INTRODUCTION
Painful temporomandibular disorders (TMDs) are characterized by localized pain in the jaw joint/s and/or masticatory muscles in conjunction with limited jaw function. According to the National Health and Interview Survey (NHIS) TMD affects 4.6% of the U.S. adult population, with prevalence higher among women than men[36]. The experience of ongoing jaw pain can evoke psychological distress as pain imposes restrictions on work and social activity[8; 21; 38]. Moreover, impairment is often compounded by the presence of other pain disorders that occur comorbidly with TMD. For example, people with TMD-like pain commonly reported comorbid headache/migraine, neck, or lower back pain; and more than half reported at least two more areas of severe pain[36].
Disability associated with TMD has been studied using a variety of related constructs: oral health related quality of life, interference in daily activities, functional limitation, and activities of daily living. We focus on the subjective experience of disability, defined by Nagi[32] (1965) as “a pattern of behavior that evolves in situations of long-term or continued impairments that are associated with functional limitations”. Nagi’s model distinguishes disability from functional limitation, pathology, and impairment while acknowledging the interrelationships between the concepts. Our conceptual model reflects concepts of disability and functional limitation that directly corresponding to Nagi’s concepts. To address pathology and impairment, we explored the role of psychological unease and experimental pain sensitivity, the latter of which has been suggested as an indicator of dysfunction in the regulatory pain pathways among people with TMD[30; 40].
We selected the most widely used measure of pain-related disability that is well validated and reliable: the Graded Chronic Pain Scale (GCPS). Two factors influenced the decision to use this measure: the generalizability and use of this scale in chronic pain populations other than TMD and the recommendation of the National Pain Strategy to use screening instruments that assess chronic pain severity and interference[44].
Among people with orofacial pain, high pain-related disability is associated with increased healthcare spending[14], underscoring the importance of understanding pain-related disability. Previous research has identified associations between psychological functioning and pain-related disability among people with TMD. Specifically, pain catastrophizing[4; 43], depression[1; 3; 15], and somatic symptoms [25; 26] were associated with increased pain-related disability in cross-sectional studies. In a longitudinal cohort, catastrophizing was predictive of higher GCPS while depression was not [46]. These studies have addressed pain-related disability in smaller populations compared to this study, definitions of pain-related disability have been varied, and the complex relationship between factors representing psychological and physical variables has not been assessed presumably due to sample size or an absence of data on a variety of participant characteristics.
Objectives
This study had two aims to explore the relationship between several factors that we believe have an effect on pain-related disability: 1) to assess the importance of a measure of presenteeism (typically defined as working while ill[22]) incorporated into the existing framework of pain-related disability using the GCPS, and 2) to explore the effect of psychological unease, experimental pain features, and jaw limitation on the outcome of pain-related disability.
MATERIALS AND METHODS
Study design
This secondary analysis was performed on cross-sectional data from 1088 adults with painful chronic TMD enrolled in the parent study, The Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study. Details of the OPPERA study protocol and procedures have been published elsewhere[41].
Setting
Between May 2006 and October 2013, community-dwelling adults with chronic TMD responded to study recruitment advertisements at one of four study sites – (University at Buffalo, NY, University of Florida in Gainesville, FL, University of Maryland in Baltimore, MD and University of North Carolina at Chapel Hill, NC).
Participants
Although OPPERA enrolled cases and controls, the current analysis is restricted to cases. Participants were aged 18–44 years and fluent in English. Exclusion criteria were: recent facial surgery or facial injury, pregnancy, orthodontic procedures, and major medical conditions[41]. Participants completed a telephone interview to assess eligibility prior to completing a battery of questionnaires and attending a 3-hour clinic visit that included a clinical examination for verification of TMD and experimental pain testing.
Chronic TMD was defined as self-reported facial pain symptoms persisting at least 6 months prior to enrollment AND meeting physical examination criteria. The latter required pain evoked by palpation in at least three masticatory muscle groups or pain in the temporomandibular joint, consistent with established guidelines of the Research Diagnostic Criteria for Temporomandibular Disorder (RDC//TMD)[41].
Human Research Ethics Committees at all study sites approved the study protocol. All participants provided signed informed consent for study participation.
Measures
Variables included self-reported questionnaire responses and experimental pain sensitivity testing results. Demographic data were collected upon entry: age, self-reported sex, self-identified racial identity, and study site.
Pain-related disability
The GCPS measures the extent to which pain is perceived as intense and/or interfering and the degree to which the pain is disabling[46]. The GCPS consists of three domains: characteristic pain intensity, interference, and disability days. Characteristic pain intensity (CPI) includes average, current, and worst pain rated using the 0–10 scale. Pain intensity is measured on a scale ranging from 0 to 10, where 0 represents ‘no pain’ and 10 represents ‘pain as bad as it could be’. Interference is rated from 0 (no interference) to 10 (unable to carry on any activities) in three areas of life: 1) daily activities, 2) social, family, and recreation, and 3) work activities. Disability days are calculated using a question about the number of days in which pain resulted in restriction of daily activities. These three domains and their subscale scores are combined to represent a “chronic pain grade” from 0 (no pain) to IV (high disability)[17]. The items from the GCPS are used to create a pain grade from 0 to IV. The GCPS has been shown to predict poorer outcomes among pain patients and higher pain grades are associated with increased healthcare spending among people with TMD. For these reasons, we believe the information collected by the GCPS items is key to understanding pain impact. In order to focus on reducing pain impact, we need to first identify impact which the GCPS is designed to do well. However, in order to step back to the conceptual underpinnings of the GCPS and instead of using the pain grade outcome, we used the individual items as observed variables contributing to latent constructs and then test the direct effect of other variables of interest on this construct of pain-related disability.
Restriction
In addition to asking about the inability to perform usual activities, it is important to assess limitations in performance, with the acknowledgement that restriction from activities and limitation in activities measure two distinct but related phenomena. Unlike other conditions such as acute pain from a broken bone, many people with TMD are able to complete their normal daily activities, although they do so while experiencing pain. This idea of presenteeism is becoming more important to understand as it relates to chronic pain in part because of economic research that has identified presenteeism as a cause of decreased productivity. The definition of presenteeism is typically showing up to work while sick[22], which people with TMD do on a daily basis.
