Abstract
The evolution of complex organ systems in metazoans has dictated that the maintenance of energy homeostasis requires coordinating local and systemic energy demands between organs with specialized functions. The gastrointestinal tract is one of many organs that is indispensable for the systemic coordination of energy substrate uptake, storage, and usage, and the spatial organization of this organ (i.e. proximity to other metabolic organs) within a complex body plan underlies its role in organ crosstalk. Studies of various arthropod intestines, and in particular insects, have shed light on the evolution and function of the gastrointestinal tract in the maintenance of energy homeostasis. This brief review focuses on studies and theories derived from the insect intestine (particularly the midgut) of adult Drosophila melanogaster to inform on the how, what, and why of the gastrointestinal tract in the systemic regulation of lipids, the most common form of stored energy in insects.
Keywords: gastrointestinal tract, intestine, midgut, Drosophila melanogaster, lipid metabolism, energy homeostasis, systemic, inter-tissue crosstalk, immune-metabolic interaction
A brief introduction:
Insect gastrointestinal tracts are complex and adaptable. The gastrointestinal tract of adult Drosophila melanogaster is composed of a foregut, midgut, and hindgut. The foregut can be subdivided into the oesophagus, crop (potentially important for food digestion and/or storage), and proventriculus (potentially important for pathogen defense), while the hindgut connects the midgut to the rectal ampulla (Fig. 1A). Finally, the midgut, which will be the primary focus of this review, is the major site of food digestion, nutrient absorption, and energy substrate storage [1]. The Drosophila midgut consists of a tube lined by an epithelial monolayer. Toward the lumen, the midgut epithelium is additionally protected by a chitin layer called the periotrophic