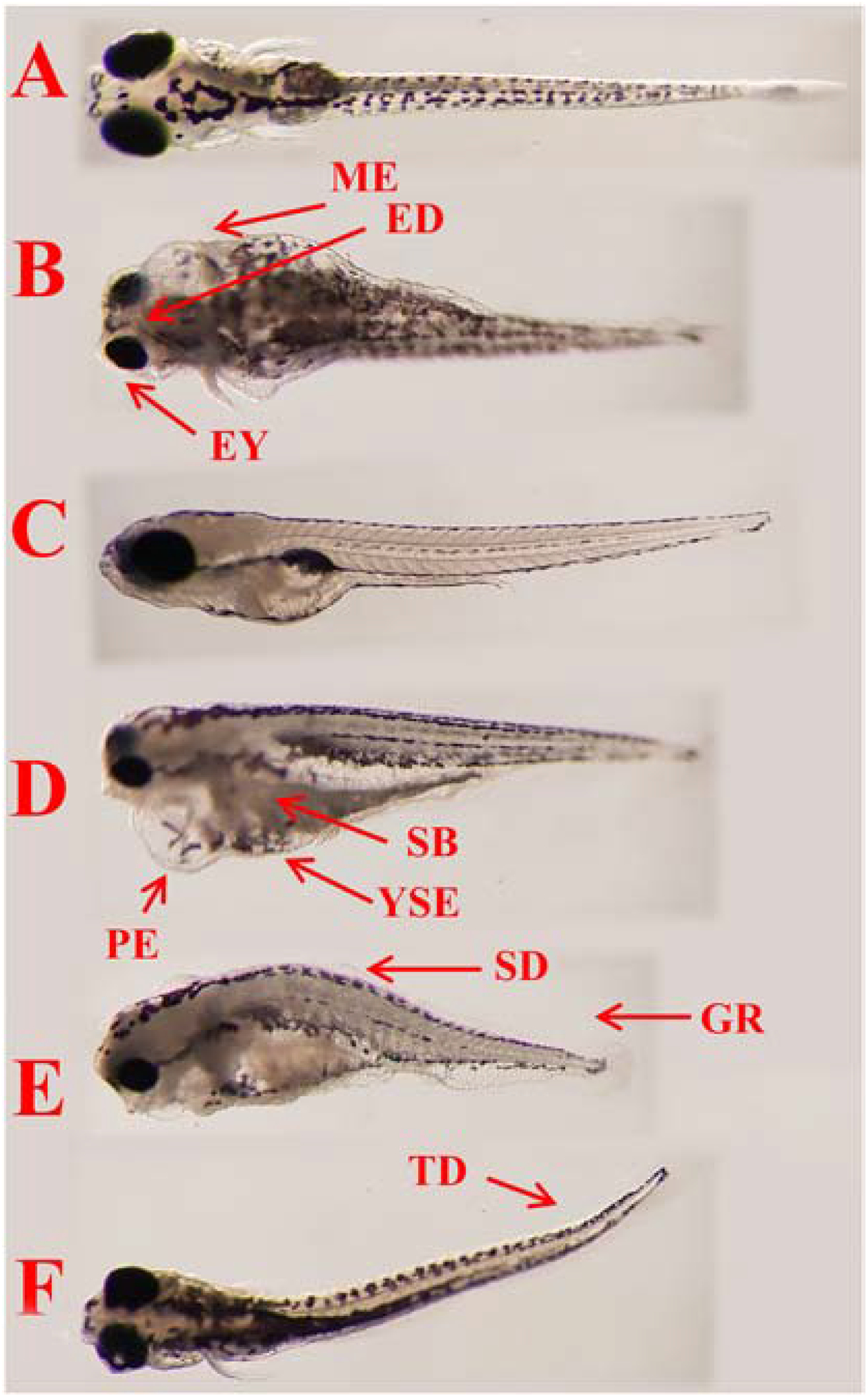
Typical teratogenic effects observed in ethanol exposed zebrafish at 6 dpf as compared with normal larva (control group). Legend: A and C – Control in the dorsal and lateral views; B, D, E, F – Fish exposed to ethanol. Arrows and abbreviations indicate main teratogenic effects as large inter-eye distance (ED), small eyes (EY), pericardial edema (PE), multiple edemas in the body (ME), (YSE), delayed swim bladder inflation (SB), spine deformation (SD), tail deformation (TD) and growth retardation (GR).