In 2004, when planning the OPPERA study, we therefore added a question asking about the number of days when a person’s efficiency dropped below 50% of what they considered normal. This allows for an assessment of the amount of time the individual is still completing their activities but in a limited capacity. At the time, it was a novel concept in TMD research, having been recognized only a few years earlier in the 2001 WHO International Classification of Functioning, Disability, and Health as participation (restriction) [47]. Because it was an untested concept, the OPPERA investigators were reluctant to add other measures of restriction to the study, and instead focused on established biopsychosocial measures when selecting the hundreds of other measures used in the study. However, those other measures are not applicable to the WHO’s rubric, whereas presenteeism is, and which accounts for our focus on presenteeism in this study.
We tested a modified version of the GCPS with the addition of a question to assess presenteeism. We created a latent variable called “restriction” which included two items: 1) the disability days question about number of days prevented from activity (called disability days in the GCPS framework) and 2) a question asking about reduction in efficiency below 50% of what is normal for the individual. This question comes the closest to addressing presenteeism, but is inclusive of restriction outside the workplace.
Psychological unease.
Psychological distress was measured using positive and negative affect scales from the Profile of Mood States-Bipolar (POMS-Bi)[28], somatization subscale from the Symptoms Checklist 90-Revised (SCL-90R)[13] (referred to here as a measure of somatic symptoms), and the catastrophizing subscale of the Coping Strategies Questionnaire (CSQ-R)[39].
Jaw limitation.
The Jaw Functional Limitation Scale (JFLS) measured limitations in three areas: chewing, opening, and verbal or emotional expression limitation[34].
Experimental pain sensitivity.
Experimental pain sensitivity included measures of thermal tolerance, pain ratings of a single application of flat-tipped probe using the 0–100 scale, mechanical temporal summation of pain measured as the change in pain rating from the first application of the probe and the pain rating after 10 applications, and pressure pain threshold (PPT) evaluated at the trapezius muscle. The trapezius PPT was selected because it is unrelated to the case definition of TMD. Full details of experimental testing procedures are described in Greenspan 2011[18].
Statistical analysis
Structural equation modeling (SEM) is an ideal method for examining the relationship between variables such as psychological functioning and pain-related disability for several reasons. SEM is unique in the ability to create latent constructs, conceptual variables comprised of observed measures that are highly correlated. SEM allows creation of a latent variable measuring a construct with at least two observed measures. If each measure of interest was included in multivariable linear regression, multicollinearity would likely occur. In contrast, creating a latent variable based on several observed measures permits illustration of a concept based on the combination of several measures while maintaining a high degree of granularity. Confirmatory factor analysis (CFA) is used to determine if observed data fit a defined model.
In testing our SEM, we first constructed measurement models and then regressed the latent factor of pain-related disability onto the measurement models, as recommended by Anderson and Gerbing [2]. All analyses were performed using the Mplus software, version 8.0[31]. After generating univariate statistics to summarize the data, we conducted three separate analyses. First, we constructed our measure of pain-related disability combining GCPS items with the question asking about decreased efficiency. Second, we performed confirmatory factor analysis (CFA) to create latent variables representing: 1) psychological unease, 2) jaw limitation, and 3) experimental pain sensitivity. Supplementary material Table S.1 shows each measurement and instrument included in measurement model building. Finally, we built a SEM to explore the relationships between the three latent variables and the variable representing pain-related disability.
Our hypothetical model is shown in Figure 1. On the left side of this model are indicators of psychological unease comprised of four self-reports using standardized instruments, jaw limitation measured by self-reports, and clinical assessments and experimental pain sensitivity assessed by four quantitative sensory tests. The right side of the model shows our model of pain-related disability comprised of three latent variables measuring pain intensity, interference, and restriction from activities. We hypothesized that pain-related disability would be positively associated with increased psychological unease, functional limitation, and experimental pain sensitivity. This finding would indicate a strong association between the variables we created based on Nagi’s model and pain-related disability.
Figure 1.
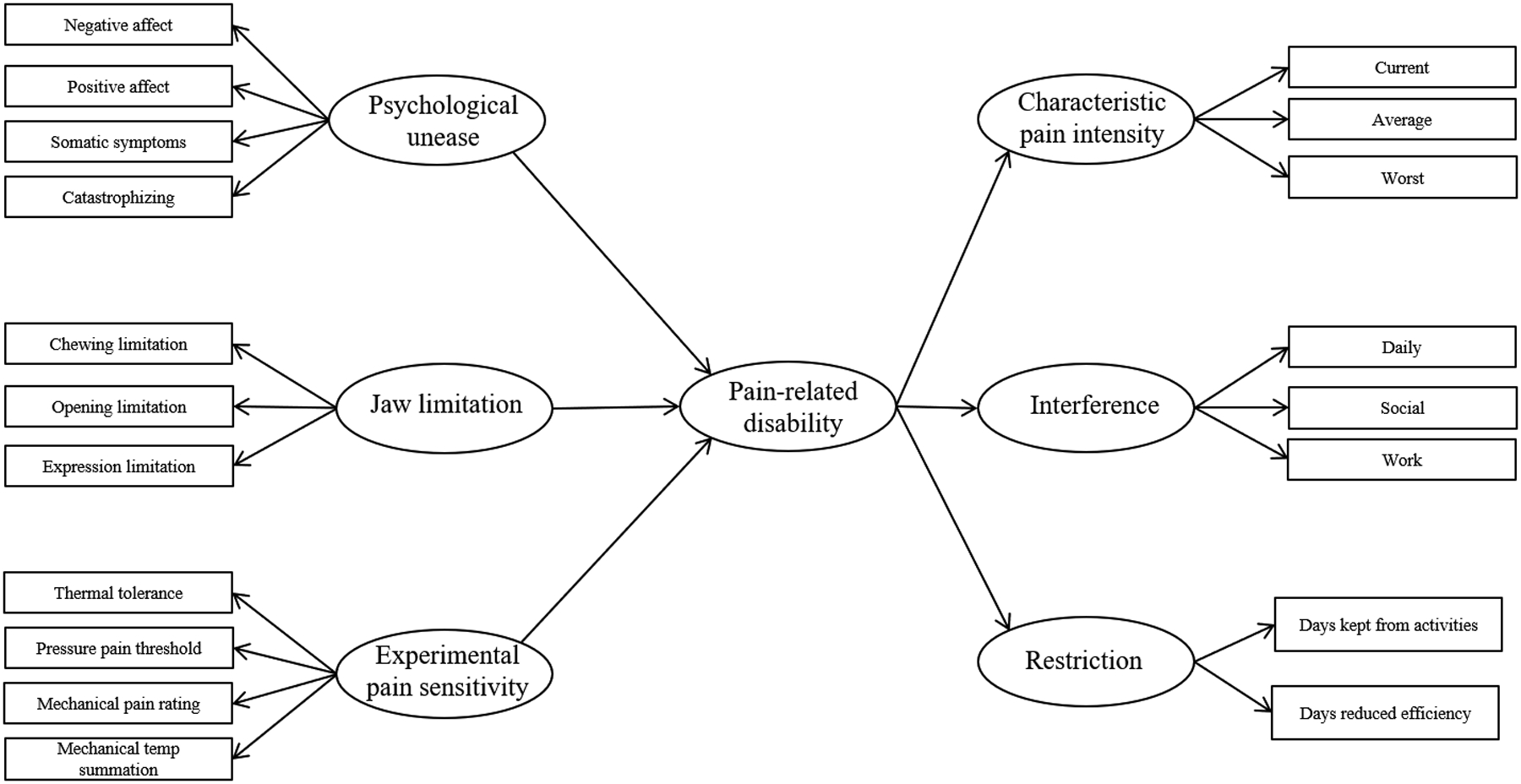
Hypothesized model of pain-related disability and constructs contributing to pain related disability. Latent variables are represented by circles while observed variables are shown as rectangles. Arrows from latent variables onto observed variables represent the variables used to create the latent construct. Arrows between latent variables represent hypothesized relationships to be tested.