matrix, and toward the basement membrane (opposite the lumen), the epithelium is surrounded by visceral muscle exposed to circulating hemolymph (arthropod blood). This midgut epithelial layer mainly contains four types of cells: intestinal stem cells (ISCs), enterocytes (ECs), secretory enteroendocrine cells (EEs), and enteroblasts (EBs, a postmitotic cell that can differentiate as an EC). The presence of ISCs promotes midgut regeneration of functional enterocytes after damage [2,3], such as that induced by external pathogens [4]. Midgut enterocytes, which are large, polyploid cells that project microvilli into the lumen, drive both nutrient absorptive functions and pathogen defense mechanisms (more on this later). In part through these ancient functions, shaped by evolution, the insect gastrointestinal tract can direct systemic metabolic responses and influence energy homeostasis.
Figure 1: Spatial Organization of Drosophila Midgut.
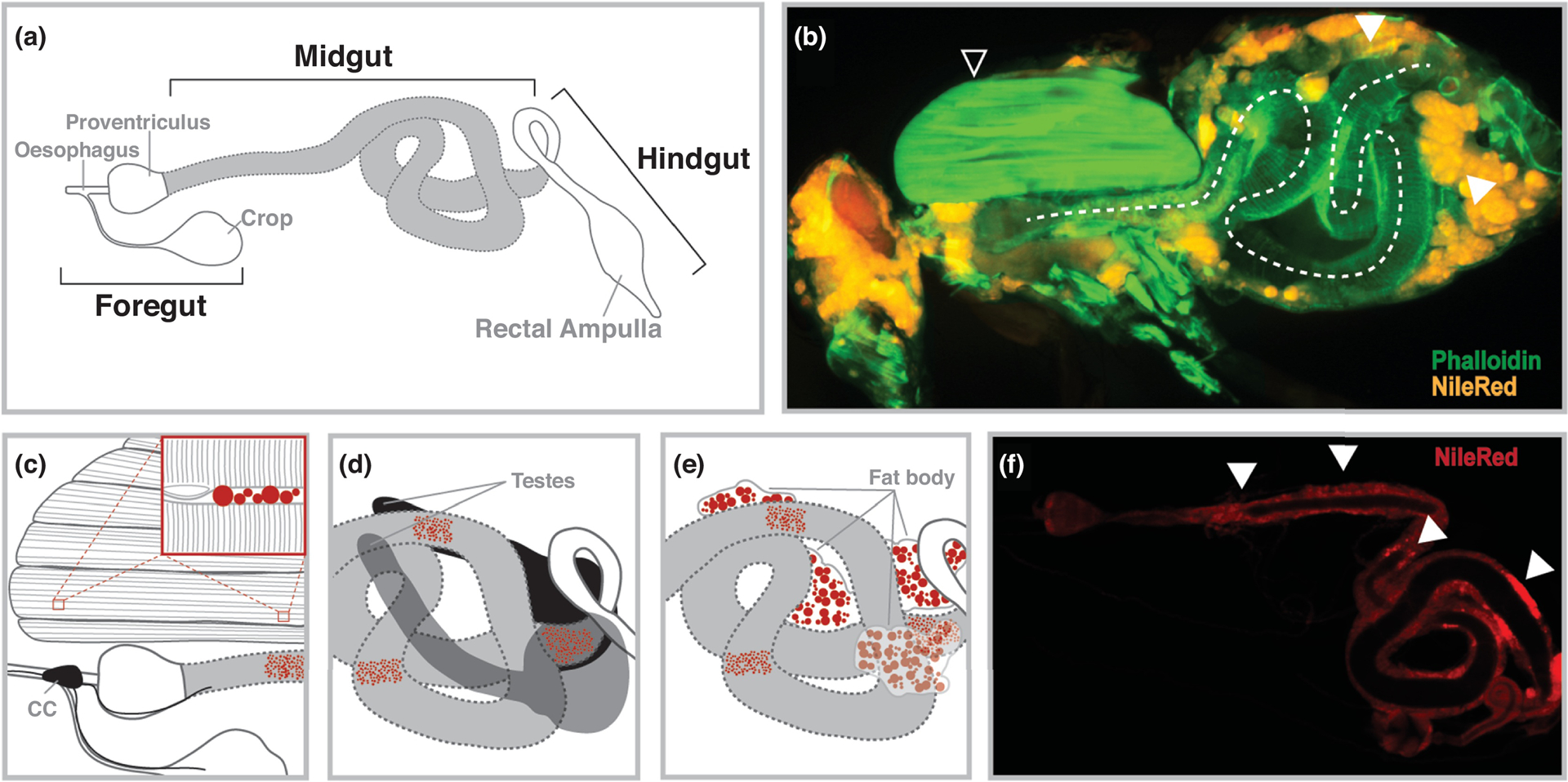
(A) The adult gastrointestinal tract; midgut is highlighted in gray. (B) Immunostaining (Phalloidin [actin, green] and NileRed [neutral lipids, orange]) of a hemisected female Drosophila highlighting the spatial organization of the midgut within the compact body plan. Select organs highlighted; midgut (dotted line), thoracic indirect flight muscle (open white arrow), and fat body (solid white arrow). (C) Proximal relationship of foregut/anterior midgut, thoracic muscle, and corpora cardiaca (CC); regions of neutral lipid storage (lipid droplets) highlighted in red. Insert highlights intermyofibrillar neutral lipid storage (D) Proximal relationship of the posterior midgut and testes. (E) Proximal relationship of the middle/posterior midgut and fat body. (F) Immunostaining (NileRed [neutral lipids, orange]) of the dissected female Drosophila midgut; monolayer epithelium surrounding the lumen. Solid white arrows highlight enrichment in neutral lipid accumulation.
