Abstract
Negative affect and poor inhibitory control are related to disinhibited eating behaviors in youth and may contribute to the development and/or maintenance of obesity. Although few studies have jointly examined these constructs in youth, it has been theorized that poor inhibitory control may be driven by negative affect. If supported, impaired inhibitory control, driven by negative affect, could represent a modifiable neurocognitive treatment target for disinhibited eating. The current study examined whether inhibitory control mediates the relationship between negative affect and eating among youth. Youth (8-17 years) participated in a Food Go/No-Go neurocognitive task to measure inhibitory control as the percentage of commission errors. A composite negative affect score was created from self-report measures of anxiety and depression. A laboratory buffet meal modeled to simulate disinhibited eating was used to measure total and snack food intake. Cross-sectional mediation models with bias-corrected bootstrap confidence intervals (CI) were conducted using negative affect as the independent variable, inhibitory control as the mediator, and intake patterns as dependent variables. One-hundred-eighty-one youths (13.2±2.7y; 55% female; BMIz 0.6±1.0) were studied. Total Go/No-Go commission errors mediated the relationship between negative affect and total intake (95%CI=[0.3, 31.6]), but not snack intake (95%CI=[−2.5, 7.3]). Commission errors for Food-Go blocks significantly mediated the relationship between negative affect and total intake (95%CI=[7.7, 44.4]), but not snack intake (95%CI=[−3.4, 9.5]). Commission errors on Neutral-Go blocks did not significantly mediate any of these relationships. Negative affect may lead to poorer inhibitory control as well as a stronger approach tendency toward food, increasing the likelihood of engaging in disinhibited eating. Future research should determine if, in combination with approaches to reduce negative affect, improved inhibitory control could help prevent overeating in youths with depressive or anxiety symptoms.
Keywords: inhibitory control, negative affect, children, adolescents, obesity, disinhibited eating
1. Introduction
Serious medical consequences are associated with pediatric overweight and obesity (Hruby et al., 2016; Yanovski, 2015), and further, children and adolescents with higher weight are more likely to develop into adults with obesity (Singh, Mulder, Twisk, Van Mechelen, & Chinapaw, 2008). As such, elucidating factors that may promote obesity is warranted in order to aim at reducing high weight in children. One potentially modifiable contributor to the development of excess weight gain and associated adverse health comorbidities among youth is a tendency to engage in disinhibited eating (Shomaker, Tanofsky-Kraff, & Yanovski, 2011). The umbrella term ‘disinhibited eating’ refers to a range of eating behaviors that involve a lack of healthy restraint over food intake (Shomaker, Tanofsky-Kraff, & Yanovski, 2011). Such eating behaviors may include, but are not limited to, loss of control eating, the key feature of binge eating disorder; emotional eating, the tendency to use food to regulate mood; and eating in the absence of hunger, the tendency to eat when one is already sated. However, the underlying mechanisms that contribute to disinhibited eating behaviors are not fully understood. Thus, a better understanding of how, and under which circumstances, disinhibited eating patterns occur and promote excess weight gain is required to identify specific treatment targets for obesity prevention.
One well-supported mechanistic theory that may help to explain which youth are at greatest risk for disinhibited eating is affect theory. Affect theory proposes that negative mood drives the onset of disinhibited eating episodes as a maladaptive attempt to reduce uncomfortable emotions, which can in turn contribute to excess weight gain (Heatherton & Baumeister, 1991; Kenardy, Arnow, & Agras, 1996). Facets of negative affect, including anxiety and depressive symptoms, have been strongly linked with disinhibited eating, particularly binge-eating behaviors (Goldschmidt, Aspen, Sinton, Tanofsky-Kraff, & Wilfley, 2008; Haedt-Matt & Keel, 2011), as well as with excess energy intake and intake of highly palatable snack foods (Byrne et al., 2020; Ranzenhofer et al., 2013) among youth across the age spectrum. Taken together, research consistently establishes a link between negative affect and disinhibited eating behaviors among youth, potentially contributing to higher risk for excess weight gain and associated health complications (Burke & Storch, 2015; Shomaker, Tanofsky-Kraff, Stern, et al., 2011). However, the nature of the relationship between negative affect and eating behaviors warrants further investigation, particularly given some pediatric studies utilizing ecological momentary assessment have not found a direct link between momentary negative affect and disinhibited eating behaviors (Goldschmidt et al., 2018; Hilbert, Rief, Tuschen-Caffier, de Zwaan, & Czaja, 2009; Ranzenhofer et al., 2014). Momentary negative mood states are considered less stable than trait levels of negative affect such as trait anxiety or depressive symptoms. It is possible that trait negative affect masks aspects of state negative affect, such that youth experiencing consistent, stable negative mood are less likely to endorse elevated levels of momentary negative mood, which may partially explain mixed findings in the literature. Thus, it is important to further elucidate the link between trait negative affect and disinhibited eating by identifying mechanisms that may explain the relationship.