3.3. Animal behavior
The results of the behavioral analysis for swimming in small and high activity is shown in Fig. 4 (A – D). A similar pattern was observed in all experimental groups for small activity during the experimental period. Moreover, all experimental groups displayed a reduction in small activity compared to DMSO and ethanol groups (Figure 4A). Ethanol exposure reduced the average small activity, but in the groups exposed to chemical compounds and their mixtures plus ethanol, we observed a greater decrease in the average small activity. Interestingly, QUE, POL and FA without ethanol exposure also affected this parameter when compared to DMSO and ethanol groups. MacPhail et al. (2009) studied the effect of light on the locomotor behavior of larval zebrafish and observed that at the beginning of the test, the swimming activity of animals was inhibited by bright light, and swimming increased again when fish were acclimatized to bright light. We observed the same pattern in the DMSO (control) group (Figure 4D), and we divided the measurements of high activity into the inhibitory (0 – 60 min) and habituation periods (60 – 80 min). Larva exposed to ethanol were more inhibited than control and did not show a habituation period until 80 min in this test (Figure 4D). By analyzing only the inhibitory period (Figure 4B) and considering swimming activity similar to DMSO group and different from ethanol group, QUE, POL, POL + FA, ternary mixture protected against ethanol-induced behavioral defects. QUE, POL and FA without ethanol exposure did not affect this parameter, being only different from the ethanol group. Analyzing only the habituation period (Figure 4C), experimental groups did not increase their swimming activity compared to the DMSO group. Only QUE and ternary mixture partially rescued ethanol-induced defects and showed an increase in the average high activity compared to the ethanol group. QUE, POL and FA without ethanol exposure also interfere in the habituation period by reducing the average high activity. In summary, QUE, POL and FA and their mixtures partially rescued behavioral ethanol-induced defects, and single compounds without ethanol exposure affected the swimming activity of the zebrafish.
Figure 4.
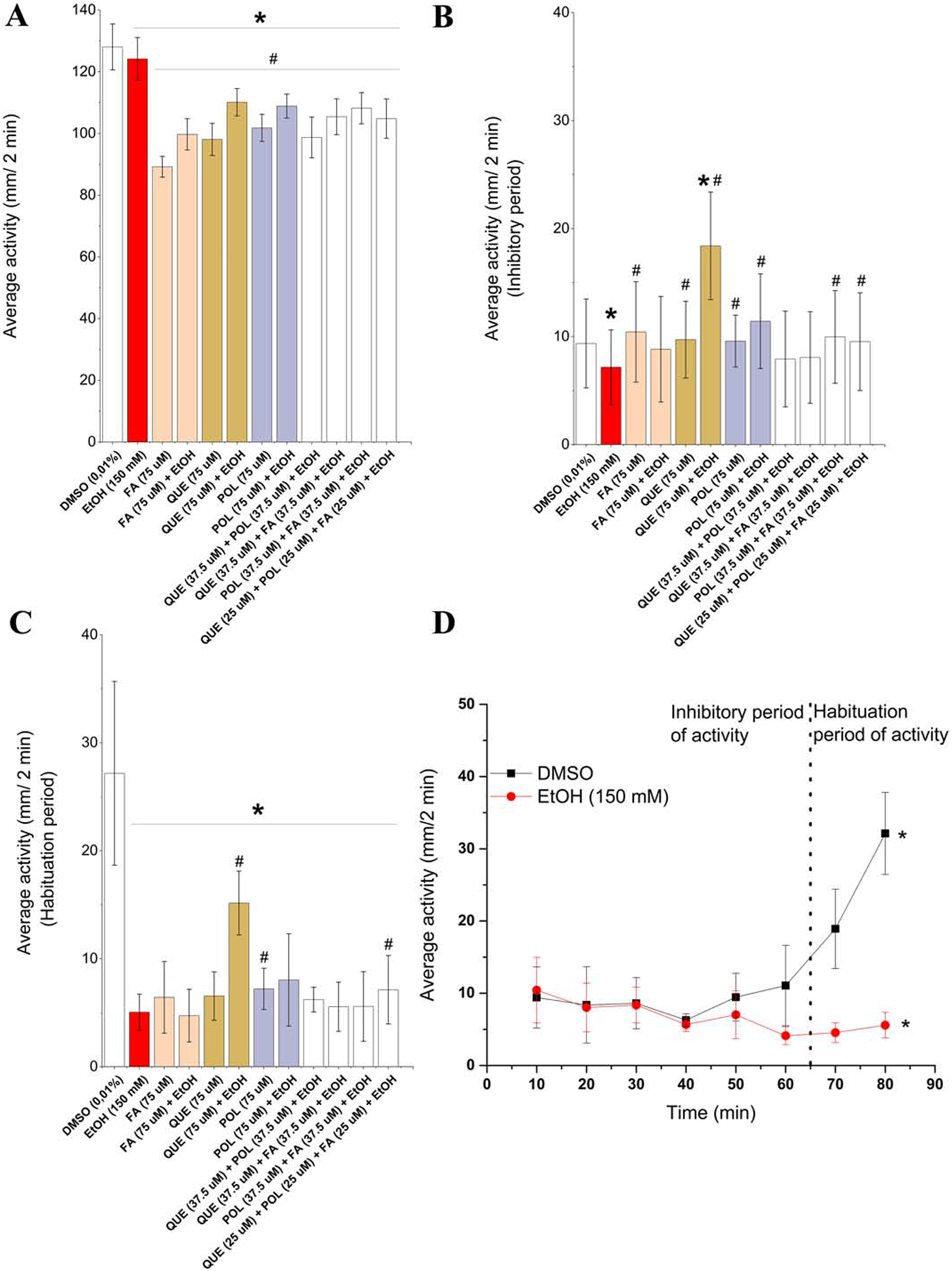
