Abstract
Purpose:
New therapies have changed the outlook for patients with multiple myeloma, but novel agents are needed for patients who are refractory or relapsed on currently approved drug classes. Novel targets other than CD38 and BCMA are needed for new immunotherapy development, as resistance to daratumumab and emerging anti-BCMA approaches appears inevitable. One potential target of interest in myeloma is ICAM1. Naked anti-ICAM1 antibodies were active in preclinical models of myeloma and safe in patients, but showed limited clinical efficacy. Here, we sought to achieve improved targeting of multiple myeloma with an anti-ICAM1 antibody-drug conjugate.
Experimental Design:
Our anti-ICAM1 human monoclonal antibody was conjugated to an auristatin derivative, and tested against multiple myeloma cell lines in vitro, orthotopic xenografts in vivo and patient samples ex vivo. The expression of ICAM1 was also measured by quantitative flow cytometry in patients spanning from diagnosis to the daratumumab-refractory state.
Results:
The anti-ICAM1 antibody-drug conjugate displayed potent anti-myeloma cytotoxicity in vitro and in vivo. In addition, we have verified that ICAM1 is highly expressed on myeloma cells and shown that its expression is further accentuated by the presence of bone marrow microenvironmental factors. In primary samples, ICAM1 is differentially overexpressed on multiple myeloma cells compared to normal cells, including daratumumab refractory patients with decreased CD38. In addition, ICAM1-ADC showed selective cytotoxicity in multiple myeloma primary samples.
Conclusion:
We propose that anti-ICAM1 antibody-drug conjugate should be further studied for toxicity, and if safe, tested for clinical efficacy in patients with relapsed or refractory multiple myeloma.
Keywords: ICAM-1, multiple myeloma, antibody-drug conjugate, targeted cancer therapy, auristatin, immunotherapy
INTRODUCTION
Multiple myeloma is a neoplastic clonal expansion of antibody producing plasma cells. Within the last 20 years, IMiDs (immunomodulatory drugs) and proteasome inhibitors have proven to be efficacious in myeloma and have extended overall survival to a median of 10 years for patients that receive autologous stem cell transplant and lenalidomide maintenance (1). In addition, monoclonal antibodies, especially daratumumab, have greatly contributed to the ability to control relapsed disease (2). However, myeloma remains incurable for most patients and inevitably the disease becomes refractory to all available agents. When proteasome inhibitor, IMiD and daratumumab resistance develops, the average survival becomes 9 months and novel therapies are still desperately needed (3). Importantly, the CD38 target is rapidly downregulated from myeloma cell surface following daratumumab treatment (4), leading to resistance (5). In fact, daratumumab-refractory patients have become the major challenge for myeloma therapy development. Of particular promise in early phase clinical trials are next-generation immunotherapies targeting BCMA including chimeric antigen receptor T-cells, bispecific T-cell engaging antibodies and antibody-drug conjugates, but preliminary data suggest that patients eventually relapse with disease resistant to these as well (6–10).
The most direct way to bypass resistance is to develop therapies against other potential targets. In the case of antibody therapy, cell surface antigens beyond CD38 and BCMA are needed. Several antigens have been characterized as highly expressed on myeloma cells, one of which is ICAM1 (Intercellular Cell Adhesion Molecule 1, aka CD54) (11). ICAM1 expression is absent on most normal hematopoietic cells, except for progenitor cells, activated immune cells and mature plasma cells (12–14). In most hematologic malignancies, ICAM1 is expressed on a subset of cases, but it is present in the vast majority of multiple myeloma and certain types of non-Hodgkin lymphoma (11,14). In myeloma, the constitutive activation of nuclear factor κB (NF-κB) may be responsible for ICAM1 overexpression through binding sites in the ICAM1 promoter (15–17). ICAM1 itself may also play a role in the pathogenesis of multiple myeloma through regulation of contacts between myeloma cells and stromal cells (18,19). Cell adhesion has a protective effect for myeloma cells and may be a mechanism of drug resistance (20). Thus, targeting ICAM1 with an antibody-based therapy may block these protective cell-cell interactions in the bone marrow (BM) microenvironment.
Multiple reports have found that anti-ICAM1 antibodies are effective against myeloma in small animal models (21–23). The mechanism of anti-myeloma activity observed for these anti-ICAM1 antibodies is diverse, including the induction of macrophage-mediated cytotoxicity (23) and antibody-dependent cellular cytotoxicity (ADCC) (22). Fab (fragment antigen binding) antibody fragments also have anti-myeloma activity in mice, demonstrating that ADCC does not completely account for mechanism of ICAM1 antibody activity and a direct functional effect is also present (21). A human anti-ICAM1 naked antibody has recently been evaluated in phase I-II clinical trials. The agent was well tolerated but little activity was observed (best response reported was stable disease) in a small number of patients treated at target-saturating dose levels (24). In addition, a small trial in smoldering myeloma (n=4) did not demonstrate efficacy (25). Thus, to exploit ICAM1 on myeloma cells, it is imperative improve on the naked antibody approach, such as next-generation modalities like “armed” antibodies termed antibody-drug conjugates (ADCs), the focus of this report.
Finding a subgroup of myeloma patients most likely to benefit from ICAM-targeted therapies based on the ICAM1 surface expression levels is also an important objective. In patients with newly diagnosed myeloma, ICAM-1 mRNA expression correlated with higher International Staging System (ISS) scores (26). This suggests that ICAM1 is more highly expressed in patients with poor prognosis that more rapidly progress to the multi-drug resistant state. Furthermore, it has been found that ICAM1 expression increased after chemotherapy, and this directly correlated to the number of prior lines of therapy (27). Consistent with this, another group found melphalan resistance to be partially mediated by the interaction of ICAM1 on myeloma cells with its ligand leukocyte function-associated antigen-1 (CD11a/CD18) on activated macrophages (28). These reports imply that myeloma patients with advanced, drug resistant disease may be especially sensitive to anti-ICAM1 antibody-based therapies due to higher target expression levels. Importantly, these are the patients represent the current unmet medical need and new treatments are paramount to improving survival.
We developed a patient specimen-based phage library selection approach to identify human antibodies against novel surface antigens overexpressed by cancer cells (29). This approach was specifically designed to identify antibodies that are rapidly internalized by malignant cells, making them ideal for the ADC format. Through this, we have identified a panel of novel human antibodies and furthermore identified ICAM1 as one of the target antigens highly overexpressed in metastatic hormone-refractory prostate cancer (30). We hereby report that ICAM1 is also a promising target in multiple myeloma, particularly for patients with advanced disease. We conjugated our fully human anti-ICAM1 antibodies to monomethyl auristatin F (MMAF), which has been described to impart selective and potent cytotoxicity through targeted delivery and ultimately microtubular catastrophe (31,32). We studied the anti-myeloma activity of this anti-ICAM1 ADC (ICAM1-ADC) in vitro using cell proliferation assays, ex vivo with patient samples and in vivo using a xenograft model. The ICAM1-ADC improved upon the naked antibody in these preclinical experiments, including a striking, long lasting anti-tumor effect in vivo.
