Primary cutaneous mucoepidermoid carcinoma (cMEC) is a rare neoplasm with mucus-secreting and epidermoid cells on histology1. Its etiopathology remains unclear and it is postulated to arise de novo or from pre-existing nevus sebaceous, sweat glands, or ectopic salivary glands2. Clinically, cMEC may mimic a basal cell carcinoma, particularly if ulcerated, and dermatologists must first rule-out metastatic disease, salivary origin, and distinguish cMEC from more aggressive adenosquamous carcinoma (cASC). Current literature on cMEC is limited to case reports and single-institution studies. Given the rarity of this tumor, lack of established treatment guidelines, and uncertain aggressiveness which may be in part due to misdiagnosis as cASC, an in-depth national study can better characterize pertinent epidemiologic and prognostic factors associated with cMEC.
After approval by the Yale Human Investigation Committee, and with adherence to STROBE guidelinesi, data on patients with a diagnosis of primary cMEC (histology code 8430/3) were obtained from the SEER database for the years 1973 to 2016. Data was collated and analyzed as reported previously3.
A total of 89 patients with cMEC were identified. The majority occurred in individuals of white race (80.0%), with slight preponderance for male sex (55.1%) and mean age of diagnosis of 63.4 years (range 23–94). Most (68.6%) cases presented with local(stage I) disease and were low grade (75.5%). The most frequent site of presentation was the face (84.3%). Treatment modalities included surgery in 81.8% of cases, radiation (15.7%), and chemotherapy (6.8%). Detailed descriptive statistics are provided in supplementary material (Mendeley doi:10.17632/3g58dntvd.1).
Five-year OS and DSS for patients with cMEC were 68.2% and 76.0%, respectively (Figure 1). Predictors of survival on univariate analysis included older age (shorter OS and DSS), high lesion grade (shorter OS), face as lesion site (longer OS and DSS) and surgical resection (longer OS and DSS). In risk-adjusted models, independent predictors of survival were older age and high grade (shorter OS and DSS), lesion location on the face (longer OS and DSS) and receipt of surgery (longer DSS)(Table 1).
