Abstract
Millions are affected by Sickle Cell Disease (SCD) worldwide with the greatest burden in sub-Saharan Africa. While its origin lies historically within the malaria belt, ongoing changes in migration patterns have shifted the burden of disease resulting in a global public health concern. We created the Consortium for the Advancement of Sickle Cell Research (CASiRe) to understand the different phenotypes of SCD across 4 countries (United States, United Kingdom, Italy and Ghana). Here, we report the multi-generational ethnic and racial background of 877 SCD patients recruited in Ghana (n=365, 41.6%), the United States (n=254, 29%), Italy (n=81, 9.2%), and the United Kingdom (n=177, 20.2%). West Africa (including Benin Gulf) (N=556, 63.4%) was the most common geographic region of origin, followed by North America (N=184, 21%), Caribbean (N=51, 5.8%), Europe (N=27, 3.1%), Central Africa (N=24, 2.7%), and West Africa (excluding Benin Gulf) (N=21, 2.4%). SCD patients in Europe were primarily West African (73%), European(10%), Caribbean(8%), and Central African(8%). In the United States, patients were largely African American (71%), Caribbean (13%) or West African (10%). Most subjects identified themselves as Black or African American; the European cohort had the largest group of Caucasian SCD patients (8%), including 21% of the Italian patients. This is the first report of a comprehensive analysis of ethnicity within an international, transcontinental group of SCD patients. The diverse ethnic backgrounds observed in our cohort raises the possibility that genetic and environmental heterogeneity within each SCD population subgroup can affect the clinical phenotype and research outcomes.
Keywords: sickle cell, race, ethnicity, phenotype, international
Introduction
Sickle Cell Disease (SCD) is one of the most common severe monogenic disorders worldwide. Its most frequent variant (Sickle Cell Anemia or homozygous SS disease) is caused by a single amino acid substitution at the sixth residue of the β-globin subunit (β6-Glu →Val) which results in the production of the characteristic sickle hemoglobin [1, 2]. Several double heterozygous forms also give rise to the clinical picture of SCD. The double heterozygous Sickle β0thalassemia (Sβ0thal, S mutation coupled with a thalassemia β° mutation) is the most severe form with a clinical picture similar to SS disease, while the double heterozygous SC disease (in which the hemoglobin composition is approximately 50% hemoglobin S and 50% hemoglobin C) generally displays intermediate severity. Other double heterozygote forms of SCD include the usually less severe Sickle β+thalassemia(Sβ+thal), Sickle Hemoglobin D-Punjab, and Sickle Hemoglobin O-Arab[2].
Every year an average of 300,000 children are born with sickle syndromes, and the prevalence of sickle cell among newborns ranges from 0.1/1000 in nonendemic countries to 20/1000 in several parts of Africa, therefore making SCD an increasing global health problem[1]. In fact, while SCD lies historically within the malaria belt of the world, changes in human migration over the last several decades have brought SCD patients from high-frequency regions like Sub-Saharan Africa (SSA) to North and South America and Europe[3, 4]. It is estimated that these trends will continue[4].
Despite being a monogenic disorder, SCD presents with extreme phenotypic variability[2, 5, 6]. While it is well known that the Central African Republic and Bantu haplotypes present the most severe clinical phenotype compared to the Senegalese and Arab ones, partially due to differences in fetal hemoglobin production, a detailed comparison of acute and chronic complications of SCD among different populations has not been performed[2]. Several studies did indeed compare mortality, acute events, alloimmunization rates, hemolysis, asthma and psychosocial burden in SCD populations living in different countries [5, 7–10] but a detailed description of clinical manifestations of SCD in various ethnic groups and environments has not been performed.
It has been shown that the incidence of certain clinical complications such as stroke,[7, 11] can vary according to ethnicity, and increasing evidence suggests that modifying factors within different ethnic groups seem to play a role in the associated clinical phenotype of SCD[12–15]. Therefore, we performed a prospective study in three continents (North America, Europe, Africa) to evaluate types of SCD and the associated complications of children and adults with SCD living in different environments. A comprehensive evaluation of ethnicity was performed.