MATERIALS AND METHODS
Patient Samples
Bone marrow aspirate specimens were collected after informed consent through the hematologic malignancies tissue bank with approval of the Investigational Review Boards at University of California at San Francisco (UCSF) and the University of Colorado Anschutz Medical Campus (CU) after informed consent and in accordance with the Declaration of Helsinki. In compliance with HIPAA (Health Insurance Portability and Accountability Act), identifying patient information was replaced with sequential numbers. In the UCSF sample cohort, myeloma cells were separated with the EasySep CD138 Positive Selection Kit (Stem Cell Technologies). In the CU sample cohort, unselected mononuclear cell (MNC) samples were used. Samples were cultured in RPMI1640 media with 100 units/ml penicillin, 100 μg/ml streptomycin, 10% fetal bovine serum and 2 ng/ml IL-6.
Cell lines
The myeloma cell lines bearing the reporter gene (firefly luciferase) were obtained from Dr. Constantine Mitsiades of Dana-Farber Cancer Institute (33) and cultured as previously described (34). The non-reporter bearing lines RPMI8226, MM1.S, MM1.R, and HS27 cell lines were obtained from American Type Culture Collection (ATCC), and maintained in in the lab according to vendor’s instructions. Cells were used within approximately 4–8 passages and were not authenticated by short tandem repeat profiling. Cells were tested negative (last test was performed in August 2020) for Mycoplasma using PCR Mycoplasma detection kit (abm, Canada).
Flow Cytometry
Flow cytometry analysis for ICAM1 was performed using M10A12 (30) biotin labeled human IgG1 antibody followed by detection with Alexa-Fluor® 647-conjugated streptavidin. The non-binding YSC10 human IgG1 (34,35) was used as the isotype control. Antibodies used to identify myeloma cells included anti-CD38-FITC, (clone AT1, Stemcell Technologies), anti-CD19-BV786 (BD), anti-CD138-BV421 (BD) and anti-CD45-BV510 (BD). Samples previously treated with daratumumab were stained for CD38 expression with multi-epitope anti-CD38-FITC (ALPCO) to prevent antigen masking. Nonspecific Fc binding was blocked with Clear Back reagent (MBL). Flow cytometry was performed on an Accuri C6 with a 96-well auto sampler (BD Biosciences) for the UCSF samples, or a FACSCelesta with a 96-well auto sampler (BD Biosciences) for the CU samples.
Cell surface Antigen Density Determination
Quantitative flow cytometry was performed to determine cell surface antigen copy number as we described previously (34). M10A12 anti-ICAM1 and AT1 anti-CD38 antibodies were labeled with Alexa-Fluor® 647 (Life Technologies/Thermo Fisher Scientific) according to manufacturer’s recommendations. Median fluorescence intensity (MFI) conversion to molecules of equivalent soluble fluorochrome (MESF) was done by generating a standard curve with Quantum™ fluorescent beads (Bangs Labs) (34,36). The fluorophore-to-antibody ratio of the labeled antibodies was determined using Simply Cellular® anti-Human (for ICAM1) or anti-mouse (for CD38) IgG beads (Bangs Labs). Finally, conversion of MESF to cell surface antigen copy number was done by division of the fluorophore-to-antibody ratio (34).
Confocal Microscopy
Alexa-Fluor® 647-labeled anti-ICAM1 antibody M10A12 was incubated with myeloma cell lines at 37 °C for 18h, washed with PBS, fixed with 4% PFA, permeabilized with PBS with 0.1% Triton X-100 and 1% bovine serum albumin (BSA), and analyzed by confocal microscopy (Olympus FluoView). A nonbinding isotype control antibody was studied in parallel. For internalization by patient samples ex vivo, mononuclear cells were incubated with Alexa Fluor® 647-labeled anti-ICAM1 or nonbinding isotype control antibodies for 18 h, processed and analyzed as described above. Myeloma cells were identified by positive ICAM1 staining and by morphology. Subcellular localization to lysosomes was assessed by co-staining with LAMP1 antibody (Cell Signaling).
Antibody-Drug Conjugate Generation
MMAF was conjugated to the anti-ICAM1 IgG1 M10A12 via the mcvcpab linker as described (34,35). Briefly, ICAM1 IgG1 was reduced by tris(2-carboxyethyl)phosphine (TCEP) at 37 °C for 2 h, purified by Zeba spin column (Pierce/Fisher), buffer-exchanged into PBS with 5mM EDTA and incubated with linker-conjugated MMAF (34) at room temperature for 1 h. Conjugation products were purified by running twice though the spin column to remove free MMAF and analyzed by HPLC using HIC with Infinity 1220 LC System (Agilent). The drug to antibody ratio (DAR) was estimated from area integration using the OpenLab CDS software (Agilent) (34,35).
ADC Potency and Specificity in vitro
For initial ADC potency assessment, we used myeloma cell lines expressing the luc reporter, and cell viability was assessed by luciferase signal post treatment using the Synergy HT microplate reader following incubation at 37 °C for 72 h as described (34). To further evaluate potency and specificity, we used non-reporter bearing the myeloma cell line RPMI8226 and a panel of normal human cell lines. Normal human CD3+ T-cells (Astarte) were also tested as a control. Cells were plated into 96-well plates at 2,000 per well and incubated with varying concentrations of ICAM1 ADC at 37 °C for 96 h, washed and further incubated with Calcein AM (1 μM) (Invitrogen) at RT for 40 min. Plates were then read on a multi-detection microplate reader (Synergy HT, Biotek) at excitation wavelength of 485 nm and emission wavelength of 530 nm. Percent cell survival was normalized against mock-treated cells. EC50 was determined by curve fitting with Prism (GraphPad).
Anti-Myeloma Activity in Primary Samples Ex Vivo
To assess ICAM1-ADC effect on myeloma patient samples, unselected MNC samples were plated in 96 well plates and treated with ICAM1-ADC or nonbinding control ADC at 37 °C for 48 h, then washed and stained for flow cytometry as described (34,37). The number of CD138-positive, CD38-positive myeloma cells and CD138-negative, CD38-negative nonplasma cells (non-PCs) were gated and counted. Treatments were performed in triplicate and normalized to untreated controls.
Conditioned Media
Bone marrow stromal cell-conditioned media containing IL-6 was collected from HS5 cells cultured under serum free conditions for 48 h as described (35,38,39). MM1.S and MM1.R cell line cell surface antigen density of ICAM1 and CD38 were measured with or without the addition of HS5 conditioned media for 72 h.
Mouse Xenograft Model of Multiple Myeloma
Mouse studies were approved by the UCSF Animal Care and Use Committee (AN142193). For in vivo assessment of ICAM1-ADC, 5 × 105 myeloma RPIM8226-Luc cells expressing firefly luciferase were injected intravenously (i.v.) into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ-(NSG) mice (4–6 weeks of age, male and female, Jackson Laboratory) to create orthometastatic myeloma xenograft models (34). Bioluminescence imaging (BLI) was used to monitor graft status. Four mouse groups were treated with either ICAM1-ADC, nonbinding control ADC (MMAF-conjugated to a non-binding human IgG1), naked anti-ICAM1 antibody, or vehicle control (PBS). The doses and schedules are described in the text. Disease status was assessed by BLI and results analyzed by Living Image (PerkinElma). Following treatment, mice were continuously monitored until death or study cessation.