Neurocognitive facets of executive functioning, namely poor inhibitory control, have also been linked to disinhibited eating behaviors (Kelly et al., 2020; Nasser, Gluck, & Geliebter, 2004; Smith, Mason, Johnson, Lavender, & Wonderlich, 2018). Further, inhibitory control has been linked to facets of negative affect (Bora, Harrison, Yücel, & Pantelis, 2013), particularly in samples of adults with symptoms of binge eating (Loeber et al., 2018; Smith, Mason, Crosby, Engel, & Wonderlich, 2019). Inhibitory control involves the deliberate suppression of automatic behaviors, emotions, or thoughts (Aron, 2011; Bari & Robbins, 2013). Youths with overweight and obesity are reported to exhibit more difficulty when inhibiting behaviors compared to their counterparts without high weight (Kamijo et al., 2012; Pauli-Pott, Albayrak, Hebebrand, & Pott, 2010). Studies have suggested that youths who possess difficulties with inhibitory control may be acutely susceptible to disinhibited eating behaviors and excess weight gain (Hartmann, Czaja, Rief, & Hilbert, 2010; Nederkoorn, Braet, Van Eijs, Tanghe, & Jansen, 2006). Negative urgency, or the tendency to have difficulty with impulse control during negative mood states (Cyders & Smith, 2008), has been consistently linked to maladaptive disinhibited eating behaviors and poorer quality of life among both youth (Rose, Nadler, & Mackey, 2018; Thomsen et al., 2018) and adult samples (Culbert, Racine, & Klump, 2015; Mallorquí-Bagué et al., 2020). The interconnected nature between negative affect and inhibitory control may be bidirectional (Bora et al., 2013), however there is some research to suggest that impairments in inhibitory control may be one mechanistic pathway through which negative affect could promote disinhibited eating. Notably, youths with disinhibited eating have been shown to report decreased inhibitory control following a negative mood induction compared to healthy youths, as well as to those with attention deficit/hyperactivity disorder, a disorder defined in part by difficulties in inhibitory control (Hartmann, Rief, & Hilbert, 2012). This research suggests that negative affect may, in fact, influence or compromise inhibitory control abilities, which in turn could lead to disinhibited eating behaviors. However, few studies have examined this potentially modifiable neurocognitive mechanism as a theoretical pathway for disinhibited eating driven by negative affect.
One study among healthy youth examining food-related inhibitory control using a computerized neurocognitive task involving pictures of food cues and neutral toy cues found that youth displayed poorer inhibition in response to food cues compared to neutral toy cues (Teslovich et al., 2014), suggesting that disinhibited eating may be driven by food-specific, as opposed to general, inhibitory control. In conjunction with this finding and past research linking negative urgency to disinhibited eating behaviors (Rose et al., 2018; Thomsen et al., 2018), it is possible that there may be a relationship between food-specific inhibitory control and negative affect that subsequently influences disinhibited eating. Using this computerized neurocognitive task, the present study aimed to improve and extend upon past research. The objective of the current study was to examine whether food-related inhibitory control mediated the relationship between negative affect and in vivo intake at a laboratory test meal in healthy, non-clinical youths across the weight spectrum. Within a cross-sectional mediation framework, it was hypothesized that 1) youth with higher levels of negative affect would exhibit poorer general inhibitory control on a food-related neurocognitive task; 2) youth with poorer general inhibitory control would consume more total and highly-palatable snack foods at a laboratory test meal; and 3) poorer general inhibitory control would mediate the relationship between negative affect and both total and snack food intake. An additional objective of the present study was to separately examine these mediation effects between food and neutral (i.e. toy) stimulus types. In line with prior research finding that youth displayed poorer inhibition in response to food cues compared to neutral toy cues (Teslovich et al., 2014), it was hypothesized that poorer inhibitory control in response to food cues would drive the mediation effect between negative affect and intake, such that the mediation effect would be present for food-related cues, but not for neutral cues.