Methods
The Consortium for the Advancement of Sickle Cell Research (CASiRe) is an international multi-institutional collaborative group evaluating the clinical severity of adults and children with SCD through a validated questionnaire and medical chart review, standardized across 4 countries (United States, United Kingdom, Italy and Ghana). The CASiRe consortium includes six sites in the United States (University of Michigan-Mott Children’s Hospital, Rainbow Babies and Children’s Hospital, Promedica Toledo Children’s Hospital, Children’s Hospital at Montefiore, Connecticut Children’s Medical Center, University of Connecticut Health Center), two sites each in Ghana (Ghana Institute of Clinical Genetics, Korle Bu Teaching Hospital and Pediatric Sickle Cell Clinic at Korle Bu Teaching Hospital), and Italy (University of Campania Luigi Vanvitelli, Azienda Ospedaliera-Università di Padova, Italy), and a single site in the United Kingdom (Guys and St. Thomas Hospital).
Between 2011 and 2017, after obtaining IRB approval at each site and written informed consent, demographic, clinical and laboratory data were collected by interviewing the patient and/or parent/guardian as well as by medical chart abstraction. At the 2 sites (Guys and St Thomas Hospital, UK; Azienda Ospedaliera-Università di Padova, Italy) with existing IRB approved SCD registries or standardized disease specific data collection, data were abstracted directly from their respective databases. Demographic data included: age, self-reported race[16, 17], sex, country of birth of patient, parents, and grandparents. Clinical data included: vital signs, height, weight, body mass index (BMI) oxygen saturation (SatO2), past medical history, past surgical history, family history, hydroxyurea use, and transfusion history. Pain crisis history was obtained from each patient including age of first pain crisis, and pain crises in the preceding 12 months requiring hospitalization or emergency department/day hospital utilization. Laboratory markers including hematological, biochemical, and electrophoretic data were obtained from the medical chart. Descriptive statistical analysis including mean, range, standard deviation, and percentages were all performed using SPSS 25.0 (IBM SPSS Statistics for Windows, Version 25.0).
Geographic Region of origin
Patients’ geographic region of origin was classified into West Africa Excluding Benin Gulf (Senegal, Mali, Mauritania, Guinea-Conakry, Guinea-Bissau, Cape Verde, Gambia), West Africa including Benin-Gulf (Ivory Coast, Ghana, Togo, Benin, Nigeria, Niger, Burkina-Faso), Central Africa (Congo, Central African Republic, Angola, Gabon, Burundi, Cameroon, Chad), Caribbean (Jamaica, Barbados, Bahamas, Cuba, Haiti, Dominican Republic, Trinidad and Tobago,Puerto Rico, and other islands), North America (U.S, Canada), Central America (Mexico, Honduras, Belize, Guatemala, Costa Rica, Panama, Nicaragua, El Salvador), South America (Brazil, Guyana, Colombia, Argentina, etc.), Europe (United Kingdom, Italy, France, Germany, Belgium, etc.), and Asia (Saudi Arabia, Vietnam, Iran, Iraq, India, China, Pakistan, etc.), partly based on Sommet et al’s classification of parents’ geographic country of origin in their prospective SCD newborn cohort study[12]. The geographic region of origin was based on parents’ country of birth.
Results
Over the six years that the study was open for participation, 877 patients were enrolled, with a mean age of 19.3 years. Table 1A and 1B show the demographic data. Four hundred fifty-one (51.4%) patients were children, and 424 (48.3%) were male. Ghanaians represented 41.6% (365) of patients, while 254 patients (29%) were recruited in the United States. Italy enrolled 81 patients (9.2%) of patients, and 177 patients (20.2%) were registered in the United Kingdom. Overall, 75% of patients (658) had Hgb SS, 168 patients (19.2%) had Sickle C disease, 29 (3.3%) had Sβ+thal and 22 patients (2.5%) had Sβ0 thal.