Statistics
All data were presented as mean and Standard Error of Measurement (SEM) unless noted. Significance was determined using GraphPad Prism. Two-tailed Student’s t-test was used when comparing 2 means. For survival (Kaplan-Meier) analysis, log-rank (Mantel-Cox) test was used (GraphPad Prism). When comparing more than 2 means, ANOVA was used with Tukey’s correction for multiple comparisons. Levels of significance are categorized as * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001. Mouse model sample sizes were determined by preliminary in vivo experience with ICAM1-ADC and other ADCs published in the literature, rather than power calculation (34,40), and statistically significant results were observed.
RESULTS
ICAM1 Expression in Multiple Myeloma Cell Lines
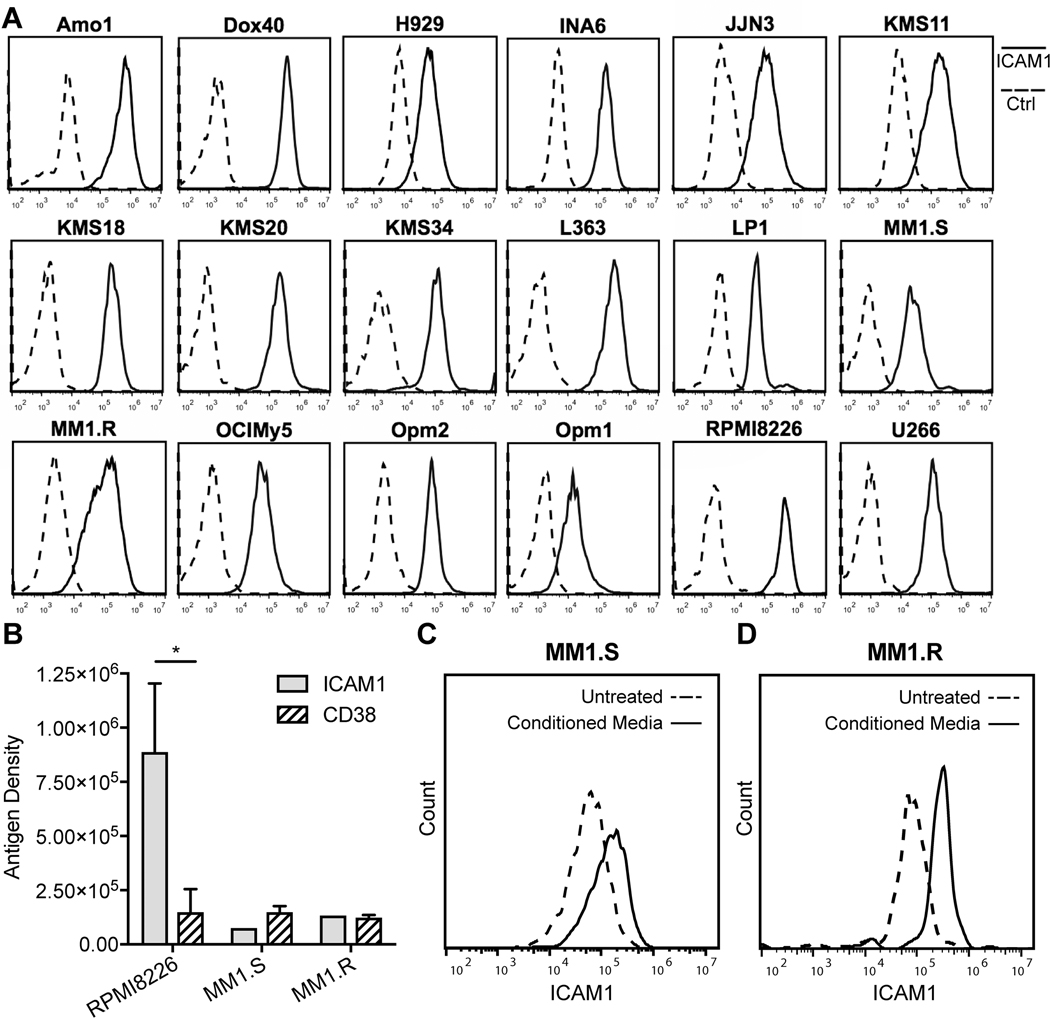
To characterize ICAM1 cell surface protein expression in multiple myeloma, we first examined a panel of commonly used myeloma cell lines. By flow cytometry using the anti-ICAM1 M10A12 human IgG1 that we discovered previously (30), all myeloma cell lines tested expressed ICAM1, when M10A12 binding was compared to a nonbinding isotype control (Fig 1A). Variability in the level of expression was present, with several lines exhibiting high levels of ICAM1 (e.g. RPMI8226), and others relatively low expression (e.g. OPM1) (Supplementary Fig S1). We next quantified the cell surface antigen density (surface antigen number per cell) on a subset of myeloma cell lines using quantitative flow cytometry (34,36). The antigen density on a high ICAM1 expressing cell line RPMI8226 was 887,835, whereas the antigen density on the medium ICAM1 expressing cell lines, MM1.S and MM1.R, was 75,341 and 133,607, respectively (Fig 1B). Interestingly, the level of ICAM1 expression on RPMI8226 was significantly higher than the clinically targetable CD38 antigen (p = 0.042). Thus, all myeloma cell lines tested in monoculture express ICAM1, with some exhibiting exceptionally high levels.
Figure 1. Assessing ICAM1 expression in myeloma cell lines by flow cytometry.
(A) Histograms showing the anti-ICAM1 IgG1 M10A12 binding (solid lines) on the cell surface of 18 myeloma cell lines, compared to a nonbinding IgG1 (Ctrl, dashed lines). (B) Cell surface antigen density of ICAM1 compared to CD38 for representative myeloma cell lines RPMI8226, MM1.S and MM1.R. Student t-test, unpaired, two tailed. *, P < 0.05. (C & D) ICAM1 cell surface expression is increased in myeloma cell lines MM1.S (C) and MM1.R (D) when incubated with HS5 conditioned media (CM) for 3 days compared to equivalent serum conditions without conditioned media (untreated).
We next examined the potential role of the BM microenvironmental factors on the level of ICAM1 expression. This was done by measuring the effect of HS5 BM stromal cell line conditioned media, containing IL-6 (38,39), on ICAM1 expression on myeloma cell lines MM1.R and MM1.S. For both lines, the addition of conditioned media further increased the level of ICAM1 expression by 2–4 fold (Fig 1C–D). Thus, although the HS5 cells are limited in their approximation of myeloma patient BM microenvironment, it does appear that ICAM1 expression is further upregulated in the presence of factors secreted from the BM microenvironment where myeloma cells typically reside.