Behavioral plots (A – C) comparing experimental groups with DMSO (*) and 150 mM ethanol (#) by one-way ANOVA followed by Tukey’s test and considered affected when p < 0.05. Legend: DMSO – 0.01% (v/v) Dimethyl sulfoxide; EtOH – Ethanol; FA – Folic acid; QUE – Quercetin; POL - Polydatin. Plot with small activity (between 4 and 8 mm/s) (A). Tukey post hoc test against DMSO (EtOH p < 0.001, FA p < 0.001, FA + EtOH p < 0.001, QUE p < 0.001, QUE + EtOH p < 0.001, POL p < 0.001, POL + EtOH p < 0.001, QUE+POL + EtOH p < 0.001, QUE+FA + EtOH p < 0.001, POL+FA + EtOH p < 0.001, QUE POL + FA + EtOH p < 0.001) and Tukey post hoc test against EtOH (FA p < 0.001, FA + EtOH p < 0.001, QUE p < 0.001, QUE + EtOH p < 0.001, POL p < 0.001, POL + EtOH p < 0.001, QUE+POL + EtOH p < 0.001, QUE+FA + EtOH p < 0.001, POL+FA + EtOH p < 0.001, QUE+POL+FA + EtOH p < 0.001). Plot with high activity (> 8 mm/s) in the inhibitory period (B). Tukey post hoc test against DMSO (EtOH p = 0.04, FA p = 0.39, FA + EtOH p = 0.70, QUE p = 0.74, QUE + EtOH p < 0.001, POL p = 0.81, POL + EtOH p = 0.09, QUE+POL + EtOH p = 0.09, QUE+FA + EtOH p = 0.29, POL+FA + EtOH p = 0.61, QUE+POL+FA + EtOH p = 0.89) and Tukey post hoc test against EtOH (FA p = 0.004, FA + EtOH p = 0.17, QUE p = 0.008, QUE + EtOH p < 0.001, POL p = 0.004, POL + EtOH p < 0.001, QUE+POL + EtOH p = 0.74, QUE+FA + EtOH p = 0.40, POL+FA + EtOH p = 0.01, QUE+POL+FA + EtOH p = 0.03). Plot with high activity (> 8 mm/s) in the habituation period (C). Tukey post hoc test against DMSO (EtOH p < 0.001, FA p < 0.001, FA + EtOH p < 0.001, QUE p < 0.001, QUE + EtOH p = 0.003, POL p < 0.001, POL + EtOH p < 0.001, QUE+POL + EtOH p < 0.001, QUE+FA + EtOH p < 0.001, POL+FA + EtOH p < 0.001, QUE+POL+FA + EtOH p < 0.001) and Tukey post hoc test against EtOH (FA p = 0.29, FA + EtOH p = 0.46, QUE p = 0.13, QUE + EtOH p < 0.001, POL p = 0.02, POL + EtOH p = 0.07, QUE+POL + EtOH p = 0.10, QUE+FA + EtOH p = 0.61, POL+FA + EtOH p = 0.67, QUE+POL+FA + EtOH p < 0.001). Results of the light test (D) in high activity (> 8 mm/s) with data expressed as the average activity and results compared by two-way ANOVA followed by Tukey’s test considering as factors affected significantly (* = p < 0.05) time (F(7,72) = 3.83 p < 0.05) and experimental group (F(1,72) = 19.16 p < 0.05). Plot with high activity (> 8 mm/s) in the inhibitory period (C).