Table 1:
1A Demographic data;1B Geographic regions of origin.
| Table 1A: Demographics | N=877 | |
| Age: Mean, range (years) | 19.3 | (2.0–73.9) |
| Age Group | N | % |
| <18 y/o ≥18 y/o |
451 426 |
51.4 48.6 |
| Sex | N | % |
| M F |
424 453 |
48.3 51.7 |
| Country of Residence | N | % |
| Ghana Italy United Kingdom USA |
365 81 177 254 |
41.6 9.2 20.2 29 |
| Sickle Cell Genotype | N | % |
| SS SC Sickle Beta Thal Plus Sickle Beta Thal Zero |
658 168 29 22 |
75 19.2 3.3 2.5 |
| Table 1B: Geographic Region of Origin | N=877 | |
| Country | N | % |
| Europe | 27 | 3.1 |
| North America | 184 | 21 |
| Central America | 1 | 0.1 |
| Caribbean | 50 | 5.7 |
| South America | 1 | 0.1 |
| Middle East | 3 | 0.3 |
| Asia | 5 | 0.6 |
| North Africa | 2 | 0.2 |
| East Africa | 3 | 0.3 |
| Central Africa | 24 | 2.7 |
| West Africa Excluding Benin Gulf | 21 | 2.4 |
| West Africa Benin Gulf (includes Ghana, Nigeria) | 556 | 63.4 |
The geographic region of West Africa including Benin Gulf represented the largest group of patients (556/63.4%), while North America represented the next largest population (184/21%). The Caribbean region (51/5.8%), Europe (27/3.1%), Central Africa (24/2.7%), and West Africa excluding Benin Gulf (21/2.4%) represented most of the rest of the patients. The remaining regions, representing smaller numbers of patients can be viewed in Table 1B.
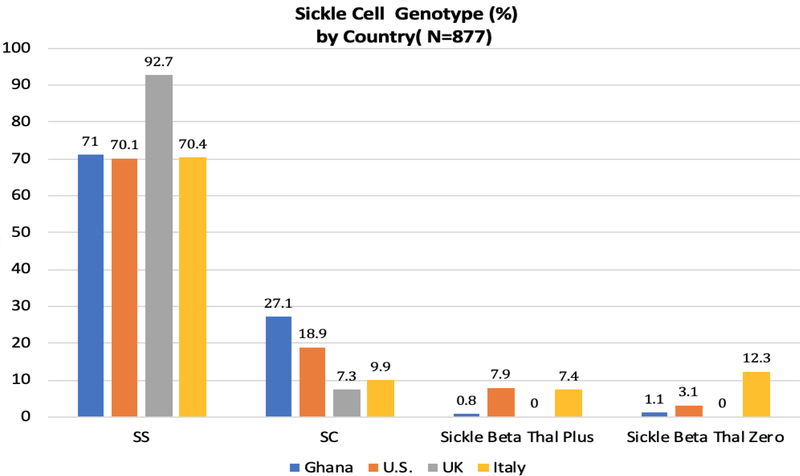
While over 70% of the patients from Ghana, the US and Italy have the SS genotype, over 92% of patients in the UK have the SS genotype. In Ghana, over 27% of patients have the SC genotype with very few patients with Sickle Beta thalassemia genotypes. There were no identified patients with sickle-beta thalassemia in the UK; in Italy, almost 20% of patients have sickle-beta thalassemia genotype (Figure 1).
Figure 1.
Disease genotype prevalence in each country
For the entire group, 820 patients (93.5%) identified themselves as African American or Black, while 30 patients (3.4%) identified themselves as Caucasian and 21 patients (2.4%) identified themselves as Latino or Hispanic. Two patients identified as both Black and Caucasian. All Ghanaians identified as Black, while in the US and UK, over 90% of patients identified themselves as Black or African American, and about 3% reported themselves as Caucasian. In comparison, in Italy, over 76% of patients reported a Black racial background, while 21% reported Caucasian background. A small number of Arab patients were identified in the US only (Table 2).