SEM has the benefit of examining multiple relationships simultaneously. Latent variables are useful tools to capture the information obtained from observed measures and concurrently account for the different influence each observed variable imparts on the underlying construct[23].
Structural equation modeling.
Robust maximum likelihood was used to address non-normality of variable distribution. Models were evaluated first by assessing fit statistics. Model fit was evaluated based on published guidelines for goodness of model fit. Absolute fit indices included the Chi-square test of model fit and root mean square error of approximation (RMSEA). The RMSEA tells us how well the model, with unknown but optimally chosen parameter estimates, would fit the population’s covariance matrix. Incremental fit indices included the comparative fit index (CFI) and Tucker-Lewis Index (TLI), both of which account for sample size and performance of the model[19]. Criteria for the model fitness was based on established values for fit indices: CFI/TLI values ≥ 0.95, an RMSEA <0.07[20].
After verifying the fit of each CFA, we built a SEM and then performed post-hoc exploratory analysis to improve the fit based on the above criteria for goodness of fit. Model modifications involved variable deletion and accommodation for correlation of errors between variables. Code for the SEM analyses is available upon request.
Assessing pain-related disability with presenteeism
In order to determine validity of the observed variable assessing presenteeism, we compared a model without the presenteeism variable and a model including the measure of presenteeism. We compared two first-order models: one with the seven traditionally used GCPS items and a second model including the variable measuring presenteeism. A second-order CFA is distinguished from a first-order in that the second-order CFA involves creation of a higher-level latent variable comprised of lower-level latent variables.
Second-order analysis is not possible unless there are at least two variables used to create each lower level latent variable. Ideally, we would test the latent restriction variable using a second-order CFA of pain-related disability with first-order variables representing constructs of pain intensity, interference and restriction. However, this is not possible with the 7-item model which contained only the single variable, ‘days kept from activity’, to measure restriction. Based on the first order CFA result, we inspected parameter estimates and goodness of fit statistics to determine the impact of including the presenteeism variable. If the fit statistics did not reflect a poorer fitting model with the additional variable, then based on the theoretical framework and conceptual model, we were justified to proceed using a second-order CFA of pain-related disability with latent variables for pain intensity, interference, and restriction.
Measurement models
Jaw limitation.
We started with a model that included the following three observed variables: the JFLS chewing limitation, opening limitation, expression limitation scores.
Psychological unease.
We began with a model that included the following four observed variables: 1) the catastrophizing subscale, 2) the somatic symptoms scale, 3) positive and 4) negative affect scales.
Experimental pain sensitivity.
We built a model using the latent variable experimental pain sensitivity with the factors of heat pain tolerance, pressure pain threshold, mechanical pain rating, and mechanical temporal summation.
Structural Equation Model
To explore the relationships between the latent constructs of jaw limitation, psychological distress and catastrophizing, experimental pain sensitivity, and pain-related disability we built a SEM regressing the measurement models onto the model of pain-related disability. Sex, age, study site, and race were regressed onto pain-related disability to statistically control for these variables. Demographic variables were regressed onto pain-related disability based on previous findings. Study site was included to control for study design. The model was evaluated based on fit indices and cutoff scores described above. Modification indices calculated by Mplus were used to guide decisions to improve model fit. Additionally, tests of the model were performed by restricting by sex and restricting to each study site. This analysis was performed using the final model to examine potential sex differences and study site differences in model performance and fit.
RESULTS
Participants
The mean age of participants was 29.2 years (7.8 SD) with pain persisting for 6.9 years (6.4 SD). Overall, 70% were white and 76% were female with participants evenly distributed across study sites. Participants rated their current facial pain at a mean of 3.8 (2.5 SD) using the 0 to 10 scale, while the mean worst facial pain in the past 6 months was 7.5 (2.0 SD). The “disability days” variables had a non-normal distribution with 50% of the sample reporting they did not experience any days when they were kept from their usual activities because of facial pain. Reduced efficiency was more common with 25% of the sample reporting 35 or more days during which their efficiency dropped below 50% of what they considered to be normal. Completeness of data, mean, minimum, and maximum variables were analyzed and are shown in Table 1.
Table 1.
