Abstract
The postsynaptic density (PSD) plays an essential role in the organization of the synaptic signaling machinery. It contains a set of core scaffolding proteins that provide the backbone to PSD protein-protein interaction networks (PINs). These core scaffolding proteins can be seen as three principal layers classified by protein family, with DLG proteins being at the top, SHANKs along the bottom, and DLGAPs connecting the two layers. Early studies utilizing yeast two hybrid enabled the identification of direct protein-protein interactions (PPIs) within the multiple layers of scaffolding proteins. More recently, mass-spectrometry has allowed the characterization of whole interactomes within the PSD. This expansion of knowledge has further solidified the centrality of core scaffolding family members within synaptic PINs and provided context for their role in neuronal development and synaptic function. Here, we discuss the scaffolding machinery of the PSD, their essential functions in the organization of synaptic PINs, along with their relationship to neuronal processes found to be impaired in complex brain disorders.
Keywords: postsynaptic density, PSD; synaptic signaling; scaffolds; protein interaction networks; interactomes
1. Introduction
The postsynaptic density (PSD) is a protein-rich specialization located at the postsynaptic membrane of excitatory synapses. Originally identified as a morphologically distinct region through electron microscopy [1, 2], a number of studies utilizing mass spectrometry have went on to characterize the protein composition of the PSD, identifying more than 2000 proteins [3–7]. This was soon followed by more detailed analyses aimed at identifying protein-protein interactions (PPIs) between individual components of the PSD [8–13]. Although challenging, these efforts have identified several PSD PPIs involving specialized signaling machinery capable of assembling large macromolecular protein complexes. These complexes work in concert with one another to interpret biochemical and electrical information received by neurotransmitter receptors along the postsynaptic membrane and induce subsequent molecular changes within the receiving neuron.
A major structural component of PSD protein complexes are scaffolding proteins that are both abundant and contain multiple protein domains specialized in PPIs (Figure 1A). This multi-domain architecture provides a platform for simultaneous interactions with multiple binding partners, can facilitate multimerization, and is a major contributing factor to the formation of macromolecular signaling complexes [14]. PPIs involving scaffolding proteins enable the presentation of their binding partners at the correct place and time. The most abundant and most highly researched scaffolding protein families of the PSD can be categorized into three main layers: a top layer represented by the discs large (DLG1–5) family of proteins which interacts with glutamate and other neurotransmitter receptors at the PSD membrane; a bottom layer composed of the SH3 and multiple Ankyrin repeat domains (SHANK1–3) protein family, which connects the scaffolding machinery to the cytoskeleton; and a middle layer composed of the DLG-associated proteins (DLGAP1–4) which connects the top and bottom layers of synaptic scaffolds (Figure 1B) [11, 13, 15]. These layers provide the core backbone of PSD protein interaction networks (PINs) through their ability to bind receptors, enzymes of diverse function, cytoskeletal proteins, and other scaffolding protein families. Each layer contains multiple corresponding family members that are essential to the organization of the PSD and can be dynamically regulated throughout development and in response to synaptic stimuli [11, 13].
Figure 1 –
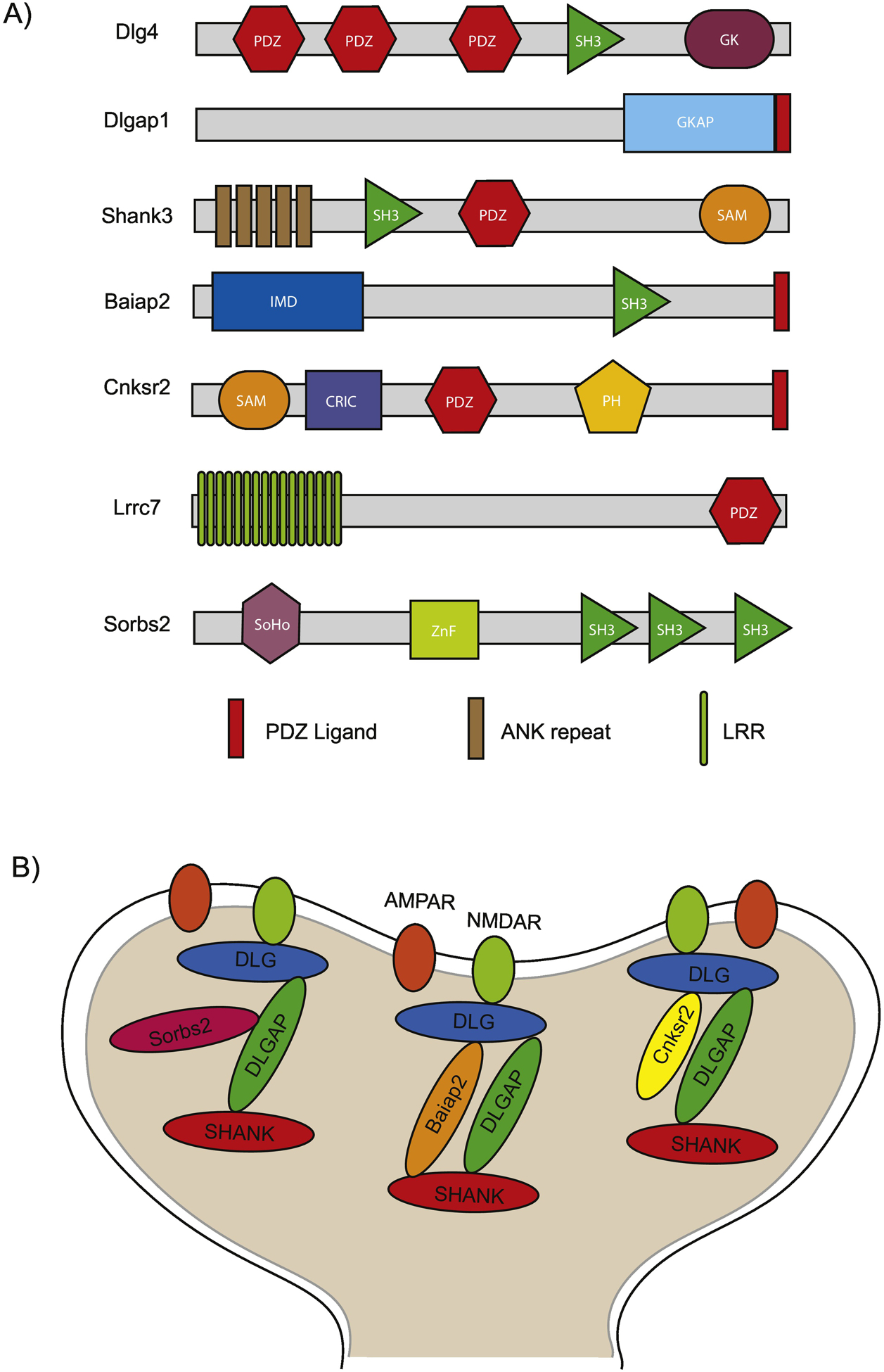
Protein domain architecture and organization of PSD scaffolding proteins. A) Figure shows protein domain composition of select PSD scaffolding proteins described in this review. Dlg4, Dlgap1, and Shank3 are representative family members of the top, middle, and bottom layers of scaffolding protein families in the PSD, respectively. B) Illustration shows the interactions between the scaffolding proteins of the PSD. DLGs, DLGAPs and SHANKs, form the three main layers while the additional scaffolds are concentrated within the middle layer.
Scaffolding proteins are the fundamental organizers of the PSD signaling machinery and PINs. Due to this, they regulate numerous cellular processes essential for synaptic function. Damaging mutations impacting the function or expression of scaffolding proteins can have detrimental effects to the flow of information within the PSD. Consequently, PSD scaffolding proteins have been associated with multiple complex brain disorders such as autism spectrum disorder (ASD), schizophrenia (SCZ), intellectual disability (ID), and developmental delay (DD) [16–24]. Because of their centrality to PSD PINs, it has been a common practice for human genetics studies to analyze associations between disease and components of the PSD or particular protein interaction datasets. This underscores the importance of characterizing PPIs involving scaffolding proteins to understand their roles in PSD signaling mechanisms. However, there are several important factors to consider when interpreting the function of PSD proteins through their respective PPIs and their role in complex brain disorders. Here, we will focus on the organization of the core signaling machinery of the PSD and not on the description of functions assigned to individual proteins. We will first describe essential concepts and technical problems when determining PPIs of the PSD scaffolds. This includes the critical importance of spatio-temporal context as PSD proteins may be differentially expressed throughout development and localized within different neuronal subcellular compartments. We will then briefly discuss and present examples of the main components of the PSD scaffolding machinery, focusing on their protein domain architecture, PPIs, and how this relates to their function and disease.
2. Determining PPIs Within the PSD
Protein complexes can be seen as functional units in which interacting proteins work together to carry out common cellular processes [25, 26]. Therefore, determining the composition of protein complexes at the PSD can provide insights into the multiple functions of a target protein. This is especially important for scaffolding proteins as they participate in the simultaneous regulation of multiple signaling pathways and cellular processes involved in neuronal development and synaptic transmission. However, PPIs are dynamic and biological variables such developmental time period, cellular location, or technical variables such as the experimental technique used for PPI characterization can all have a significant impact on the protein interactions identified.
A number of methods have been used for the identification of PPIs, each with their own set of advantages and disadvantages. The identification of direct, binary interactions between two proteins has been historically addressed through yeast two-hybrid systems [27]. Although they provide evidence of direct protein interactions, both the cellular environment and stoichiometric expression levels between the interacting proteins do not represent endogenous conditions. Thus, interactions identified through this method require further in-vivo validation. Apart from yeast two-hybrid, Immunoprecipitation (IP) of a protein of interest can be followed by immunoblotting (IP-IB) or mass-spectrometry (IP-MS) for either low- or high-throughput PPI identification, respectively. PPIs identified using IP-MS may include secondary and tertiary interactions in addition to direct interactions. These datasets are usually described as protein complexes, but they can be better described as a collection of direct and indirect protein interactions comprising protein interactomes.
Recent large-scale studies have characterized tens of thousands of PPIs through either yeast two-hybrid [28] or IP-MS following protein expression in cultured mammalian cell lines (e.g. HEK 293T or HCT116 cells) [29, 30]. While these studies have greatly expanded the knowledge of protein interactomes, the identification of PPIs involving synaptic proteins has proven to be more difficult. These studies are helpful in identifying interactions between two expressed components within the particular assay conditions as opposed to whether they are interactors within endogenous conditions. Therefore, they may contain large numbers of false positive and negative PPIs. Furthermore, comparisons of PPIs involving synaptic proteins determined under endogenous conditions versus PPIs for the same protein in systems such as HEK 293T cells have shown minimal PPI overlap between the two systems [8–11].