Table 2:
Racial demographics of the whole group(2A) and each country(2B), respectively.
| Table 2A: Demographics: Race | N=877 | |||
| Black or African American* | 820 | 93.5 | ||
| Caucasian* | 30 | 3.4 | ||
| Asian | 3 | 0.3 | ||
| Latino or Hispanic | 21 | 2.4 | ||
| Native American | 3 | 0.3 | ||
| Middle Eastern | 2 | 0.2 | ||
| Table 2B: Racial Background of Each Country | ||||
| Race | GHANA | US | UK | ITALY |
| N=877 | % | % | % | % |
| Black | 100 | 90.9 | 91.5 | 76.5 |
| Asian | 0 | 0.4 | 0 | 2.5 |
| Caucasian | 0 | 3.1 | 2.9 | 21 |
| Arab | 0 | 0.8 | 0 | 0 |
| Hispanic | 0 | 4.3 | 5.6 | 0 |
| Other | 0 | 1.2 | 0 | 0 |
2 Subjects checked both Races, N=879 total
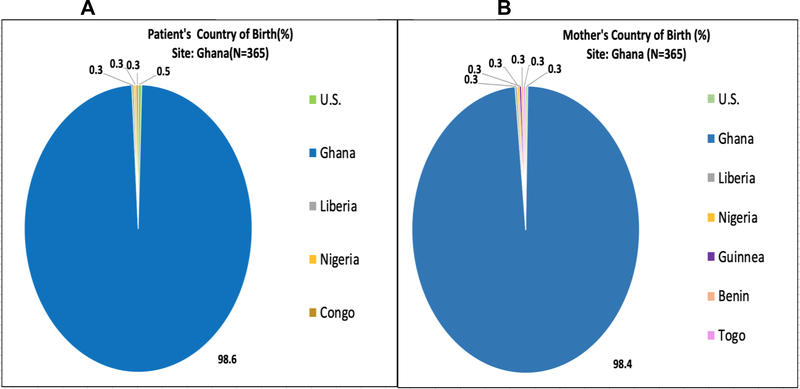
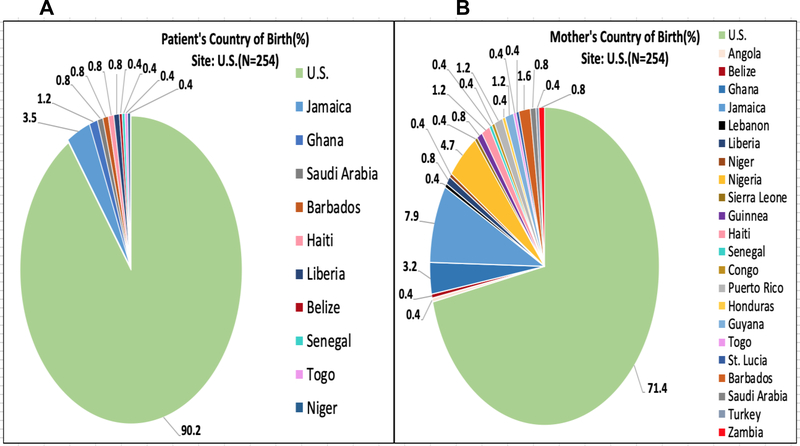
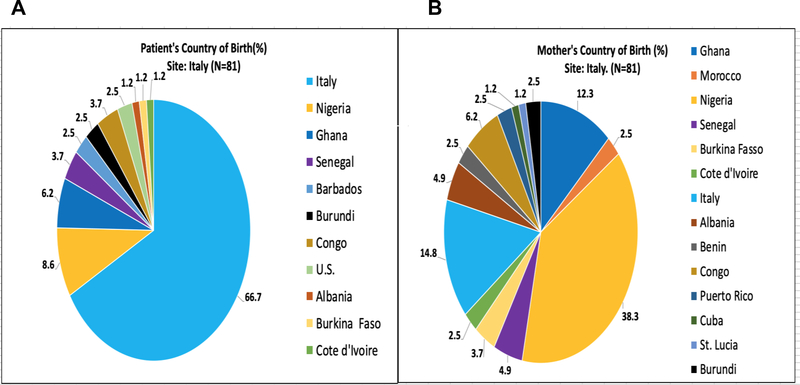
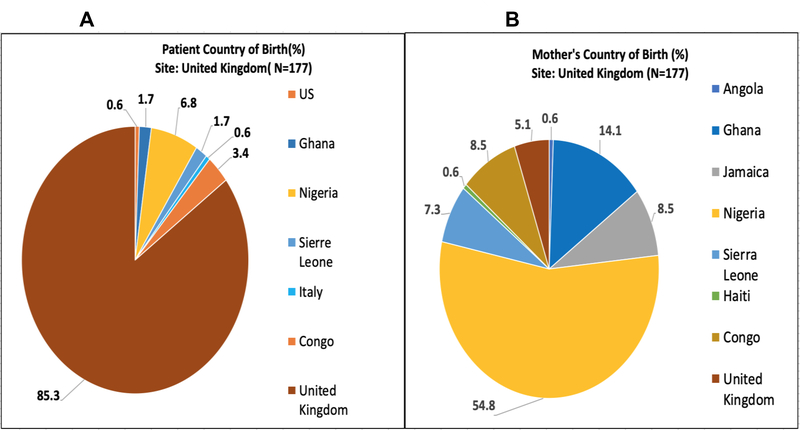
Figures 2–5 represent the country of birth for patients(A) and mothers(B) from Ghana (Figures 2A and 2B), Italy (3A and 3B), the UK (4A and 4B), and the USA (5A and 5B). Of note, the vast majority (>98%) of Ghanaian patients were born in Ghana, and mothers of Ghanaian patients were also almost all (>98%) from Ghana. In contrast, 66.7% of patients enrolled in the Italian sites were born in Italy, while only 14.6% of mothers of Italian patients were born in Italy. Most of the mothers of Italian patients were born in West Africa (primarily from Nigeria and Ghana). Over 85% of patients enrolled in the UK were born in the UK, while only 5.1% of their mothers were born in the UK (54% in Nigeria). In the US, over 90% of patients enrolled were born within the US; Mothers of patients enrolled in the US were born in America over 71% of the time. Jamaica (13%) and West African countries were the next highest maternal countries of origin. Fathers of patients mirrored similar country of birth patterns in Europe and the US. Nigeria was the paternal country of origin in 36% and 56% of patients from Italy and United Kingdom respectively (Supplementary Figures 1–4).
Figure 2:
(A)Patient’s Country of Birth %; (B)Mother’s Country of Birth %; Pie Charts of each CASiRe country are represented (Ghana)
Figure 5:
(A)Patient’s Country of Birth %; (B)Mother’s Country of Birth %; Pie Charts of each CASiRe country are represented (United States)
Figure 3:
(A)Patient’s Country of Birth %; (B)Mother’s Country of Birth %; Pie Charts of each CASiRe country are represented (Italy)
Figure 4:
(A)Patient’s Country of Birth %; (B)Mother’s Country of Birth %; Pie Charts of each CASiRe country are represented (United Kingdom)
Discussion
In recent decades, ethnic migration patterns have contributed to a more global spread of SCD outside of its origins in the malaria belt of Africa[2, 18]. This study is the first to describe the geographic distribution of these migrations in a very large cohort of nearly 900 patients with SCD. A small retrospective study of 70 SCD Italian refugees reported on their country of origins. Similar to our study, they found the majority of SCD patients emanated from SSA with the remaining patients from the Caribbean, Middle East and Asia[18]. Unlike other reports combining results of previous clinical trials and cohort studies[6, 15] which highlight differences in clinical presentation of SCD across continents, this paper’s specific focus was the geographic distribution of patients of different ethnic origins in the US, Europe and Africa. Another study by Akingbola et al [5] reported direct comparisons of SCD patients from Nigeria and US highlighting differences in hydroxyurea use, body mass index and blood pressure. The multinational DOVE study revealed geographic differences in pain reporting and analgesic use among sub-Saharan African, North American and European SCD patients[15, 19]. While these findings were novel, our cohort study suggests that differences in clinical report and management of pain cannot be fully explained by geography alone. It is more likely that variations in composition of a variety of ethnic groups in different countries impacts disease manifestation, perceptions and disease self-management[20].
