Abstract
There are differential risk relationships between parity and breast cancer according to estrogen receptor (ER) status, with an increased risk of ER− disease reduced by breastfeeding. This may be particularly relevant for understanding the higher incidence of ER− tumors in Black women, who are more likely to be parous and less likely to breastfeed than other US groups. Potential mechanisms for these relationships may include effects of disordered breast involution on inflammatory milieu in the breast as well as epigenetic reprogramming in the mammary gland, which can affect cell fate decisions in progenitor cell pools. In normal breast tissue, parity has been associated with hypermethylation of FOXA1, a pioneer transcription factor which promotes the luminal phenotype in luminal progenitors, while repressing the basal phenotype. In breast tumors, relationships between FOXA1 methylation and parity were strongest among women who did not breastfeed. Here we summarize the epidemiologic literature regarding parity, breastfeeding and breast cancer subtypes, and review potential mechanisms whereby these factors may influence breast carcinogenesis, with a focus on effects on progenitor cell pools in the mammary gland.
Keywords: breast cancer, breastfeeding, estrogen receptor, luminal progenitor
Parity, breastfeeding and breast cancer subtypes: the epidemiological evidence
A large body of literature over the years has demonstrated that a number of breast cancer risk factors are related to reproductive and hormonal characteristics, with summaries generally noting that both nulliparity and late age at first full-term pregnancy are associated with increased risk (1), with some reduction with breastfeeding (2). A meta-analysis of data from 47 epidemiological studies including more than 50,000 women with breast cancer and almost twice as many controls showed that having children was associated with decreased risk of breast cancer, with the decreased risk with parity greatest among women who breastfed (3). Relative risks for women with 1, 2, 3, 4 and 5 children were 1.0, 0.94, 0.86, 0.84, and 0.73, respectively, for women who never breastfed, compared to risks of 0.97, 0.93, 0.83, 0.73 and 0.64 among women who breastfed.
Even from very early studies, however, it was clear that relationships between reproductive risk factors and breast cancer etiology were complex. For example, there were observations of transient increases in risk following a pregnancy (4-7), and the effects of parity appeared to vary depending upon the age at breast cancer diagnosis. Studies by both Brinton (8) and Mayberry (9) found that for African-American/Black women, parity was protective only for those diagnosed after age 40. In fact, among Black women less than 40 years of age, parity actually increased risk of breast cancer (8). As reviewed by Pathak (10), it was thought that this dual effect of parity could be related to effects on proliferation of initiated cells (to increase risk), countered by inducing terminal differentiation of terminal endbuds, yielding them less susceptible to DNA damage over time, as shown by Jose and Irma Russo (11,12). According to this model, the association of breastfeeding with reduced risk could be attributed to its role in the maximal differentiation of the mammary gland.
Relationships between parity, breastfeeding and breast cancer risk were put into clearer context with advances in understanding the molecular underpinnings of breast tumors, first with classification of cancers by estrogen receptor (ER) status, and then with gene expression and subsequent immunohistochemical (IHC) profiling to distinguish five distinct subtypes: luminal A (ER+ or PR+ and HER2−), luminal B (ER+ or PR+ and HER2+), HER2 overexpressing, (ER−, PR−, HER2+), basal-like (ER−, PR−, HER2− and EGFR+ or CK 5/6+) or triple negative (ER−, PR−, HER2−) breast cancer (TNBC), which has poorer prognosis than other subtypes, and unclassified (negative for all 5 markers) (13,14). Classification by five or more intrinsic subtypes may have important implications for cancer treatment and its outcomes, but as proposed by Anderson and colleagues, in studies of breast cancer risk, it is likely more appropriate to consider breast cancer as having two distinct etiologic subtypes, which essentially correspond to ER+ and ER− disease (15). Of interest, a recent analysis of data from 15 prospective cohort studies addressed the question of a crossover effect of childbirth after pregnancy on risk, with consideration of breastfeeding and according to ER status (16). In that study, the investigators found that risk for ER− breast cancer was highest 2.2 years after birth (HR=1.77; 95% CI, 1.34-2.33). Risk decreased to a HR of 1.38 (95%CI, 1.01-1.88) at 34.5 years after birth but did not cross over to inverse associations. Risk for ER− breast cancer was highest for parous women, regardless of breastfeeding. With longer follow-up, risk with parity was reduced for ER+ breast cancer, but remained elevated for ER− disease. This is somewhat consistent with the early studies that observed crossover according to age at diagnosis, in that women diagnosed before age 40 are more likely to have ER− breast cancer than older women (17,18).
ER− and TNBC are more common in Black women than in other US populations; until recently, there had been few explanations for this phenomenon. It was not until there were fairly large studies with sufficient numbers of Black women with ER− breast tumors that associations between risk factors and higher incidence of ER− breast cancer could be clearly elucidated. A landmark paper from the Carolina Breast Cancer Study (CBCS) in 2008, which included Black as well as White women with breast cancer and non-cancer controls, was one of the first to draw attention to these differences in risk factors by breast cancer subtypes (19). In that study, Millikan and colleagues found that compared to women who were nulliparous, having children was associated with reduced risk of luminal A (ER+/PR+/HER2−) breast cancer, with similar odds ratios (ORs) of 0.70 for 1, 2, and 3 or more children. ORs remained the same regardless of breastfeeding. However, for basal-like breast cancer, similar to TNBC, there was almost a two-fold increase in risk with parity (OR for 1-2 children =1.8 (95% CI, 1.1-1.3); OR for 3 or more children=1.9 (95% CI, 1.1-3.3)). Importantly, this risk was only observed among women who had not breastfed; increased risk associated with parity was ameliorated if women breastfed (OR for 1-2 children=1.1 (95% CI, 0.6-1.3); OR for 3 or more children=1.3 (95% CI, 0.7-2.3)).