ICAM1 Expression in Multiple Myeloma Patient Samples
Myeloma patients who have been previously treated with daratumumab downregulate CD38 expression (5), and therefore may benefit from therapies against alternative targets. Thus, we compared ICAM1 and CD38 on myeloma cells from patient BM aspirate samples spanning from diagnosis, to relapsed, to daratumumab-refractory clinical settings. Flow cytometric analysis of BM MNCs showed that ICAM1 was co-expressed with CD38 and another classic plasma cell/myeloma cell marker CD138. Representative examples of the expression of ICAM1 on MM cells compared to the background expression on the remaining normal non-plasma cells (non-PCs) are shown from a patient at diagnosis and another patient with relapsed, daratumumab naïve disease (Fig 2A–B). In samples from patients that were daratumumab-refractory, ICAM1 remained high and differentially identified myeloma cells from non-PCs, whereas CD38 was downregulated, and no longer clearly separated MM cells from non-PCs (Fig 2C). In a series of myeloma patient samples, we calculated the fold-overexpression of ICAM1 and CD38 on myeloma cells relative to non-PCs using the dividing the MFI of myeloma cells by the MFI of non-PCs. The mean ICAM1 fold overexpression was 27.2 for newly diagnosed patients, 102.6 for relapsed, daratumumab naïve patients, and 234.5 for daratumumab-relapsed/refractory patients (Fig 2D, Supplementary Table S1). By comparison, the mean CD38 fold overexpression was 64.8 in newly diagnosed patients, 50.7 in relapsed, daratumumab naïve patients, and only 6.2 in daratumumab-relapsed/refractory patients. As activated immune populations such as T-cells, express ICAM1 (14), we also compared MM cells to the normal BM T-cells in four patients, and found little to no overlap in the expression between these populations (Supplementary Fig S2). Overall, these data suggest ICAM1 is a particularly attractive target in patients with relapsed, daratumumab-refractory myeloma.
Figure 2. ICAM1 is overexpressed on cell surface of primary myeloma cells from patients with advanced disease.
(A-C) FACS analysis of patient samples separated by MM cells by CD138+ (events in red) and non-PCs by CD138- (events in blue) and co-labeled with CD38 and ICAM1 showing ICAM1 overexpression in a sample with relapsed (B) compared to a patient with newly diagnosed (A), and preserved high ICAM1 expression and downregulated CD38 in a patient previously treated with daratumumab (C). (D) In myeloma patients, the ratio of ICAM1 and CD38 MFI was comparably high in the newly diagnosed and daratumumab-naïve/relapsed settings, whereas only ICAM1 remained elevated to that degree in the daratumumab-refractory setting. (E) In a cohort of 10 myeloma patients, the mean ICAM1 and CD38 antigen densities were ~1×106/cell. Comparisons made by two-tailed student t-tests. * P < 0.05; ** P < 0.01. MM – multiple myeloma cells, non-PCs – nonplasma cells, Dara-RR – relapsed/refractory to daratumab.
Next, to measure the cell surface antigen density levels of ICAM1 on myeloma cells from patient samples, a second cohort of 10 patients was evaluated by quantitative flow cytometry (Table 1). The ICAM1 mean antigen density on primary myeloma cells was 1,037,571, whereas it was 561,974 for CD38. The ICAM1 levels were significantly higher on myeloma cells compared to non-PCs (p = 0.003). Notably, as for myeloma cell lines, certain patients’ myeloma cells exhibited extraordinarily high antigen densities > 1,000,000 (Table 1, 3/10 patients). Thus, the antigen densities of ICAM1 are high on MM cells and comparable to CD38, the gold-standard antibody therapy target for multiple myeloma.
Table 1.
ICAM1 Antigen Density on Multiple Myeloma Cells from Patient Samples.
| Pt# | Age/Sex | Setting | Prior Tx | MM Antigen Density | non-PC Antigen Density |
|---|---|---|---|---|---|
| UCSF132 | 56F | New Dx | 0 | 190,233 | 1,779 |
| UCSF135 | 65F | New Dx | 0 | 107,822 | 8,862 |
| UCSF134 | 57M | Relapse | 1 | 5,615,253 | 22,151 |
| UCSF102 | 57M | Relapse | 9 | 553,900 | 52,208 |
| UCSF131 | 58M | New Dx | 0 | 95,305 | 2,152 |
| UCSF133 | 59F | New Dx | 0 | 2,531,196 | 2,479 |
| UCSF126 | 70M | Relapse | 4 | 100,665 | 28,073 |
| UCSF006 | 36F | New Dx | 0 | 1,025,182 | 92,406 |
| UCSF079 | 69F | Relapse | 1 | 54,014 | 6,201 |
| UCSF145 | 61M | Relapse | 10 | 102,148 | 24,815 |
Dx – newly diagnosed, F – female, M – male, Prior Tx – prior lines of treatment, Pt – patient
ICAM1 Antibody Internalization
To evaluate the potential of ICAM1 as a target for ADC, we evaluated internalization of our ICAM1 antibody into myeloma cells. MM1.R and RPMI8226 cells were cultured in the presence of M10A12 IgG1, fixed, permeabilized and visualized by confocal microscopy. To evaluate for lysosomal trafficking, cells were co-stained with anti-LAMP1 antibody. ICAM1 antibody was observed to be internalized and present in lysosomes after 18 h incubation (Fig 3A, Supplemental Fig S3). We next studied ICAM1 antibody internalization ex vivo using patient samples. As shown in Fig 3B, myeloma patient cells incubated with anti-ICAM1 antibody for 18 h were also visualized to internalize and traffic the antibody to lysosomes. These data support the approach of myeloma targeting with ICAM1-ADC.
Figure 3. Confocal microscopic analysis of anti-ICAM1 antibody internalization by myeloma cell line and patient myeloma cells.
(A) MM1.R cells were incubated with Alexa Fluor-647 conjugated anti-ICAM1 antibody for 18 h, then fixed, permeabilized and costained for LAMP1 to show internalization and partial colocalization to late lysosomes. Scale bar: 30 μm. (B) Anti-ICAM1 antibody internalization after 18 h and colocalization with LAMP1 in myeloma cells from a patient BM aspirate. Scale bar: 30 μm.