2. Methods
2.1. Participants and Procedure
This analysis was conducted using data from a larger ongoing observational longitudinal trial (ClinicalTrials.gov ID# NCT02390765); other findings from this cohort have been previously reported (Byrne et al., 2019; Kelly et al., 2020; LeMay-Russell et al., 2019; Shank et al., 2019). Participants were boys and girls, 8-17 years old. Youth were excluded if they had a Full Scale Intelligent Quotient ≤ 70 (Wechsler, 2011), a serious major medical or full-threshold DSM psychiatric condition, current presence of an eating disorder other than binge eating disorder, were underweight (i.e., BMI < 5th percentile) (Kuczmarski et al., 2002), had a history of significant or recent brain injury, a significant reduction in weight during the past three months (>5% of body weight), or reported current and regular use of medications or substances known to impact weight and/or eating behavior. Exclusionary psychiatric conditions and eating disorder diagnoses were determined by semi-structured clinical interviews, respectively, the Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997) and the Eating Disorder Examination adult or child version (Bryant-Waugh, Cooper, Taylor, & Lask, 1996; Fairburn & Cooper, 1993).
All youth and a parent/guardian attended two visits at the National Institutes of Health Hatfield Clinical Research Center. Written assent and consent, respectively, were obtained. At the first visit, children and adolescents completed questionnaires and had eligibility assessed. Approximately two weeks later, eligible participants completed the second visit, which was conducted after an overnight fast. Anthropometric measurements were collected in the fasted state. At approximately 10:00 AM, participants consumed a standardized breakfast shake (17% protein, 16% fat, 67% carbohydrate) containing 21% of daily energy needs, as estimated by measured body weight, height, age, and average activity level within the previous week (Craig et al., 2003; Institute of Medicine, 2006). At approximately 12:30 PM, participants completed a laboratory test meal. After the test meal, cognitive tasks were administered. The study procedure was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development institutional review board.
2.2. Measures
2.2.1. Anthropometric Measurements
Fasting weight was measured on a calibrated scale to the nearest 0.1kg and height was measured in triplicate on a calibrated stadiometer. BMI-z was calculated to adjust BMI for age and sex accordingly to the Centers for Disease Control and Prevention growth standards (Kuczmarski et al., 2002). Dual energy x-ray absorptiometry (GE Lunar iDXA, GE Healthcare, Madison WI; software GE encore 15), a validated measure of body composition in youth (Sopher et al., 2004), was used to assess body lean (kg) and fat (%) mass.
2.2.2. Depressive Symptoms
The Children’s Depression Inventory (Kovacs, 1992), a 27-item self-report questionnaire, was used to assess depressive symptoms over the previous two weeks. Total scores range from 0-54, with higher scores representing more depressive symptoms. The questionnaire has previously shown good reliability and validity in youth (Carey, Faulstich, Gresham, Ruggiero, & Enyart, 1987; Smucker, Craighead, Craighead, & Green, 1986). A cut-off score of 19-20 screens for clinical depression (Kovacs, 1992). In this sample, the Children’s Depression Inventory demonstrated good internal consistency (α = .85).
2.2.3. Anxiety Symptoms
Participants completed the trait subscale of the State-Trait Anxiety Inventory for Children (Spielberger, 1973), a 20-item self-report questionnaire. Total scores range from 20-60, with higher scores representing more trait anxiety symptoms. The questionnaire has demonstrated good psychometric properties in youth (Barnes, Harp, & Jung, 2002; Muris, Merckelbach, Ollendick, King, & Bogie, 2002; Spielberger, 1973). In this sample, the trait subscale of the State-Trait Anxiety Inventory for Children showed very good internal consistency (α = .90).