In addition to the experimental method used for PPI identification, characterization of PPIs within a distinct spatio-temporal context is essential. This is because proteins may have unique interacting partners dependent on both the developmental time period and the spatial location in which the protein resides. Unfortunately, variations of spatio-temporal context are still lacking for PSD PPIs as a large majority of studies focus solely on interactions within PSD fractions of adult rodent models. This has been the standard approach because the role of PSD proteins has been highly studied in the context of learning, memory and synaptic plasticity within mature synapses. However, the protein complex composition of the core-scaffolding proteins have been shown to change throughout neuronal development, with differential binding partners at embryonic stages compared to adulthood [11]. In addition, the spatial location of scaffolding proteins influences their function on many different levels, including anatomical brain region, cell type, subcellular location, and tissue expression. In line with this, the use of biochemical fractionation has been able to show differential PPIs for a number of proteins within the PSD and alternative neuronal locations such as the dendrite or soma [11, 12].
Several efforts have been made to characterize the protein interactions that provide the architectural framework for PSD function. Early studies using methods such as yeast two-hybrid assays were able to delineate individual PPIs involving the core PSD scaffolding proteins and were the first indication of these proteins forming “layers” within the PSD [14]. More recently, characterization of endogenous protein complexes via mass-spectrometry has both verified early yeast two-hybrid studies and greatly expanded the number of known intracellular binding partners of PSD scaffolding proteins by hundreds of interactors [8–13]. These large interactomes likely reflect the involvement of PSD scaffolding proteins in multiple protein complexes. At the same time, they provide context for the numerous cellular processes regulated by core PSD scaffolds and the phenotypes that arise in mutant mouse models.
3. Core Scaffolding Components of the PSD
3.1. The DLG family (Dlg4)
The DLG family forms the top layer of scaffolding proteins through their ability to stabilize glutamate receptors at the postsynaptic membrane and simultaneously bind several signaling molecules. DLGs belong to the MAGUK superfamily of proteins and have four family members commonly found at the PSD, including Dlg1 (SAP97), Dlg2 (PSD93/Chapsyn-110), Dlg3 (SAP102), and Dlg4 (PSD95/SAP90) [31]. A fifth family member, Dlg5, has also been observed to function in early neural development and synaptogenesis, but its function at the PSD has not been extensively studied [32, 33]. A conserved set of protein domains specialized in PPIs defines the protein domain architecture of the DLG family. These include three PDZ domains, an SH3 domain, and a GK domain towards the C-terminus (Figure 1A) [31, 34]. Through the use of yeast two-hybrid screening, initial PPI studies were able to show that each protein domain is involved in specific PPIs. For example, while the first two PDZ domains of Dlg4 have been shown to interact with C-terminal PDZ ligands of Shaker-type potassium channels and the NMDA receptor subunits, GluR2A and GluR2B [35–37], the third PDZ domain has been shown to specifically interact with neuroligins [38]. In contrast, Syngap1 and TARPs may interact with all three PDZ domains of Dlg4 [39–41]. The GK domain of the DLG family does not have the ability to bind or phosphorylate GMP [42] but instead acts as a protein interaction module with proteins such as Dlgap1 and Map1a [39, 43–45]. The SH3 domain has been shown to form both intra- and intermolecular interactions with the GK domain to regulate protein-protein interactions and MAGUK oligomerization [46, 47]. Furthermore, the SH3-GK intramolecular interaction has been proposed to undergo conformational changes upon ligand binding to the third PDZ domain which allows for alternate PPIs of the GK domain [48, 49].
Dlg4 is the most highly studied DLG family member and has been shown to have hundreds of binding partners through IP-MS studies utilizing either anti-Dlg4 antibodies [11, 13, 50] or endogenous Dlg4 TAP-TAG affinity purification [8]. TAP-TAG affinity purification is performed in two individual steps which allowed for the identification of proteins with less affinity in the initial purification step and those with increased affinity towards Dlg4 in the final step, termed the core Dlg4 complex [8]. Studies that have characterized Dlg4 interactors through IP-MS have identified a largely overlapping set of Dlg4 interacting proteins including NMDA and AMPA glutamate receptors, potassium channels, multiple DLG and DLGAP family members, cytoskeletal proteins, cell adhesion proteins, and signaling molecules. This illustrates the diverse set of cellular processes scaffolding proteins facilitate at the PSD including cytoskeletal dynamics and regulation of synaptic activity. As protein domains can also indicate protein function, protein domain profiling of Dlg4 complexes has shown an abundance of domains involved in PPIs, along with a specific enrichment in GKAP and PBPe domains (Figure 2) [11]. With GKAP and PBPe domains being specific to DLGAP family members and ionotropic glutamate receptors, respectively, this reflects the role of Dlg4 in facilitating the connection between glutamate receptors and the internal signaling machinery of the PSD.
Figure 2 –

Interactomes of the PSD Core scaffolding proteins and protein domain enrichment. (Left) Figure shows protein-protein interactions (PPIs) involving representative proteins of the three main layers of the postsynaptic density in adult mouse cortex. (Right) Protein domains found to be enriched in the interactomes of each corresponding core-scaffolding protein. All data is derived from [11].
In line with DLG proteins interacting with glutamate receptors, DLGs have been shown to be important for both AMPA receptor targeting to the synapse and NMDA receptor stabilization [34, 51, 52]. In addition, Dlg2 and Dlg4 have recently been shown to act cooperatively with NMDA receptors to form large supercomplexes involved in synaptic transmission. Interestingly, this large complex depends on the presence of Dlg2, Dlg4 and GluN2B as shown by its absence in mouse models lacking either Dlg2, Dlg4, or the C-terminal domain of Glun2B [10]. While Dlg2 and Dlg4 may act cooperatively in forming associated protein complexes, mutations in these proteins have been shown to have opposite effects on synaptic transmission. Here, Dlg4 knockout mice have been found to display increased LTP and impaired LTD while Dlg2 knockout mice exhibit impaired LTP and normal LTD [53]. Like Dlg4, mice lacking Dlg3 have been shown to have increased LTP, while conditional knockout of Dlg1 shows no effect on LTP [54, 55]. In addition to changes in electrophysiology, a lack of Dlg3 has been shown to increase the association of Dlg4 with NMDA receptor complexes indicating altered protein complex composition [54]. Through development, western blot analysis has shown Dlg3 to be more abundant than Dlg4 during early postnatal stages and then the opposite expression pattern during adulthood [56]. While mRNA expression profiling and mass spectrometry have been able to confirm their differential expression patterns throughout development, these studies have also shown both Dlg3 and Dlg4 to be similarly expressed and present in equal amounts in protein complexes during embryonic developmental stages [11, 57]. This, along with compensatory expression of alternate Dlg family members observed in Dlg4 mutant mice, illustrates the highly interconnected nature of the DLG family of proteins [54, 58, 59].
3.2. The DLGAP Family (Dlgap1)
The DLGAP family of proteins form the middle-layer of the PSD, connecting the DLG family of scaffolding proteins to the SHANK family. DLGAPs are the least studied family of PSD core scaffolds. Yeast two-hybrid studies originally identified DLGAPs as directly binding to the GK domain of the DLG family through multiple 14 amino acid repeats at the N-terminal region of DLGAP proteins [39, 43]. However, after these initial reports, the nature of DLG/DLGAP interactions have not been further investigated. DLGAPs were also characterized by their ability to bind to the PDZ domain of SHANKs through the PDZ ligand motif of Dlgap1 [60], effectively connecting the top and bottom layers of the PSD. DLGAPs have been shown to interact directly with the motor protein, dynein light chain (DLC), in association with Dlg4 and Myosin-V. This has been proposed to serve as a complex to regulate transport of DLGAP and DLG proteins [61]. Compared to the DLG family, DLGAPs are not rich in conserved protein domains. Instead, they contain a single conserved sequence of 160 amino acids in the C-terminal region termed the GKAP homology domain of unknown function 1 [62] (GH1 or GKAP domain) along with protein interaction motifs responsible for binding DLGs and SHANKs (Figure 1A).
Among the DLGAP family, Dlgap1 is the only member whose endogenous protein interactions have been identified through IP-MS [11, 13]. Proteins involved in Dlgap1-associated complexes are largely overlapping with those of Dlg4 including glutamate receptors, calcium channels, and signaling molecules. However, they do have distinct differences indicating the clustering of specific proteins via DLGAPs (Figure 2). Profiling of protein domain architecture within Dlgap1 complexes shows an enrichment of proteins containing domains involved in PPIs such as PDZ and SH3 domains (Figure 2) [11]. This highlights the centrality of Dlgap1 to PSD PINs as it interacts with scaffolding and adaptor proteins in both the upper and lower layers of the PSD. In line with yeast two-hybrid studies delineating the role of DLGAPs in connecting the two other major scaffolding layers of the PSD, functional studies have shown a disconnect between these layers in Dlgap1 knockout mice [15]. Here, mass spectrometry of immunoisolated DLG family members using a pan-MAGUK antibody was able to show decreased association with SHANK family members. Furthermore, immunoprecipitation of Shank3 followed by immunoblotting against Dlg4 was able to show a 60% decrease in association. The remaining fraction of Shank3 associated with Dlg4 is presumed to be connected through the other DLGAP family members [15]. This highlights the essential role of DLGAPs in maintaining the structure of the PSD.
The DLGAP family members have been shown to have both overlapping and distinct spatio-temporal expression throughout development. For example, while there are minor differences between the expression of the family members in the hippocampus and cortex, Dlgap3 has increased expression in the striatum relative to the other family members [63, 64]. With respect to development, Dlgap2 has the lowest expression in early post-natal development followed by relatively similar expression levels between the family members into adulthood [65]. In addition, different laboratories have reported a variety of phenotypes in individual mouse models lacking Dlgap 1–4. These phenotypes include behavioral abnormalities, deficits in synaptic transmission, and dysregulation of synaptic proteins [15, 64, 66, 67]. For example, a lack of Dlgap2 has been reported to decrease overall PSD thickness and length along with a decrease in the quantity of GluR1 and Camk2a PSD proteins. In contrast, a lack of Dlgap4 resulted in an overall increase in PSD size and no observed effects in the total protein levels of GluR1 and Camk2a [66, 67]. Although only investigated in the Dlgap4 mutant mouse model, elimination of Dlgap4 did not result in compensatory changes in the protein levels of other DLGAP family members [66]. The observed phenotypes reinforce the relevance of the DLGAP family of scaffolding proteins on the PSD structure and composition.