These results were subsequently replicated in additional studies of breast cancer in Black women. In the Black Women’s Health Study (BWHS), risk of ER+/PR+ breast cancer was reduced with parity, regardless of breastfeeding; however, there was a 50% increase in risk of ER−/PR− disease among women who had 3 or more children and did not breastfeed (OR =1.5; 95% CI, 1.1-2.2) (20). This increased risk was greatly reduced with breastfeeding (OR =1.1; 95% CI, 0.8-1.7). Similarly, among Black women in the Women’s Circle of Health Study (WCHS), parity reduced risk of ER+ breast cancer, but increased risk of ER− and TNBC, with ORs similar to those observed in CBCS among women who did not breastfeed (OR =1.9; 95% CI, 0.99-3.72) (21). Although these findings were consistent, sample sizes were small and some risk estimates were unstable, making it difficult to draw strong conclusions. Thus, the lead investigators of CBCS, BWHS, WCHS and the MultiEthnic Cohort (MEC) formed the AMBER Consortium to pool data and samples to have adequate statistical power to be able to investigate risk factors for aggressive breast cancer in Black women (17). Analysis in AMBER confirmed and extended results from smaller studies; with data from almost 4,000 Black women with breast cancer and 14,000 controls, results showed that, while having children was associated with reduced risk of ER+ breast cancer, it actually increased risk of ER− and TNBC (22). Importantly, breastfeeding appeared to greatly reduce the increased risk of ER− cancer with parity. For example, having 4 or more children was associated with more than 60% increased risk of TNBC among women who did not breastfeed (OR=1.68; 95% CI, 1.15-2.44), but no increased risk among women who breastfed (OR=1.08; 95% CI, 0.65-1.77).
More recent large studies in other populations have also observed reduced parity-associated risk of ER− breast cancer with breastfeeding. For example, analyses from the Nurses’ Health Study, including 12,452 women with breast cancer (8,235 ER+ and 1,978 ER−) showed that having children reduced risk of ER+ (HR=0.82; 95% CI, 0.77-0.98) but not ER− disease (HR=0.99; 95% CI, 0.94-1.05) (23). Relationships with ER+ breast cancer were observed regardless of breastfeeding. Basal-like breast cancer was highest among women with higher parity (> 2 children) who never breastfed (HR=1.43; 95% CI, 0.92-2.23), with no asssociations for those who did breast feed their infants (HR=1.05; 95% CI, 0.70-1.57). A pooled analysis of nine cohort studies found that parity was also associated with reduced risk of ER+ but increased risk of ER− and TNBC (24). Data were not available for breastfeeding in that pooled analysis, but an earlier review and meta-analysis of 27 studies with 36,881 breast cancer cases found that there were inverse associations with breastfeeding for ER− (OR=0.90; 95% CI, 0.82-0.99) and TNBC (OR=0.78; 95% CI, 0.66-0.91), in addition to ER+ or PR+ subtypes (OR=0.89; 95% CI, 0.80-0.99) (25). Similar results were observed in a large case-case analysis, with reductions in odds of TNBC with breastfeeding compared to hormone-responsive (HR+) cancers (OR=0.90; 95% CI, 0.82-0.99) (26), and a large pooled analysis with 558 TNBC and more than 5000 controls found that for younger parous women (< 50 years), odds of TNBC was twofold higher for women who never breastfed (OR=2.02; 95% CI,1.12-3.63) (27). Risk estimates were greatest for women with 3 or more pregnancies and no or fewer months of breastfeeding (OR=2.56; 95% CI, 1.22-5.35). Similar relationships with TNBC were observed in a pooled analysis, with 82% lower risk for Black women < 44 years of age who breastfed compared to those who had not (28).
Potential Mechanisms underlying the etiology of ER− breast cancer.
In our research in AMBER, in addition to findings regarding parity and breastfeeding, we also found differential relationships by ER status with age at menarche (29). Earlier age at menarche was associated with increased risk of ER+ breast cancer only among parous women, and the risk was greatest for those with the longest period of time between menarche and first full term birth. However, early menarche increased risk of ER− disease regardless of whether or not women were parous. These findings support the paradigms of etiologic heterogeneity discussed above in relation to age at first full-term pregnancy. Relationships of early menarche with ER+ breast cancer only among women with children and dependent upon time between menarche and childbirth is consistent with the Russo model of susceptibility of the breast to damage before pregnancy-induced differentiation of terminal end buds in the ducts. However, for ER− disease, it appears that menarche alone may be a critical event for the development of ER− breast cancer vs. the importance of time between menarche and reproduction for ER+ disease.
There is reason to consider how events in early years, such as menarche, parity and breastfeeding could affect later development of ER− vs. ER+ breast cancer. One potential mechanism is possibly through effects on progenitor cells in the mammary gland. There is substantive evidence that most breast tumors, both luminal (ER+) and basal-like (ER−), arise from luminal progenitor cells (reviewed in (30)). It has been proposed that cancer risk factors may alter the number and/or properties of progenitor cells (31), and that some cell populations may be exquisitely sensitive to damage during puberty. It is clear from data from survivors of atomic bombings in Japan in World War II that exposure to ionizing radiation during adolescence, not adulthood, led to subsequent breast cancer, similar to breast cancer following radiation to the chest for Hodgkin’s disease in young adults (32,33). Importantly, breast cancers arising from childhood treatment with radiation tend to be ER− and/or TNBC and also to have more aggressive characteristics (34). Investigating mechanisms behind this phenomenon, Barcellos-Hoff’s group showed with computational modeling and mouse studies that irradiation during puberty increases stem cell self-renewal and increases susceptibility to developing ER− breast cancer (35). Hormonal exposures at a young age may similarly affect progenitor cells and play a role in the etiology of ER− breast cancer. However, factors that influence the genesis of distinct ER subtypes from this common pool of progenitors are not well defined. Similarly, knowledge of how breastfeeding ameliorates the observed increased risk of ER− breast cancer is in its infancy.