List of observed variables comprising latent variables for measurement models
| Variable notaStion | Latent variable name | Observed variable | N | Mean | Min | Max |
|---|---|---|---|---|---|---|
| UNEASE | Psychological unease | Somatic symptoms scale | 1073 | 0.7 | 0.0 | 3.1 |
| Catastrophizing scale | 1079 | 1.5 | 0.0 | 6.0 | ||
| Positive affect score | 1082 | 80.3 | 35.0 | 118.0 | ||
| Negative affect score | 1082 | 58.6 | 30.0 | 111.0 | ||
| JAW | Jaw function | JFLS chewing limitation score | 1069 | 2.5 | 0.0 | 10.0 |
| JFLS opening limitation score | 1069 | 2.7 | 0.0 | 10.0 | ||
| JFLS expression limitation score | 1069 | 1.2 | 0.0 | 10.0 | ||
| EXP | Experimental pain sensitivity | Thermal tolerance (°Celsius) | 1013 | 45.4 | 33.5 | 51.5 |
| Pressure pain threshold Trapezius (kPa) | 1013 | 277.6 | 101.6 | 600.0 | ||
| Mechanical probe pain rating | 1047 | 12.0 | 0.0 | 94.8 | ||
| Mechanical temporal summation (change in pain rating) | 1047 | 13.3 | −13.7 | 85.0 | ||
| PRD | Pain-related disability | |||||
| CPI | Characteristic pain intensity | Current pain intensity | 1062 | 3.8 | 0.0 | 10.0 |
| Average pain intensity | 1062 | 5.4 | 0.0 | 10.0 | ||
| Worst pain intensity | 1062 | 7.5 | 0.0 | 10.0 | ||
| INTER | Pain interference | Interference in daily activities | 1062 | 2.5 | 0.0 | 10.0 |
| Interference in social activities | 1062 | 2.4 | 0.0 | 10.0 | ||
| Interference in work activities | 1063 | 2.2 | 0.0 | 10.0 | ||
| RES | Restriction | Days kept from activities (past 6 months) | 1054 | 17.5 | 0.0 | 180.0 |
| Days with reduced efficiency (past 6 months) | 1049 | 29.0 | 0.0 | 180.0 | ||
kPa=kilopascals; mm=millimeters
Pain-related disability CFA model
Results from the measurement models of pain-related disability with and without the variable measuring presenteeism were compared. Results from the measurement models of pain-related disability with and without the variable measuring presenteeism were compared. The first-order seven-variable model had poor fit (RMSEA=0.219, 90% CI for RMSEA (0.207, 0.234), CFI=0.754, TLI=0.631, SRMR=0.108). The first-order model with eight variables demonstrated slightly better fit (RMSEA=0.197, 90% CI for RMSEA (0.186, 0.209), CFI =0.751, TLI=0.652, SRMR=0.105) than the model without the presenteeism variable. Despite fit indices supporting the conclusion that neither model demonstrates good fit with the data, based on the less desirable RMSEA for the model without presenteeism compared to the model with presenteeism (0.219 versus 0.197 respectively), the slight increase in the TLI and the slight decrease in the SRMR, indicates inclusion of presenteeism in the first-order CFA improves model fit [5].
The standardized parameters show the highest contribution to variance in the pain-related disability latent variable from the interference latent variable (0.88). The restriction latent variable had a high loading (0.82) and the pain intensity latent variable was statistically significant but had a lower standardized value (0.66) compared to the other two variables. The high direct effect of the restriction latent variable suggests restriction is relevant to pain-related disability.
This second order model of pain-related disability demonstrated excellent fit (RMSEA=0.048, 90% CI for RMSEA (0.035, 0.062), CFI =0.987, TLI=0.979, SRMR=0.021). These results support the conclusion that the addition of a restriction latent variable provides useful information in understanding the construct of pain-related disability. (Supplementary materials, Figure S.1)
Measurement models
The measurement model based on our hypothesized model assessing jaw limitation demonstrated a poor fit (Table 2, Model 1). First, the positive affect variable was eliminated from the model because the negative affect variable was deemed sufficient (Model 2). Next, correlation between errors was permitted between the following variable pairs: somatic symptoms and negative affect and chewing and opening limitation (Model 3). The model building process with parameter estimates and model fit indices is shown in Table 2.
Table 2.
Standardized parameter estimates from 3 models starting with the full model and then performing exploratory model alterations to obtain the best-fitting model (n=1088). Model 1 includes all variables shown in Figure 1, the original conceptual model. Model 2 does not include positive affect variable. In Model 3, error variances for the following variable pairs were allowed to correlate: 1) negative affect and somatic symptoms and 2) chewing and opening limitation.
| Model 1* | Model 2+ | Model 3ǂ | |
|---|---|---|---|
| Psychological unease | |||
| Somatic symptoms | 0.660 | 0.793 | 0.605 |
| Catastrophizing | 0.555 | 0.608 | 0.777 |
| Positive affect | −0.595 | ||
| Negative affect | 0.783 | 0.615 | 0.448 |
| Jaw limitation | |||
| Chewing limitation | 0.771 | 0.773 | 0.604 |
| Opening limitation | 0.887 | 0.882 | 0.707 |
| Expression limitation | 0.716 | 0.722 | 0.899 |
| Experimental pain sensitivity | |||
| Thermal tolerance | 0.548 | 0.542 | 0.552 |
| Pressure pain threshold | 0.413 | 0.413 | 0.412 |
| Mechanical pain rating | −0.512 | −0.517 | −0.509 |
| Mechanical temporal summation | −0.451 | −0.453 | −0.454 |
| Chi2 (df) | 314 (41) | 166 (32) | 93 (30) |
| p-value | <0.001 | <0.001 | <0.001 |
| RMSEA (90% CI) | 0.078 (0.070, 0.086) | 0.062 (0.053, 0.071) | 0.044 (0.034, 0.054) |
| CFI | 0.886 | 0.934 | 0.969 |
| TLI | 0.847 | 0.907 | 0.953 |
| SRMR | 0.065 | 0.051 | 0.036 |
Model 1: full model including all variables from proposed conceptual model ;
Model 2: dropped variable measuring positive affect;
Model 3: allowed correlation between negative affect and somatic symptoms and correlation between chewing and opening limitation; df=degrees of freedom; RMSEA= Root mean squared error; CI=confidence intervals; CFI=Comparative Fit Index; TLI=Tucker Lewis Index; SRMR=Standardized Root Mean Square Residual
A single factor model with four indicator variables was proposed to measure psychological unease defined by the following indicators: somatic symptoms, catastrophizing, positive and negative affect. The fit of this model was improved after the removal of the positive affect variable.
A single factor model with three indicator variables was proposed to measure jaw limitation using the following indicators: opening limitation, chewing limitation, expressional limitation (all from the JFLS).
Fit indices for the final exploratory measurement model demonstrated very good fit shown in Table 2 (Chi2=93 (p<0.001), df=30, RMSEA=0.044, 90% CI for RMSEA (0.034, 0.054), CFI=0.969, TLI=0.953, SRMR=0.036). Psychological unease and jaw limitation variables were positively associated at 0.50. Both psychological unease and jaw limitation variables were negatively associated with experimental pain sensitivity but the relationship was not strong (standardized parameter estimates −0.24 and −0.23 for psychological unease and jaw limitation, respectively).