2.2.4. Inhibitory Control
Inhibitory control was assessed using a computerized 12-minute Food Go/No-Go neurocognitive task (Teslovich et al., 2014). The task instructed the participant to press the space ! bar when a specific stimulus-type appeared (“Go” trials), but inhibit pressing the space bar when a different stimulus-type appeared (“No-Go” trials). Each stimulus was presented for 500 ms, with 2000-4000 ms inter-trial intervals. The task contained four randomized, counterbalanced i blocks. Two of the four blocks contained neutral toy stimuli as Go cues and food stimuli as No-Go cues (i.e., Neutral Toy = Go cue/ Food = No-Go cue, hereafter referred to as “Neutral-Go” blocks), while the other two blocks contained food stimuli as Go cues and neutral toy stimuli as No-Go cues (i.e., Food = Go cue/ Neutral Toy = No-Go cue, hereafter referred to as “Food-Go” I blocks). Depending on the block, instructions stated either “Press the spacebar when you see [food/toy]. Don’t press for any other pictures, only [food/toy]. Go as fast as you can without making any mistakes.” The food cue stimuli included images of low- and high-calorie-density foods (See Supplement in Teslovich et al., 2014). Within each block, approximately 73% of trials required a Go response and 27% required a No-Go response, in order to develop a prepotent “Go” response tendency. Participants completed a 3-minute practice round before beginning the scored portion of the task. The variable of interest on this task is the percentage of commission errors, which is characterized by inaccurate No-Go trial responses, or in other terms, failing to inhibit pressing the space bar on a trial when participants are instructed to not press. A higher percentage of commission errors represents worse inhibitory control (Batterink, Yokum, & Stice, 2010; Teslovich et al., 2014). Commission errors were first summed in total across all four blocks as a measure of general inhibitory control (i.e. commission errors on Neutral-Go blocks + commission errors on Food-Go blocks, hereafter referred to as “total percentage commission errors”), and were then analyzed separately for Neutral-Go blocks only and Food-Go blocks only (Teslovich et al., 2014) in order to examine more specific measures of food-related inhibitory control.
2.2.5. Food intake
Participants were presented with a multi-item buffet meal in the laboratory that included >10,000 kcal (55% carbohydrate, 12% protein, 33% fat). The buffet meal included both high- and low-energy dense foods, including foods typically considered palatable by youths (Tanofsky-Kraff et al., 2009). Before the buffet meal, participants listened to a standardized recording that stated, “Let yourself go and eat as much as you want.” This validated test meal paradigm is designed to promote disinhibited eating (Mirch et al., 2006; Tanofsky-Kraff et al., 2009). The amount of food consumed was determined by weighing each food item on an electronic balance scale to the nearest gram before and after the meal. Nutrient content data were based on the USDA National Nutrient Database Standard reference database and nutrition labels. The total amount consumed (as kcal) and the consumption of palatable snack foods (as kcal) was then calculated for each participant. Palatable snack foods, including both sweet- (e.g. cookies, jellybeans, chocolate candy) and salty- (e.g. tortilla chips, pretzels) snack foods, were characterized by those foods thought to be representative of highly palatable foods that are energy dense, high in sugar and fat, and low in protein (Ranzenhofer et al., 2013; Tanofsky-Kraff et al., 2009).
2.3. Data Analytic Plan
Analyses were conducted using IBM SPSS 25.0 (IBM Corp., Armonk, NY). Data were screened for outliers and normality. Fat mass percentage was arcsine square-root transformed to improve normality. Extreme but plausible outliers across all variables (defined a priori as at least three standard deviations from the mean; n = 12) were recoded to that threshold in order to minimize their influence on findings while retaining these cases (Behrens, 1997; Osborne & Overbay, 2004). A composite negative affect score was created by averaging standardized scores for depressive and anxiety symptoms, a method that has been used in prior research (Byrne et al., 2020; Elliott et al., 2010; Nelson, Aylward, & Steele, 2007). A paired samples t-test was conducted to compare commission errors between the two conditions (Neutral-Go versus Food-Go). Participant characteristics by sex were examined using independent samples t-tests or chi-quare tests, as appropriate.
Six cross-sectional mediation models with bias-corrected bootstrap confidence intervals (CI) were conducted using the Preacher and Hayes Indirect Mediation macro v3.4 for SPSS (Hayes, 2017). For each mediation model, the relationship between negative affect and inhibitory control (a pathways) and the relationship between inhibitory control and energy intake (b pathways) are reported. In addition, bootstrapping with 10,000 resamples estimated the 95% bias-corrected confidence interval (CI) for the indirect pathway (ab). Both unstandardized and standardized indirect effects were calculated and reported (Hayes, 2017; Preacher & Kelley, 2011) in order to facilitate comparison of effects across models. The composite negative affect score was the independent variable for all models. The mediators were 1) percentage of total commission errors, 2) percentage commission errors on Neutral-Go blocks only, and 3) percentage commission errors on Food-Go blocks only. The dependent variables were total energy intake (kcal) and snack food intake (kcal). Covariates for the mediation models were age (continuous), sex (coded as 0 = female; 1 = male), race (coded as 0 = non-Hispanic White; 1 = other), lean mass (kg; continuous), and fat mass (%; continuous). All tests were two-tailed. Findings were considered significant when p ≤ .05. For these exploratory analyses, no adjustment for multiplicity of testing was applied.