Involution following Pregnancy
Although parity has an overall protective effect against breast cancer in later life, studies have shown that childbirth at any age bestows an increased risk of cancer in the first decade postpartum, as discussed above. Moreover, these postpartum breast cancers (PPBC) are often more aggressive with poorer outcomes (36,37). Following lactation, the mammary gland undergoes a massive remodeling process including programmed cell death of most epithelial cells, regeneration of adipose tissue, and infiltration of multipe immune cell types (38,39). Through a number of elegant studies over the last two decades, Schedin, Lyons and colleagues have dissected the role that involution plays in the generation of more aggressive breast cancers within the decade following childbirth (reviewed in (40)). These include increased inflammation and lymphangiogenesis and modification of the extracellular matrix (ECM), all characteristics of “wound healing”. In addition, they provided evidence that cells from very early stage tumors cells can be released into the stroma during involution where they have access to vasculature (41,42), and also demonstrated that breast involution is accompanied by remodeling in the liver, making this organ more amenable to cancer cell seeding and overt metastasis. Gene expression studies of mouse mammary glands undergoing involution have shown differential expression consistent with many of the observed biochemical changes and tissue remodeling (39,43,44). Thus far, however, these gene expression profiles show only moderate correlation with any particular aggressive subtype of cancer, specifically with inflammatory breast cancer (IBC) (45).
DNA methylation and mammary gland development
As discussed above, parity has long been known to reduce lifetime risk of breast cancer (46,47) as well as enhance the efficiency of milk production in second pregnancies (48,49). These observations suggest the existence of a long-term memory effect induced in mammary tissue by pregnancy. As proposed by Russo and Russo, the most likely mechanism for this memory effect is epigenetic reprograming in the mammary gland as a result of pregnancy hormone-induced differentiation and tissue remodeling (50,51). For example, DNA methylation in the mouse mammary gland is affected by the milieu of hormonal changes that occur during puberty, development, pregnancy and lactation (52-54). Using a targeted bisulfite sequencing approach and DNA from total mammary gland, Katz et al. identified hundreds of genes that were differentially methylated between parous and nulliparous mice, some of which persisted over time (52). Importantly, this study identified parity-associated increased methylation at the insulin-like growth factor receptor (Igf1r) and other members of the IGF signaling pathway, supporting the notion that suppression of this pathway is related to the parity associated protective effect against breast cancer. Using reduced representation bisulfite sequencing (RRBS), and flow-sorted mammary epithelial cells, Huh et al. showed that pregnancy had the most prominent effect on cell populations enriched for mammary epithelial stem cells and luminal progenitors, and that many of these methylation changes were associated with downregulation of genes and pathways important in stem cell function (e.g. Hedgehog, TGFβ), a finding also possibly relevant to pregnancy-induced reduction in breast cancer risk (53). By carrying out whole genome bisulfite sequencing of flow-sorted mammary epithelial cells, dos Santos et al. demonstrated that pregnancy results in long term methylation changes, some of which affect genes important in mammary gland development, lactation, and involution, suggesting that a first pregnancy “primes” the mammary gland for a more rapid response to subsequent pregnancies (54). Changes in DNA methylation accompany and are critical to most differentiation processes (55). Hence, the vast majority of these methylation changes probably reflect normal differentiation-associated modifications in the epigenetic state of mammary epithelial cells and/or differences in the relative proportions of distinct cell populations brought about by the pregnancy and lactation cycles. However, none of these studies evaluated the effect of suckling on these methylation changes, since all animals were allowed to nurse normally.
Aberrant DNA methylation of luminal progenitor genes and ER− breast cancer
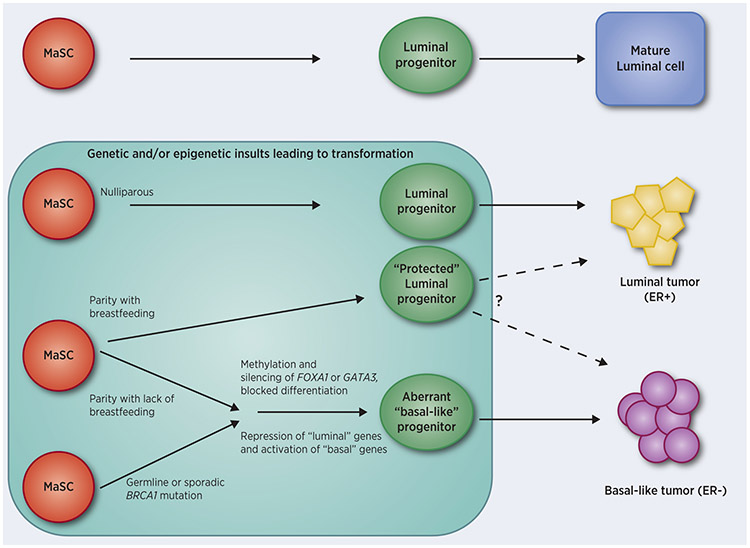
One possible mechanism whereby reproductive events could influence whether luminal progenitor cells give rise to ER− versus ER+ breast tumors is through errors in the establishment and maintenance of differentiation-associated DNA methylation states. We performed a two-stage genome-wide methylation study using the Illumina Infinium Human methylation 450K platform, first in a small analysis using fresh frozen samples from Black and White women (56), and then in FFPE tissues from 733 patients (57). In both studies, hierarchical clustering separated groups by ER status. The number of differentially methylated loci (DML) between Whites and Blacks was almost twofold higher in ER− breast tumors compared to ER+, and there were more DMLs by ER status among Blacks. One of the top DMLs by ER subtype in both studies, which was highly correlated with gene expression, was FOXA1. This pioneer transcription factor, important in mammary gland development, induces expression of a multitude of downstream genes that promote the luminal phenotype in luminal progenitors, while also directly repressing the basal phenotype (58). In our data, FOXA1 was hypermethylated and significantly downregulated in ER− tumors, with the highest methylation and lowest expression in ER− breast cancers from Black women. Moreover, the degree of methylation at FOXA1 in ER− tumors was positively associated with the number of children born to these women; importantly, this increase in methylation at FOXA1 was significantly reduced in women who had breastfed (57). Consistent with methylation results, we found that FOXA1 protein expression in breast tumors was lowest among parous Black women who had not breastfed, with associations most pronounced for ER− breast cancer (59). Additionally, in two studies of breast tissue from women undergoing reduction mammoplasty, FOXA1 was hypermethylated in normal breast tissue in parous compared to nulliparous women (60,61), suggesting that the observations made in breast tumors (56,57) may be derived from changes in pre-neoplastic cells. It is interesting that BRCA1-deficient breast cancers, most of which are of the ER− basal-like phenotype, are also thought to derive from an expanded population of aberrant luminal progenitors (62). Moreover, wildtype BRCA1 protein has been shown to positively regulate FOXA1 by preventing its DNA methylation and silencing (63). As illustrated in Figure 1, it is therefore plausible that BRCA1-mutated breast tumors are ER− because mutation/inactivation of BRCA1 leads to methylation-induced silencing of FOXA1 in luminal progenitors, and that parity-associated methylation of FOXA1 has similar consequences.