It is generally accepted that midgut enterocytes are the key cell type for food digestion and nutrient absorption in Drosophila, especially the major caloric energy substrates; protein, carbohydrate, and lipid (thoroughly reviewed in [1]). Neutral lipids in particular, such as sterol esters, triacylglycerides (TAG), and diacylglycerides (DAG), play an ancestral role in shaping energy homeostasis and organismal lipid metabolism, and TAG specifically is the most abundant stored energy (calorie) source in insects ([5]and thoroughly reviewed in [6,7]). The products of lipid digestion in the midgut lumen (mainly free fatty acids, glycerol, and DAG; along with dietary sterols) can be absorbed into enterocytes via diffusion or specific mechanisms that couple sterol and lipid uptake (note: Drosophila are cholesterol auxotrophs, and thus require refined mechanisms to promote efficient uptake of dietary sterols [8,9]). Ingested lipids can be reassembled into TAG, and stored in lipid droplets within enterocytes. Midgut TAGs can further be mobilized to other energy usage or storage organs (such as the insect fat body, the major site of TAG storage) through insect lipoproteins via hemolymph [10,11]. While lipid (and sterol) absorption is critical, natural Drosophila diets generally consist of decaying plant, fruit, or fungal material. These diets are general low in TAG, but enriched in complex carbohydrates. To this end, most adult insects rapidly convert simple sugars into stored lipids (TAG) via de novo lipogenesis, and the midgut and fat body are the two major insect organs where this process occurs [5,6].
The Drosophila melanogaster gastrointestinal tract, and midgut in particular, thus plays a critical role in the balance of lipid energy substrate uptake, usage, storage, and mobilization.
How…Does the Drosophila midgut influence systemic lipid-dependent energy homeostasis?:
While the midgut’s role in nutritive lipid uptake and synthesis is a threshold for balancing energy supply and demand for other organs, the diverse cellular composition of this intestinal tract also relays signals to coordinate lipid metabolism through organ crosstalk. This crosstalk is also likely to be defined by the spatial organization of the midgut within the insect’s compact body plan (which also lacks a closed or direct circulatory system). Specific parts of the tubular midgut make direct or proximal contact with other energy storage/usage tissues, such as the thoracic flight muscle, the fat body, and sex organs (some of which is visualized in the cross-sectional image presented in Fig. 1B) that may facilitate spatio-temporal regulation of energy homeostasis through unique functional compartmentalization of the gastrointestinal tract. For example, a large section of the anterior midgut, as well as the foregut, are embedded within the indirect flight muscle of the thorax (Fig. 1B–C). Specialized neuroendocrine cells, called the corpora cardiaca (CC), are also attached to the foregut, and these cells secrete circulating peptide hormones capable of shaping organismal TAG metabolism (Fig. 1C and [12,13]). The flight muscle of insects requires abundant energy sources, including the usage of both stored and mobilized TAG, highlighting a potentially unique proximal relationship between the anterior midgut/foregut, physically anchored endocrine cells, and muscle in the control energy homeostasis [5]. Another example of midgut spatial organization dictating signal relays with other organs is the relationship between the posterior midgut and testes (Fig. 1D). In part of a complex mechanism, specific cell types in the male (but not female) posterior midgut can secrete citrate, and proximal cells within the testes can uptake this metabolite to promote spermatogenesis [14]. Thus, it is possible other metabolites or energy substrates (such as lipids) are also proximally ‘transferred’ between these organs. Finally, parts of the middle midgut and posterior midgut are embedded within the fat body (attached to the abdominal cuticle, Fig. 1B and E), which is the major of site of TAG storage and de novo lipid synthesis in most insects. To this end, the fat body is also a depot for lipid mobilization to energy usage tissues [6,11]. The proximal relationship between sections of the midgut and fat storage depots again highlights the potential for unique crosstalk mechanisms (i.e. a direct link between nutrient uptake and storage) between specific regions of midgut enterocytes and the fat body in order to coordinate energy homeostasis. Furthermore, the fat body acts as an essential secretory organ in insects, and direct midgut-fat body crosstalk might promote a unique feed-forward loop to enhance secretion of fat body-derived signals to drive systemic communication within the entire organism (reviewed in [6]).