3.3. The SHANK Family (Shank3)
The SHANK family of proteins (Shank1–3) form the bottom layer of the PSD core scaffolding machinery through the direct interaction with the DLGAP family [60]. Like the DLG family, SHANKs contain a variety of protein domains and motifs specialized in PPIs. From the N-to C-terminal, these include multiple ankyrin repeat domains, an SH3 domain, A PDZ domain, a proline rich region, and a SAM domain (Figure 1A). Each member of the SHANK family has isoforms that lack particular domains and therefore influence their function. For example, the Shank3b isoform lacks the SAM domain and proline rich region which results in differential subcellular localization [68], whereas particular isoforms of Shank1 and Shank2 lack the N-terminal ankyrin repeats and SH3 domain [69, 70]. Profiling SHANK PPIs via yeast two-hybrid has been able to highlight their role in regulating cytoskeletal dynamics and glutamate receptor activity. Here, the ankyrin repeats were shown to directly interact with Sharpin and Sptan1 [71, 72] while the proline rich region has been shown to interact with the Homer family of adaptor proteins and cortactin [60, 73]. Sptan1 and Cortactin are both regulators of the actin cytoskeleton, while Homer proteins couple SHANKs to metabotropic glutamate receptors (mGluRs) [60, 72, 74]. The association of SHANKs to mGluRs, combined with their coupling to NMDA receptors through the DLGAP and DLG families, enable crosstalk between different classes of glutamate receptors [73], although it is not known if these protein interactions are part of one or multiple protein complexes. In addition, the SAM domain of SHANKs has been shown to facilitate SHANK multimerization [60] and regulate the localization of Shank2 and Shank3 [75]. These processes have been found to be regulated by Zn2+ binding to Shank2 and Shank3, while Shank1 does not appear to bind Zn2+ [76, 77]. In line with this, the interactome of Shank3 has been shown to be modulated by Zn2+ concentration [78].
The three SHANK family members have both overlapping and unique patterns of expression dependent on spatial location and developmental time period. It has been described that Shank1 and Shank2 have increased expression during early postnatal development [79]. However, all three family members have also been identified in embryonic day 14 mouse cortex through mass spectrometry. While Shank1 and Shank2 interactomes have not been characterized at this developmental stage, it has been shown that Shank3 associates to a variety of cytoskeletal proteins, synaptic adaptors, and scaffolds such as Homer 1–3, Baiap2 and Lrrc7 in embryonic day 14 mouse cortex [11]. In common with interactions found at the adult PSD, the DLGAP family members have been reported to associate with DLGs at early embryonic developmental stages. In contrast, the interaction between SHANK and DLGAP family members has not been observed during this same time period, illustrating how scaffolding proteins can have differential binding partners dependent on specific developmental stages and how the layers of PPIs within the PSD build on one another throughout time [11]. With respect to regional expression, SHANK proteins do not appear to have major differential expression between the family members in the cortex and hippocampus. However, similar to Dlgap3, Shank3 has increased expression in the striatum relative to Shank1 and Shank2 [64, 79, 80].
All three SHANK family members have been implicating in contributing to complex brain disorders along with regulating processes such as spine morphology and synaptic transmission [16, 81, 82]. Several mouse models have been generated that knockout particular isoforms or the proteins completely. As with the DLG family of proteins, deletion of SHANKs can result in compensatory mechanisms. For example, Shank3 protein expression is increased in Shank2 knockout mice, and in mice lacking Shank3, there is a significant upregulation of Shank2 and trend of increased Shank1 protein [83, 84]. However, as with other components of the PSD, how this compensatory differential protein expression of PSD scaffolds might affect the stoichiometry of postsynaptic protein complexes is unknown. Changes in total protein levels of NMDA receptors (Shank1, 2, and 3), AMPA receptors (Shank2 and 3), DLGAPs (Shank1 and 3), and Homer proteins (Shank1 and 3) are common within the several different SHANK mouse models, showing alterations in the protein binding partners of the SHANK family [82, 85].
Shank3 is the most widely studied member of the SHANK family due to its strong association to complex brain disorders and Phelan-McDermid syndrome. Multiple studies have identified endogenous Shank3 interactomes [11, 13, 78, 86, 87]. These studies have confirmed the presence of multiple members of the DLGAP and HOMER families associated with SHANKs at the PSD. Furthermore, the protein domain architecture shows an increased presence of proteins with GKAP, PDZ, PH, and S_TKc domains at the PSD (Figure 2). While GKAP domains are indicative of multiple DLGAP family members, the enrichment in the S_TKc domain shows that Shank3 is associated with an abundance of kinases likely involved in the propagation of cellular information relayed from the upper layers of the postsynaptic signaling machinery.
Haploinsufficiency of SHANK3 has been shown to be causative in Phelan-McDermid syndrome, which is characterized by global developmental delay, autistic behavior, hypotonia, and either absent or delayed speech [88–90]. In addition, mutations in SHANK3 have been shown to be associated to ASD, ID, and SCZ [16, 91–94]. Numerous Shank3 mouse models have been generated to examine the effects of disruptions within specific isoforms, loss of the whole protein, and specific mutations found in patients affected by complex developmental brain disorders [82]. The comparison of mutant mouse models containing a SCZ-associated mutation and an ASD-associated mutation has been able to show how different mutations can result in different effects on the translated protein product of Shank3 and phenotypic characteristics related to complex brain diseases. It has been reported that the mouse model containing the ASD-associated mutation resulted in a near complete loss of Shank3 accompanied by defects in synaptic transmission within the striatum and impairments in social behavior during early developmental stages. In contrast, the SCZ-associated mutation resulted in expression of a truncated Shank3 protein, defects in synaptic transmission within the prefrontal cortex, and social dominance behavior during adulthood [84]. Thus, Shank3 mutants exhibited numerous phenotypes related to those observed in patients with SHANK3 mutations and different complex brain disorders. Recently, CRISPR/Cas9 genome engineering has been used to generate cynomolgus macaques with indels that disrupt expression of SHANK3 [95]. This allowed the study of SHANK3-related phenotypes in brain structure, cognitive function, and social behavior in a model organism closer to humans. As seen in Shank3 mutant mice, impaired social interactions and other behavioral abnormalities were also observed. In addition, functional magnetic resonance imaging was able to show large scale changes in brain connectivity with global hypoconnectivity and local hyperconnectivity in areas such as the somatosensory cortex [95]. This represents the first successful study of exploring the functional consequences of mutations in a core scaffolding protein of the PSD in a cohort of non-human primates.
4. Expanded Families of Scaffolding Proteins
4.1. Baiap2 (IRSp53)
Baiap2 (also known as IRSp53) is an abundant PSD scaffolding protein implicated in regulating actin dynamics through its interactions with F-actin bundles and small GTPases [96]. From the N- to the C-terminus, all isoforms of Baiap2 contain an IMD (IRSp53 and MIM homology) domain, a CRIB-PR (Cdc42/Rac interactive binding-proline rich) region, and a SH3 domain (Figure 1A). At the C-terminus, alternate isoforms of Baiap2 may either contain a WH2 (WASP homology 2) domain or a PDZ ligand [96]. Similar to the DLGAP family of proteins, Baiap2 interacts with both DLGs and SHANKs, albeit through different domains. Here, Baiap2 has been shown to directly interact with the first two PDZ domains of the DLG family through the its C-terminal PDZ ligand and interact with a proline-rich region in SHANKs through its N-terminal SH3 domain [97–99]. Akin to the intramolecular interaction of the GK and SH3 domains of the DLG family, the CRIB-PR region has been shown to interact with the SH3 domain of Baiap2, effectively reducing its capacity to interact with other proteins. Binding of the small GTPase Cdc42 has been shown to reverse this inhibition by inducing a conformation change [100]. Baiap2 has been shown to interact with a host of cytoskeletal proteins through its SH3 domain in order to regulate actin dynamics including dynamin and Wasf2, a member of the WAVE complex [101, 102]. In addition, the IMD domain binds F-actin to regulate cytoskeleton dynamics and is involved in Baiap2 dimerization [103, 104]. Although Baiap2 has not been directly subjected to high-throughput protein interaction identification at the PSD, it has been identified in Dlg4, Dlgap1, and Shank3 interactomes at different developmental stages within mouse cortex [11]. In addition, co-fractionation of protein complexes in whole brain has identified Baiap2 in complex with Cdc42 [105]. These studies confirm previously identified interactions and provide further support for the central role of Baiap2 as a PSD scaffold.
Baiap2−/− mice display a variety of abnormalities consistent with the protein-protein interactions of Baiap2. Apical Baiap2−/− pyramidal neurons in the prelimbic area of the medial prefrontal cortex have reduced complexity and decreased number of dendritic spines [106]. However, hippocampal neurons in Baiap2−/− mice showed no morphological abnormalities, illustrating a potential region-specific role of Baiap2 [107, 108]. In addition, Baiap2−/− mice have enhanced LTP and decreased NMDA-dependent LTD. The decrease in LTD was shown to be due to a stabilization of F-actin and a subsequent decrease in NMDA receptor trafficking due to a lack of Baiap2 [106, 107]. Abnormalities in LTP and LTD, coupled with behavioral phenotypes illustrating deficits in learning and memory, shows the importance of Baiap2 in organizing PSD PINs.
4.2. Cnksr2 (MAGUIN)
Cnksr2 (also known as MAGUIN) is a multi-domain scaffolding protein located at the PSD directly implicated in psychiatric disorders. From the N- to C- terminus, Cnksr2 contains a SAM domain, a conserved region in CNK (CRIC) domain, a PDZ domain, a PH domain surrounded by poly-proline regions on either side, and a PDZ ligand at the N-terminus (Figure 1A). Through the PDZ ligand, Cnksr2 has been shown to directly interact with the DLG family along with other scaffolding molecules including Magi2 (S-SCAM) and Lrrc7 (Densin-180) [109, 110]. The PH domain has been attributed to synaptic targeting of Cnksr2 [111]. Unlike the SHANKs, the SAM domain has been proposed to not contribute to multimerization of Cnksr2, with a leucine rich region at the C-terminus instead being responsible for this [109]. Through immunoprecipitation, Cnksr2 has been shown to interact with Raf1 implicating it in Ras/MAPK signaling [112] and multiple proteins regulating the Rac/Cdc42 GTP signaling pathway, including Arhgap39 [11, 113]. The interaction between Arhgap39 and Cnksr2 has been shown to mediate spine maturation. Overexpression of Cnksr2 lacking a binding site for Arhgap39 failed to rescue an increased protrusion number but decreased mature synapse number observed in hippocampal neurons post knockdown of Cnksr2. In contrast, overexpression of wildtype Cnksr2 was able to rescue this phenotype, illustrating the importance of Cnksr2 in targeting Arhgap39 activity to the PSD [113]. High throughput characterization of endogenous Cnksr2 PPIs has been carried out in two separate studies [11, 113]. These studies were able to confirm scaffolding interactions identified through yeast two-hybrid such as Dlg4 and Lrrc7, but also expand this to including additional scaffolding proteins such as Baiap2, Dlgap1, and Shank3 [11, 113], showing the integral role Cnksr2 may have in PSD PINs involving the scaffolding proteins of PSD.
In line with Cnksr2 being central to PSD PINs, Cnksr2 has also been implicated in contributing to multiple complex brain disorders. Cnksr2 is found within a single-gene, genome-wide significant locus, contributing to schizophrenia risk [20]. In addition, mutations resulting in a loss of expression in Cnksr2 have been shown to be causative for X-linked intellectual disability. Over 9 families have been identified with individuals affected by a deficiency in Cnksr2 [114–117]. Furthermore, large scale exome sequencing efforts to characterize genetic causes of developmental disorders have identified several additional damaging mutations in Cnksr2 [22]. With common variation contributing to schizophrenia and loss of function contributing to intellectual disability, this illustrates how different types of mutations in a single gene can result in differing outcomes related to complex brain disorders.