Figure 1. Putative pathways to ER+ and ER− breast tumors.
During normal breast/mammary gland development, some mammary gland stem cells divide and become committed to the luminal cell lineage. These luminal progenitor cells undergo further differentiation into mature luminal cells of the ducts and alveoli. Expression of FOXA1 and GATA3 are pivotal to the proper differentiation of these cells (58,64). Current data (reviewed in (30)) suggest that luminal progenitors are also the cells-of-origin for both ER+ and ER− breast tumors following genetic and epigenetic insults (shaded area indicates timeframe when these event may occur). If transformation occurs in a luminal progenitor cell that expresses FOXA1 and GATA3, differentiation continues along the luminal lineage resulting in luminal (ER+) tumors. Breastfeeding following pregnancy either prevents methylation and silencing of FOXA1 and/or GATA3, or somehow reduces the effects of silencing; in addition, parity and breastfeeding alters chromatin structure of luminal progenitors making them less likely to become transformed or to progress to cancer. On the other hand, if BRCA1 is inactivated by mutation or epigenetic silencing in a stem cell or luminal progenitor, FOXA1 may become methylated and silenced resulting in repression of luminal signature genes and activation of basal genes; transformation of these differentially-arrested (“basal-like”) progenitors can lead to tumor cells of the basal (ER−) phenotype (63). Likewise, similarly arrested progenitors may be generated by abnormal methylation and silencing of FOXA1 and/or GATA3 as a result of parity and lack of breastfeeding. Transformation of these aberrant progenitors could also lead to ER− breast tumors.
GATA3 has also been shown to be pivotal in the differentiation of luminal progenitors to mature luminal cells (64). Together, GATA3 and FOXA1 proteins bind estrogen-responsive target genes to promote ERα function (65). GATA3 has been shown to promote differentiation and suppress metastasis in breast cancer, at least in part by inducing expression of microRNA-29b (66). Recently, in a TCGA cohort of breast tumors, GATA3 was shown to be downregulated and hypermethylated in ER− breast cancers compared to ER+ cases (65), a finding similar to our independent cohort (57). Interestingly, genetic depletion of either Gata3 (67) or Foxa1 (Sribenja et al., unpublished) in the murine mammary gland results in a similar phenotype, skewing epithelial cell types towards luminal progenitors. It will be interesting to determine whether parity-associated methylation at FOXA1 and GATA3 occur in the same tumor or are mutually exclusive events. Importantly, a recent report by Basree et al. demonstrated a similar increase in the proportion of luminal progenitors in glands from mice that had their pups removed prematurely after only one week of nursing (termed abrupt involution, AI) as opposed to the typical 3-4 weeks (gradual involution, GI), however, the methylation status of Foxa1 or any genes was not assessed (68).
Mechanisms of aberrant DNA methylation of pivotal luminal progenitor genes
One can speculate how epigenetic reprogramming could occur by lack of breastfeeding and result in aberrant DNA methylation of FOXA1 and other genes in breast luminal progenitor cells. The methylation status of FOXA1/Foxa1 in mammary epithelial stem cells (MaSC), the cells that give rise to mammary luminal progenitor cells, is not known. However, Foxa1 is expressed at very low levels in the basal/MaSC compartment of MECs (53), and similar to Elf5, another gene pivotal to luminal cell differentiation, may be methylated in MaSC cells but is demethylated during the transition to luminal progenitors (69). If an active demethylation mechanism is involved in this process (70), it is unlikely to be 100% efficient, and it is possible that some luminal progenitors are generated with Foxa1 still methylated and resistant to transcriptional activation and luminal differentiation. Expression of Myc proto-oncogene is essential for the proper function of mammary stem and progenitor cells (71). ElShamay has proposed the “Oncogene Elimination Hypothesis” which posits that fully differentiated mammary gland cells expressing proto-oncogenes also express immune cell enlisting factors, as well as tumor-specific peptides presented in the context of MHC class, and that these cells would be detected and eliminated by infiltrating immune cells during involution (72). If terminal differentiation is blocked, for example by methylation associated silencing of FOXA1 due to abrupt involution brought about by shortened or a lack of breastfeeding, this putative “seek and destroy” mechanism may be impaired allowing these cells to escape immune surveillance and destruction, and survive as potential precursors to ER− breast cancer.
A second possibility, supported by the Basree et al. study (68), is that lack of, or short term, breastfeeding results in more rapid involution of the mammary gland with accompanying increased inflammation ( (73,74), a condition previously shown to cause aberrant DNA methylation (reviewed in (75)); therefore, even if Foxa1 is in an unmethylated state in early luminal progenitor cells, abrupt involution could result in inflammation-induced aberrant methylation potentially leading to its silencing and differentiation arrest of luminal progenitor cells.