3. Results
3.1. Participant Characteristics
One hundred and eighty-one children and adolescents (55% female; 13.2±2.7 years; BMI-z: 0.6±1.0) completed the study procedures and had valid cognitive task data. The racial/ethnic breakdown was 45% non-Hispanic White, 26% non-Hispanic Black, 12% non-Hispanic Asian, 8% Hispanic or Latino, and 9% other, multiple races, or unknown. Participant characteristics by sex are shown in Table 1. Girls reported more anxiety symptoms [t(180) = 6.1, p = .01], committed fewer total [t(180) = −2.7, p = .02] and Neutral-Go [t(180) = −3.0, p = .01] commission errors, and had a higher percentage of fat mass [t(180) = 5.9, p < .001] compared to boys. Participants made significantly more commission errors on Neutral-Go blocks (40.5±19.9) than Food-Go blocks [33.1±19.5; t(180) = −7.0, p < .001; Figure 1].
Table 1:
Participant Characteristics by Sex
| Total Sample (n = 181) |
Boys (n = 82) |
Girls (n = 99) |
p | |
|---|---|---|---|---|
| Age (years) | 13.2 (2.7) | 13.0 (2.7) | 13.4 (2.7) | .29 |
| Age range, n (%) | .20 | |||
| Pre-Adolescent (8-12) | 66 (36) | 34 (42) | 32 (32) | |
| Adolescent (13-17) | 115 (64) | 48 (59) | 67 (68) | |
| Race, n (%) | .06 | |||
| Non-Hispanic White | 81 (45) | 43 (52) | 38 (38) | |
| Non-Hispanic Black | 47 (26) | 16 (20) | 31 (31) | |
| Hispanic/Latino | 15 (8) | 8 (10) | 7 (7) | |
| Other/Unknown | 38 (21) | 15 (18) | 23 (23) | |
| BMI (kg/m2) | 21.8 (5.5) | 20.9 (5.0) | 22.6 (5.9) | .05 |
| BMI-z | 0.6 (1.0) | 0.5 (1.0) | 0.7 (1.0) | .18 |
| Weight status, n (%) | .88 | |||
| Healthy Weight | 120 (66) | 56 (68) | 64 (65) | |
| Overweight | 28 (16) | 12 (15) | 16 (16) | |
| Obese | 33 (18) | 14 (17) | 19 (19) | |
| Lean mass (kg) | 37.3 (11.7) | 39.0 (13.2) | 35.8 (9.9) | .06 |
| Fat mass (%)a | 28 (9) | 24 (9) | 31 (7) | <.001* |
| Depressive symptoms, median (IQR)b | 6 (3, 10) | 5.5 (3, 9) | 7 (3, 10) | .20 |
| Anxiety, median (IQR)b | 30 (26, 37) | 29 (24, 34) | 31.5 (27, 38) | .01* |
| LOC eating presence, n (%)c | 16 (8.8) | 5 (6.1) | 11 (11.1) | .19 |
| Emotional eating, n (%)d | 76 (42.0) | 32 (39.0) | 44 (44.4) | .28 |
| Eating in the absence of hunger, n (%)e | 87 (48.1) | 33 (40.2) | 54 (54.5) | .02* |
| Total commission errors (%) | 37 (18) | 41 (17) | 34 (19) | .02* |
| Neutral-Go commission errors (%) | 41 (20) | 45 (19) | 37 (20) | .01* |
| Food-Go commission errors (%) | 33 (20) | 36 (19) | 31 (20) | .08 |
| Total intake (kcal) | 967 (419) | 994 (410) | 926 (423) | .28 |
| Snack intake (kcal) | 224 (184) | 221 (168) | 213 (189) | .78 |
Data presented as M (SD) unless otherwise noted.
Un-transformed mean and standard deviation shown.
IQR = interquartile range; median (25% IQR, 75% IQR) shown.