Neutral lipid storage, and likely TAG storage, within enterocytes is also heterogenic throughout the midgut, with enrichment found in sections of the anterior and posterior midgut regions (as well as small section of the middle midgut, Fig. 1F and [15]). The diversity in lipid storage reflects the metabolic functional compartmentalization of the midgut (based on cellular morphology/heterogeneity and metabolic gene expression, [15–17]). While the biological relevance for some of these highly-specific regional differences in metabolic functionality remain unclear, it is likely that the spatial organization of the gastrointestinal tract within the insect body plan (i.e. proximity to other metabolic organs) dictates some of this regionality (Fig. 1B). Thus, it is to be expected that the evolution of unique organ systems in unique insects has shaped local and systemic communication networks to coordinate energy homeostasis.
Furthermore, the Drosophila midgut is innervated by a complex enteric nervous system, which through neuronal relays can also direct local and systemic metabolic responses. This enteric nervous system underlies a crucial midgut-brain communication axis that governs many aspects of systemic physiology and feeding behavior (not discussed here but thoroughly reviewed in [1]).
What…Does the Drosophila midgut utilize to coordinate systemic lipid-dependent energy homeostasis?:
There are a variety of midgut-derived signals, secreted metabolites or peptides, which can directly or indirectly (or putatively) regulate lipid-dependent energy homeostasis through controlling various cellular/systemic signaling networks. These signals can be local (paracrine, that in turn dictate organismal physiology) or systemic (endocrine), and are derived from diverse cell types/regions (summarized in Table 1).
Table 1:
Drosophila Midgut-derived Signals
| Name | Location | Cell type | Target | Target tissue | Target pathway | Type of signal | Reference |
|---|---|---|---|---|---|---|---|
| Tachykinin | EE | TkR99D | Midgut (ECs) | SREBP inhibition | Paracrine | [19] | |
| Neurotensin | Midgut | EE | NTR | Multiple tissues | AMPK | Paracrine/endocrine | [20] |
| Allatostatin A | Midgut | EE | AstA-R2 | CC and brain (IPCs) | AKH and insulin secretion | Paracrine/endocrine | [21,22] |
| Bursicon alpha | Midgut | EE | Lgr2 | CC | AKH secretion | Paracrine/endocrine | [23] |
| CCHamide2 | Midgut | EE | CCHa2-R | Brain (IPCs) | Insulin production | Endocrine | [24,25] |
| IMPL2 | Midgut | – | Insulin-like peptide | Multiple tissues | Insulin signaling | Endocrine | [27,28] |
| Limostatin | Foregut | CC | LstR | Brain (IPCs) | Insulin secretion | Endocrine | [13] |
| AKH | Foregut | CC | AKHR | Multiple tissues | AKHR signaling | Endocrine | [5,12] |
| Acetic acid | Midgut lumen | Acetobacter p. | – | Multiple tissues | Insulin signaling | – | [30] |
| Acetate | Midgut lumen | U/l microbiota | IMD/tachykinin | Multiple tissues | Insulin signaling | – | [31•] |
| Amino acids | Midgut lumen | LactobacMus P. | – | Multiple tissues | TOR signaling | – | [32] |
IMPL2 - Imaginal morphogenesis protein-late 2; AKH - Adipokenetic hormone; EE – Enteroendocrine; EC - Enterocyte; CC - Corpora cardiaca: TkR99O - Tachykinin receptor 99D; NTR - Neurotensin receptor; AstA-R2 - Allatostatin A receptor R2; LstR - Limostatin receptor; AKHR -Adipokenetic hormone receptor; IMD - Immune deficiency pathway; IPC - Insulin producing cells; SREBP - Sterol regulatory element binding protein; Acetobacter p. – pomorum; U/I – Unidentified; Lactobacillus p. - plantarum: AMPK - 5′ AMP activated kinase; TOR - Target of rapamycin.