Within the US CASiRe sites, 22 different countries of origin were represented based on mother’s country of birth emanating from the Caribbean, South America, Central America, Middle East and SSA. Europe reported at least 8 SSA nations as maternal countries of origin as well as other nations from the Caribbean and North Africa, yet at least 2/3 of patients recruited in Europe and 9/10 of patients enrolled in the US were born in those countries. The striking difference between maternal and patient country of origin seen in the US, Ghana and Europe suggests that information on ethnic background could be lost in one generation. It also suggests that genetic, ethnic, environmental factors, such as diet and nutritional status, cultural and national factors including health care delivery systems and norms of disease self-management, and concomitant diseases like obesity and diabetes could play different roles in different settings, thereby explaining some of the differences in disease phenotype that are beginning to emerge[15]. There will be even more increased variability of the SB compound mutations in Europe as more ethnic groups access Europe and have an in-depth diagnostic work up completed [18, 21]. The above considerations could target health service planning depending on the country and setting.
SCD displays extreme variability; while some can be explained by individual characteristics, others could be common to ethnic groups, like different blood group phenotypes[22, 23] which deeply impact blood compatibility, blood donation and risk of alloimmunization, or ethnic linked-polymorphisms like endothelial nitric oxide synthase (eNOS) or interleukin polymorphisms which could influence vasculopathy and inflammation differently in Caucasians and African SCD patients[24–28]. Large longitudinal cohorts enrolling SCD patients in comprehensive care programs that include ethnicity when evaluating clinical and hematological outcomes can aid in filling the gaps in the knowledge of SCD regarding incomplete identification of disease modifiers to enable development of comprehensive prediction models for patient stratification and targeted interventions[29, 30].
The results of our study can therefore support the need to perform longitudinal cohort studies considering ethnicity variability in order to aid clinical experts and political stakeholders in prioritizing SCD health related interventions according to the population in each setting.
In conclusion, the CASiRe cohort study is a global snapshot of approximately 900 international SCD patients with varied family migration patterns in SSA, North America and Europe reflecting the diversity of SCD patients in each country. New insights into the geographic origins of SCD patients may lead to an improved understanding of SCD phenotypic diversity and perhaps novel approaches to disease management within subpopulations of SCD patients, introducing the concept of approaching individual patients or subgroups of patients utilizing ethnicity in a broader sense [20].
The recent resurgence in clinical trials was based on improved understanding of the pathophysiology of SCD leading to promising disease modifying therapies [31–34]. Obtaining geographic origins and ethnicity of SCD patients may be essential to discovering the underlying mechanisms of the discordant phenotypic expression observed in SCD and could guide public health interventions in different settings. Moreover, the ethnicity data of our international cohort underscores the need for global partnership to further understand the epidemiology of sickle cell disease and optimize health interventions.
Supplementary Material
Acknowledgements
We would like to thank the following individuals for their contributions to this work: Students of the University of Michigan Minority Health and Health Disparities International Research Training (MHIRT)program (Funding support: Grant # NIMHD T37MD001425) Ahmed Owda, Duna Buttner, Biana D. Oteng, Sophia Akatue, Ashya Smith, Austin Novarra, Clementine Fu, Fitz Tavernier, Lewis Graham, Rose Bamfo, Esther Kim, Haikel Halle, Rebekah Urbonya, Fatimah Farooq, Sheri Van Omen, Marianna Yamamoto. Thanks to Adetola Kassim, Vishwas Sakhalkar for their help in developing the questionnaire and protocol.