LOC eating presence within the past month measured by the Eating Disorder Examination adult or child version (Bryant-Waugh et al., 1996; Fairburn & Cooper, 1993).
Emotional eating measured by the Emotional Eating Scale for Children (Tanofsky-Kraff et al., 2007); n and % reported for those who endorsed high emotional eating (total score > 13) (Vannucci et al., 2012).
Eating in the absence of hunger measured by the Eating in the Absence of Hunger Questionnaire for Children (Tanofsky-Kraff et al., 2008); n and % reported for those who reported high eating in the absence of hunger (>median cut-off of 21).
Group differences are significant at p < .05 for independent samples t-tests or Chi-square analyses, as appropriate. Abbreviations: BMI-z, body mass index adjusted for age and sex; LOC, loss of control; Neutral-Go, blocks in which images of neutral toy images were the Go target and food images were the No-Go target; Food-Go, blocks in which food images were the Go target and neutral toy images were the No-Go target.
Figure 1.
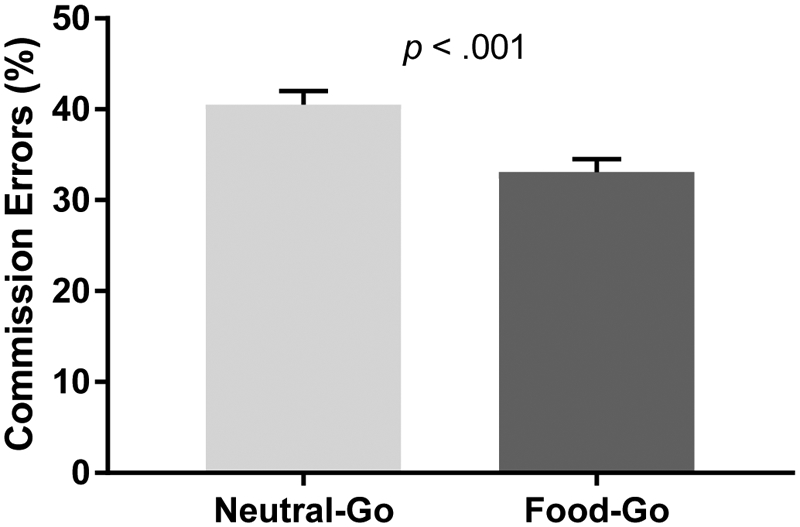
Total percentage commission errors on Neutral-Go versus Food-Go blocks. Participants committed a greater percentage of commission errors on Neutral-Go blocks than Food-Go blocks (p < .001). Mean ± standard error are shown.
3.2. Total Percentage Commission Errors
Mediation findings for total commission errors are shown in Table 2. Negative affect was not significantly associated with total commission errors (a = 0.03, SE = 0.01, p = .06). Total commission errors was significantly positively associated with total energy intake (b = 544.3, SE = 163.0, p < .01), but not snack energy intake (b = 71.7, SE = 78.8, p = .36). Total commission errors significantly mediated the relationship between negative affect and total energy intake (ab = 14.0, SE = 8.0, unstandardized 95% CI = [0.3, 31.6], standardized 95% CI = [<0.01, 0.07]; Figure 2). Total commission errors did not mediate the relationship between negative affect and snack energy intake (ab = 1.8, SE = 2.4, unstandardized 95% CI = [−2.5, 7.3]. standardized 95% CI = [−0.01, 0.04]).
Table 2:
Mediation Analyses by Model
| a (SE) | b (SE) | c (SE) | c’ (SE) | ab (SE) |
Unstandardized 95% CI (ab) |
Standardized 95% CI (ab) |
|
|---|---|---|---|---|---|---|---|
| Total Commission Errors (%) | |||||||
| Total Energy (kcal) | 0.03 (0.01) | 544.3 (163.0)* | 38.3 (29.8) | 24.3 (29.2) | 14.0 (8.0)* | 0.3, 31.6 | <0.01, 0.07 |
| Snack Intake (kcal) | 0.03 (0.01) | 71.7 (78.8) | 22.6 (14.0) | 20.7 (14.1) | 1.8 (2.4) | −2.5, 7.3 | −0.01, 0.04 |
| Commission Errors Neutral-Go Blocks (%) | |||||||
| Total Energy (kcal) | 0.01 (0.02) | 342.7 (148.2)* | 38.3 (29.8) | 34.5 (29.4) | 3.9 (5.5) | −7.1, 15.6 | −0.02, 0.04 |
| Snack Intake (kcal) | 0.01 (0.02) | 54.7 (70.6) | 22.6 (14.0) | 22.0 (14.0) | 0.6 (1.5) | −2.4, 4.2 | −0.01, 0.02 |
| Commission Errors Food-Go Blocks (%) | |||||||
| Total Energy (kcal) | 0.04 (0.01)* | 599.5 (153.0)* | 38.3 (29.8) | 14.4 (29.2) | 23.9 (9.5)* | 7.7, 44.4 | 0.02, 0.10 |
| Snack Intake (kcal) | 0.04 (0.01)* | 71.7 (74.8) | 22.6 (14.0) | 19.7 (14.3) | 2.9 (3.2) | −3.4, 9.5 | −0.02, 0.05 |
Significant at p < .05. Abbreviations: Neutral-Go, blocks in which images of neutral toys were the Go target and food images were the No-Go target; Food-Go, blocks in which food images were the Go target and neutral toys were the No-Go target; CI, confidence interval.