Numerous neuropeptides are secreted from distinct or regional midgut enteroendocrine cells [18], and can impact various aspects of lipid metabolism and lipid-dependent energy homeostasis. Tachykinin, for example, locally directs de novo lipid synthesis in enterocytes, and further influences systemic lipid homeostasis in other organs [19], while Neurotensin can promote lipid accumulation in various lipid storage tissues through modulating 5’-AMP-activated protein kinase (AMPK) signaling [20]. Neuropeptides Allatostatin A and Bursicon alpha can regulate insect adipokinetic hormone (AKH, a functional homolog of glucagon) secretion from the corpora cardiaca in the foregut, and AKH can subsequently shape lipid synthesis and mobilization from the fat body and midgut [12,21–23]. CCHamide2, and potentially Allatostatin A as well, can putatively impact the production/secretion of insulin-like peptides (ILPs, functional homologs of insulin) from insulin-producing cells (IPCs) in the insect brain [24,25]. Circulating ILPs are critical systemic signals that drives lipid storage in insects, and the antagonism between AKH and ILPs acts to balance lipid catabolism/anabolism in organs with specialized metabolic functions to systemically coordinate energy homeostasis [5,26]. Many of these neuropeptides are also expressed in the central nervous system, such as Allatostatin A, highlighting a potential systemic ‘metabolic balance’ shaped by ILPs (controlled locally by brain neuropeptides targeting IPCs) and AKH (controlled locally by enteroendocrine-derived neuropeptides targeting the corpora cardiaca). Midgut-derived IMPL2, a circulating insulin-like peptide binding protein that inhibits ligand/receptor interactions, can also limit ILP function and promote systemic lipid wasting [27,28].
The spatial organization of the corpora cardiaca within the foregut provides a route for local midgut signals to control circulating signals, and thus the ability to systemically coordinate lipid-dependent energy homeostasis. Beyond AKH, these neuroendocrine cells also secrete Limostatin, which is a decretin hormone that suppresses insulin-like peptide production in IPCs [13].
Finally, the Drosophila midgut lumen also hosts transient and colonizing resident commensal bacteria. These microbiota promote nutritional symbiosis, utilizing dietary nutrients in the lumen but also aiding in nutrient uptake for the insect host [29]. Some of these bacteria also direct systemic physiology through bacteria-derived metabolites [30–32]. For example, Acetobacter pomorum can secrete acetic acid from the midgut lumen in the developing insect larvae, which in turn can influence insulin signaling pathway activity throughout the organism [30]. While some of these microbe-dependent control mechanisms have only been described in the larval midgut, it is likely similar mechanisms exist in the adult insect. To this end, midgut bacteria act as additional source of systemic or local signals that can likely direct lipid-dependent energy homeostasis.
Why…Does the Drosophila midgut play a central role in the systemic coordination of lipid-dependent energy homeostasis?:
The evolution of these midgut-derived signals and systemic signaling networks, likely further influenced by the spatial organization of the gastrointestinal tract within the insect body plan, is driven by ancient lipid-metabolic adaptive responses. First, some of these signals aid in the inter-organ rationing and regulation of lipid usage, storage, uptake, and mobilization during nutrient deprivation or in response to dietary changes/macronutrient imbalances [19]. This includes microbiota-dependent control of metabolic responses, as adaptation to dietary changes constitutes a selective pressure influencing gastrointestinal tract bacterial diversity across taxa [33–35]. Secondly, some of these signals aid in coordinating insect feeding behavior with nutrient uptake (lipids, sugars, and amino acids), along with further balancing inter-organ lipid usage, storage, and mobilization [21–23]. This coordination may be linked to diurnal changes in food intake [5], or reproduction (and sexually dimorphic local or systemic cues [14]).