A Campbell: Research Funding and Consultancy from Global Blood Therapeutics (GBT), Novartis, Cyclerion; Consultancy from BluebirdBio; D Manwani: Research funding from Grifols; Consultancy for Novartis, Pfizer, Global Blood Therapeutics; B Andemariam: Consultancy for Novartis, Pfizer, NovoNordisk, Emmaus, Cyclerion, Terumo, Sanofi; DSMB: Global Blood Therapeutics; Research Funding: Imara; B Inusa:
Education Funding: Novartis AstraZeneca, Global Blood Therapeutics, Celgene, Vertex; C. Strunk: Consultancy Global Blood Therapeutics and Novartis; R Colombatti: Research Funding: Global Blood Therapeutics, Novartis.
Footnotes
Disclosure of potential conflicts of interest
No disclosures to declare from the other co-authors.
Compliance with Ethical Standards
Informed consent and Research involving human participants and/or animals
Except at two sites (Guys and St Thomas Hospital, United Kingdom; Azienda Ospedaliera-Università di Padova, Italy) that had a previously Institutional Review Board approved Sickle Cell Disease Registry, Institutional Review Board approval was obtained at each site and written informed consent was obtained by each patient and/or parent/guardian.
This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Weatherall DJ, The inherited diseases of hemoglobin are an emerging global health burden. Blood, 2010. 115(22): p. 4331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piel FB, Steinberg MH, and Rees DC, Sickle Cell Disease. N Engl J Med, 2017. 377(3): p. 305. [DOI] [PubMed] [Google Scholar]
- 3.Inusa BPD and Colombatti R, European migration crises: The role of national hemoglobinopathy registries in improving patient access to care. Pediatr Blood Cancer, 2017. 64(7). [DOI] [PubMed] [Google Scholar]
- 4.Piel FB, et al. , Global migration and the changing distribution of sickle haemoglobin: a quantitative study of temporal trends between 1960 and 2000. Lancet Glob Health, 2014. 2(2): p. e80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akingbola TS, et al. , Comparison of patients from Nigeria and the USA highlights modifiable risk factors for sickle cell anemia complications. Hemoglobin, 2014. 38(4): p. 236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraf SL, et al. , Differences in the clinical and genotypic presentation of sickle cell disease around the world. Paediatr Respir Rev, 2014. 15(1): p. 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Njamnshi AK, et al. , Stroke may appear to be rare in Saudi-Arabian and Nigerian children with sickle cell disease, but not in Cameroonian sickle cell patients. Br J Haematol, 2006. 133(2): p. 210; author reply 211. [DOI] [PubMed] [Google Scholar]
- 8.Montanaro M, et al. , Intellectual function evaluation of first generation immigrant children with sickle cell disease: the role of language and sociodemographic factors. Ital J Pediatr, 2013. 39: p. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitra P, et al. , Risk factors for mortality in adult patients with sickle cell disease: a meta-analysis of studies in North America and Europe. Haematologica, 2017. 102(4): p. 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y and Maitta RW, Alloimmunisation rates of sickle cell disease patients in the United States differ from those in other geographical regions. Transfus Med, 2016. 26(3): p. 225–30. [DOI] [PubMed] [Google Scholar]
- 11.Bavarsad Shahripour R, et al. , Can STOP Trial Velocity Criteria Be Applied to Iranian Children with Sickle Cell Disease? J Stroke, 2014. 16(2): p. 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommet J, et al. , Clinical and haematological risk factors for cerebral macrovasculopathy in a sickle cell disease newborn cohort: a prospective study. Br J Haematol, 2016. 172(6): p. 966–77. [DOI] [PubMed] [Google Scholar]