Figure 1.
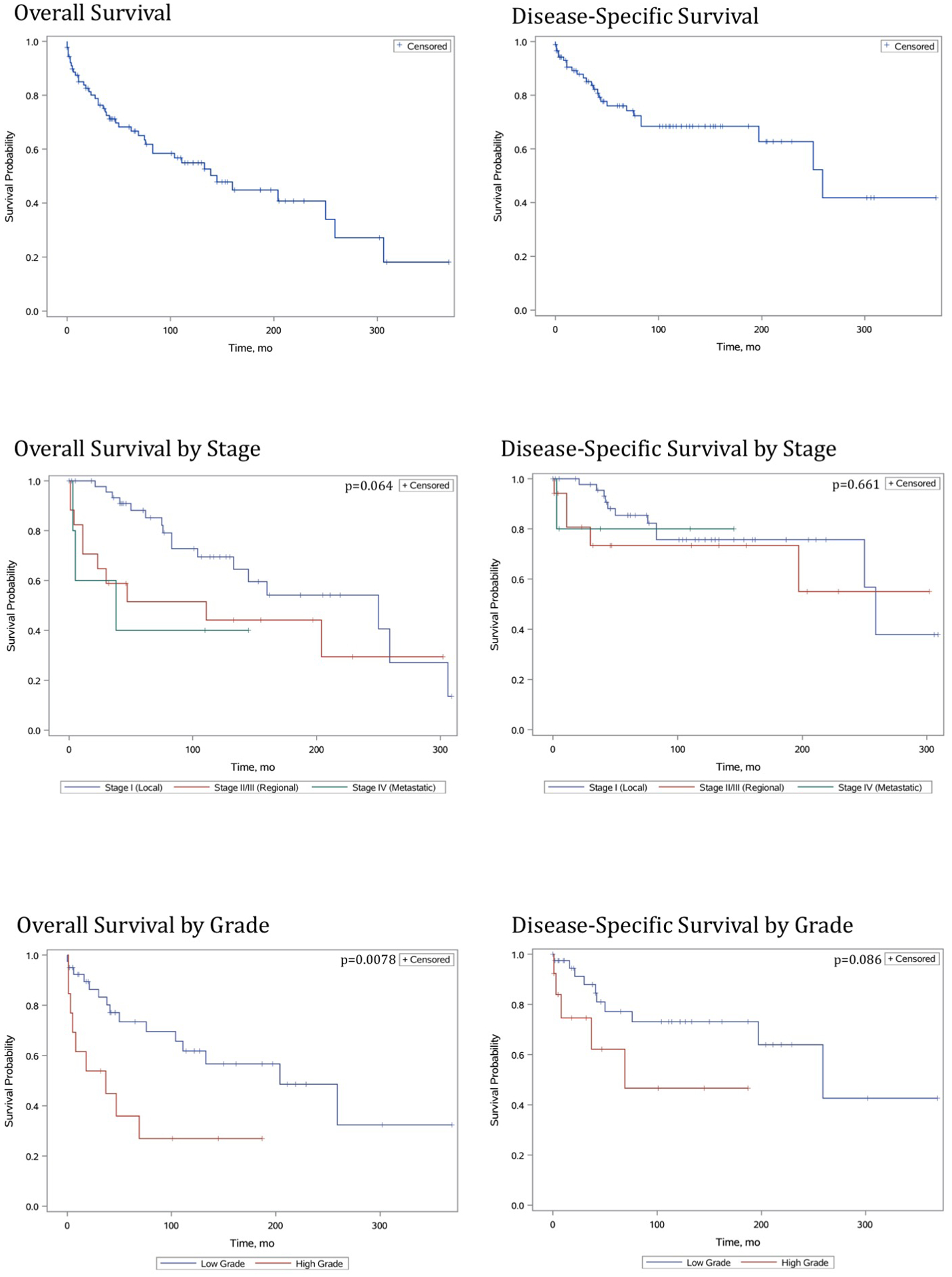
Malignant cutaneous mucoepidermoid carcinoma. Survival analysis using Kaplan-Meier analysis.
Table 1.
Univariate and multivariate analysis of overall and disease-specific survival.
| Overall | Disease-Specific | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Univariatea | ||||
| Year of diagnosis (Advanced) | 1.01 (0.98–1.05) | 0.60 | 1.03 (0.98–1.08) | 0.27 |
| Age (Older) | 1.07 (1.04–1.10) | <0.0001 | 1.07 (1.03–1.10) | <0.0001 |
| Sex (Male) | 1.35 (0.73–2.51) | 0.34 | 1.20 (0.54–2.65) | 0.65 |
| Race (White) | 1.03 (0.43–2.50) | 0.94 | 0.98 (0.33–2.88) | 0.96 |
| Residency demographic (Rural) | 1.00 (Reference) | 1.00 (Reference) | ||
| Urban | 0.47 (0.21–1.06) | 0.06 | 0.60 (0.24–1.51) | 0.27 |
| Metro | 0.66 (0.32–1.37) | 0.27 | 0.31 (0.11–0.89) | 0.03 |
| Stage (Higher) | 1.50 (1.04–2.17) | 0.03 | 1.24 (0.70–2.19) | 0.46 |
| Grade (High) | 3.02 (1.28–7.14) | 0.01 | 2.62 (0.85–8.07) | 0.09 |
| Body site (Trunk or Extremities) | 1.00 (Reference) | 1.00 (Reference) | ||
| Face | 0.33 (0.14–0.76) | 0.009 | 0.27 (0.10–0.72) | 0.009 |
| Surgery (Performed) | 0.38 (0.19–0.77) | 0.007 | 0.22 (0.10–0.53) | 0.0006 |
| Radiation therapy (Received) | 1.61 (0.77–3.40) | 0.21 | 1.12 (0.38–3.30) | 0.83 |
| Chemotherapy (None) | 0.21 (0.08–0.56) | 0.002 | 0.15 (0.05–0.47) | 0.001 |
| Multivariatea* | ||||
| Year of diagnosis (Advanced) | 1.01 (0.95–1.07) | 0.76 | 1.05 (0.96–1.14) | 0.28 |
| Age (Older) | 1.09 (1.05–1.14) | <0.01 | 1.08 (1.03–1.14) | <0.01 |
| Sex (Male) | 0.48 (0.17–1.35) | 0.17 | 0.40 (0.11–1.54) | 0.18 |
| Grade (High) | 8.49 (2.46–29.3) | <0.01 | 6.86 (1.40–33.63) | 0.02 |
| Body site (Face) | 0.11 (0.03–0.45) | <0.01 | 0.08 (0.01–0.45) | <0.01 |
| Surgery (Performed) | 0.56 (0.19–1.64) | 0.29 | 0.23 (0.06–0.86) | 0.03 |
Category in parentheses defines the strata the hazard ratio represents.