Finally, ancestral metabolic signaling pathways have co-evolved with ancestral innate immune signaling pathways, eliciting integrated responses. As the primary barrier to external pathogens, the insect gastrointestinal tract has distinct cell types that promote both nutrient-sensing and bacterial-sensing [36], allowing for bidirectional communication between signaling pathways that respond to a variety of external cues (such as bacteria or dietary macronutrients). To this end, innate immune signaling pathways can locally (and subsequently throughout the organism) direct midgut lipid metabolism in response to diverse microbes and/or pathogens, often utilizing midgut- or microbe-derived signals that coordinate lipid-metabolic responses [31,37–40]. This discrete control of lipid metabolism (and often TAG catabolism) helps shape energy homeostasis and limit pathogenesis in response to enteropathogenic infections that require both a complex balance of energy usage and maintenance, as well as a complex integration of a multitude of signaling networks. While the insect midgut senses enteropathogens, this external cue is quickly communicated to other immuno-metabolic organs (such as the fat body, [41]) in order to systemically coordinate a broader response, controlling both systemic innate immune responses and, likely, lipid-metabolic responses.
Thus, beyond feeding, diet, and reproduction, immune-metabolic interactions have likely played a significant part in shaping the Drosophila midgut’s role in the systemic coordination of lipid-dependent energy homeostasis.
Materials and Methods:
Whole Fly and Midgut Imaging:
Whole flies were first fixed in Eppendorf tubes with 4% PFA for 2–3 days at 4°C. Fixed flies were then washed with 1X PBS and then hemisected with a scalpel. The hemisected samples were again fixed with 4% PFA for 20 min at room temperature.
Midguts were dissected in PBS and fixed with 4% PFA for 5 min at room temperature. Fixed samples were then washed 3X with PBST(0.1% TritonX-100) for 10 mins. each, and then incubated with fresh Nile Red solution (2μl of 0.004% Nile Red Solution in 500 μl PBST) overnight at 4°, followed by washing with PBST and then staining with DAPI (Hoechst Stain) and Alexa Fluor 488 phalloidin (Thermo Fisher Scientific).
Images were collected using a Leica M165 FluoCombi Stereoscope system and processed using Leica software and Adobe Photoshop.
Highlights.
The spatial organization of the Drosophila midgut in the systemic coordination of lipid homeostasis
Midgut-derived signals that promote local and systemic lipid-metabolic responses
Immune-metabolic interactions shape midgut-dependent regulation of lipid-metabolic responses
Acknowledgments:
This work was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases (Grant R01 DK108930 to J.K.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: None
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
- 1.Miguel-Aliaga I, Jasper H, Lemaitre B: Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics 2018, 210:357–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohlstein B, Spradling A: The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 2005, 439:470–474. [DOI] [PubMed] [Google Scholar]
- 3.Micchelli CA, Perrimon N: Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2005, 439:475–479. [DOI] [PubMed] [Google Scholar]
- 4.Biteau B, Hochmuth CE, Jasper H: Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 2011, 9:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Karpac J: Muscle Directs Diurnal Energy Homeostasis through a Myokine-Dependent Hormone Module in Drosophila. Curr Biol 2017, 27:1941–1955.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heier C, Kühnlein RP: Triacylglycerol Metabolism in Drosophila melanogaster. Genetics 2018, 210:1163–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kühnlein RP: Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. Lipid droplet-based storage fat metabolism in Drosophila. J Lipid Res 2012, 53:1430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieber MH, Thummel CS: Coordination of triacylglycerol and cholesterol homeostasis by DHR96 and the Drosophila LipA homolog magro. Cell Metab 2011, 15:122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieber MH, Thummel CS: The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab 2009, 10:481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho M, Sampaio JL, Palm W, Brankatschk M, Eaton S, Shevchenko A: Effects of diet and development on the Drosophila lipidome. Mol Syst Biol 2012, 8:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palm W, Sampaio JL, Brankatschk M, Carvalho M, Mahmoud A, Shevchenko A, Eaton S: Lipoproteins in Drosophila melanogaster—Assembly, Function, and Influence on Tissue Lipid Composition. Plos Genet 2012, 8:e1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gáliková M, Diesner M, Klepsatel P, Hehlert P, Xu Y, Bickmeyer I, Predel R, Kühnlein RP: Energy Homeostasis Control in Drosophila Adipokinetic Hormone Mutants. Genetics 2015, 201:665–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfa RW, Park S, Skelly K-R, Poffenberger G, Jain N, Gu X, Kockel L, Wang J, Liu Y, Powers AC, et al. : Suppression of insulin production and secretion by a decretin hormone. Cell Metab 2015, 21:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 14.Hudry B, Goeij E de, Mineo A, Gaspar P, Hadjieconomou D, Studd C, Mokochinski JB, Kramer HB, Plaçais P-Y, Preat T, et al. : Sex Differences in Intestinal Carbohydrate Metabolism Promote Food Intake and Sperm Maturation. Cell 2019, 178:901–918.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show here that a specific subset of posterior midgut cells in males, proximally located to the male sex organs, directs midgut carbohydrate metabolism, spermatogenesis in the testes, and food intake through cytokine and citrate signaling. This is one of the first studies to highlight how the spatial organization of the midgut can direct (sexually dimorphic) organ crosstalk and systemic physiology.