4. Discussion
According to WHO (WHO, 2018), at least half of the world’s adults have consumed alcohol in the year of 2018. This statement raises concerns about the abuse of alcohol, and research is needed to identify new therapeutic agents to protect against ethanol-induced defects. In our study, embryonic ethanol exposure affected zebrafish epiboly, embryonic development and behavior. Early development in mammalian systems occurs in utero, and it is difficult to evaluate the effects of chemical compounds using these models. However, the measurement of epiboly was useful for screening the activity of QUE, POL and FA and their mixtures in our zebrafish FASD model. QUE, FA and some binary mixtures effectively protected against ethanol-induced epiboly progression defects. The protective effects of retinoic acid on ethanol-induced epiboly defects were already known (Marrs et al., 2010), and here, we show QUE and FA also reduced these defects.
QUE and FA probably reduced defects induced by ethanol exposure through different pathways because their mixture did not reduce epiboly defects. Disruption of the folate pathway causes developmental defects in zebrafish (Lee et al., 2012), and ethanol exposure interferes with the folate absorption (Muralidharan et al., 2013). The exogenous FA administered in our study probably restored levels of FA that reduced the toxic effect of ethanol. On the other hand, QUE reduced liver injuries on the mice, a different model, by PI3K/Akt/nuclear factor-κB and STAT3 signaling pathways (Zhu et al., 2017). These properties of QUE may have reduced epiboly defects in our FASD model. Lai et al. (2018) studied the protective effect of POL against alcoholic liver injury using 6.25 – 25 μg/L POL in larvae with 96 hpf (we used 20 – 40 μg/L POL in embryos with 2 – 24 hpf). Possibly the chorion membrane interferes in the absorption of POL, or the protective activity was less as compared to QUE and FA in our study. Interestingly, when POL was in a mixture with QUE and FA, the results were different. The binary mixtures QUE+POL, POL+FA and the ternary mixture also protect against epiboly defects (Figure 1A). This protective effect on epiboly was seen with single QUE and FA treatment rather than a mixture of the 2 compounds (Figure 1B). However, our results suggest a synergistic effect of these chemical compounds against ethanol-induced defects.
In our previous work (Cadena et al., 2020), 100 mM ethanol exposure produced teratogenic effects at a level of 25% affected larvae. However, when we increased the ethanol concentration for 150 mM, we observed 75% of larvae with teratogenic effects. Chemical compounds and their mixtures used in our work reduced the percentage of affected animals (Figure 2A). However, compared to the epiboly results, the mixtures, specifically QUE+POL were effective in reducing ethanol-induced teratogenesis. QUE and resveratrol combinations were more effective than single compounds to reduce edema and leukocyte infiltration in a mouse model of a skin lesion with inhibition of edema and inflammatory cell activation (Caddeo et al., 2016). Despite the low effect of POL, a glucoside of resveratrol, to reduce epiboly defects and teratogenic effects, QUE+POL was effective in reducing ethanol-induced defects, showing a possible synergistic effect of these polyphenol mixtures (Figure 2B). As mentioned above, QUE and POL have anti-inflammatory and antioxidant properties (Huang et al., 2017; Lai et al., 2018; Lee et al., 2019; Ince, 2020), and several teratogenic effects (Figure 3) observed in our work as pericardial (PE), multiple (ME) and yolk sac (YSE) edemas may be related to inflammatory action of ethanol exposure (Zhu et al., 2017; Ince, 2020). Moreover, QUE can pass through the placenta and reach the fetal tissues by fetal circulation. QUE could also be available through breast milk (Ince, 2020). These effects can be important considerations in whether QUE is used in humans. Finally, FA was also effective in reducing the ethanol-induced defects at the same level as QUE and QUE+POL. FA protective effects against ethanol-induced defects were already known (Sarmah and Marrs, 2013; Muralidharan et al., 2015; Cadena et al., 2020). Despite the FA effects, mixtures with QUE and POL were not effective, indicating that FA may act through a different pathway, as mentioned above.