- 13.Duru KC, et al. , Extensive genomic variability of knops blood group polymorphisms is associated with sickle cell disease in Africa. Evol Bioinform Online, 2015. 11: p. 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noble JA, et al. , Interethnic diversity of the CD209 (rs4804803) gene promoter polymorphism in African but not American sickle cell disease. PeerJ, 2015. 3: p. e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanter J, et al. , Novel findings from the multinational DOVE study on geographic and age-related differences in pain perception and analgesic usage in children with sickle cell anaemia. Br J Haematol, 2019. 184(6): p. 1058–1061. [DOI] [PubMed] [Google Scholar]
- 16.Health, N.I.o. National Institutes of Health (2001) NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2001; Available from: https://grants.nih.gov/policy/inclusion/women-and-minorities.htm
- 17.National Center on Birth Defects and Developmental Disabilities, C.f.D.C.a.P. 2019; Available from: https://www.cdc.gov/ncbddd/sicklecell/data.html. [DOI] [PMC free article] [PubMed]
- 18.De Franceschi L, et al. , Access to emergency departments for acute events and identification of sickle cell disease in refugees. Blood, 2019. 133(19): p. 2100–2103. [DOI] [PubMed] [Google Scholar]
- 19.Inusa BPD, et al. , Geographic Differences in Phenotype and Treatment of Children with Sickle Cell Anemia from the Multinational DOVE Study. J Clin Med, 2019. 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun L, et al. , Racial categories in medical practice: how useful are they? PLoS Med, 2007. 4(9): p. e271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moussa EY, Yassine NM, and Borjac JM, New variants in beta globin gene among the Palestinian refugees with sickle cell disease in Lebanon. Saudi Med J, 2018. 39(12): p. 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nasr A, et al. , Antibody responses to P. falciparum Apical Membrane Antigen 1(AMA-1) in relation to haemoglobin S (HbS), HbC, G6PD and ABO blood groups among Fulani and Masaleit living in Western Sudan. Acta Trop, 2018. 182: p. 115–123. [DOI] [PubMed] [Google Scholar]
- 23.Boateng LA, et al. , Red blood cell alloimmunization and minor red blood cell antigen phenotypes in transfused Ghanaian patients with sickle cell disease. Transfusion, 2019. 59(6): p. 2016–2022. [DOI] [PubMed] [Google Scholar]
- 24.Armenis I, et al. , Data on eNOS T786 and G894T polymorphisms and peripheral blood eNOS mRNA levels in Sickle Cell Disease. Data Brief, 2017. 10: p. 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afifi RA, et al. , CD209–336A/G promotor polymorphism and its clinical associations in sickle cell disease Egyptian Pediatric patients. Hematol Oncol Stem Cell Ther, 2018. 11(2): p. 75–81. [DOI] [PubMed] [Google Scholar]
- 26.Armenis I, et al. , Prognostic value of T786C and G894T eNOS polymorphisms in sickle cell disease. Nitric Oxide, 2017. 62: p. 17–23. [DOI] [PubMed] [Google Scholar]
- 27.Afifi RAA, et al. , IL-Ibeta+3954 C/T Polymorphism and Its Clinical Associations in Egyptian Sickle Cell Disease Patients. Int J Hematol Oncol Stem Cell Res, 2019. 13(1): p. 35–41. [PMC free article] [PubMed] [Google Scholar]
- 28.Antwi-Boasiako C, et al. , Association between eNOS Gene Polymorphism (T786C and VNTR) and Sickle Cell Disease Patients in Ghana. Diseases, 2018. 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira F, et al. , A systematic literature review on the European, African and Amerindian genetic ancestry components on Brazilian health outcomes. Sci Rep, 2019. 9(1): p. 8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wonkam A and Makani J, Sickle cell disease in Africa: an urgent need for longitudinal cohort studies. Lancet Glob Health, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ataga KI, et al. , Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med, 2017. 376(5): p. 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heeney MM, et al. , A Multinational Trial of Prasugrel for Sickle Cell Vaso-Occlusive Events. N Engl J Med, 2016. 374(7): p. 625–35. [DOI] [PubMed] [Google Scholar]
- 33.Martins GLS, et al. , Generation of integration-free iPS cell lines from three sickle cell disease patients from the state of Bahia, Brazil. Stem Cell Res, 2018. 33: p. 10–14. [DOI] [PubMed] [Google Scholar]
- 34.Vichinsky E, et al. , A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N Engl J Med, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.