4.3. Sorbs2 (nArgBP2)
Sorbs2 is an abundant scaffolding protein at the PSD that has multiple isoforms, including nARGBP2, an isoform specifically expressed in neural tissues. Sorbs2 contains several conserved protein domains, including a sorbin homology (SoHo) domain at the C-terminus, a C2H2-type zinc finger, and three SH3 domains at the N-terminus (Figure 1A) [118]. Sorbs2 directly interacts with the DLGAP family of proteins through its third SH3 domain, exemplifying its association with the core scaffolding components of the PSD [119]. Although Sorbs2 complexes have not been subject to high-throughput identification, characterization of Dlgap1 complexes at the PSD have confirmed this association [11]. By using specific domains of Sorbs2 for immunoprecipitation, the SoHo domain was shown to interact with alpha2-spectrin and the SH3 domains were shown to interact with multiple proteins involved in regulation of cytoskeleton dynamics such as dynamin, synaptojanin, and the WAVE complex [120]. These interactions are identical to what is observed with the SH3 domain of Baiap2, indicating the possibility of a functional complex. The role for Sorbs2 in the regulation of cytoskeletal dynamics has been confirmed in excitatory neurons as the Sorbs2 knockout mouse model has decreased dendritic complexity and knockdown of Sorbs2 results in abnormal spine morphology [121, 122]. In addition, Sorbs2 knockout mice display defects in excitatory synapse transmission and behavioral tasks associated with learning and memory [122].
4.4. Lrrc7 (Densin-180)
Lrrc7 (also known as Densin-180) is central to the network of PSD scaffolding proteins as it has been shown to directly interact with both Cnksr2 and SHANKs [109, 123]. Lrrc7 is concentrated at the PSD and contains 16 leucine rich repeats (LRRs) and a PDZ domain (Figure 1A) [124]. Proteins containing this domain architecture have been collectively termed LAP (leucine rich repeat and PDZ domain) proteins [125]. While the LRR domain has been implicated in targeting Lrrc7 to the plasma membrane [126], the PDZ domain has been shown to directly interact with delta-catenin and Cnksr2 [109, 127]. Lrrc7 has also been shown to directly interact with alpha-actinin, a cytoskeletal protein that binds to F-actin to anchor structures within the PSD [128]. With both Cnksr2 and alpha-actinin binding to Dlg4, these interactions provide a link between Lrrc7 and Dlg4 [109, 129]. In addition, Lrrc7 interacts with key proteins involved in calcium signaling and synaptic plasticity including Camk2a and the L-type calcium channels, Cav1.2 and Cav1.3 [128, 130, 131]. Here, Lrrc7 has been shown to recruit Camk2a to calcium channels in order to facilitate the modulation of neuronal excitability [130, 131].
In line with Lrrc7 interacting with both cytoskeletal proteins and facilitating the regulation of synaptic plasticity, Lrrc7 mutant mice display reduced levels of both alpha-actinin and mGluR5 in PSD fractions [132]. As alpha-actinin directly interacts with mGluR5, this has been postulated to be contributing factor to the reduction in mGluR5 [133]. Mutant mice also displayed deficiencies in both NMDA and mGluR-dependent forms of LTD along with abnormalities in spine morphology. While a lack of Lrrc7 results in decreased dendritic complexity, overexpression of Lrrc7 in cultured hippocampal neurons has been shown to produce the opposite effect [123, 134]. In addition, mutant mouse models have been shown to exhibit abnormal behavioral traits such as impaired short-term memory tasks, aggressive behavior during juvenile developmental stages, and social dysfunction in adulthood [132, 134]. Positive allosteric modulation of mGluR5 has recently been shown to rescue both behavioral abnormalities and neuronal morphology, highlighting the critical link between metabotropic glutamate receptors and Lrrc7 [134].
5. PSD scaffolds in Complex Brain Disorders
Complex brain disorders such as ASD, SCZ, ID, and DD are polygenic neurodevelopmental disorders that are influenced through a variety of genetic and environmental risk factors [135]. Substantial progress has been made in identifying the genetic underpinnings of complex brain disorders with respect to single nucleotide polymorphisms (SNPs) identified through Genome Wide Association Studies (GWAS), inherited or de novo single nucleotide variants (SNVs), and copy number variations (CNVs) [16–24]. Together, these studies have identified thousands of genetic risk factors associated to complex brain disorders. Although these risk factors contribute through several different forms of genetic abnormalities and with varying levels of penetrance, a number of them have been found to converge on postsynaptic proteins involved in neuronal development and synaptic function.
A common theme amongst studies identifying genetic risk factors for complex brain disorders is statistically testing the enrichment of risk factors in particular gene sets that may be relevant to the disease. Common lists include targets of the fragile X mental retardation 1 (FMR1) protein, protein complexes (such as NMDA or ARC), and the proteins found to be at the PSD [18, 20, 136]. However, these enrichment analyses test a small number of PSD interactomes, creating a limited view of synaptic PINs within the PSD. Moreover, there is a large degree of overlap between the proteins found to harbor damaging mutations within different neurodevelopmental disorders [11, 16–24]. This is not surprising since the core scaffold machinery of the PSD is a highly connected component within PSD PPI networks. In accordance with this, interactomes of Dlg4, Dlgap1, and Shank3 have all been shown to be highly enriched in proteins found to harbor de novo coding mutations in patients affected by ASD, ID, and DD, with several risk factors shared between the three complexes (Figure 3A). In addition, numerous risk factors found to interact with all three core scaffolding proteins were also implicated in contributing to multiple disorders through different mutations (Figure 3B) [11]. This emphasizes both the degree of overlap within individual risk factors contributing to multiple complex brain disorders and the centrality of the core scaffolds in PSD PINs affected by them. Supporting their essential role in synaptic function, highly connected proteins in the PSD PINs that are risk factors for complex brain disorders have also been shown to be differentially regulated by phosphorylation upon the induction of LTP. Here, gene-set enrichment analysis has been able to show that while the PSD as a whole is enriched in risk factors for complex brain disorders, the depletion of the phospho-regulated binding partners of Dlg4, Dlgap1, and Shank3 completely eliminates this enrichment [13]. This shows that the core-scaffolding components not only serve as a hub for PPIs within the PSD but also for the regulation of multiple processes involved in synaptic function and complex brain disorders.
Figure 3 –

Core scaffolding PSD interactomes and complex brain disorders. (A) Distribution of risk factors found to harbor nonsynonymous mutations in patients affected by autism spectrum disorder (ASD), intellectual disability (ID), developmental delay (DD), schizophrenia (SCZ), or multiple disorders within the interactomes of Dlg4, Dlgap1, and Shank3. Multiple risk factors are common interactors between the proteins representing the three main layers of the PSD. (B) Heatmap showing proteins found to interact with all three core scaffolding proteins (Dlg4, Dlgap1, and Shank3). Columns indicate if the protein has been implicated in contributing to complex brain disorders in the Online Mendelian Inheritance in Man (OMIM) database and through de novo nonsynonymous mutations in ASD, ID, DD, and SCZ. The abbreviations in the OMIM column are as follows: ASD – susceptibility to autism spectrum disorder, CNH – congenital hypomyelinating neuropathy, DD – related to developmental disorders, ID – Related to intellectual disability, E – related to epilepsy, MC – microcephaly. All data for PPIs and nonsynonymous mutations are derived from [11].
6. Conclusion
Scaffolding proteins within the PSD are essential for the organization of synaptic PINs and play key roles in regulating synaptic function. The core-scaffolding components provide the architectural framework for signaling mechanisms within the PSD and share several characteristics such as possessing an abundance of protein domains specialized in PPIs. These domains allow for the occurrence of multiple PPIs simultaneously throughout the length of the protein and dependent on the scaffold, can also allow for protein multimerization. Multimerization can result in multiple copies of a scaffold within a complex and macromolecular complex assembly. In line with this, characterization of endogenous PPIs involving the core scaffolding proteins have identified hundreds of interactors. These interactomes have a core of highly connected proteins within the PSD PIN that contain the “PSD-risk” for complex brain disease. While significant progress has been made in determining the PPIs of PSD scaffolding proteins, there is still a need for increased quantitative analyses to determine stoichiometric ratios between binding partners and increased coverage of multiple developmental time points to determine the differential association of PPIs throughout time. With the advent of cell-type neuronal differentiation techniques in induced pluripotent stem cells, this may provide a platform to determine PPIs of PSD proteins during early development and model patient-specific mutations in complex brain disorders at a cell-type specific resolution. In this regard, it will be important to develop a correspondent bioinformatic pipeline and database to provide a clear annotation of cell-type specific protein interactions. In addition, the determination of interactomes following the induction of synaptic activity will allow the characterization of PSD PINs during these dynamic processes. With the scaffolding components of the PSD organizing multiple cellular processes and signaling pathways essential for synaptic function, increased characterization of their interactomes under multiple cellular and functional contexts will further elucidate their relationship with complex brain disorders.
Acknowledgements
This work was supported by the National Institutes of Health (R01MH115005, R21MH115404) to MPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests: None.