ICAM1-ADC has Potent and Specific Anti-Myeloma Cytotoxicity
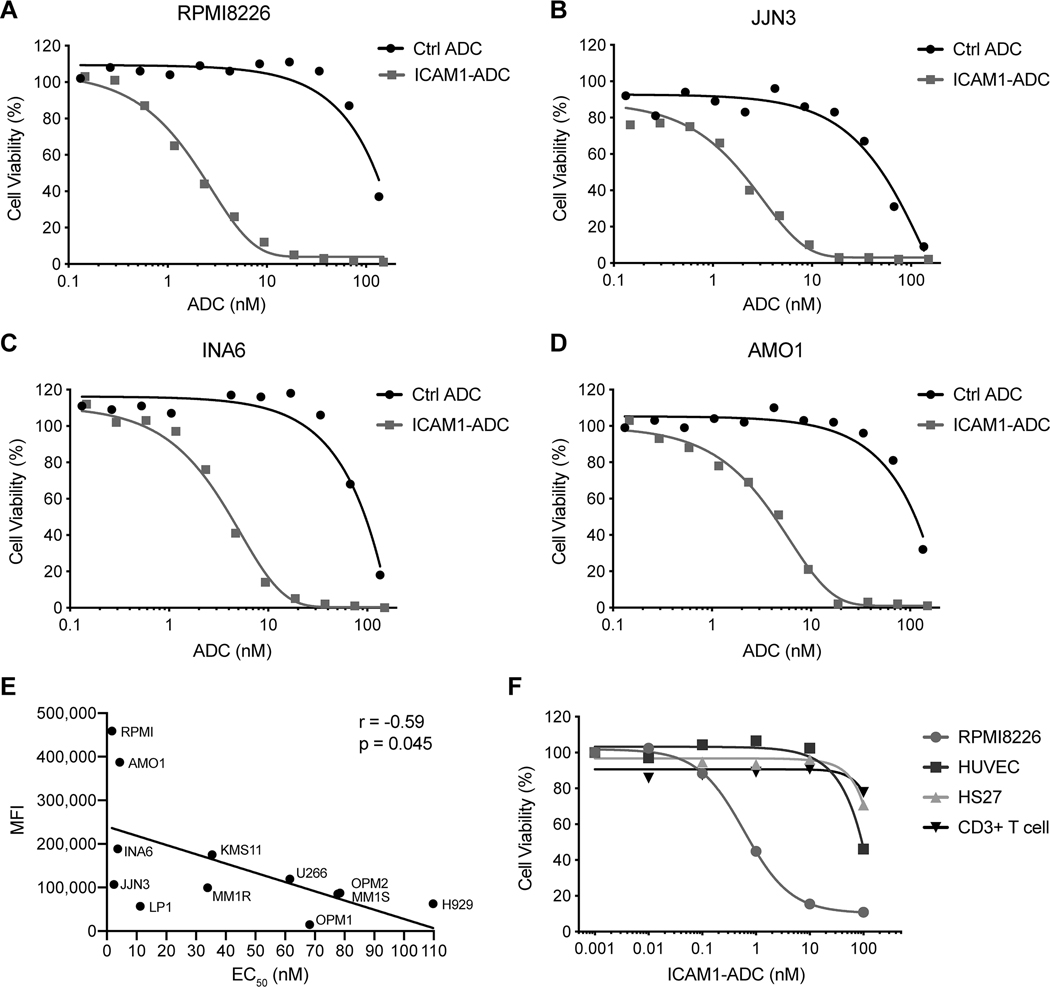
We constructed an anti-ICAM1 ADC using methods that we have employed for developing our other ADCs (34). The M10A12 IgG1 was conjugated to the microtubule polymerization inhibitor monomethyl auristatin F (MMAF) via the lysosomal protease cleavable valine-citrulline linker that was utilized in Brentuximab vedotin (41). The conjugate is henceforth referred to as ICAM1-ADC. High Performance Liquid Chromatography (HPLC) analysis with Hydrophobic Interaction Chromatography (HIC) of the ICAM1-ADC showed an average drug-to-antibody ratio of 3.9 (Supplemental Figure S4). A nonbinding human IgG1 was conjugated using the same method to create the isotype control ADC (34) to MMAF as a control. The ICAM1-ADC was first studied multiple myeloma cell lines by analyzing cytotoxicity after a 72 h incubation. RPMI8226, JJN3, INA6 and AMO1 were the most sensitive, with potent cytotoxicity observed of EC50 of 1.9 – 4.2 nM. By contrast, the control ADC showed minimal cytotoxicity (typically EC50 > 100 nM) (Fig 4A–D). MM cell lines expressing low levels of ICAM1 (H929, OPM1 and LP1) showed little or no therapeutic window compared to the control ADC (Supplementary Fig 5A–C). Overall, the EC50 after ICAM1-ADC treatment was lower in cell lines with a higher ICAM1 expression (r = −0.59, P = 0.045) (Figure 4E). In RPMI8226 cells, the unconjugated MMAF-Hydrochloride without antibody showed nearly identical cytotoxicity to the ICAM1-ADC, showing that potency is not sacrificed through ICAM1-mediated targeting (Supplementary Fig S5D). To further study specificity, we measured the ICAM1-ADC effect on a panel of non-tumorigenic cell lines following 96 h incubation. As shown in Fig 4E, while the ICAM1 ADC is potently cytotoxic against the MM line RPMI8226 (EC50 = 0.60 nM), it showed minimal cytotoxicity against the non-tumorigenic ICAM1-expressing HS27 and normal donor T-cells and low-negative ICAM1-expressing HUVEC cells (Fig 4F, Supplementary Fig S6).
Figure 4. Activity of ICAM1-ADC on cell lines in vitro.
(A-D) ICAM1-ADC selectively inhibits cell proliferation of myeloma cell lines RPMI8226, JJN3, INA6 and AMO1 in vitro. Percent cell viability after 72 h treatment shown. EC50 values were estimated by curve-fitting and are as follow: RPMI8226 1.9 nM, JJN3 2.2 nM, INA6 3.6 nM, and AMO1 4.2 nM. (E) The MFI of ICAM1 expression correlated inversely with cell line EC50 for sensitivity to ICAM1-ADC. (F) Tested against RPMI8226 for comparison after 96 h incubation (EC50 = 0.6 nM), ICAM1-ADC has relatively little effect on cells with low ICAM1 expression, including normal T cells, HUVEC and Hs27 cells. Ctrl ADC – nonbinding isotype control ADC.
Going beyond cell lines, we next evaluated the effect on ICAM1-ADC in primary samples from patients with multiple myeloma. ICAM1-ADC was incubated with unfractionated BM aspirate MNCs from myeloma patients ex vivo, selective decrease in myeloma cells, but not normal non-PCs was observed by flow cytometry (Fig 5A). When treated with graded concentrations, primary myeloma cells showed reduced viability in the presence of ICAM1-ADC starting at 0.1 nM, whereas no anti-myeloma effect was observed for control ADC up to 100 nM (Fig 5B). To investigate the ex vivo response frequency to ICAM1-ADC, we screened patients at the single concentration of 10 nM (Supplementary Table S2). From this, 3/5 patients displayed a significant decrease in viable myeloma cells, with no effect in non-PCs (Fig 5C). To investigate if ICAM1-ADC retained activity in daratumumab-refractory patients, we tested additional samples from this population and 2/4 showed significant decrease in the viable myeloma cell population in a manner that was partially accounted for by target antigen expression levels, and accentuated over naked ICAM1 antibody effect (Fig 5D, Supplementary Fig S7). Thus, ICAM1-ADC has broad anti-myeloma activity at low concentrations in primary samples from myeloma patients, including those that are daratumumab refractory, with relatively little effect on normal cells.
Figure 5. Activity of ICAM1-ADC on primary myeloma cells ex vivo.
(A) In a representative example shown, ICAM1-ADC selectively decreased the number of viable patient myeloma cells measured by flow cytometry after 48 h treatment, with minimal effect on the normal MNC population (non-PC, black bars). (B) When exposed to graded concentrations for 48 h, ICAM1-ADC but not the control ADC showed potent anti-myeloma activity even at the lowest concentration tested (0.1 nM). (C) In patients screened with 10 nM ICAM1-ADC treatment for 48 h, 3/5 showed a significant reduction of viable myeloma cells, with no observed toxicity in non-PC. (D) Additional samples from patients who were clinically daratumumab-refractory were tested with 10 nM ICAM1-ADC treatment for 48 h, with 2/4 showing a significant reduction of viable myeloma cells. Our anti-ICAM1 monoclonal antibody had a smaller effect. Comparisons in (D) and (D) were made by ANOVA with Tukey’s correction for multiple comparisons. * P < 0.05; ** P < 0.01, *** P < 0.001, **** P < 0.0001. Ctrl ADC – nonbinding isotype control ADC.