- 15.Marianes A, Spradling AC: Physiological and stem cell compartmentalization within the Drosophila midgut. Elife 2013, 2:e00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchon N, Osman D, David FPA, Fang HY, Boquete J-P, Deplancke B, Lemaitre B: Morphological and Molecular Characterization of Adult Midgut Compartmentalization in Drosophila. Cell Reports 2013, 3:1725–1738. [DOI] [PubMed] [Google Scholar]
- 17.Hung R-J, Hu Y, Kirchner R, Liu Y, Xu C, Comjean A, Tattikota SG, Li F, Song W, Sui SH, et al. : A cell atlas of the adult Drosophila midgut. Proc National Acad Sci 2020, 117:1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Yin C, Yang F, Zhang Y, Huang H, Wang J, Deng B, Cai T, Rao Y, Xi R: The Cellular Diversity and Transcription Factor Code of Drosophila Enteroendocrine Cells. Cell Reports 2019, 29:4172–4185.e5. [DOI] [PubMed] [Google Scholar]
- 19.Song W, Veenstra JA, Perrimon N: Control of lipid metabolism by tachykinin in Drosophila. Cell Reports 2014, 9:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, Starr ME, Kim JT, Harris JW, Yiannikouris FB, et al. : An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature 2016, 533:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hentze JL, Carlsson MA, Kondo S, Nässel DR, Rewitz KF: The Neuropeptide Allatostatin A Regulates Metabolism and Feeding Decisions in Drosophila. Sci Rep-uk 2015, 5:11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hergarden AC, Tayler TD, Anderson DJ: Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. P Natl Acad Sci Usa 2012, 109:3967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scopelliti A, Bauer C, Yu Y, Zhang T, Kruspig B, Murphy DJ, Vidal M, Maddocks ODK, Cordero JB: A Neuronal Relay Mediates a Nutrient Responsive Gut/Fat Body Axis Regulating Energy Homeostasis in Adult Drosophila. Cell Metab 2019, 29:269–284.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sano H, Nakamura A, Texada MJ, Truman JW, Ishimoto H, Kamikouchi A, Nibu Y, Kume K, Ida T, Kojima M: The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of Drosophila melanogaster. Plos Genet 2015, 11:e1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren GR, Hauser F, Rewitz KF, Kondo S, Engelbrecht AF, Didriksen AK, Schjøtt SR, Sembach FE, Li S, Søgaard KC, et al. : CCHamide-2 Is an Orexigenic Brain-Gut Peptide in Drosophila. Plos One 2015, 10:e0133017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad M, He L, Perrimon N: Regulation of insulin and adipokinetic hormone/glucagon production in flies. Wiley Interdiscip Rev Dev Biology 2019, doi: 10.1002/wdev.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, Perrimon N: Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev Cell 2015, 33:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figueroa-Clarevega A, Bilder D: Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev Cell 2015, 33:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storelli G, Strigini M, Grenier T, Bozonnet L, Schwarzer M, Daniel C, Matos R, Leulier F: Drosophila Perpetuates Nutritional Mutualism by Promoting the Fitness of Its Intestinal Symbiont Lactobacillus plantarum. Cell Metab 2018, 27:362–377.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin SC, Kim S-H, You H, Kim B, Kim AC, Lee K-A, Yoon J-H, Ryu J-H, Lee W-J: Drosophila Microbiome Modulates Host Developmental and Metabolic Homeostasis via Insulin Signaling. Science 2011, 334:670–674. [DOI] [PubMed] [Google Scholar]
- • 31.Kamareddine L, Robins WP, Berkey CD, Mekalanos JJ, Watnick PI: The Drosophila Immune Deficiency Pathway Modulates Enteroendocrine Function and Host Metabolism. Cell Metab 2018, 28:449–462.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show here that microbiota in the midgut lumen control Tachykinin levels in enteroendocrine cells through secreted acetate. Tachykinin can subsequently control neutral lipid accumulation in the midgut and systemic metabolic responses (such as insulin signaling). Thus, innate immune signaling within unique cells in the midgut is promotes microbiota-dependent control of organismal physiology.