Structural Equation Model
The above measurement model result met the criteria specified in the methods section for goodness of fit, permitting the next step of testing the model. Based on the measurement model results, the next step was to fit a SEM of latent variables representing psychological unease, jaw limitation, and experimental pain sensitivity regressed onto pain-related disability. Table 3 outlines the model-fitting process and the respective fit indices for each alternative model. The initial model had modification indices indicating improvements in the model specification might improve the fit of the model. Removal of the experimental pain sensitivity latent variable was based on the low the direct effect on pain-related disability balanced with the clinical burden on patients and clinicians performing this test that is time consuming, uncomfortable, and requires sophisticated equipment.
Table 3.
Standardized parameter estimates from 3 models starting with the full model and then performing exploratory model alterations to obtain the best-fitting model. Model 1 includes all variables included in the measurement model. Model 2 dropped the experimental pain sensitivity latent variable. In Model 3, to improve model fit, correlation among variable pairs were permitted between the following pairs: Interference and Restriction, chewing and opening limitation, negative affect and somatic symptoms, and Characteristic pain intensity variables measuring average and worst pain.
| Model 1* | Model 2+ | Model 3ǂ | |
|---|---|---|---|
| N | 1088 | 1085 | 1085 |
| Characteristic pain intensity | |||
| Current | 0.719 | 0.718 | 0.771 |
| Average | 0.866 | 0.866 | 0.791 |
| Worst | 0.775 | 0.774 | 0.683 |
| Interference | |||
| Daily | 0.883 | 0.883 | 0.882 |
| Social | 0.907 | 0.907 | 0.907 |
| Work | 0.904 | 0.903 | 0.903 |
| Restriction | |||
| Days kept from activity | 0.757 | 0.758 | 0.756 |
| Days less efficient | 0.875 | 0.873 | 0.874 |
| Jaw limitation | |||
| Chewing limitation | 0.780 | 0.781 | 0.643 |
| Opening limitation | 0.859 | 0.858 | 0.733 |
| Expression limitation | 0.740 | 0.740 | 0.859 |
| Psychological unease | |||
| Somatic symptoms | 0.742 | 0.743 | 0.619 |
| Catastrophizing | 0.684 | 0.684 | 0.764 |
| Negative affect | 0.571 | 0.571 | 0.421 |
| Experimental pain sensitivity | |||
| Thermal tolerance | 0.5357 | ||
| Pressure pain threshold | 0.419 | ||
| Mechanical pain rating | −0.518 | ||
| Mechanical temporal summation | −0.453 | ||
| Structural model | |||
| UNEASE→PRD | 0.484 | 0.519 | 0.579 |
| JAW→PRD | 0.413 | 0.445 | 0.406 |
| EXP→PRD | −0.201 | ||
| Age→PRD | 0.135 | 0.128 | 0.131 |
| Study site→PRD | 0.131 | 0.131 | 0.123 |
| Race→PRD | 0.029 | 0.058 | 0.059 |
| Sex→ PRD | −0.041 | 0.001 | 0.007 |
| Model fit statistics | |||
| Chi2 (df) | 842 (193) | 564 (122) | 408 (119) |
| P value | <0.001 | <0.001 | <0.001 |
| RMSEA (90% CI) | 0.056 (0.052, 0.059) | 0.058 (0.053, 0.063) | 0.047 |
| CFI | 0.912 | 0.935 | 0.958 |
| TLI | 0.898 | 0.922 | 0.948 |
| SRMR | 0.057 | 0.054 | 0.040 |
Model 1: full model including all variables from measurement model ;
Model 2: dropped experimental pain sensitivity variable;
Model 3: permitted correlation between ; UNEASE=Psychological unease; JAW=jaw limitation, EXP= experimental pain sensitivity; PRD=pain-related disability
Next, we allowed correlation among the error terms for the following pairs of variables: negative affect and somatic symptoms, chewing limitation and opening limitation, average and worst pain intensity ratings, and the interference and restriction latent variables. These exploratory changes resulted in a final model, the results of which are shown in Figure 2 and supplementary material Table S.2. The final model is depicted in Figure 2. This is a well-fitting model (fit indices: RMSEA=0.048, 90% CI=0.043, 0.053, CFI=0.956, TLI=0.946, SRMR=0.040). The model explained 78% of variation in pain-related disability.
Figure 2.
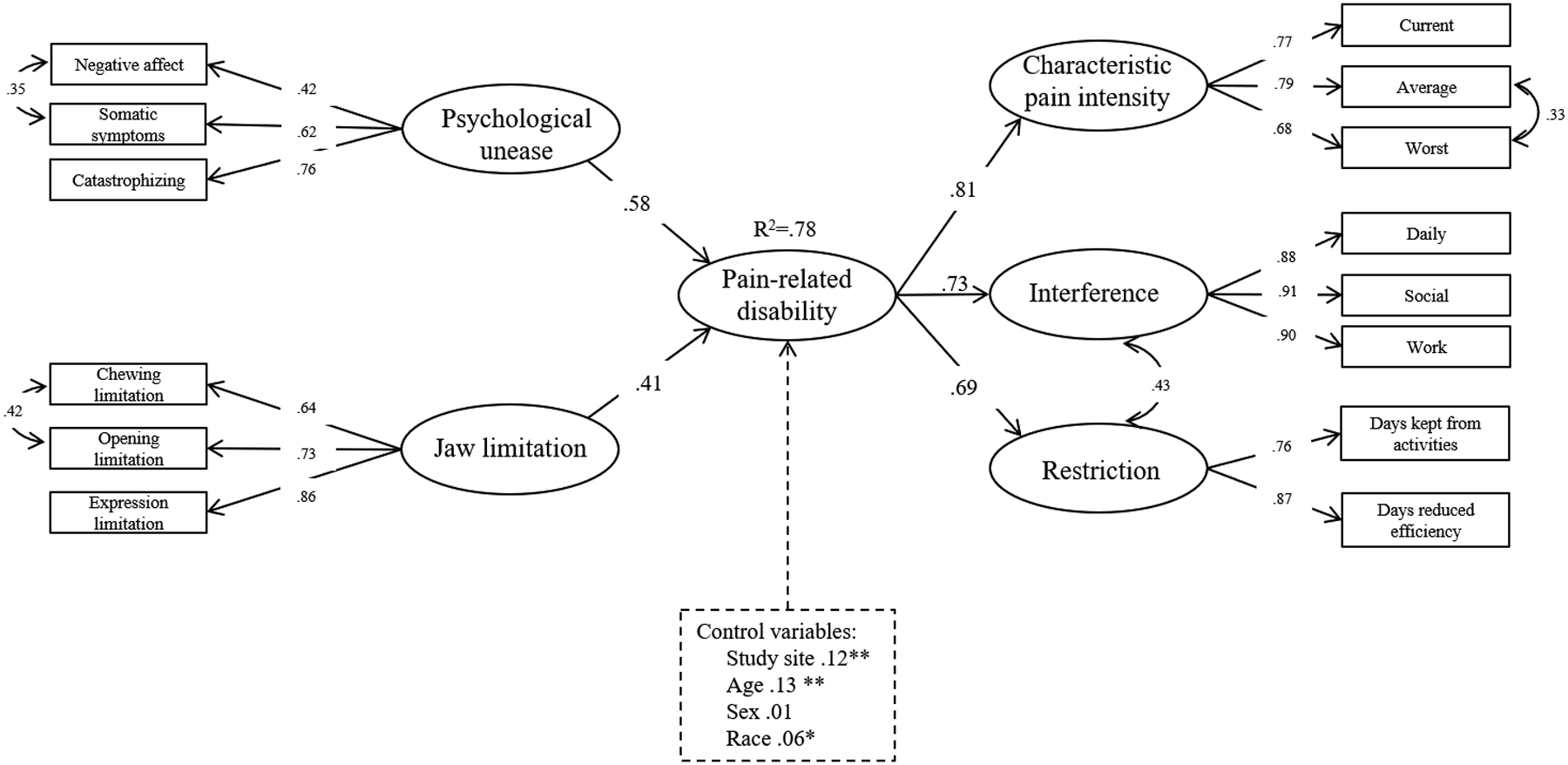
Final model and standardized parameter estimates of pain-related disability, psychological unease, and jaw limiation. Latent variables are shown in circles while observed variables are in rectangles. Control variables are shown surrounded by a dashed line. Curved arrows refer to covariance.