A third possibility concerns differences in the length of time that mammary epithelial cells are exposed to breast milk in women who breastfeed opposed to those who do not. Breast milk contains exosomes, “nanosized” membrane-bound vesicles secreted by various cell types that carry several types of macromolecules including proteins, lipids and RNAs including microRNAs (miRNAs)(reviewed in (76)). Exosomes have been implicated in several physiological and pathological processes and are thought be important for intercellular communication (77). In the mammary gland, exosomes are thought to be involved in regulating lactation and involution (78). DNA methylation is regulated, in part, by fluctuating levels of DNA methyltransferases (DNMTs) and proteins that remove methyl moieties such as TET1, TET2, and TET3 (70). Intriguingly, milk exosomes carrying miR-29s and miR-148a negatively regulate DNMT3a/b and DNMT1, respectively (79,80). Moreover, inhibition of miR-29s in primary bovine mammary epithelial cells resulted in global DNA hypermethylation, as well as increased promoter methylation of several lactation-related genes including Elf5 (79). Since milk exosomes can be taken up by cultured mammary ductal epithelial cells and remain functional (81), it is conceivable that decreased exposure of mammary epithelial cells to milk exosomes in vivo could result in increased expression of DMNTs and consequent aberrant methylation.
Other potential mechanisms
Another potential mechanism by which parity and breastfeeding could be related to development of ER− breast cancer is derived from the identification of pappalysin-1 (PAPPA) as a pregnancy-dependent oncogene (82). Using a transgenic mouse model (MMTV-PAPPA), the authors showed that overexpression of PAPPA during lactation and involution results in decreased IGFBP5a levels and increased IGF signaling in the mammary gland, as well as the occurrence of mammary tumors with a tumor-associated collagen signature (TACS-3). They went on to show that extended lactation (> 2 weeks) allowed accumulation of high levels of glycoproteins stanniocalcin-1 and −2 (STC1 and STC2), which inhibit PAPPA protease activity, resulting in abrogation of the phenotype due to aberrant PAPPA expression. To determine the significance of these finding in humans, Takabatake et al. also analyzed expression levels of PAPPA and IGFBP-5 by IHC in breast tumors from 46 premenopausal women (82). The analysis demonstrated expression of PAPPA in tumors from 79% of parous women (n = 28) but only 11% of nulliparous women (n = 18), with a significantly higher proportion of tumors with a TACS-3 signature in the parous group (p < 0.0001). Most relevant was the finding of an increased incidence of TNBC in the parous group (32%) relative to the nulliparous group (5.2%). The effect of breastfeeding in the parous group could not be determined since the duration of breastfeeding was not available.
Interestingly, Atkinson et al. (83) reported that normal adjacent tissue from women with TNBC contained a disproportionally higher level of cells with breast cancer stem cell characteristics in both ipsilateral and contralateral breast tissue as determined by both IHC staining and gene expression analysis (83). Importantly, these authors found that patients with these putative stem cells in their normal breast tissue were less likely to have breastfed or had a shorter duration of breastfeeding. Whether these potentially tumorigenic stem cells are related to our proposed differentiation-arrested progenitors remain to be determined.
Future Directions.
There is still much to be learned about the role of breastfeeding in reduction of aggressive breast cancer. For example, how much breastfeeding is required to reduce risk of ER− and TNBC? Are three months sufficient? Six? Is the first or last pregnancy the most important or is the total months breastfeeding what is key for risk reduction? These questions can be addressed in large epidemiologic studies with detailed data on reproductive and breastfeeding histories, and also through studies in the laboratory to understand the effects of these variables on cell populations in the breast. It will be important in future population-based studies to also consider the potential confounding effect of age at menarche on relationships with breastfeeding, as well as potential interactions with other lifestyle factors, such as use of oral contraceptives, alcohol consumption and body mass index.
Advances in technology may also lead to new avenues of investigation. In the future it is likely that, because of its exceptional resolution, application of new single-cell techniques will shed light on the mechanism(s) by which pregnancy without breastfeeding contributes to the increased occurrence of ER− breast cancer in Black women. For example, single-cell RNA-seq (scRNA-seq) should facilitate the identification of specific cell clusters within the larger luminal progenitor fraction of mammary epithelial cells (84) whose presence is associated with not suckling, and whose gene expression signature suggests a differentiation arrested phenotype that may be predisposed to development of ER− cancer upon additional genetic and epigenetic insult. A better understanding of the pathways involved in making luminal progenitors prone to ER− tumors when transformed, and information on the mechanisms involved in mediating the differences in breast tissue involution and remodeling, will facilitate the identification of potential pharmacologic preventive measures for women who are unable or unwilling to breastfeed, particularly Black women, thereby reducing the prevalence of ER− breast cancer and its inherent mortality.