References
- [1].Gray EG, Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study, J Anat 93 (1959) 420–33. [PMC free article] [PubMed] [Google Scholar]
- [2].Palay SL, Synapses in the central nervous system, J Biophys Biochem Cytol 2(4 Suppl) (1956) 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bayes A, Collins MO, Croning MD, van de Lagemaat LN, Choudhary JS, Grant SG, Comparative study of human and mouse postsynaptic proteomes finds high compositional conservation and abundance differences for key synaptic proteins, PLoS One 7(10) (2012) e46683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA, Identification and verification of novel rodent postsynaptic density proteins, Mol Cell Proteomics 3(9) (2004) 857–71. [DOI] [PubMed] [Google Scholar]
- [5].Li KW, Hornshaw MP, Van Der Schors RC, Watson R, Tate S, Casetta B, Jimenez CR, Gouwenberg Y, Gundelfinger ED, Smalla KH, Smit AB, Proteomics analysis of rat brain postsynaptic density. Implications of the diverse protein functional groups for the integration of synaptic physiology, J Biol Chem 279(2) (2004) 987–1002. [DOI] [PubMed] [Google Scholar]
- [6].Peng J, Kim MJ, Cheng D, Duong DM, Gygi SP, Sheng M, Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry, J Biol Chem 279(20) (2004) 21003–11. [DOI] [PubMed] [Google Scholar]
- [7].Trinidad JC, Thalhammer A, Specht CG, Lynn AJ, Baker PR, Schoepfer R, Burlingame AL, Quantitative analysis of synaptic phosphorylation and protein expression, Mol Cell Proteomics 7(4) (2008) 684–96. [DOI] [PubMed] [Google Scholar]
- [8].Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, Zografos L, Armstrong JD, Choudhary JS, Grant SG, Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins, Mol Syst Biol 5 (2009) 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fernandez E, Collins MO, Frank RAW, Zhu F, Kopanitsa MV, Nithianantharajah J, Lempriere SA, Fricker D, Elsegood KA, McLaughlin CL, Croning MDR, McLean C, Armstrong JD, Hill WD, Deary IJ, Cencelli G, Bagni C, Fromer M, Purcell SM, Pocklington AJ, Choudhary JS, Komiyama NH, Grant SGN, Arc Requires PSD95 for Assembly into Postsynaptic Complexes Involved with Neural Dysfunction and Intelligence, Cell Rep 21(3) (2017) 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Frank RA, Komiyama NH, Ryan TJ, Zhu F, O’Dell TJ, Grant SG, NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation, Nat Commun 7 (2016) 11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li J, Zhang W, Yang H, Howrigan DP, Wilkinson B, Souaiaia T, Evgrafov OV, Genovese G, Clementel VA, Tudor JC, Abel T, Knowles JA, Neale BM, Wang K, Sun F, Coba MP, Spatiotemporal profile of postsynaptic interactomes integrates components of complex brain disorders, Nat Neurosci 20(8) (2017) 1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilkinson B, Li J, Coba MP, Synaptic GAP and GEF Complexes Cluster Proteins Essential for GTP Signaling, Sci Rep 7(1) (2017) 5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li J, Wilkinson B, Clementel VA, Hou J, O’Dell TJ, Coba MP, Long-term potentiation modulates synaptic phosphorylation networks and reshapes the structure of the postsynaptic interactome, Sci Signal 9(440) (2016) rs8. [DOI] [PubMed] [Google Scholar]
- [14].Sheng M, Kim E, The postsynaptic organization of synapses, Cold Spring Harb Perspect Biol 3(12) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Coba MP, Ramaker MJ, Ho EV, Thompson SL, Komiyama NH, Grant SGN, Knowles JA, Dulawa SC, Dlgap1 knockout mice exhibit alterations of the postsynaptic density and selective reductions in sociability, Sci Rep 8(1) (2018) 2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, Stevens C, Reichert J, Mulhern MS, Artomov M, Gerges S, Sheppard B, Xu X, Bhaduri A, Norman U, Brand H, Schwartz G, Nguyen R, Guerrero EE, Dias C, Autism Sequencing C, i P-BC, Betancur C, Cook EH, Gallagher L, Gill, Sutcliffe JS, Thurm A, Zwick ME, Borglum AD, State MW, Cicek AE, Talkowski ME, Cutler DJ, Devlin B, Sanders SJ, Roeder K, Daly MJ, Buxbaum JD, Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism, Cell 180(3) (2020) 568–584 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, Pallesen J, Agerbo E, Andreassen OA, Anney R, Awashti S, Belliveau R, Bettella F, Buxbaum JD, Bybjerg-Grauholm J, Baekvad-Hansen M, Cerrato F, Chambert K, Christensen JH, Churchhouse C, Dellenvall K, Demontis D, De Rubeis S, Devlin B, Djurovic S, Dumont AL, Goldstein JI, Hansen CS, Hauberg ME, Hollegaard MV, Hope S, Howrigan DP, Huang H, Hultman CM, Klei L, Maller J, Martin J, Martin AR, Moran JL, Nyegaard M, Naerland T, Palmer DS, Palotie A, Pedersen CB, Pedersen MG, dPoterba T, Poulsen JB, Pourcain BS, Qvist P, Rehnstrom K, Reichenberg A, Reichert J, Robinson EB, Roeder K, Roussos P, Saemundsen E, Sandin S, Satterstrom FK, Davey Smith G, Stefansson H, Steinberg S, Stevens CR, Sullivan PF, Turley P, Walters GB, Xu X, Autism C Spectrum Disorder Working Group of the Psychiatric Genomics, Bupgen, C. Major Depressive Disorder Working Group of the Psychiatric Genomics, T. andMe Research, Stefansson K, Geschwind DH, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Neale BM, Daly MJ, Borglum AD, Identification of common genetic risk variants for autism spectrum disorder, Nat Genet 51(3) (2019) 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, Georgieva L, Rees E, Palta P, Ruderfer DM, Carrera N, Humphreys I, Johnson JS, Roussos P, Barker DD, Banks E, Milanova V, Grant SG, Hannon E, Rose SA, Chambert K, Mahajan M, Scolnick EM, Moran JL, Kirov G, Palotie A, McCarroll SA, Holmans P, Sklar P, Owen MJ, Purcell SM, O’Donovan MC, De novo mutations in schizophrenia implicate synaptic networks, Nature 506(7487) (2014) 179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Howrigan DP, Rose SA, Samocha KE, Fromer M, Cerrato F, Chen WJ, Churchhouse C, Chambert K, Chandler SD, Daly MJ, Dumont A, Genovese G, Hwu HG, Laird N, Kosmicki JA, Moran JL, Roe C, Singh T, Wang SH, Faraone SV, Glatt SJ, McCarroll SA, Tsuang M, Neale BM, Exome sequencing in schizophrenia-affected parent-offspring trios reveals risk conferred by protein-coding de novo mutations, Nat Neurosci 23(2) (2020) 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schizophrenia C Working Group of the Psychiatric Genomics, Biological insights from 108 schizophrenia-associated genetic loci, Nature 511(7510) (2014) 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lelieveld SH, Reijnders MR, Pfundt R, Yntema HG, Kamsteeg EJ, de Vries P, de Vries BB, Willemsen MH, Kleefstra T, Lohner K, Vreeburg M, Stevens SJ, van der Burgt I, Bongers EM, Stegmann AP, Rump P, Rinne T, Nelen MR, Veltman JA, Vissers LE, Brunner HG, Gilissen C, Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability, Nat Neurosci 19(9) (2016) 1194–6. [DOI] [PubMed] [Google Scholar]
- [22].Deciphering Developmental Disorders S, Prevalence and architecture of de novo mutations in developmental disorders, Nature 542(7642) (2017) 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, Antaki D, Shetty A, Holmans PA, Pinto D, Gujral M, Brandler WM, Malhotra D, Wang Z, Fajarado KVF, Maile MS, Ripke S, Agartz I, Albus M, Alexander M, Amin F, Atkins J, Bacanu SA, Belliveau RA Jr., Bergen SE, Bertalan M, Bevilacqua E, Bigdeli TB, Black DW, Bruggeman R, Buccola NG, Buckner RL, Bulik-Sullivan B, Byerley W, Cahn W, Cai G, Cairns MJ, Campion D, Cantor RM, Carr VJ, Carrera N, Catts SV, Chambert KD, Cheng W, Cloninger CR, Cohen D, Cormican P, Craddock N, Crespo-Facorro B, Crowley JJ, Curtis D, Davidson M, Davis KL, Degenhardt F, Del Favero J, DeLisi LE, Dikeos D, Dinan T, Djurovic S, Donohoe G, Drapeau E, Duan J, Dudbridge F, Eichhammer P, Eriksson J, Escott-Price V, Essioux L, Fanous AH, Farh KH, Farrell MS, Frank J, Franke L, Freedman R, Freimer NB, Friedman JI, Forstner AJ, Fromer M, Genovese G, Georgieva L, Gershon ES, Giegling I, Giusti-Rodriguez P, Godard S, Goldstein JI, Gratten J, de Haan L, Hamshere ML, Hansen M, Hansen T, Haroutunian V, Hartmann AM, Henskens FA, Herms S, Hirschhorn JN, Hoffmann P, Hofman A, Huang H, Ikeda M, Joa I, Kahler AK, Kahn RS, Kalaydjieva L, Karjalainen J, Kavanagh D, Keller MC, Kelly BJ, Kennedy JL, Kim Y, Knowles JA, Konte B, Laurent C, Lee P, Lee SH, Legge SE, Lerer B, Levy DL, Liang KY, Lieberman J, Lonnqvist J, Loughland CM, Magnusson PKE, Maher BS, Maier W, Mallet J, Mattheisen M, Mattingsdal M, McCarley RW, McDonald C, McIntosh AM, Meier S, Meijer CJ, Melle I, Mesholam-Gately RI, Metspalu A, Michie PT, Milani L, Milanova V, Mokrab Y, Morris DW, Muller-Myhsok B, Murphy KC, Murray RM, Myin-Germeys I, Nenadic I, Nertney DA, Nestadt G, Nicodemus KK, Nisenbaum L, Nordin A, O’Callaghan E, O’Dushlaine C, Oh SY, Olincy A, Olsen L, O’Neill FA, Van Os J, Pantelis C, Papadimitriou GN, Parkhomenko E, Pato MT, Paunio T, Psychosis Endophenotypes International C, Perkins DO, Pers TH, Pietilainen O, Pimm J, Pocklington AJ, Powell J, Price A, Pulver AE, Purcell SM, Quested D, Rasmussen HB, Reichenberg A, Reimers MA, Richards AL, Roffman JL, Roussos P, Ruderfer DM, Salomaa V, Sanders AR, Savitz A, Schall U, Schulze TG, Schwab SG, Scolnick EM, Scott RJ, Seidman LJ, Shi J, Silverman JM, Smoller JW, Soderman E, Spencer CCA, Stahl EA, Strengman E, Strohmaier J, Stroup TS, Suvisaari J, Svrakic DM, Szatkiewicz JP, Thirumalai S, Tooney PA, Veijola J, Visscher PM, Waddington J, Walsh D, Webb BT, Weiser M, Wildenauer DB, Williams NM, Williams S, Witt SH, Wolen AR, Wormley BK, Wray NR, Wu JQ, Zai CC, Adolfsson R, Andreassen OA, Blackwood DHR, Bramon E, Buxbaum JD, Cichon S, Collier DA, Corvin A, Daly MJ, Darvasi A, Domenici E, Esko T, Gejman PV, Gill M, Gurling H, Hultman CM, Iwata N, Jablensky AV, Jonsson EG, Kendler KS, Kirov G, Knight J, Levinson DF, Li QS, McCarroll SA, McQuillin A, Moran JL, Mowry BJ, Nothen MM, Ophoff RA, Owen MJ, Palotie A, Pato CN, Petryshen TL, Posthuma D, Rietschel M, Riley BP, Rujescu D, Sklar P, St Clair D, Walters JTR, Werge T, Sullivan PF, O’Donovan MC, Scherer SW, Neale BM, Sebat J, Cnv C Schizophrenia Working Groups of the Psychiatric Genomics, Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects, Nat Genet 49(1) (2017) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, Thiruvahindrapuram B, Xu X, Ziman R, Wang Z, Vorstman JA, Thompson A, Regan R, Pilorge M, Pellecchia G, Pagnamenta AT, Oliveira B, Marshall CR, Magalhaes TR, Lowe JK, Howe JL, Griswold AJ, Gilbert J, Duketis E, Dombroski BA, De Jonge MV, Cuccaro M, Crawford EL, Correia CT, Conroy J, Conceicao IC, Chiocchetti AG, Casey JP, Cai G, Cabrol C, Bolshakova N, Bacchelli E, Anney R, Gallinger S, Cotterchio M, Casey G, Zwaigenbaum L, Wittemeyer K, Wing K, Wallace S, van Engeland H, Tryfon A, Thomson S, Soorya L, Roge B, Roberts W, Poustka F, Mouga S, Minshew N, McInnes LA, McGrew SG, Lord C, Leboyer M, Le Couteur AS, Kolevzon A, Jimenez Gonzalez P, Jacob S, Holt R, Guter S, Green J, Green A, Gillberg C, Fernandez BA, Duque F, Delorme R, Dawson G, Chaste P, Cafe C, Brennan S, Bourgeron T, Bolton PF, Bolte S, Bernier R, Baird G, Bailey AJ, Anagnostou E, Almeida J, Wijsman EM, Vieland VJ, Vicente AM, Schellenberg GD, Pericak-Vance M, Paterson AD, Parr JR, Oliveira G, Nurnberger JI, Monaco AP, Maestrini E, Klauck SM, Hakonarson H, Haines JL, Geschwind DH, Freitag CM, Folstein SE, Ennis S, Coon H, Battaglia A, Szatmari P, Sutcliffe JS, Hallmayer J, Gill M, Cook EH, Buxbaum JD, Devlin B, Gallagher L, Betancur C, Scherer SW, Convergence of genes and cellular pathways dysregulated in autism spectrum disorders, Am J Hum Genet 94(5) (2014) 677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hartwell LH, Hopfield JJ, Leibler S, Murray AW, From molecular to modular cell biology, Nature 402(6761 Suppl) (1999) C47–52. [DOI] [PubMed] [Google Scholar]