Figure 2.
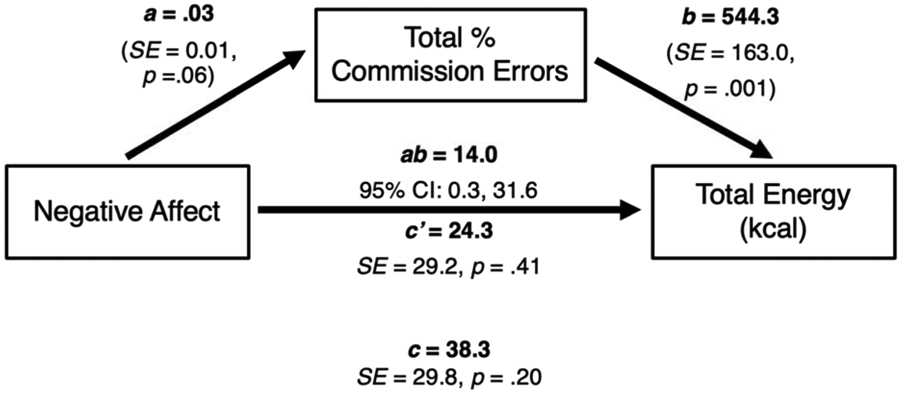
Cross-sectional mediation models with negative affect as the independent variable, total percent commission errors as the mediator, and total energy intake (kcal) as the dependent variable. The a pathway represents the relationship between negative affect and total commission errors, the b pathway represents the relationship between total commission errors and total energy intake, and the c pathway represents the total relationship between negative affect and total energy intake. The c’ pathway represents the direct relationship between negative affect and total energy intake, after adjusting for total commission errors. The ab pathway represents the unstandardized indirect relationship between negative affect and total energy intake, through the total commission errors pathway. Models adjusted for age, sex, race, lean mass, and percentage fat mass. Total commission errors significantly mediated the relationship between negative affect and total energy intake.
3.3. Percentage Commission Errors on Neutral-Go Blocks
Mediation findings for commission errors on Neutral-Go blocks are shown in Table 2. Negative affect was not significantly associated with Neutral-Go commission errors (a = 0.01, SE = 0.02, p = .46). Neutral-Go commission errors were significantly positively associated with total energy intake (b = 342.7, SE = 148.2, p = .02), but not snack energy intake (b = 54.7, SE = 70.6, p = .44). Neutral-Go commission errors did not mediate the relationship between negative affect and total energy intake (ab = 3.9, SE = 5.5, unstandardized 95% CI = [−7.1, 15.6], standardized 95% CI = [−0.02, 0.04]), or snack energy intake (ab = 0.6, SE = 1.5, unstandardized 95% CI = [−2.4, 4.2], standardized 95% CI = [−0.01, 0.02]).
3.4. Percentage Commission Errors on Food-Go Blocks
Mediation findings for commission errors on Food-Go blocks are shown in Table 2. Negative affect was significantly associated with Food-Go commission errors (a = 0.04, SE = 0.01, p = .01). Food-Go commission errors were significantly associated with total energy intake (b = 599.5, SE = 153.0, p < .001), but not snack food energy intake (b = 71.7, SE = 74.8, p = .34). Food-Go commission errors significantly mediated the relationship between negative affect and total energy intake (ab = 23.9, SE = 9.5, unstandardized 95% CI = [7.7, 44.4], standardized 95% CI = [0.02, 0.10]; Figure 3), but not snack energy intake (ab = 2.9, SE = 3.2, unstandardized 95% CI = [−3.4, 9.5], standardized 95% CI = [−0.02, 0.05]).