Larvae without detectable morphological defects were also affected by ethanol exposure as shown in behavioral analysis. Epiboly and teratogenic effects showed that ethanol exposure affected larvae developmentally. However, the behavioral analysis showed that larve with undetectable teratogenic effects were also affected. In addition, Cleal and Parker (2018) studied the effects of early ethanol exposure (2 – 9 dpf) on zebrafish and found behavioral defects on 3 month adult fish leading to concerns that fish with undetectable teratogenic effects may have potential to develop early or later behavioral defects. We observed a reduction in the small swimming activity in ethanol group compared to control (Figure 4A). Ethanol exposure was also studied in a binge drinking model using larvae at 6 dpf exposed to higher concentrations (4% ethanol) in light-dark test (MacPhail et al., 2009), producing motionless larvae. Comparing with our results, the different methodology of ethanol exposure produced different behavioral effects. However in both cases, a reduction in the swimming activity in the fish was observed. The embryonic ethanol exposure also affected the habituation period (Figure 4D), where the swimming activity during light periods gradually increased (MacPhail et al., 2009). The habituation period is caused by a repeated stimulus, leading to an attenuated response, in this case, inhibition of swimming activity followed by an increase of swimming activity (Basnet et al., 2019). Animals exposed to 150 mM ethanol were more inhibited (Figure 4B) and did not increase their swimming activity during the habituation period (Figure 4C). Prenatal alcohol exposure affects human behavior (WHO, 2018; Basnet et al., 2019) and zebrafish behavior. The zebrafish has brain organization and cellular morphology that is homologous with other vertebrates (Basnet et al., 2019), which suggests that our findings may be found in other animal models.
The experimental groups exposed to chemical compounds and their mixtures reduced the small swimming activity, as compared to DMSO and ethanol groups, revealing the complexity and sensitivity of behavioral data. In our previous work (Cadena et al., 2020), FA also reduced small and high activity in the excitatory light-dark locomotion test, as shown here in the light test. Also, QUE had the lowest toxicity when compared with gallic acid and curcumin in zebrafish embryos (Harishkumar et al., 2019), but these authors did not study the behavioral effects. QUE, POL and FA treatment without ethanol did not affect the epiboly or development of the larvae, but embryonic exposure to QUE, POL and FA affected the larval behavior. Additional experiments are needed to understand whether these behavioral effects of QUE, POL, and FA are related to toxicity or another effect on the behavior of zebrafish. Low concentrations of these chemical compounds do not protect against the epiboly defects (Figure 1A), and higher concentrations are difficult to solubilize in embryo medium or water, leading to the proposal of a new approach using pharmaceutical nanotechnology (Cadena et al., 2013; Caddeo et al., 2016). QUE, POL, FA and their mixtures partially protected against ethanol behavioral defects in our results, demonstrating that ethanol at high concentrations is very toxic and it is difficult to mitigate its effects.
5. Conclusion
In conclusion, this study presents new evidence that QUE, POL, FA, and their mixtures partially protect against epiboly, teratogenic, and behavioral defects induced by ethanol exposure. QUE, FA, and QUE+POL were most effective in reducing these defects. Further studies are needed to evaluate the protective effects of mixtures. Unfortunately, alcohol abuse and FASD remain too common, and at this moment, we do not have therapeutics or protective treatments for all ethanol-induced defects.
Research Highlights.
Folic acid and quercetin protected against ethanol-induced epiboly defects.
Quercetin, folic acid and quercetin+polydactin reduced ethanol-induced morphological defects.
Larvae exposed to ethanol were more strongly affected and did not show a normal habituation period in behavior assays.
Quercetin, polydactin, folic acid and their mixtures partially reduced developmental and behavioral defects induced by ethanol-exposure.