Acknowledgments
Financial support: The Breast Cancer Research Foundation (Ambrosone); R01CA225947 (MPIs: Ambrosone, Higgins, Palmer); R01CA133264 (MPIs: Ambrosone, Higgins, Demissie); P30 CA 016056
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest
References
- 1.Harris JR, Lippman ME, Veronesi U, Willett W. Breast Cancer (1). N Engl J Med 1992;327:319–28. [DOI] [PubMed] [Google Scholar]
- 2.Newcomb PA. Lactation and Breast Cancer Risk. J Mammary Gland Biol Neoplasia 1997;2:311–8. [DOI] [PubMed] [Google Scholar]
- 3.Breast Cancer and Breastfeeding: Collaborative Reanalysis of Individual Data from 47 Epidemiological Studies in 30 Countries, Including 50302 Women with Breast Cancer and 96973 Women without the Disease. Lancet 2002;360:187–95. [DOI] [PubMed] [Google Scholar]
- 4.Wohlfahrt J, Andersen PK, Mouridsen HT, Melbye M. Risk of Late-Stage Breast Cancer after a Childbirth. Am J Epidemiol 2001;153:1079–84. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient Increase in Breast Cancer Risk after Giving Birth: Postpartum Period with the Highest Risk (Sweden). Cancer Causes Control 2002;13:299–305. [DOI] [PubMed] [Google Scholar]
- 6.Lyons TR, Schedin PJ, Borges VF. Pregnancy and Breast Cancer: When They Collide. J Mammary Gland Biol Neoplasia 2009;14:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kvale G, Heuch I. A Prospective Study of Reproductive Factors and Breast Cancer. Ii. Age at First and Last Birth. Am J Epidemiol 1987;126:842–50. [DOI] [PubMed] [Google Scholar]
- 8.Brinton LA, Benichou J, Gammon MD, Brogan DR, Coates R, Schoenberg JB. Ethnicity and Variation in Breast Cancer Incidence. Int J Cancer 1997;73:349–55. [DOI] [PubMed] [Google Scholar]
- 9.Mayberry RM, Stoddard-Wright C. Breast Cancer Risk Factors among Black Women and White Women: Similarities and Differences. Am J Epidemiol 1992;136:1445–56. [DOI] [PubMed] [Google Scholar]
- 10.Pathak DR. Dual Effect of First Full Term Pregnancy on Breast Cancer Risk: Empirical Evidence and Postulated Underlying Biology. Cancer Causes Control 2002;13:295–8. [DOI] [PubMed] [Google Scholar]
- 11.Russo J, Russo IH. Biological and Molecular Bases of Mammary Carcinogenesis. Lab Invest 1987;57:112–37. [PubMed] [Google Scholar]
- 12.Russo IH, Koszalka M, Russo J. Comparative Study of the Influence of Pregnancy and Hormonal Treatment on Mammary Carcinogenesis. Br J Cancer 1991;64:481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated Observation of Breast Tumor Subtypes in Independent Gene Expression Data Sets. Proc Natl Acad Sci U S A 2003;100:8418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and Clinical Characterization of the Basal-Like Subtype of Invasive Breast Carcinoma. Clin Cancer Res 2004;10:5367–74. [DOI] [PubMed] [Google Scholar]
- 15.Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How Many Etiological Subtypes of Breast Cancer: Two, Three, Four, or More? J Natl Cancer Inst 2014;106:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols HB, Schoemaker MJ, Cai J, Xu J, Wright LB, Brook MN, et al. Breast Cancer Risk after Recent Childbirth: A Pooled Analysis of 15 Prospective Studies. Ann Intern Med 2019;170:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer JR, Ambrosone CB, Olshan AF. A Collaborative Study of the Etiology of Breast Cancer Subtypes in African American Women: The Amber Consortium. Cancer Causes Control 2014;25:309–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast Cancer Statistics, 2019. CA Cancer J Clin 2019;69:438–51. [DOI] [PubMed] [Google Scholar]
- 19.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of Basal-Like Breast Cancer. Breast Cancer Res Treat 2008;109:123–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L. Parity and Lactation in Relation to Estrogen Receptor Negative Breast Cancer in African American Women. Cancer Epidemiol Biomarkers Prev 2011;20:1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrosone CB, Zirpoli G, Ruszczyk M, Shankar J, Hong CC, McIlwain D, et al. Parity and Breastfeeding among African-American Women: Differential Effects on Breast Cancer Risk by Estrogen Receptor Status in the Women's Circle of Health Study. Cancer Causes Control 2014;25:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, Lactation, and Breast Cancer Subtypes in African American Women: Results from the Amber Consortium. J Natl Cancer Inst 2014;106:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortner RT, Sisti J, Chai B, Collins LC, Rosner B, Hankinson SE, et al. Parity, Breastfeeding, and Breast Cancer Risk by Hormone Receptor Status and Molecular Phenotype: Results from the Nurses' Health Studies. Breast Cancer Res 2019;21:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudet MM, Gierach GL, Carter BD, Luo J, Milne RL, Weiderpass E, et al. Pooled Analysis of Nine Cohorts Reveals Breast Cancer Risk Factors by Tumor Molecular Subtype. Cancer Res 2018;78:6011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, et al. Breastfeeding and Breast Cancer Risk by Receptor Status--a Systematic Review and Meta-Analysis. Ann Oncol 2015;26:2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Li CI, Tang MT, Porter P, Hill DA, Wiggins CL, et al. Reproductive Factors and Risk of Luminal, Her2-Overexpressing, and Triple-Negative Breast Cancer among Multiethnic Women. Cancer Epidemiol Biomarkers Prev 2016;25:1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.John EM, Hines LM, Phipps AI, Koo J, Longacre TA, Ingles SA, et al. Reproductive History, Breast-Feeding and Risk of Triple Negative Breast Cancer: The Breast Cancer Etiology in Minorities (Bem) Study. Int J Cancer 2018;142:2273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H, Ursin G, Xu X, Lee E, Togawa K, Duan L, et al. Reproductive Factors and the Risk of Triple-Negative Breast Cancer in White Women and African-American Women: A Pooled Analysis. Breast Cancer Res 2017;19:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, et al. Important Role of Menarche in Development of Estrogen Receptor-Negative Breast Cancer in African American Women. J Natl Cancer Inst 2015;107:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross K, Wronski A, Skibinski A, Phillips S, Kuperwasser C. Cell Fate Decisions During Breast Cancer Development. J Dev Biol 2016;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Visvader JE. Cells of Origin in Cancer. Nature 2011;469:314–22. [DOI] [PubMed] [Google Scholar]