*p<.05 **p<0.001
We controlled for study site, sex, age, and race in the model even though sex was not significantly associated with pain-related disability. Sex is frequently a variable of interest in chronic pain research due to the higher prevalence of chronic pain among women compared with men and therefore was retained in the final model. Jaw limitation and psychological unease had significant direct effects on the latent pain-related disability variable (.41 and .58 respectively). Parameter estimates, standard errors and p-values for all variables in the final measurement model are shown in supplementary materials (Table S.2 and Figure S.2).
Restricting the models by sex resulted in a decrease in the effect of jaw limitation on pain-related disability (0.41 to 0.35) and an increase in psychological unease on pain-related disability (0.58 to 0.61) when the sample was restricted to the 253 males. The R2 for the male only model was 0.77 with no other changes in results. Among 832 females, the direct effect of jaw limitation on pain-related disability was only slightly lower (0.58 and 0.56) and the effect of jaw limitation was slightly higher (0.41 and 0.43). The R2 value for the model restricted to females only was 0.78, which was identical to that of the combined model. This is consistent with the lack of statistical significance of the sex variable in the earlier. Although study site was statistically significant in the larger model, the results of running the models restricted to each study site showed small differences in model fit or direct effects with R2 ranging from 0.66 in the sample from UNC to 0.81 when the sample was restricted to only participants from the UB study site (results not shown).
DISCUSSION
Key Results
This study demonstrated the importance of measuring reduced efficiency when understanding pain-related disability among people with chronic painful TMD. Furthermore, the SEM revealed strong positive relationships between self-reported jaw limitation and psychological unease and pain-related disability. In the SEM, age and race were significantly associated with pain-related disability. Pain-related disability increased with age and was more common among people of color. These parameter estimates were much lower than estimates for the variables of interest (all less than 0.10). Sex was the only sociodemographic variable that was not significantly associated with pain-related disability.
Three studies have, to our knowledge, included latent variables in conceptual models of psychological distress and jaw function along with an indicator of pain-related disability among people with TMD[6; 9; 11]. One study combined jaw functional limitation and the two summary scores from the GCPS as a measure of “TMD impact”[6] whereas our model differentiated between disability and functional limitation. While their finding supports our conclusion that items from the GCPS and JFLS are useful tools to measure the impact of TMD, our structural model illustrates that GCPS and JFLS are measuring different constructs. All previous studies involved a smaller number of participants than our study and pain-related disability was measured inconsistently, using other measures such as the Multidimensional Pain Inventory[11] and the Integrated Multidimensional Patient Assessment Tool for Health[9]. However, the measurement models tested in a cross-sectional sample of 251 masticatory muscle pain patients resulted in a well-fitting model with significant associations between variables representing psychological distress and pain symptoms [9] which is consistent with our finding.
Interpretation
As psychological unease and jaw limitation increased, pain-related disability also increased. Low factor loadings support the conclusion that several variables had no association with pain-related disability. Experimental pain sensitivity, jaw limitation including oral parafunction behavior, pain chronicity, number of comorbid conditions, and the number of painful body sites were found to not contribute to the model and were removed in further analyses.
Catastrophizing loaded slightly higher than somatic symptoms on the psychological unease latent variable indicating that the impact of catastrophizing may be very important in understanding the construct of pain-related disability. This is consistent with previous research about the association between catastrophizing and TMD pain[4; 10; 16; 27; 43]. In a cross-sectional study of a rural chronic pain population, pain catastrophizing was reportedly associated with pain intensity, interference and perceived disability. The relationship between catastrophizing and perceived disability persisted even after controlling for demographics and depression[12]. Our study sites represent a mix of urban and suburban areas with the Baltimore site likely being the most urban. Our restricted analysis by study site resulted in no differences in model fit by study site.
Both psychological unease and functional limitation have been found to be associated with high pain-related disability [15; 24; 26]. This work is the first to model experimental pain sensitivity as a latent construct and examine the relationship between experimental pain sensitivity and pain-related disability. The inclusion of experimental pain sensitivity was based on the biopsychosocial model of pain[42] and the hypothesis that experimental pain testing may measure biological processes that underlie chronic painful TMD. Research identified somatosensory amplification (increased perception of normal sensation as intensity and or distressing) among women with chronic orofacial pain[37]. Somatosensory amplification is thought to be a feature common among many pain conditions including fibromyalgia and also among several psychiatric conditions such as anxiety[33]. Although people with TMD have lower pressure pain thresholds[7; 18] and thermal tolerance[35] when compared to pain-free individuals, our finding supports the conclusion that experimental pain sensitivity did not have a strong direct effect on pain-related disability. This model performed well when restricted to only female or male participants, but conclusions cannot be made about the role of demographic characteristics in the individual observed or latent variables because age, sex, race, and study site in the final model were regressed only on the pain-related disability variable.