ICAM1-ADC Eliminates Multiple Myeloma Cell Line Xenografts In Vivo.
We next studied anti-myeloma activity of ICAM1-ADC in vivo using an orthometastatic myeloma mouse xenograft model. The study design is shown in Fig 6A. RPMI8226-Luc cells expressing the firefly luciferase were administered i.v. by tail vein injection. After a 10-day period of tumor cell engraftment, 5 mg/kg ICAM1-ADC was given twice a week for a total of 4 doses. For comparison, vehicle (PBS), naked ICAM1 antibody and nonbinding ADC were administered at the same dose and schedule. As shown in Fig 6B, disease activity steadily progressed in vehicle and control ADC groups as measured by BLI. Mice in the naked ICAM1 antibody treated group showed inhibition of myeloma progression (Fig 6B). Mice in the ICAM1-ADC treated group had complete elimination of detectable disease by day 14 that was maintained throughout the study period (Fig 6B). Kaplan-Meier analysis was performed and the result is shown in Fig 6C. All animals in the ICAM1-ADC treated group survived until sacrifice at day 200 (200 days post implant). In contrast, mice in the vehicle control group died by day 47 (median survival = 43 days), control ADC-treated group died by day 53 (median survival = 48 days), and naked anti-ICAM1 antibody improved survival, but all died by day 125 (median survival = 84 days) (Fig 6C). The hazard ratio was 22.87 for ICAM1-ADC vs. control ADC (p = 0.0019), and 21.79 for ICAM1-ADC vs ICAM1-mAb (p = 0.0018). Of note, we have previously found that the M10A12 antibody blocks ICAM1 interaction with the extracellular matrix and does not bind murine ICAM1 (30). Thus, the naked antibody may have anti-myeloma efficacy in NSG mice through disruption of myeloma cell interaction with the bone marrow microenvironment, but this experiment does not provide information about toxicity due to lack of binding to murine ICAM1.
Figure 6. In vivo ICAM1-ADC anti-myeloma activity in the RPMI8226-Luc line myeloma xenograft model.
(A) The firefly luciferase reporter bearing RPMI8226-Luc cells were i.v. injected and allowed to establish grafts for 10 days. Starting on the 11th day post implant (treatment day 1) a total of 4 injections were given twice a week of PBS, control nonbinding ADC (5 mg/kg), naked ICAM1-mAb (5 mg/kg) and ICAM1-ADC (5 mg/kg). (B) Disease status was monitored by BLI (top rows – dorsal views, bottom rows – ventral views). Tx – treatment, mAb – naked antibody. The study was done as part of a single experiment where we also studied CD46 ADC (34), and as such data for the control arms (PBS Vehicle and nonbinding ADC) from that study was re-used. (C) Kaplan-Meier survival curves of NSG mice carrying xenografts transplanted with RPMI8226-Luc and treated with ICAM1-ADC, ICAM1-mAb or controls. The study was terminated on the 200th day post tumor implant. These survival curves are significantly different (P < 0.0001, log rank test (Mantel-Cox)). The hazard ratios (HR (Mantel-Haenszel)) and p values (log rank test (Mantel-Cox)) for two group comparison are as follows: ICAM1-ADC vs. Nonbinding ADC, HR = 0.043, P = 0.0019; ICAM1-ADC vs. ICAM1-mAb, HR = 0.046, P = 0.0018; and naked ICAM1 mAb vs. Vehicle control, HR = 0.044, P = 0.0019. mAb – monoclonal antibody, Tx – treatment, Ctrl ADC – nonbinding isotype control ADC.
DISCUSSION
Here, we have shown that ICAM1 is overexpressed in a similar degree to CD38 in multiple myeloma cell lines and primary myeloma cells derived from patients at diagnosis and at advanced stages. In addition, ICAM1 may be a potential target in the daratumumab-refractory setting when resistant cells demonstrate CD38 expression. We developed a novel anti-ICAM1-ADC and showed that it has potent anti-myeloma activity in vitro, ex vivo and in vivo. In an orthometastatic myeloma xenograft model, ICAM1-ADC completely eliminated disease cells and resulted in 100% survival for the duration of the experiment (~200 days), a striking activity not seen for the anti-ICAM1 naked antibody studied in the same experiment.
Currently, several myeloma therapies are effective to control disease at diagnosis and for years thereafter. However, no treatment appears curative and virtually all patients’ disease eventually becomes resistant to the available agents. These patients with multi-drug resistant disease are in dire need of novel therapies. Immunotherapies with potential in advanced disease are thus particularly attractive. Although daratumumab has seen great success in the clinic, all patients eventually develop resistance (5). The anti-BCMA-based immunotherapies including a novel ADC, bispecific T-cell engaging antibodies and CAR-T cells have shown very promising results in clinical trials (8–10,42–44). The CAR-T approach is particularly effective with complete response observed for a high percentage of treated patients (7,45,46). Nonetheless, resistance to CAR-T has been observed for CD19 CAR-T following initial response, as tumor develops evasion mechanisms including down-regulation of the target antigen on the cell surface (47). It appears that BCMA CAR-T will also encounter the same issue, as patients are relapsing even after deep initial response (48). Generally speaking, a way to overcome resistance to current treatment is to explore additional tumor-associated cell surface targets that are functionally important to tumor growth and survival so that they cannot be easily down-regulated. Our study confirms that ICAM1 is highly expressed in multiple myeloma patients at diagnosis and advanced stages (11,14), therefore is an attractive target for new therapy development that has potential to overcome resistance to current therapies.
Previous studies have shown that ICAM1 is a potential target for multiple myeloma and there have been reports of preclinical anti-myeloma activity of naked anti-ICAM1 antibodies (21–23). A naked anti-ICAM1 human antibody (BI-505) has entered into multiple clinical trials (24,25). A phase I study in multiple myeloma patients showed that the agent is well tolerated at a dose (10 mg/kg) that saturates the ICAM1 binding site in humans, but has limited clinical activity with the best response being stable disease (24). A single-arm, open-label, phase 2 clinical trial in smoldering multiple myeloma found BI-505 to be safe, but showed no clinically relevant efficacy (25). Another phase 2 trial was conducted to explore the addition of BI-505 to high dose melphalan and autologous stem cell transplantation, but it has encountered safety issues and was terminated (www.clinicaltrials.gov Identifier: NCT02756728). Overall, this particular naked anti-ICAM1 antibody has not demonstrated efficacy in clinical trials. Nonetheless, ICAM1 remains an important target to consider for the development of next-generation immunotherapies in multiple myeloma due to its nearly ubiquitous high-level expression and demonstrated safety profile as a single agent in human trials. Our finding that high ICAM1 expression is seen in patients in the daratumumab-refractory setting supports the continued exploration of ICAM1 targeting for relapsed/refractory multiple myeloma.