- [26].Spirin V, Mirny LA, Protein complexes and functional modules in molecular networks, Proc Natl Acad Sci U S A 100(21) (2003) 12123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Suter B, Kittanakom S, Stagljar I, Two-hybrid technologies in proteomics research, Curr Opin Biotechnol 19(4) (2008) 316–23. [DOI] [PubMed] [Google Scholar]
- [28].Luck K, Kim DK, Lambourne L, Spirohn K, Begg BE, Bian W, Brignall R, Cafarelli T, Campos-Laborie FJ, Charloteaux B, Choi D, Cote AG, Daley M, Deimling S, Desbuleux A, Dricot A, Gebbia M, Hardy MF, Kishore N, Knapp JJ, Kovacs IA, Lemmens I, Mee MW, Mellor JC, Pollis C, Pons C, Richardson AD, Schlabach S, Teeking B, Yadav A, Babor M, Balcha D, Basha O, Bowman-Colin C, Chin SF, Choi SG, Colabella C, Coppin G, D’Amata C, De Ridder D, De Rouck S, Duran-Frigola M, Ennajdaoui H, Goebels F, Goehring L, Gopal A, Haddad G, Hatchi E, Helmy M, Jacob Y, Kassa Y, Landini S, Li R, van Lieshout N, MacWilliams A, Markey D, Paulson JN, Rangarajan S, Rasla J, Rayhan A, Rolland T, San-Miguel A, Shen Y, Sheykhkarimli D, Sheynkman GM, Simonovsky E, Tasan M, Tejeda A, Tropepe V, Twizere JC, Wang Y, Weatheritt RJ, Weile J, Xia Y, Yang X, Yeger-Lotem E, Zhong Q, Aloy P, Bader GD, De Las Rivas J, Gaudet S, Hao T, Rak J, Tavernier J, Hill DE, Vidal M, Roth FP, Calderwood MA, A reference map of the human binary protein interactome, Nature 580(7803) (2020) 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huttlin EL, Bruckner RJ, Paulo JA, Cannon JR, Ting L, Baltier K, Colby G, Gebreab F, Gygi MP, Parzen H, Szpyt J, Tam S, Zarraga G, Pontano-Vaites L, Swarup S, White AE, Schweppe DK, Rad R, Erickson BK, Obar RA, Guruharsha KG, Li K, Artavanis-Tsakonas S, Gygi SP, Harper JW, Architecture of the human interactome defines protein communities and disease networks, Nature 545(7655) (2017) 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, Tam S, Zarraga G, Colby G, Baltier K, Dong R, Guarani V, Vaites LP, Ordureau A, Rad R, Erickson BK, Wuhr M, Chick J, Zhai B, Kolippakkam D, Mintseris J, Obar RA, Harris T, Artavanis-Tsakonas S, Sowa ME, De Camilli P, Paulo JA, Harper JW, Gygi SP, The BioPlex Network: A Systematic Exploration of the Human Interactome, Cell 162(2) (2015) 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Won S, Levy JM, Nicoll RA, Roche KW, MAGUKs: multifaceted synaptic organizers, Curr Opin Neurobiol 43 (2017) 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang SH, Celic I, Choi SY, Riccomagno M, Wang Q, Sun LO, Mitchell SP, Vasioukhin V, Huganir RL, Kolodkin AL, Dlg5 regulates dendritic spine formation and synaptogenesis by controlling subcellular N-cadherin localization, J Neurosci 34(38) (2014) 12745–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chang Y, Klezovitch O, Walikonis RS, Vasioukhin V, LoTurco JJ, Discs large 5 is required for polarization of citron kinase in mitotic neural precursors, Cell Cycle 9(10) (2010) 1990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oliva C, Escobedo P, Astorga C, Molina C, Sierralta J, Role of the MAGUK protein family in synapse formation and function, Dev Neurobiol 72(1) (2012) 57–72. [DOI] [PubMed] [Google Scholar]
- [35].Niethammer M, Kim E, Sheng M, Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases, J Neurosci 16(7) (1996) 2157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kornau HC, Schenker LT, Kennedy MB, Seeburg PH, Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95, Science 269(5231) (1995) 1737–40. [DOI] [PubMed] [Google Scholar]
- [37].Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M, Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases, Nature 378(6552) (1995) 85–8. [DOI] [PubMed] [Google Scholar]
- [38].Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC, Binding of neuroligins to PSD-95, Science 277(5331) (1997) 1511–5. [DOI] [PubMed] [Google Scholar]
- [39].Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M, GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules, J Cell Biol 136(3) (1997) 669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Walkup WG, Mastro TL, Schenker LT, Vielmetter J, Hu R, Iancu A, Reghunathan M, Bannon BD, Kennedy MB, A model for regulation by SynGAP-alpha1 of binding of synaptic proteins to PDZ-domain ‘Slots’ in the postsynaptic density, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dakoji S, Tomita S, Karimzadegan S, Nicoll RA, Bredt DS, Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases, Neuropharmacology 45(6) (2003) 849–56. [DOI] [PubMed] [Google Scholar]
- [42].Olsen O, Bredt DS, Functional analysis of the nucleotide binding domain of membrane-associated guanylate kinases, J Biol Chem 278(9) (2003) 6873–8. [DOI] [PubMed] [Google Scholar]
- [43].Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y, SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density, J Biol Chem 272(18) (1997) 11943–51. [DOI] [PubMed] [Google Scholar]
- [44].Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, Ralston HJ, Bredt DS, Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A, J Neurosci 18(21) (1998) 8805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reese ML, Dakoji S, Bredt DS, Dotsch V, The guanylate kinase domain of the MAGUK PSD-95 binds dynamically to a conserved motif in MAP1a, Nat Struct Mol Biol 14(2) (2007) 155–63. [DOI] [PubMed] [Google Scholar]
- [46].McGee AW, Dakoji SR, Olsen O, Bredt DS, Lim WA, Prehoda KE, Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins, Mol Cell 8(6) (2001) 1291–301. [DOI] [PubMed] [Google Scholar]
- [47].Tavares GA, Panepucci EH, Brunger AT, Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95, Mol Cell 8(6) (2001) 1313–25. [DOI] [PubMed] [Google Scholar]
- [48].Rademacher N, Kuropka B, Kunde SA, Wahl MC, Freund C, Shoichet SA, Intramolecular domain dynamics regulate synaptic MAGUK protein interactions, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zeng M, Ye F, Xu J, Zhang M, PDZ Ligand Binding-Induced Conformational Coupling of the PDZ-SH3-GK Tandems in PSD-95 Family MAGUKs, J Mol Biol 430(1) (2018) 69–86. [DOI] [PubMed] [Google Scholar]
- [50].Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP, Composition of the synaptic PSD-95 complex, Mol Cell Proteomics 6(10) (2007) 1749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA, Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins, Neuron 52(2) (2006) 307–20. [DOI] [PubMed] [Google Scholar]
- [52].Timkovich R, 15N NMR spectroscopy of Pseudomonas cytochrome c-551, Biochemistry 29(33) (1990) 7773–80. [DOI] [PubMed] [Google Scholar]
- [53].Carlisle HJ, Fink AE, Grant SG, O’Dell TJ, Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity, J Physiol 586(24) (2008) 5885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cuthbert PC, Stanford LE, Coba MP, Ainge JA, Fink AE, Opazo P, Delgado JY, Komiyama NH, O’Dell TJ, Grant SG, Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies, J Neurosci 27(10) (2007) 2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA, The role of SAP97 in synaptic glutamate receptor dynamics, Proc Natl Acad Sci U S A 107(8) (2010) 3805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sans N, Petralia RS, Wang YX, Blahos J 2nd, Hell JW, Wenthold RJ, A developmental change in NMDA receptor-associated proteins at hippocampal synapses, J Neurosci 20(3) (2000) 1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fukaya M, Ueda H, Yamauchi K, Inoue Y, Watanabe M, Distinct spatiotemporal expression of mRNAs for the PSD-95/SAP90 protein family in the mouse brain, Neurosci Res 33(2) (1999) 111–8. [DOI] [PubMed] [Google Scholar]
- [58].Winkler D, Daher F, Wustefeld L, Hammerschmidt K, Poggi G, Seelbach A, Krueger-Burg D, Vafadari B, Ronnenberg A, Liu Y, Kaczmarek L, Schluter OM, Ehrenreich H, Dere E, Hypersocial behavior and biological redundancy in mice with reduced expression of PSD95 or PSD93, Behav Brain Res 352 (2018) 35–45. [DOI] [PubMed] [Google Scholar]
- [59].Coley AA, Gao WJ, PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment, Sci Rep 9(1) (2019) 9486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M, Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin, Neuron 23(3) (1999) 569–82. [DOI] [PubMed] [Google Scholar]
- [61].Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M, Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein, J Neurosci 20(12) (2000) 4524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tong J, Yang H, Eom SH, Chun C, Im YJ, Structure of the GH1 domain of guanylate kinase-associated protein from Rattus norvegicus, Biochem Biophys Res Commun 452(1) (2014) 130–5. [DOI] [PubMed] [Google Scholar]
- [63].Welch JM, Wang D, Feng G, Differential mRNA expression and protein localization of the SAP90/PSD-95-associated proteins (SAPAPs) in the nervous system of the mouse, J Comp Neurol 472(1) (2004) 24–39. [DOI] [PubMed] [Google Scholar]