Variables were chosen for the multivariate model using forward and backwards stepwise selection using an entry of 0.3 and stay of 0.15.
Our study provides insight into nation-wide epidemiology, prognosis, and treatment for cMEC. On risk-adjusted model surgical resection was a predictor of DSS, supporting its use in management, whereas the understanding of the utility of chemotherapy and/or radiation therapy is limited based on unmeasured biases in coding this specific data. Our data also support literature demonstrating that cMEC is an overall low-grade neoplasm distinguishable from more aggressive cASC, and that it may benefit from surgical resection4,5. In particular, Nouri et al. have reported success with the use of Moh’s micrographic surgery for treatment of cMEC on the face4.
Limitations in this study design include a potential for absent or incorrect reporting of retrospective data, including misclassification bias from potentially overlapping cancer terms, migration of patients in and out of SEER registry areas, potential over-representation of data from academic centers and changes in coding practices over time. Despite such limitations, our study presents the first available population-level data on cMEC. Determinants of survival include: age, cancer grade, lesion location, and receipt of surgical intervention. Although a rare tumor, physicians should be cognizant of the pertinent epidemiologic, therapeutic, and prognostic factors which may guide management.
Supplementary Material
Abbreviations
- cMEC
Cutaneous mucoepidermoid carcinoma
- DSS
Disease-specific survival
- HR
Hazard ratio
- OS
Overall survival
- PY
Person-years
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- SEER
Surveillance, Epidemiology and End Results
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplement(s): RECORD Checklist, IRB Exemption, Mendeley (doi:10.17632/3gg58dntvd.1)
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
As age is defined as a continuous variable in this dataset, the hazard ratio reflects the increased risk of death for each additional year of life.
References
- 1.Friedman KJ. Low-Grade Primary Cutaneous Adenosquamous (Mucoepidermoid) Carcinoma Report of a Case and Review of the Literature. Am J Dermatopathol. 1989;11(1):43. doi: 10.1097/00000372-198902000-00007 [DOI] [PubMed] [Google Scholar]
- 2.Yen A, Sanchez RL, Fearneyhough P, Tschen J, Wagner RF. Mucoepidermoid carcinoma with cutaneous presentation. J Am Acad Dermatol. 1997;37(2):340–342. doi: 10.1016/s0190-9622(97)80387-8 [DOI] [PubMed] [Google Scholar]
- 3.Mirza F, Tuggle C, Zogg C, Mirza H, Narayan D. Epidemiology of malignant cutaneous granular cell tumors: A US population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database. J Am Acad Dermatol. 2017;78(3):490–497.e1. doi: 10.1016/j.jaad.2017.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nouri K, Trent JT, Lowell B, Vaitla R, Jimenez GP. Mucoepidermoid carcinoma (adenosquamous carcinoma) treated with Mohs micrographic surgery. Int J Dermatol. 2003;42(12):957–959. doi: 10.1111/j.1365-4632.2003.02076.x [DOI] [PubMed] [Google Scholar]
- 5.Riedlinger W, Hurley M, Dehner L, Lind A. Mucoepidermoid carcinoma of the skin: a distinct entity from adenosquamous carcinoma: a case study with a review of the literature. Am J Surg Pathology. 2005;29(1):131–135. doi: 10.1097/01.pas.0000147397.08853.6f [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