Strengths of the study include the large sample size of community dwelling participants with chronic painful TMD. The sample size and recruitment of people from surrounding communities as opposed to specialty pain clinics supports the generalizability of findings to people with chronic painful TMD.
A study of pain-related disability among people with chronic pain conditions including low back pain[29] utilized SEM to model relationships between factors related to disability. Among 156 patients with low back pain, predictors of increased disability included female sex, depression, and fear avoidance beliefs while fulltime employment predicted decreased disability[29]. Our finding contrasts this finding that sex was associated with disability but supports the conclusion that increased negative affect is associated with increased disability.
Limitations
The primary limitation of this study is the cross-sectional design, which precludes causal inference. The SEM approach appropriately assumes causality by the use of one-way arrows between exogenous and endogenous variables, but within endogenous variables, causality cannot be inferred. When selecting variables to identify in regression models, a casual structure is imposed on the data. However, the strength of evidence reported here for a conceptual model requires testing in a longitudinal setting for true causality.
SEM has multiple strengths that made the approach the optimal choice for this analysis. SEM, as implemented via MPlus, permits complex continuous outcome variables, accounts for correlated variables, measurement error, handles missing data well, and performs simultaneous examination of multiple relationships between variables. However, SEM requires strong assumptions that cannot be overlooked. These include the assumption of linear relationships between variables and the assumption of no unmeasured confounding. In this study, there is the possibility that an unmeasured variable is the cause of increased psychological unease, jaw limitation, and pain-related disability. These assumptions have been cited as a reason for caution when using SEM, particularly the recommendation that SEM is best used when there are many effects being explored for hypothesis generation[45].
Interpreting the latent variables also requires caution. Although we began with a variable to describe jaw limitation including body pain sensitivity and comorbid conditions, after testing the measurement model and performing exploratory changes, our variable changed. The final variable originally called “jaw limitation” was reduced to specifically measuring jaw limitations. This change resulted in our final model not including the broader intended jaw limitation. This doesn’t mean the excluded variables are not important for considering pain-related disability among people with TMD, but they did not load together with functional limitation variables. Another model might include these variables in the form of an additional construct.
Conclusion
We specified and estimated a model based on theory and previous literature to examine factors conceptually important to understanding pain-related disability as well as factors potentially associated with pain-related disability. This model included latent constructs measuring psychological unease, jaw functional limitation, experimental pain sensitivity, and pain-related disability.
The results of this cross-sectional study of people with chronic TMD suggest psychological unease and jaw functional limitations are important factors to assess in order to understand pain-related disability. Results also demonstrate that experimental pain sensitivity, maximum unassisted jaw opening, chronicity of pain, and oral parafunction behaviors are not relevant to pain-related disability.
Future studies should explore this model longitudinally in order to determine causes of pain-related disability. Application of this model to other pain populations such as fibromyalgia and migraine headache would elucidate commonalities and differences among pain populations.
Supplementary Material
Acknowledgements:
The authors extend gratitude to OPPERA research staff and the study participants.
Funding: This work was supported by the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) U01-DE017018. VM is supported by T32 AT003378.
Abbreviations
- CFI
Comparative fit index
- CFA
Confirmatory factor analysis
- CI
Confidence interval
- GCPS
Graded Chronic Pain Scale
- JFLS
Jaw Functional Limitation Scale
- NHIS
National Health Interview Survey
- PPT
Pressure pain threshold
- RMSEA
Root mean square error of approximation
- SEM
Structural equation model
- SRMR
Standardized Root Mean Square Residual
- TLI
Tucker Lewis Index
- TMD
Temporomandibular disorder
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES
- [1].Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain--results of the North Cheshire oro-facial pain prospective population study. Pain 2010;149(2):354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson JC, Gerbing DW. Structural equation modeling in practice: A review and recommended two-step approach. Psychological bulletin 1988;103(3):411. [Google Scholar]
- [3].Auerbach SM, Laskin DM, Frantsve LM, Orr T. Depression, pain, exposure to stressful life events, and long-term outcomes in temporomandibular disorder patients. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2001;59(6):628–633; discussion 634. [DOI] [PubMed] [Google Scholar]
- [4].Buenaver LF, Quartana PJ, Grace EG, Sarlani E, Simango M, Edwards RR, Haythornthwaite JA, Smith MT. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: The mediating role of sleep disturbance. Pain (03043959) 2012;153(6):1159–1166 1158p. [DOI] [PubMed] [Google Scholar]
- [5].Byrne BM. Structural equation modeling with Mplus: Basic concepts, applications, and programming: routledge, 2013.
- [6].Chantaracherd P, John MT, Hodges JS, Schiffman EL. Temporomandibular Joint Disorders’ Impact on Pain, Function, and Disability. Journal of Dental Research 2015;94(3):86S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen H, Slade G, Lim PF, Miller V, Maixner W, Diatchenko L. Relationship between temporomandibular disorders, widespread palpation tenderness, and multiple pain conditions: a case-control study. J Pain 2012;13(10):1016–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dahlstrom L, Carlsson GE. Temporomandibular disorders and oral health-related quality of life. A systematic review. Acta odontologica Scandinavica 2010;68(2):80–85. [DOI] [PubMed] [Google Scholar]
- [9].Davis CE, Carlson CR, Studts JL, Curran SL, Hoyle RH, Sherman JJ, Okeson JP. Use of a structural equation model for prediction of pain symptoms in patients with orofacial pain and temporomandibular disorders. J Orofac Pain 2010;24(1):89–100. [PubMed] [Google Scholar]
- [10].Davis CE, Stockstill JW, Stanley WD, Qiang W. Pain-related worry in patients with chronic orofacial pain. Journal of the American Dental Association (JADA) 2014;145(7):722–730 729p. [DOI] [PubMed] [Google Scholar]
- [11].Davis PJ, Reeves JL, Hastie BA, Graff-Radford SB, Naliboff BD. Depression determines illness conviction and pain impact: A structural equation modeling analysis. Pain Medicine 2000;1(3):238–246. [DOI] [PubMed] [Google Scholar]
- [12].Day MA, Thorn BE. The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. PAIN® 2010;151(2):467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Derogatis LR. SCL-90-R: Administration, sssscoring and procedures manual for the R (evised) version and other instruments of the psychopathology rating scale series: Clinical Psychometric Research, 1992.