Given the limited efficacy of naked anti-ICAM1 antibody as a single agent in clinical trials, therapeutic targeting of ICAM1 may require arming naked mAbs with more potent anti-tumor functions. In one example, an Fc modified ICAM1 antibody has shown enhanced ADCC and anti-tumor activity in preclinical studies (22). In another example, ICAM1 has been pursued as a target of viral-based immunotherapy in preclinical models of myeloma, by way of its function as a receptor for the oncolytic coxsackievirus A21 (49). An ICAM1-targeted CAR-T showed potent anti-tumor activity against thyroid cancer in preclinical studies (50). In this study, we created an ICAM1-ADC by conjugating MMAF to our novel ICAM1 antibody and evaluated its preclinical anti-tumor activity. We found that our anti-ICAM1 ADC showed significantly enhanced anti-myeloma activity compared to naked anti-ICAM1 antibody and completely eliminated myeloma cells in vivo in xenograft models.
Antibodies that block interaction of ICAM1 with its ligand may interfere with various immune functions that depend on that interaction (18). Potential on-target side effects could come from ICAM1 expression in activated vascular endothelium, type 1 alveolar epithelial cells, hematopoietic progenitors and activated immune cells including macrophages, T-cells and B-cells (12–14,51). Of note, the naked anti-ICAM1 antibody as a single agent was well tolerated in clinical trials (24,25). Recently, an anti-ICAM1 antibody-based chimeric antigen receptor T cells have been developed and evaluated for efficacy and toxicity in preclinical models of thyroid cancer (50,52). The agent was found to be efficacious against malignant cells without significant toxicity, and is being advanced into clinical trials (52). On the other hand, ADCs are an attractive treatment modality for multiple myeloma, as most disease is localized in BM and the large molecule drugs can readily diffuse through sinusoids with relatively limited access to other tissues (53,54). Ultimately, toxicities of the ICAM1-ADC will need to be evaluated in a nonhuman primate model study before advancing to clinical trials in multiple myeloma patients who have progressed beyond current therapies.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Multiple myeloma is incurable, and although treatment advances have prolonged life expectancy, patients inevitably develop resistance. Thus, development of new drugs and immunotherapies remains a critical need. Targeting novel antigens other than CD38 and BCMA will be important for patients that have already received and become resistant to daratumumab and BCMA targeted therapies. One such alternative target is ICAM1. A naked anti-ICAM1 antibody has been tested in several clinical trials, showing good safety profile but no efficacy as a single agent. Here, we provide the first evidence that an anti-ICAM1 antibody-drug conjugate may greatly improve upon the efficacy of naked antibodies. In primary myeloma samples from patients with relapsed disease, the expression of ICAM1 appears especially high. Thus, the anti-ICAM1 antibody-drug conjugate has potential to be a new type of therapeutic for multiple myeloma patients who are either refractory to or have progressed beyond current treatments.
ACKNOWLEDGEMENTS
We thank Drs. Scott Bidlingmaier, Nam-Kyung Lee and Beau Idler for help with experiments, Dr. Aaron C. Logan for help with tissue acquisition, Dr. Constantine Mitsiades for providing cell lines, and Dr. Marc A. Shuman for general advice. DWS was supported by a K08CA222704 from the National Cancer Institute and a Young Investigator award from the UCSF Stephen and Nancy Grand Multiple Myeloma Translational Initiative (MMTI), BTA a Grand Scholar award from the UCSF Stephen and Nancy Grand MMTI. The work was supported in part by grants from the National Cancer Institute (R01 CA118919, R01 CA171315, and R01 CA129491 to BL).
Financial Support: NCI R01 CA118919 (BL), NCI R01 CA171315 (BL), NCI R01 CA129491 (BL), NCI K08CA222704 (DWS), UCSF Stephen and Nancy Grand Multiple Myeloma Translational Initiative (MMTI) Young Investigator Award (DWS) and Grand Scholar Award (BTA)
Footnotes
BL is an inventor of a patent covering the anti-ICAM1 antibody. The other authors have declared that no conflict of interest exists.
REFERENCES
- 1.Holstein SA, Suman VJ, McCarthy PL. Update on the role of lenalidomide in patients with multiple myeloma. Ther Adv Hematol. 2018;9:175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sherbenou DW, Mark TM, Forsberg P. Monoclonal Antibodies in Multiple Myeloma: A New Wave of the Future. Clin Lymphoma Myeloma Leuk. 2017;17:545–54. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi UH, Cornell RF, Lakshman A, Gahvari ZJ, McGehee E, Jagosky MH, et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghose J, Viola D, Terrazas C, Caserta E, Troadec E, Khalife J, et al. Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology. 2018;7:e1486948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijhof IS, Casneuf T, Velzen J van, Kessel B van, Axel AE, Syed K, et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood. 2016;128:959–70. [DOI] [PubMed] [Google Scholar]
- 6.Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol. 2018;36:2267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Evaluation of AMG 420, an anti-BCMA bispecific T-cell engager (BiTE) immunotherapy, in R/R multiple myeloma (MM) patients: Updated results of a first-in-human (FIH) phase I dose escalation study. J Clin Oncol. 2019;37:8007–8007. [Google Scholar]
- 9.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa L. First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. ASH; 2019. [cited 2020 Jan 23]. Available from: https://ash.confex.com/ash/2019/webprogram/Paper122895.html [Google Scholar]
- 11.van Riet I, van Camp B. The Involvement of Adhesion Molecules in the Biology of Multiple Myeloma. Leuk Lymphoma. 1993;9:441–52. [DOI] [PubMed] [Google Scholar]
- 12.Boyd AW, Dunn SM, Fecondo JV, Culvenor JG, Duhrsen U, Burns GF, et al. Regulation of expression of a human intercellular adhesion molecule (ICAM-1) during lymphohematopoietic differentiation. Blood. 1989;73:1896–903. [PubMed] [Google Scholar]
- 13.Arkin S, Naprstek B, Guarini L, Ferrone S, Lipton JM. Expression of intercellular adhesion molecule-1 (CD54) on hematopoietic progenitors. Blood. 1991;77:948–53. [PubMed] [Google Scholar]
- 14.Maio M, Del Vecchio L. Expression and functional role of CD54/Intercellular Adhesion Molecule-1 (ICAM-1) on human blood cells. Leuk Lymphoma. 1992;8:23–33. [DOI] [PubMed] [Google Scholar]
- 15.Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM. Classical and/or alternative NF-κB pathway activation in multiple myeloma. Blood. 2010;115:3541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-κB as a Therapeutic Target in Multiple Myeloma. J Biol Chem. 2002;277:16639–47. [DOI] [PubMed] [Google Scholar]
- 17.Ni H, Ergin M, Huang Q, Qin J-Z, Amin HM, Martinez RL, et al. Analysis of expression of nuclear factor κB (NF-κB) in multiple myeloma: downregulation of NF-κB induces apoptosis. Br J Haematol. 2001;115:279–86. [DOI] [PubMed] [Google Scholar]
- 18.Boyd AW, Wawryk SO, Burns GF, Fecondo JV. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988;85:3095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–98. [DOI] [PubMed] [Google Scholar]