- 32.Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD Jr. Radiation Effects on Breast Cancer Risk: A Pooled Analysis of Eight Cohorts. Radiat Res 2002;158:220–35. [DOI] [PubMed] [Google Scholar]
- 33.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-Specific Late Mortality among 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 2008;100:1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castiglioni F, Terenziani M, Carcangiu ML, Miliano R, Aiello P, Bertola L, et al. Radiation Effects on Development of Her2-Positive Breast Carcinomas. Clin Cancer Res 2007;13:46–51. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Fernandez-Garcia I, Vijayakumar S, Martinez-Ruis H, Illa-Bochaca I, Nguyen DH, et al. Irradiation of Juvenile, but Not Adult, Mammary Gland Increases Stem Cell Self-Renewal and Estrogen Receptor Negative Tumors. Stem Cells 2014;32:649–61. [DOI] [PubMed] [Google Scholar]
- 36.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum Diagnosis Demonstrates a High Risk for Metastasis and Merits an Expanded Definition of Pregnancy-Associated Breast Cancer. Breast Cancer Res Treat 2013;138:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goddard ET, Bassale S, Schedin T, Jindal S, Johnston J, Cabral E, et al. Association between Postpartum Breast Cancer Diagnosis and Metastasis and the Clinical Features Underlying Risk. JAMA Netw Open 2019;2:e18699.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson CJ. Involution: Apoptosis and Tissue Remodelling That Convert the Mammary Gland from Milk Factory to a Quiescent Organ. Breast Cancer Res 2006;8:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, et al. Involution of the Mouse Mammary Gland Is Associated with an Immune Cascade and an Acute-Phase Response, Involving LBP, CD14 and STAT3. Breast Cancer Res 2004;6:R75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borges VF, Lyons TR, Germain D, Schedin P. Postpartum Involution and Cancer: An Opportunity for Targeted Breast Cancer Prevention and Treatments? Cancer Res 2020;80:1790–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyons TR, O'Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum Mammary Gland Involution Drives Progression of Ductal Carcinoma in Situ through Collagen and COX-2. Nat Med 2011;17:1109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2-Dependent Lymphangiogenesis Promotes Nodal Metastasis of Postpartum Breast Cancer. J Clin Invest 2014;124:3901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene Expression Profiling of Mammary Gland Development Reveals Putative Roles for Death Receptors and Immune Mediators in Post-Lactational Regression. Breast Cancer Res 2004;6:R92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein T, Salomonis N, Nuyten DS, van de Vijver MJ, Gusterson BA. A Mouse Mammary Gland Involution Mrna Signature Identifies Biological Pathways Potentially Associated with Breast Cancer Metastasis. J Mammary Gland Biol Neoplasia 2009;14:99–116. [DOI] [PubMed] [Google Scholar]
- 45.Bambhroliya A, Van Wyhe RD, Kumar S, Debeb BG, Reddy JP, Van Laere S, et al. Gene Set Analysis of Post-Lactational Mammary Gland Involution Gene Signatures in Inflammatory and Triple-Negative Breast Cancer. PLoS One 2018;13:e0192689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, et al. Timing of Menarche and First Full-Term Birth in Relation to Breast Cancer Risk. Am J Epidemiol 2008;167:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schonfeld SJ, Pfeiffer RM, Lacey JV Jr., de Gonzalez A Berrington, Doody MM, Greenlee RT, et al. Hormone-Related Risk Factors and Postmenopausal Breast Cancer among Nulliparous Versus Parous Women: An Aggregated Study. Am J Epidemiol 2011;173:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Amici D, Gasparoni A, Guala A, Klersy C. Does Ethnicity Predict Lactation? A Study of Four Ethnic Communities. Eur J Epidemiol 2001;17:357–62. [DOI] [PubMed] [Google Scholar]
- 49.Zuppa AA, Tornesello A, Papacci P, Tortorolo G, Segni G, Lafuenti G, et al. Relationship between Maternal Parity, Basal Prolactin Levels and Neonatal Breast Milk Intake. Biol Neonate 1988;53:144–7. [DOI] [PubMed] [Google Scholar]
- 50.Russo IH, Russo J. Pregnancy-Induced Changes in Breast Cancer Risk. J Mammary Gland Biol Neoplasia 2011;16:221–33. [DOI] [PubMed] [Google Scholar]
- 51.Barton M, Santucci-Pereira J, Russo J. Molecular Pathways Involved in Pregnancy-Induced Prevention against Breast Cancer. Front Endocrinol (Lausanne) 2014;5:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katz TA, Liao SG, Palmieri VJ, Dearth RK, Pathiraja TN, Huo Z, et al. Targeted DNA Methylation Screen in the Mouse Mammary Genome Reveals a Parity-Induced Hypermethylation of Igf1r That Persists Long after Parturition. Cancer Prev Res (Phila) 2015;8:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huh SJ, Clement K, Jee D, Merlini A, Choudhury S, Maruyama R, et al. Age- and Pregnancy-Associated DNA Methylation Changes in Mammary Epithelial Cells. Stem Cell Reports 2015;4:297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dos Santos CO, Dolzhenko E, Hodges E, Smith AD, Hannon GJ. An Epigenetic Memory of Pregnancy in the Mouse Mammary Gland. Cell Rep 2015;11:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith ZD, Meissner A. DNA Methylation: Roles in Mammalian Development. Nat Rev Genet 2013;14:204–20. [DOI] [PubMed] [Google Scholar]