6. Acknowledgments
This work was supported by NIH/NIAAA 1 R21 AA026711 to James A. Marrs, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES (PRInt #88887.364263/2019-00) for the fellowship to Pabyton G. Cadena. The authors thank members of the Marrs laboratory and students of Laboratório de Ecofisiologia e Comportamento Animal - LECA (Brazil) for inspiration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been acscepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Basnet RM, Zizioli D, Taweedet S, Finazzi D, Memo M, 2019. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddeo C, Nacher A, Vassallo A, Armentano MF, Pons R, Fernàndez-Busquets X, Carbone C, Valenti D, Fadda AM, Manconi M, 2016. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. International Journal of Pharmaceutics 513, 153–163. [DOI] [PubMed] [Google Scholar]
- Cadena PG, Cadena MRS, Sarmah S, Marrs JA, 2020. Folic acid reduces the ethanol-induced morphological and behavioral defects in embryonic and larval zebrafish (Danio rerio) as a model for fetal alcohol spectrum disorder (FASD). Reproductive Toxicology 96, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena PG, Pereira MA, Cordeiro RB, Cavalcanti IM, Barros Neto B, Pimentel Mdo C, Lima Filho JL, Silva VL, Santos-Magalhaes NS, 2013. Nanoencapsulation of quercetin and resveratrol into elastic liposomes. Biochimica et Biophysica Acta (BBA) - Biomembranes 1828, 309–316. [DOI] [PubMed] [Google Scholar]
- Christou M, Kavaliauskis A, Ropstad E, Fraser TWK, 2020. DMSO effects larval zebrafish (Danio rerio) behavior, with additive and interaction effects when combined with positive controls. Science of the Total Environment 709. [DOI] [PubMed] [Google Scholar]
- Cleal M, Parker MO, 2018. Moderate developmental alcohol exposure reduces repetitive alternation in a zebrafish model of fetal alcohol spectrum disorders. Neurotoxicology and teratology 70, 1–9. [DOI] [PubMed] [Google Scholar]
- Collier AD, Min SS, Campbell SD, Roberts MY, Camidge K, Leibowitz SF, 2020. Maternal ethanol consumption before paternal fertilization: Stimulation of hypocretin neurogenesis and ethanol intake in zebrafish offspring. Progress in neuro-psychopharmacology & biological psychiatry 96, 109728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubińska-Magiera M, Daczewska M, Lewicka A, Migocka-Patrzałek M, Niedbalska-Tarnowska J, Jagla K, 2016. Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function. International journal of molecular sciences 17, 1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Buckley DM, Eberhart JK, 2018. Diving into the world of alcohol teratogenesis: a review of zebrafish models of fetal alcohol spectrum disorder. Biochemistry and cell biology = Biochimie et biologie cellulaire 96, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harishkumar R, Reddy LPK, Karadkar SH, Murad MA, Karthik SS, Manigandan S, Selvaraj CI, Christopher JG, 2019. Toxicity and Selective Biochemical Assessment of Quercetin, Gallic Acid, and Curcumin in Zebrafish. Biological & pharmaceutical bulletin 42, 1969–1976. [DOI] [PubMed] [Google Scholar]
- Huang QH, Xu LQ, Liu YH, Wu JZ, Wu X, Lai XP, Li YC, Su ZR, Chen JN, Xie YL, 2017. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF- B p65 Pathway. Evidence-based Complementary and Alternative Medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince E, 2020. The protective effect of quercetin in the alcohol-induced liver and lymphoid tissue injuries in newborns. Molecular biology reports 47, 451–459. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H, Zebrafish Neuroscience Research C, 2013. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10, 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF, 1995. Stages of embryonic development of the zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Lai Y, Zhou C, Huang P, Dong Z, Mo C, Xie L, Lin H, Zhou Z, Deng G, Liu Y, Chen Y, Huang S, Wu Z, Sun X, Gao L, Lv Z, 2018. Polydatin alleviated alcoholic liver injury in zebrafish larvae through ameliorating lipid metabolism and oxidative stress. Journal of Pharmacological Sciences 138, 46–53. [DOI] [PubMed] [Google Scholar]