- 20.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell Adhesion Mediated Drug Resistance (CAM-DR): Role of Integrins and Resistance to Apoptosis in Human Myeloma Cell Lines. Blood. 1999;93:1658–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y-W, Richardson JA, Vitetta ES. Anti-CD54 (ICAM-1) Has Antitumor Activity in SCID Mice with Human Myeloma Cells. Cancer Res. 1995;55:610–6. [PubMed] [Google Scholar]
- 22.Klausz K, Cieker M, Kellner C, Oberg H-H, Kabelitz D, Valerius T, et al. A novel Fc-engineered human ICAM-1/CD54 antibody with potent anti-myeloma activity developed by cellular panning of phage display libraries. Oncotarget. 2017;8:77552–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veitonmäki N, Hansson M, Zhan F, Sundberg A, Löfstedt T, Ljungars A, et al. A human ICAM-1 antibody isolated by a function-first approach has potent macrophage-dependent antimyeloma activity in vivo. Cancer Cell. 2013;23:502–15. [DOI] [PubMed] [Google Scholar]
- 24.Hansson M, Gimsing P, Badros A, Niskanen TM, Nahi H, Offner F, et al. A Phase I Dose-Escalation Study of Antibody BI-505 in Relapsed/Refractory Multiple Myeloma. Clin Cancer Res. 2015;21:2730–6. [DOI] [PubMed] [Google Scholar]
- 25.Wichert S, Juliusson G, Johansson Å, Sonesson E, Teige I, Wickenberg AT, et al. A single-arm, open-label, phase 2 clinical trial evaluating disease response following treatment with BI-505, a human anti-intercellular adhesion molecule-1 monoclonal antibody, in patients with smoldering multiple myeloma. PloS One. 2017;12:e0171205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampaio MSS, Vettore AL, Yamamoto M, Chauffaille M de LLF, Zago MA, Colleoni GWB. Expression of eight genes of nuclear factor-kappa B pathway in multiple myeloma using bone marrow aspirates obtained at diagnosis. Histol Histopathol. 2009;24:991–7. [DOI] [PubMed] [Google Scholar]
- 27.Schmidmaier R, Mrsdorf K, Baumann P, Emmerich B, Meinhardt G. Evidence for cell adhesion mediated drug resistance of multiple myeloma cells in vivo. Int J Biol Markers. 2008;21:218–22. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia. 2013;27:702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan W, Sassoon A, An F, Simko JP, Liu B. Identification of Clinically Significant Tumor Antigens by Selecting Phage Antibody Library on Tumor Cells in Situ Using Laser Capture Microdissection. Mol Cell Proteomics. 2006;5:2364–73. [DOI] [PubMed] [Google Scholar]
- 30.Conrad F, Zhu X, Zhang X, Chalkley RJ, Burlingame AL, Marks JD, et al. Human antibodies targeting cell surface antigens overexpressed by the hormone refractory metastatic prostate cancer cells: ICAM-1 is a tumor antigen that mediates prostate cancer cell invasion. J Mol Med. 2009;87:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003;21:778–84. [DOI] [PubMed] [Google Scholar]
- 32.Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced Activity of Monomethylauristatin F through Monoclonal Antibody Delivery: Effects of Linker Technology on Efficacy and Toxicity. Bioconjug Chem. American Chemical Society; 2006;17:114–24. [DOI] [PubMed] [Google Scholar]
- 33.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherbenou DW, Aftab BT, Su Y, Behrens CR, Wiita A, Logan AC, et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J Clin Invest. 2016;126:4640–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Y, Liu Y, Behrens CR, Bidlingmaier S, Lee N-K, Aggarwal R, et al. Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight [Internet]. 2018. [cited 2020 Jan 22];3. Available from: https://insight.jci.org/articles/view/121497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kay S, Herishanu Y, Pick M, Rogowski O, Baron S, Naparstek E, et al. Quantitative flow cytometry of ZAP-70 levels in chronic lymphocytic leukemia using molecules of equivalent soluble fluorochrome. Cytometry B Clin Cytom. 2006;70:218–26. [DOI] [PubMed] [Google Scholar]
- 37.Walker ZJ, VanWyngarden MJ, Stevens BM, Abbott D, Hammes A, Langouët-Astrie C, et al. Measurement of ex vivo resistance to proteasome inhibitors, IMiDs, and daratumumab during multiple myeloma progression. Blood Adv. American Society of Hematology; 2020;4:1628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C, Soori M, Miles FL, Sikes RA, Carson DD, Chung LWK, et al. Paracrine factors produced by bone marrow stromal cells induce apoptosis and neuroendocrine differentiation in prostate cancer cells. The Prostate. 2011;71:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delk NA, Farach-Carson MC. Interleukin-6: a bone marrow stromal cell paracrine signal that induces neuroendocrine differentiation and modulates autophagy in bone metastatic PCa cells. Autophagy. 2012;8:650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tai Y-T, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123:3128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30:631–7. [DOI] [PubMed] [Google Scholar]
- 42.Cho S-F, Anderson KC, Tai Y-T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front Immunol. 2018;9:1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. Effective Targeting of Multiple B-Cell Maturation Antigen–Expressing Hematological Malignances by Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T Cells. Hum Gene Ther. 2018;29:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ormhøj M, Bedoya F, Frigault MJ, Maus MV. CARs in the Lead Against Multiple Myeloma. Curr Hematol Malig Rep. 2017;12:119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mailankody S, Htut M, Lee KP, Bensinger W, Devries T, Piasecki J, et al. JCARH125, Anti-BCMA CAR T-cell Therapy for Relapsed/Refractory Multiple Myeloma: Initial Proof of Concept Results from a Phase 1/2 Multicenter Study (EVOLVE). Blood. 2018;132:957–957. [Google Scholar]
- 46.Zhao W-H, Liu J, Wang B-Y, Chen Y-X, Cao X-M, Yang Y, et al. Updated Analysis of a Phase 1, Open-Label Study of LCAR-B38M, a Chimeric Antigen Receptor T Cell Therapy Directed Against B-Cell Maturation Antigen, in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2018;132:955–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruella M, Maus MV. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput Struct Biotechnol J. 2016;14:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Q, Zhao J, Song Y, Liu D. Recent updates on CAR T clinical trials for multiple myeloma. Mol Cancer. 2019;18:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Au GG, Lincz LF, Enno A, Shafren DR. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br J Haematol. 2007;137:133–41. [DOI] [PubMed] [Google Scholar]
- 50.Min IM, Shevlin E, Vedvyas Y, Zaman M, Wyrwas B, Scognamiglio T, et al. CAR T Therapy Targeting ICAM-1 Eliminates Advanced Human Thyroid Tumors. Clin Cancer Res. 2017;23:7569–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paine R, Christensen P, Toews GB, Simon RH. Regulation of alveolar epithelial cell ICAM-1 expression by cell shape and cell-cell interactions. Am J Physiol-Lung Cell Mol Physiol. American Physiological Society; 1994;266:L476–84. [DOI] [PubMed] [Google Scholar]
- 52.Vedvyas Y, McCloskey JE, Yang Y, Min IM, Fahey TJ, Zarnegar R, et al. Manufacturing and preclinical validation of CAR T cells targeting ICAM-1 for advanced thyroid cancer therapy. Sci Rep. 2019;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990;50:814s–9s. [PubMed] [Google Scholar]
- 54.Tabrizi M, Bornstein GG, Suria H. Biodistribution Mechanisms of Therapeutic Monoclonal Antibodies in Health and Disease. AAPS J. 2009;12:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.