- 56.Ambrosone CB, Young AC, Sucheston LE, Wang D, Yan L, Liu S, et al. Genome-Wide Methylation Patterns Provide Insight into Differences in Breast Tumor Biology between American Women of African and European Ancestry. Oncotarget 2014;5:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Espinal AC, Buas MF, Wang D, Cheng DT, Sucheston-Campbell L, Hu Q, et al. FOXA1 Hypermethylation: Link between Parity and ER-Negative Breast Cancer in African American Women? Breast Cancer Res Treat 2017;166:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernardo GM, Bebek G, Ginther CL, Sizemore ST, Lozada KL, Miedler JD, et al. FOXA1 Represses the Molecular Phenotype of Basal Breast Cancer Cells. Oncogene 2013;32:554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng T-Y, Yao S, Omilian A, Khoury T, Buas M, Ondracek-Payne R, et al. FOXA1 Protein Expression in ER+ and ER− Breast Cancer in Relation to Parity and Breastfeeding in Black and White Women. Cancer Epidemiol, Biomarkers Prev 2020;29:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh S, Gu F, Wang CM, Lin CL, Liu J, Wang H, et al. Genome-Wide DNA Methylation Profiling Reveals Parity-Associated Hypermethylation of FOXA1. Breast Cancer Res Treat 2014;147:653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zendehbad Z, Izadi P, Daraei A, Yekaninejad MS, Nafissi N, Younosi N, et al. Early Parity Epigenetic Footprint of FOXA1 Gene Body in Normal Breast Tissue of Iranian Women. Iran Biomed J 2019;23:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant Luminal Progenitors as the Candidate Target Population for Basal Tumor Development in BRCA1 Mutation Carriers. Nat Med 2009;15:907–13. [DOI] [PubMed] [Google Scholar]
- 63.Gong C, Fujino K, Monteiro LJ, Gomes AR, Drost R, Davidson-Smith H, et al. FOXA1 Repression Is Associated with Loss of BRCA1 and Increased Promoter Methylation and Chromatin Silencing in Breast Cancer. Oncogene 2015;34:5012–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 Maintains the Differentiation of the Luminal Cell Fate in the Mammary Gland. Cell 2006;127:1041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang G, Wang X, Sheng D, Zhou L, Liu Y, Xu C, et al. Cooperativity of Co-Factor Nr2f2 with Pioneer Factors GATA3, FOXA1 in Promoting ERα Function. Theranostics 2019;9:6501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 Suppresses Metastasis and Modulates the Tumour Microenvironment by Regulating Microrna-29b Expression. Nat Cell Biol 2013;15:201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, et al. Gata-3 Is an Essential Regulator of Mammary-Gland Morphogenesis and Luminal-Cell Differentiation. Nat Cell Biol 2007;9:201–9. [DOI] [PubMed] [Google Scholar]
- 68.Basree MM, Shinde N, Koivisto C, Cuitino M, Kladney R, Zhang J, et al. Abrupt Involution Induces Inflammation, Estrogenic Signaling, and Hyperplasia Linking Lack of Breastfeeding with Increased Risk of Breast Cancer. Breast Cancer Res 2019;21:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee HJ, Hinshelwood RA, Bouras T, Gallego-Ortega D, Valdes-Mora F, Blazek K, et al. Lineage Specific Methylation of the Elf5 Promoter in Mammary Epithelial Cells. Stem Cells 2011;29:1611–9. [DOI] [PubMed] [Google Scholar]
- 70.Wu H, Zhang Y. Reversing DNA Methylation: Mechanisms, Genomics, and Biological Functions. Cell 2014;156:45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moumen M, Chiche A, Deugnier MA, Petit V, Gandarillas A, Glukhova MA, et al. The Proto-Oncogene Myc Is Essential for Mammary Stem Cell Function. Stem Cells 2012;30:1246–54. [DOI] [PubMed] [Google Scholar]
- 72.ElShamy WM. The Protective Effect of Longer Duration of Breastfeeding against Pregnancy-Associated Triple Negative Breast Cancer. Oncotarget 2016;7:53941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively Activated Macrophages and Collagen Remodeling Characterize the Postpartum Involuting Mammary Gland across Species. Am J Pathol 2010;176:1241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum Breast Involution Reveals Regression of Secretory Lobules Mediated by Tissue-Remodeling. Breast Cancer Res 2014;16:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang ZH, Dang YQ, Ji G. Role of Epigenetics in Transformation of Inflammation into Colorectal Cancer. World J Gastroenterol 2019;25:2863–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melnik BC, Schmitz G. Milk's Role as an Epigenetic Regulator in Health and Disease. Diseases 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016;164:1226–32. [DOI] [PubMed] [Google Scholar]
- 78.Hendrix A, Hume AN. Exosome Signaling in Mammary Gland Development and Cancer. Int J Dev Biol 2011;55:879–8.7 [DOI] [PubMed] [Google Scholar]
- 79.Bian Y, Lei Y, Wang C, Wang J, Wang L, Liu L, et al. Epigenetic Regulation of Mir-29s Affects the Lactation Activity of Dairy Cow Mammary Epithelial Cells. J Cell Physiol 2015;230:2152–63. [DOI] [PubMed] [Google Scholar]
- 80.Braconi C, Huang N, Patel T. Microrna-Dependent Regulation of DNA Methyltransferase-1 and Tumor Suppressor Gene Expression by Interleukin-6 in Human Malignant Cholangiocytes. Hepatology 2010;51:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin W, Tsukasaki Y, Dasgupta S, Mukhopadhyay N, Ikebe M, Sauter ER. Exosomes in Human Breast Milk Promote Emt. Clin Cancer Res 2016;22:4517–24. [DOI] [PubMed] [Google Scholar]
- 82.Takabatake Y, Oxvig C, Nagi C, Adelson K, Jaffer S, Schmidt H, et al. Lactation Opposes Pappalysin-1-Driven Pregnancy-Associated Breast Cancer. EMBO Mol Med 2016;8:388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atkinson RL, Yang WT, Rosen DG, Landis MD, Wong H, Lewis MT, et al. Cancer Stem Cell Markers Are Enriched in Normal Tissue Adjacent to Triple Negative Breast Cancer and Inversely Correlated with DNA Repair Deficiency. Breast Cancer Res 2013;15:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bach K, Pensa S, Grzelak M, Hadfield J, Adams DJ, Marioni JC, et al. Differentiation Dynamics of Mammary Epithelial Cells Revealed by Single-Cell Rna Sequencing. Nat Commun 2017;8:2128. [DOI] [PMC free article] [PubMed] [Google Scholar]