- [64].Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G, Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice, Nature 448(7156) (2007) 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kindler S, Rehbein M, Classen B, Richter D, Bockers TM, Distinct spatiotemporal expression of SAPAP transcripts in the developing rat brain: a novel dendritically localized mRNA, Brain Res Mol Brain Res 126(1) (2004) 14–21. [DOI] [PubMed] [Google Scholar]
- [66].Schob C, Morellini F, Ohana O, Bakota L, Hrynchak MV, Brandt R, Brockmann MD, Cichon N, Hartung H, Hanganu-Opatz IL, Kraus V, Scharf S, Herrmans-Borgmeyer I, Schweizer M, Kuhl D, Wohr M, Vorckel KJ, Calzada-Wack J, Fuchs H, Gailus-Durner V, Hrabe de Angelis M, Garner CC, Kreienkamp HJ, Kindler S, Cognitive impairment and autistic-like behaviour in SAPAP4-deficient mice, Transl Psychiatry 9(1) (2019) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jiang-Xie LF, Liao HM, Chen CH, Chen YT, Ho SY, Lu DH, Lee LJ, Liou HH, Fu WM, Gau SS, Autism-associated gene Dlgap2 mutant mice demonstrate exacerbated aggressive behaviors and orbitofrontal cortex deficits, Mol Autism 5 (2014) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang X, Xu Q, Bey AL, Lee Y, Jiang YH, Transcriptional and functional complexity of Shank3 provides a molecular framework to understand the phenotypic heterogeneity of SHANK3 causing autism and Shank3 mutant mice, Mol Autism 5 (2014) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].McWilliams RR, Gidey E, Fouassier L, Weed SA, Doctor RB, Characterization of an ankyrin repeat-containing Shank2 isoform (Shank2E) in liver epithelial cells, Biochem J 380(Pt 1) (2004) 181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lim S, Naisbitt S, Yoon J, Hwang JI, Suh PG, Sheng M, Kim E, Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development, J Biol Chem 274(41) (1999) 29510–8. [DOI] [PubMed] [Google Scholar]
- [71].Lim S, Sala C, Yoon J, Park S, Kuroda S, Sheng M, Kim E, Sharpin, a novel postsynaptic density protein that directly interacts with the shank family of proteins, Mol Cell Neurosci 17(2) (2001) 385–97. [DOI] [PubMed] [Google Scholar]
- [72].Bockers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ, Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin, J Biol Chem 276(43) (2001) 40104–12. [DOI] [PubMed] [Google Scholar]
- [73].Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF, Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins, Neuron 23(3) (1999) 583–92. [DOI] [PubMed] [Google Scholar]
- [74].Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF, Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins, Neuron 21(4) (1998) 707–16. [DOI] [PubMed] [Google Scholar]
- [75].Boeckers TM, Liedtke T, Spilker C, Dresbach T, Bockmann J, Kreutz MR, Gundelfinger ED, C-terminal synaptic targeting elements for postsynaptic density proteins ProSAP1/Shank2 and ProSAP2/Shank3, J Neurochem 92(3) (2005) 519–24. [DOI] [PubMed] [Google Scholar]
- [76].Baron MK, Boeckers TM, Vaida B, Faham S, Gingery M, Sawaya MR, Salyer D, Gundelfinger ED, Bowie JU, An architectural framework that may lie at the core of the postsynaptic density, Science 311(5760) (2006) 531–5. [DOI] [PubMed] [Google Scholar]
- [77].Grabrucker AM, Knight MJ, Proepper C, Bockmann J, Joubert M, Rowan M, Nienhaus GU, Garner CC, Bowie JU, Kreutz MR, Gundelfinger ED, Boeckers TM, Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation, EMBO J 30(3) (2011) 569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lee Y, Ryu JR, Kang H, Kim Y, Kim S, Zhang Y, Jin C, Cho HM, Kim WK, Sun W, Han K, Characterization of the zinc-induced Shank3 interactome of mouse synaptosome, Biochem Biophys Res Commun 494(3–4) (2017) 581–586. [DOI] [PubMed] [Google Scholar]
- [79].Bockers TM, Segger-Junius M, Iglauer P, Bockmann J, Gundelfinger ED, Kreutz MR, Richter D, Kindler S, Kreienkamp HJ, Differential expression and dendritic transcript localization of Shank family members: identification of a dendritic targeting element in the 3’ untranslated region of Shank1 mRNA, Mol Cell Neurosci 26(1) (2004) 182–90. [DOI] [PubMed] [Google Scholar]
- [80].Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G, Shank3 mutant mice display autistic-like behaviours and striatal dysfunction, Nature 472(7344) (2011) 437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, Giuliano F, Stordeur C, Depienne C, Mouzat K, Pinto D, Howe J, Lemiere N, Durand CM, Guibert J, Ey E, Toro R, Peyre H, Mathieu A, Amsellem F, Rastam M, Gillberg IC, Rappold GA, Holt R, Monaco AP, Maestrini E, Galan P, Heron D, Jacquette A, Afenjar A, Rastetter A, Brice A, Devillard F, Assouline B, Laffargue F, Lespinasse J, Chiesa J, Rivier F, Bonneau D, Regnault B, Zelenika D, Delepine M, Lathrop M, Sanlaville D, Schluth-Bolard C, Edery P, Perrin L, Tabet AC, Schmeisser MJ, Boeckers TM, Coleman M, Sato D, Szatmari P, Scherer SW, Rouleau GA, Betancur C, Leboyer M, Gillberg C, Delorme R, Bourgeron T, Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments, PLoS Genet 10(9) (2014) e1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Monteiro P, Feng G, SHANK proteins: roles at the synapse and in autism spectrum disorder, Nat Rev Neurosci 18(3) (2017) 147–157. [DOI] [PubMed] [Google Scholar]
- [83].Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen AL, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck S, Smalla KH, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd AM, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM, Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2, Nature 486(7402) (2012) 256–60. [DOI] [PubMed] [Google Scholar]
- [84].Zhou Y, Kaiser T, Monteiro P, Zhang X, Van der Goes MS, Wang D, Barak B, Zeng M, Li C, Lu C, Wells M, Amaya A, Nguyen S, Lewis M, Sanjana N, Zhou Y, Zhang M, Zhang F, Fu Z, Feng G, Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects, Neuron 89(1) (2016) 147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sala C, Vicidomini C, Bigi I, Mossa A, Verpelli C, Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders, J Neurochem 135(5) (2015) 849–58. [DOI] [PubMed] [Google Scholar]
- [86].Lee Y, Kang H, Lee B, Zhang Y, Kim Y, Kim S, Kim WK, Han K, Integrative Analysis of Brain Region-specific Shank3 Interactomes for Understanding the Heterogeneity of Neuronal Pathophysiology Related to SHANK3 Mutations, Front Mol Neurosci 10 (2017) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Han K, Holder JL Jr., Schaaf CP, Lu H, Chen H, Kang H, Tang J, Wu Z, Hao S, Cheung SW, Yu P, Sun H, Breman AM, Patel A, Lu HC, Zoghbi HY, SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties, Nature 503(7474) (2013) 72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kolevzon A, Angarita B, Bush L, Wang AT, Frank Y, Yang A, Rapaport R, Saland J, Srivastava S, Farrell C, Edelmann LJ, Buxbaum JD, Phelan-McDermid syndrome: a review of the literature and practice parameters for medical assessment and monitoring, J Neurodev Disord 6(1) (2014) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wilson HL, Wong AC, Shaw SR, Tse WY, Stapleton GA, Phelan MC, Hu S, Marshall J, McDermid HE, Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms, J Med Genet 40(8) (2003) 575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Phelan K, McDermid HE, The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome), Mol Syndromol 2(3–5) (2012) 186–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hassani Nia F, Woike D, Kloth K, Kortum F, Kreienkamp HJ, Truncating mutations in SHANK3 associated with global developmental delay interfere with nuclear beta-catenin signaling, J Neurochem (2020). [DOI] [PubMed] [Google Scholar]
- [92].Gauthier J, Champagne N, Lafreniere RG, Xiong L, Spiegelman D, Brustein E, Lapointe M, Peng H, Cote M, Noreau A, Hamdan FF, Addington AM, Rapoport JL, Delisi LE, Krebs MO, Joober R, Fathalli F, Mouaffak F, Haghighi AP, Neri C, Dube MP, Samuels ME, Marineau C, Stone EA, Awadalla P, Barker PA, Carbonetto S, Drapeau P, Rouleau GA, Team SD, De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia, Proc Natl Acad Sci U S A 107(17) (2010) 7863–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T, Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders, Nat Genet 39(1) (2007) 25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Boccuto L, Lauri M, Sarasua SM, Skinner CD, Buccella D, Dwivedi A, Orteschi D, Collins JS, Zollino M, Visconti P, Dupont B, Tiziano D, Schroer RJ, Neri G, Stevenson RE, Gurrieri F, Schwartz CE, Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders, Eur J Hum Genet 21(3) (2013) 310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhou Y, Sharma J, Ke Q, Landman R, Yuan J, Chen H, Hayden DS, Fisher JW 3rd, Jiang M, Menegas W, Aida T, Yan T, Zou Y, Xu D, Parmar S, Hyman JB, Fanucci-Kiss A, Meisner O, Wang D, Huang Y, Li Y, Bai Y, Ji W, Lai X, Li W, Huang L, Lu Z, Wang L, Anteraper SA, Sur M, Zhou H, Xiang AP, Desimone R, Feng G, Yang S, Atypical behaviour and connectivity in SHANK3-mutant macaques, Nature 570(7761) (2019) 326–331. [DOI] [PubMed] [Google Scholar]
- [96].Kang J, Park H, Kim E, IRSp53/BAIAP2 in dendritic spine development, NMDA receptor regulation, and psychiatric disorders, Neuropharmacology 100 (2016) 27–39. [DOI] [PubMed] [Google Scholar]
- [97].Soltau M, Richter D, Kreienkamp HJ, The insulin receptor substrate IRSp53 links postsynaptic shank1 to the small G-protein cdc42, Mol Cell Neurosci 21(4) (2002) 575–83. [DOI] [PubMed] [Google Scholar]
- [98].Soltau M, Berhorster K, Kindler S, Buck F, Richter D, Kreienkamp HJ, Insulin receptor substrate of 53 kDa links postsynaptic shank to PSD-95, J Neurochem 90(3) (2004) 659–65. [DOI] [PubMed] [Google Scholar]
- [99].Choi J, Ko J, Racz B, Burette A, Lee JR, Kim S, Na M, Lee HW, Kim K, Weinberg RJ, Kim E, Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases, J Neurosci 25(4) (2005) 869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kast DJ, Yang C, Disanza A, Boczkowska M, Madasu Y, Scita G, Svitkina T, Dominguez R, Mechanism of IRSp53 inhibition and combinatorial activation by Cdc42 and downstream effectors, Nat Struct Mol Biol 21(4) (2014) 413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Miki H, Takenawa T, WAVE2 serves a functional partner of IRSp53 by regulating its interaction with Rac, Biochem Biophys Res Commun 293(1) (2002) 93–9. [DOI] [PubMed] [Google Scholar]