- Lee MS, Bonner JR, Bernard DJ, Sanchez EL, Sause ET, Prentice RR, Burgess SM, Brody LC, 2012. Disruption of the folate pathway in zebrafish causes developmental defects. BMC developmental biology 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee J, Lee H, Sung J, 2019. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. Journal of food biochemistry 43, e13002. [DOI] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S, 2009. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 30, 52–58. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Clendenon SG, Ratcliffe DR, Fielding SM, Liu Q, Bosron WF, 2010. Zebrafish fetal alcohol syndrome model: effects of ethanol are rescued by retinoic acid supplement. Alcohol (Fayetteville, N.Y.) 44, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE, 2018. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. Jama 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, Marrs JA, 2015. Zebrafish retinal defects induced by ethanol exposure are rescued by retinoic acid and folic acid supplement. Alcohol (Fayetteville, N.Y.) 49, 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, Marrs JA, 2018. Retinal Wnt signaling defect in a zebrafish fetal alcohol spectrum disorder model. PLoS One 13, e0201659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, Zhou FC, Marrs JA, 2013. Fetal Alcohol Spectrum Disorder (FASD) Associated Neural Defects: Complex Mechanisms and Potential Therapeutic Targets. Brain sciences 3, 964–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2013. Education at a Glance 2013: OECD Indicators. OECD Publishing. [Google Scholar]
- Pace MC, Passavanti MB, Aurilio C, Sansone P, Aurilio R, De Maria S, Lama S, Federico A, Ravagnan G, Caraglia M, Stiuso P, 2015. Polydatin administration improves serum biochemical parameters and oxidative stress markers during chronic alcoholism: A pilot study. In Vivo 29, 405–408. [PubMed] [Google Scholar]
- Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, Baile CA, 2008. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. Journal of medicinal food 11, 773–783. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Probst C, Gmel G, Rehm J, 2017. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. The Lancet Global Health 5, e290–e299. [DOI] [PubMed] [Google Scholar]
- Sarmah S, Marrs JA, 2013. Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: prevention with folic acid. Developmental dynamics : an official publication of the American Association of Anatomists 242, 1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Marrs JA, 2017. Embryonic Ethanol Exposure Affects Early- and Late-Added Cardiac Precursors and Produces Long-Lasting Heart Chamber Defects in Zebrafish. Toxics 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Muralidharan P, Curtis CL, McClintick JN, Buente BB, Holdgrafer DJ, Ogbeifun O, Olorungbounmi OC, Patino L, Lucas R, Gilbert S, Groninger ES, Arciero J, Edenberg HJ, Marrs JA, 2013. Ethanol exposure disrupts extraembryonic microtubule cytoskeleton and embryonic blastomere cell adhesion, producing epiboly and gastrulation defects. Biology open 2, 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmah S, Muralidharan P, Marrs JA, 2016. Embryonic Ethanol Exposure Dysregulates BMP and Notch Signaling, Leading to Persistent Atrio-Ventricular Valve Defects in Zebrafish. PLoS One 11, e0161205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwend T, Loucks EJ, Snyder D, Ahlgren SC, 2011. Requirement of Npc1 and availability of cholesterol for early embryonic cell movements in zebrafish. J Lipid Res 52, 1328–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MCG, Silva JFD, Santos TP, Silva NPC, Santos ARD, Andrade ALC, Souza EHLS, Sales Cadena MR, Sa FB, Silva Junior VAD, Cadena PG, 2019. The complexation of steroid hormones into cyclodextrin alters the toxic effects on the biological parameters of zebrafish (Danio rerio). Chemosphere 214, 330–340. [DOI] [PubMed] [Google Scholar]
- Westerfield M, 2000. The zebrafish book: A guide for the laboratory use of zebrafish (Danio rerio), 4th ed. University of Oregon Press, Eugene, OR. [Google Scholar]
- WHO, 2018. Global status report on alcohol and health 2018. World Health Organization. World Health Organization. [Google Scholar]
- Zhang Y, Li S, Wang W, Xu C, Liang S, Liu M, Hao W, Zhang R, 2015. Beneficial effects of polydatin on learning and memory in rats with chronic ethanol exposure. International journal of clinical and experimental pathology 8, 11116–11123. [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Zhou X, Zhao J, 2017. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-kappaB and STAT3 signaling pathway. Experimental and therapeutic medicine 14, 6169–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]