- [14].Durham J, Shen J, Breckons M, Steele JG, Araujo-Soares V, Exley C, Vale L. Healthcare Cost and Impact of Persistent Orofacial Pain: The DEEP Study Cohort. J Dent Res 2016;95(10):1147–1154. [DOI] [PubMed] [Google Scholar]
- [15].Dworkin SF. Temporomandibular Disorder (TMD) Pain–Related Disability Found Related to Depression, Nonspecific Physical Symptoms, and Pain Duration at 3 International Sites. The Journal of Evidence-Based Dental Practice 2011;11(3):143–144. [DOI] [PubMed] [Google Scholar]
- [16].Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain 2011;12(11 Suppl):T46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garofalo JP, Gatchel RJ, Wesley AL, Ellis E 3rd. Predicting chronicity in acute temporomandibular joint disorders using the research diagnostic criteria. Journal of the American Dental Association (1939) 1998;129(4):438–447. [DOI] [PubMed] [Google Scholar]
- [18].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. The Journal of Pain 2011;12(11):T61–T74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hooper D, Coughlan J, Mullen M. Structural equation modelling: Guidelines for determining model fit. Articles 2008:2. [Google Scholar]
- [20].Lt Hu, PM Bentler. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural equation modeling: a multidisciplinary journal 1999;6(1):1–55. [Google Scholar]
- [21].John MT, Reissmann DR, Schierz O, Wassell RW. Oral health-related quality of life in patients with temporomandibular disorders. J Orofac Pain 2007;21(1):46–54. [PubMed] [Google Scholar]
- [22].Johns G Presenteeism in the workplace: A review and research agenda. Journal of Organizational Behavior 2010;31(4):519–542. [Google Scholar]
- [23].Kline RB. Principles and Practice of Structural Equation Modeling. New York: Guilford Publications, 2016. [Google Scholar]
- [24].Kotiranta U, Suvinen T, Kauko T, Le Bell Y, Kemppainen P, Suni J, Forssell H. Subtyping patients with temporomandibular disorders in a primary health care setting on the basis of the research diagnostic criteria for temporomandibular disorders axis II pain-related disability: a step toward tailored treatment planning? Journal of oral & facial pain and headache 2015;29(2):126–134. [DOI] [PubMed] [Google Scholar]
- [25].Manfredini D, Borella L, Favero L, Ferronato G, Guarda-Nardini L. Chronic pain severity and depression/somatization levels in TMD patients. The International journal of prosthodontics 2010;23(6):529–534. [PubMed] [Google Scholar]
- [26].Manfredini D, Winocur E, Ahlberg J, Guarda-Nardini L, Lobbezoo F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. Journal of Dentistry 2010;38(10):765–772. [DOI] [PubMed] [Google Scholar]
- [27].Martin A, Auerbach S, Ness G, Zoghby G. Pain-related catastrophizing is a predictor of pain following medical intervention for temporomandibular disorders. Annals of Behavioral Medicine 2014;47:S276–S276. [Google Scholar]
- [28].McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services, 1971. [Google Scholar]
- [29].Melton BL, Moqbel M, Kanaan S, Sharma NK. Structural equation model of disability in low back pain. Spine 2016;41(20):1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mohn C, Vassend O, Knardahl S. Experimental pain sensitivity in women with temporomandibular disorders and pain-free controls: the relationship to orofacial muscular contraction and cardiovascular responses. Clin J Pain 2008;24(4):343–352. [DOI] [PubMed] [Google Scholar]
- [31].Muthén LK, Muthén BO. Mplus: Statistical analysis with latent variables: User’s guide: Muthén & Muthén Los Angeles, 2010.
- [32].Nagi S Some conceptual issues in disability and rehabilitation. In: M Sussman, editor Sociology and Rehabilitation Washington, DC: American Sociological Association, 1965. pp. 100–113. [Google Scholar]
- [33].Nakao M, Barsky AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosocial Medicine 2007;1:17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ohrbach R, Larsson P, List T. The jaw functional limitation scale: Development, reliability, and validity of 8-item and 20-item versions. Journal of Orofacial Pain 2008;22(3):219–230. [PubMed] [Google Scholar]
- [35].Park JW, Clark GT, Kim YK, Chung JW. Analysis of thermal pain sensitivity and psychological profiles in different subgroups of TMD patients. International journal of oral and maxillofacial surgery 2010;39(10):968–974. [DOI] [PubMed] [Google Scholar]
- [36].Plesh O, Adams SH, Gansky SA. Temporomandibular Joint and muscle disorder-type pain and comorbid pains in a national US sample. Journal of orofacial pain 2011;25(3):190–198. [PMC free article] [PubMed] [Google Scholar]
- [37].Raphael KG, Marbach JJ, Gallagher RM. Somatosensory amplification and affective inhibition are elevated in myofascial face pain. Pain Medicine 2000;1(3):247–253. [DOI] [PubMed] [Google Scholar]
- [38].Reisine ST, Weber J. The effects of temporomandibular joint disorders on patients’ quality of life. Community dental health 1989;6(3):257–270. [PubMed] [Google Scholar]
- [39].Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983;17(1):33–44. [DOI] [PubMed] [Google Scholar]
- [40].Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temporomandibular disorders patients. Pain 2003;102(3):221–226. [DOI] [PubMed] [Google Scholar]
- [41].Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. J Pain 2011;12(11 Suppl):T12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suvinen TI, Reade PC, Kemppainen P, Kononen M, Dworkin SF. Review of aetiological concepts of temporomandibular pain disorders: towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factors. European journal of pain (London, England) 2005;9(6):613–633. [DOI] [PubMed] [Google Scholar]
- [43].Turner JA, Brister H, Huggins K, Mancl L, Aaron LA, Truelove EL. Catastrophizing is associated with clinical examination findings, activity interference, and health care use among patients with temporomandibular disorders. J Orofac Pain 2005;19(4):291–300. [PubMed] [Google Scholar]
- [44].US Department of Health and Human Services. National Pain Strategy: A comprehensive population health-level strategy for pain. Washington, DC: US Department of Health and Human Services 2016. [Google Scholar]
- [45].VanderWeele TJ. Invited commentary: structural equation models and epidemiologic analysis. American journal of epidemiology 2012;176(7):608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain (03043959) 1992;50(2):133–149 117p. [DOI] [PubMed] [Google Scholar]
- [47].World Health Organization. International Classification of Functioning, Disability and Health: World Health Organization,, 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