- [102].Chou AM, Sem KP, Wright GD, Sudhaharan T, Ahmed S, Dynamin1 is a novel target for IRSp53 protein and works with mammalian enabled (Mena) protein and Eps8 to regulate filopodial dynamics, J Biol Chem 289(35) (2014) 24383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, Futterer K, Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53, EMBO J 24(2) (2005) 240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yamagishi A, Masuda M, Ohki T, Onishi H, Mochizuki N, A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein, J Biol Chem 279(15) (2004) 14929–36. [DOI] [PubMed] [Google Scholar]
- [105].Pourhaghighi R, Ash PEA, Phanse S, Goebels F, Hu LZM, Chen S, Zhang Y, Wierbowski SD, Boudeau S, Moutaoufik MT, Malty RH, Malolepsza E, Tsafou K, Nathan A, Cromar G, Guo H, Abdullatif AA, Apicco DJ, Becker LA, Gitler AD, Pulst SM, Youssef A, Hekman R, Havugimana PC, White CA, Blum BC, Ratti A, Bryant CD, Parkinson J, Lage K, Babu M, Yu H, Bader GD, Wolozin B, Emili A, BraInMap Elucidates the Macromolecular Connectivity Landscape of Mammalian Brain, Cell Syst 10(4) (2020) 333–350 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chung W, Choi SY, Lee E, Park H, Kang J, Park H, Choi Y, Lee D, Park SG, Kim R, Cho YS, Choi J, Kim MH, Lee JW, Lee S, Rhim I, Jung MW, Kim D, Bae YC, Kim E, Social deficits in IRSp53 mutant mice improved by NMDAR and mGluR5 suppression, Nat Neurosci 18(3) (2015) 435–43. [DOI] [PubMed] [Google Scholar]
- [107].Kim MH, Choi J, Yang J, Chung W, Kim JH, Paik SK, Kim K, Han S, Won H, Bae YS, Cho SH, Seo J, Bae YC, Choi SY, Kim E, Enhanced NMDA receptor-mediated synaptic transmission, enhanced long-term potentiation, and impaired learning and memory in mice lacking IRSp53, J Neurosci 29(5) (2009) 1586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sawallisch C, Berhorster K, Disanza A, Mantoani S, Kintscher M, Stoenica L, Dityatev A, Sieber S, Kindler S, Morellini F, Schweizer M, Boeckers TM, Korte M, Scita G, Kreienkamp HJ, The insulin receptor substrate of 53 kDa (IRSp53) limits hippocampal synaptic plasticity, J Biol Chem 284(14) (2009) 9225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ohtakara K, Nishizawa M, Izawa I, Hata Y, Matsushima S, Taki W, Inada H, Takai Y, Inagaki M, Densin-180, a synaptic protein, links to PSD-95 through its direct interaction with MAGUIN-1, Genes Cells 7(11) (2002) 1149–60. [DOI] [PubMed] [Google Scholar]
- [110].Yao I, Hata Y, Ide N, Hirao K, Deguchi M, Nishioka H, Mizoguchi A, Takai Y, MAGUIN, a novel neuronal membrane-associated guanylate kinase-interacting protein, J Biol Chem 274(17) (1999) 11889–96. [DOI] [PubMed] [Google Scholar]
- [111].Iida J, Nishimura W, Yao I, Hata Y, Synaptic localization of membrane-associated guanylate kinase-interacting protein mediated by the pleckstrin homology domain, Eur J Neurosci 15(9) (2002) 1493–8. [DOI] [PubMed] [Google Scholar]
- [112].Yao I, Ohtsuka T, Kawabe H, Matsuura Y, Takai Y, Hata Y, Association of membrane-associated guanylate kinase-interacting protein-1 with Raf-1, Biochem Biophys Res Commun 270(2) (2000) 538–42. [DOI] [PubMed] [Google Scholar]
- [113].Lim J, Ritt DA, Zhou M, Morrison DK, The CNK2 scaffold interacts with vilse and modulates Rac cycling during spine morphogenesis in hippocampal neurons, Curr Biol 24(7) (2014) 786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Houge G, Rasmussen IH, Hovland R, Loss-of-Function CNKSR2 Mutation Is a Likely Cause of Non-Syndromic X-Linked Intellectual Disability, Mol Syndromol 2(2) (2012) 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Vaags AK, Bowdin S, Smith ML, Gilbert-Dussardier B, Brocke-Holmefjord KS, Sinopoli K, Gilles C, Haaland TB, Vincent-Delorme C, Lagrue E, Harbuz R, Walker S, Marshall CR, Houge G, Kalscheuer VM, Scherer SW, Minassian BA, Absent CNKSR2 causes seizures and intellectual, attention, and language deficits, Ann Neurol 76(5) (2014) 758–64. [DOI] [PubMed] [Google Scholar]
- [116].Sun Y, Liu YD, Xu ZF, Kong QX, Wang YL, CNKSR2 mutation causes the X-linked epilepsy-aphasia syndrome: A case report and review of literature, World J Clin Cases 6(12) (2018) 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Polla DL, Saunders HR, de Vries BBA, van Bokhoven H, de Brouwer APM, A de novo variant in the X-linked gene CNKSR2 is associated with seizures and mild intellectual disability in a female patient, Mol Genet Genomic Med 7(10) (2019) e00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lee SE, Kim JA, Chang S, nArgBP2-SAPAP-SHANK, the core postsynaptic triad associated with psychiatric disorders, Exp Mol Med 50(4) (2018) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kawabe H, Hata Y, Takeuchi M, Ide N, Mizoguchi A, Takai Y, nArgBP2, a novel neural member of ponsin/ArgBP2/vinexin family that interacts with synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP), J Biol Chem 274(43) (1999) 30914–8. [DOI] [PubMed] [Google Scholar]
- [120].Cestra G, Toomre D, Chang S, De Camilli P, The Abl/Arg substrate ArgBP2/nArgBP2 coordinates the function of multiple regulatory mechanisms converging on the actin cytoskeleton, Proc Natl Acad Sci U S A 102(5) (2005) 1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lee SE, Kim Y, Han JK, Park H, Lee U, Na M, Jeong S, Chung C, Cestra G, Chang S, nArgBP2 regulates excitatory synapse formation by controlling dendritic spine morphology, Proc Natl Acad Sci U S A 113(24) (2016) 6749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang Q, Gao X, Li C, Feliciano C, Wang D, Zhou D, Mei Y, Monteiro P, Anand M, Itohara S, Dong X, Fu Z, Feng G, Impaired Dendritic Development and Memory in Sorbs2 Knock-Out Mice, J Neurosci 36(7) (2016) 2247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Quitsch A, Berhorster K, Liew CW, Richter D, Kreienkamp HJ, Postsynaptic shank antagonizes dendrite branching induced by the leucine-rich repeat protein Densin-180, J Neurosci 25(2) (2005) 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Apperson ML, Moon IS, Kennedy MB, Characterization of densin-180, a new brain-specific synaptic protein of the O-sialoglycoprotein family, J Neurosci 16(21) (1996) 6839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Santoni MJ, Pontarotti P, Birnbaum D, Borg JP, The LAP family: a phylogenetic point of view, Trends Genet 18(10) (2002) 494–7. [DOI] [PubMed] [Google Scholar]
- [126].Jiao Y, Robison AJ, Bass MA, Colbran RJ, Developmentally regulated alternative splicing of densin modulates protein-protein interaction and subcellular localization, J Neurochem 105(5) (2008) 1746–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Izawa I, Nishizawa M, Ohtakara K, Inagaki M, Densin-180 interacts with delta-catenin/neural plakophilin-related armadillo repeat protein at synapses, J Biol Chem 277(7) (2002) 5345–50. [DOI] [PubMed] [Google Scholar]
- [128].Walikonis RS, Oguni A, Khorosheva EM, Jeng CJ, Asuncion FJ, Kennedy MB, Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin, J Neurosci 21(2) (2001) 423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Matt L, Kim K, Hergarden AC, Patriarchi T, Malik ZA, Park DK, Chowdhury D, Buonarati OR, Henderson PB, Gokcek Sarac C, Zhang Y, Mohapatra D, Horne MC, Ames JB, Hell JW, alpha-Actinin Anchors PSD-95 at Postsynaptic Sites, Neuron 97(5) (2018) 1094–1109 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Wang S, Stanika RI, Wang X, Hagen J, Kennedy MB, Obermair GJ, Colbran RJ, Lee A, Densin-180 Controls the Trafficking and Signaling of L-Type Voltage-Gated Cav1.2 Ca(2+) Channels at Excitatory Synapses, J Neurosci 37(18) (2017) 4679–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A, Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II, J Neurosci 30(15) (2010) 5125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Carlisle HJ, Luong TN, Medina-Marino A, Schenker L, Khorosheva E, Indersmitten T, Gunapala KM, Steele AD, O’Dell TJ, Patterson PH, Kennedy MB, Deletion of densin-180 results in abnormal behaviors associated with mental illness and reduces mGluR5 and DISC1 in the postsynaptic density fraction, J Neurosci 31(45) (2011) 16194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Cabello N, Remelli R, Canela L, Soriguera A, Mallol J, Canela EI, Robbins MJ, Lluis C, Franco R, McIlhinney RA, Ciruela F, Actin-binding protein alpha-actinin-1 interacts with the metabotropic glutamate receptor type 5b and modulates the cell surface expression and function of the receptor, J Biol Chem 282(16) (2007) 12143–53. [DOI] [PubMed] [Google Scholar]
- [134].Chong CH, Li Q, Mak PHS, Ng CCP, Leung EHW, Tan VH, Chan AKW, McAlonan G, Chan SY, Lrrc7 mutant mice model developmental emotional dysregulation that can be alleviated by mGluR5 allosteric modulation, Transl Psychiatry 9(1) (2019) 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gandal MJ, Leppa V, Won H, Parikshak NN, Geschwind DH, The road to precision psychiatry: translating genetics into disease mechanisms, Nat Neurosci 19(11) (2016) 1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, Singh T, Klei L, Kosmicki J, Shih-Chen F, Aleksic B, Biscaldi M, Bolton PF, Brownfeld JM, Cai J, Campbell NG, Carracedo A, Chahrour MH, Chiocchetti AG, Coon H, Crawford EL, Curran SR, Dawson G, Duketis E, Fernandez BA, Gallagher L, Geller E, Guter SJ, Hill RS, Ionita-Laza J, Jimenz Gonzalez P, Kilpinen H, Klauck SM, Kolevzon A, Lee I, Lei I, Lei J, Lehtimaki T, Lin CF, Ma’ayan A, Marshall CR, McInnes AL, Neale B, Owen MJ, Ozaki N, Parellada M, Parr JR, Purcell S, Puura K, Rajagopalan D, Rehnstrom K, Reichenberg A, Sabo A, Sachse M, Sanders SJ, Schafer C, Schulte-Ruther M, Skuse D, Stevens C, Szatmari P, Tammimies K, Valladares O, Voran A, Li-San W, Weiss LA, Willsey AJ, Yu TW, Yuen RK, Study DDD, Homozygosity A Mapping Collaborative for, Consortium UK, Cook EH, Freitag CM, Gill M, Hultman CM, Lehner T, Palotie A, Schellenberg GD, Sklar P, State MW, Sutcliffe JS, Walsh CA, Scherer SW, Zwick ME, Barett JC, Cutler DJ, Roeder K, Devlin B, Daly MJ, Buxbaum JD, Synaptic, transcriptional and chromatin genes disrupted in autism, Nature 515(7526) (2014) 209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