- 32.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F: Lactobacillus plantarum Promotes Drosophila Systemic Growth by Modulating Hormonal Signals through TOR-Dependent Nutrient Sensing. Cell Metab 2011, 14:403–414. [DOI] [PubMed] [Google Scholar]
- 33.Martino ME, Joncour P, Leenay R, Gervais H, Shah M, Hughes S, Gillet B, Beisel C, Leulier F: Bacterial Adaptation to the Host’s Diet Is a Key Evolutionary Force Shaping Drosophila-Lactobacillus Symbiosis. Cell Host Microbe 2018, 24:109–119.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher RM, Henry LM, Cornwallis CK, Kiers ET, West SA: The evolution of host-symbiont dependence. Nat Commun 2017, 8:ncomms15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karasov WH, Douglas AE: Comprehensive Physiology. Compr Physiol 2013, 3:741–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K-A, Lee W-J: Immune-metabolic interactions during systemic and enteric infection in Drosophila. Curr Opin Insect Sci 2018, 29:21–26. [DOI] [PubMed] [Google Scholar]
- 37.Harsh S, Heryanto C, Eleftherianos I: Intestinal lipid droplets as novel mediators of host-pathogen interaction in Drosophila. Biol Open 2019, 8:bio039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 38.Lee K-A, Cho K-C, Kim B, Jang I-H, Nam K, Kwon YE, Kim M, Hyeon DY, Hwang D, Seol J-H, et al. : Inflammation-Modulated Metabolic Reprogramming Is Required for DUOX-Dependent Gut Immunity in Drosophila. Cell Host Microbe 2018, 23:338–352.e5. [DOI] [PubMed] [Google Scholar]; The NADPH oxidase DUOX is critical in the Drosophila midgut to generate ROS (reactive oxygen species) for defense against external pathogens. The authors show here that pathogen infection can stimulate a complex signaling network in midgut enterocytes that limits lipid synthesis and enhances lipid catabolism, which further promotes DUOX function, highlighting innate immune-induced lipid-metabolic reprogramming of the midgut.
- 39.Hang S, Purdy AE, Robins WP, Wang Z, Mandal M, Chang S, Mekalanos JJ, Watnick PI: The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe 2014, 16:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakrabarti S, Poidevin M, Lemaitre B: The Drosophila MAPK p38c regulates oxidative stress and lipid homeostasis in the intestine. Plos Genet 2014, 10:e1004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang S, Zhao Y, Yu J, Fan Z, Gong S-T, Tang H, Pan L: Sugar Alcohols of Polyol Pathway Serve as Alarmins to Mediate Local-Systemic Innate Immune Communication in Drosophila. Cell Host Microbe 2019, 26:240–251.e8. [DOI] [PubMed] [Google Scholar]; The authors show here that the systemic coordination of infection responses between the Drosophila midgut and fat body requires secretion of unique metabolites (sugar alcohols) from circulating hemocytes.