Figure 3.
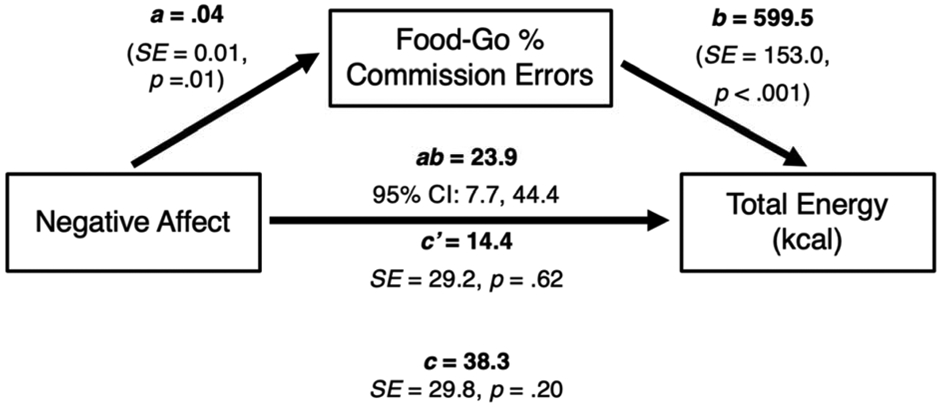
Cross-sectional mediation models with negative affect as the independent variable, Food-Go percent commission errors as the mediator, and total energy intake (kcal) as the dependent variable. The a pathway represents the relationship between negative affect and Food-Go commission errors, the b pathway represents the relationship between Food-Go commission errors and total energy intake, and the c pathway represents the total relationship between negative affect and total energy intake. The c’ pathway represents the direct relationship between negative affect and total energy intake, after adjusting for Food-Go commission errors. The ab pathway represents the unstandardized indirect relationship between negative affect and total energy intake, through the Food-Go commission errors pathway. Models adjusted for age, sex, race, lean mass, and percentage fat mass. Food-Go commission errors significantly mediated the relationship between negative affect and total energy intake.
Given the cross-sectional nature of the present mediation analyses, alternate exploratory follow-up analyses were conducted to examine whether negative affect mediated a relationship between inhibitory control and energy intake variables. Results from these analyses indicated that negative affect was not a significant mediator between any inhibitory control and energy intake variables (ps > .05), including between total commission errors and total energy (CI = [24.2, 115.8]) and snack intake (CI = [−4.6, 68.1]); commission errors on Neutral-Go blocks and total energy (CI = [−11.2, 89.6]) and snack intake (CI = [−5.6, 45.4]); and commission errors on Food-Go blocks and total energy (CI = [−46.5, 117.99]) and snack intake [CI = [−9.6, 79.0]).
4. Discussion
In our sample, negative affect was significantly positively associated with percent Food-Go commission errors in all models; however, negative affect was not associated with total commission errors or Neutral-Go commission errors. All types of commission errors were positively associated with total energy intake, while no type of commission error was significantly associated with snack energy intake. Total commission errors significantly mediated the relationship between negative affect and total intake, but not snack intake. More specifically, and contrary to hypotheses, this relationship appeared to be primarily driven by significant mediation from Food-Go commission errors, as the effect of Neutral-Go commission errors did not reach significance. Findings suggest that negative affect potentially contributes to poorer inhibitory control, which in turn influences overall energy intake.
Poorer inhibitory control has shown a link to disinhibited eating in adults (Nasser et al., 2004) and among pediatric samples, in that youth with obesity have shown decreased food-related inhibition in several studies (Batterink et al., 2010; Nederkoorn, Coelho, Guerrieri, Houben, & Jansen, 2012). Findings from the current study further support this relationship among healthy children and adolescents: all types of commission errors (i.e., total, Food-Go blocks, and Neutral-Go blocks), indicating poorer overall inhibitory control, were associated with greater energy intake, regardless of negative affect. Additionally, prior research utilizing the same neurocognitive task as the present study found that participants committed a greater percentage of commission errors on Neutral-Go (vs. food) blocks, compared to Food-Go (vs. neutral toys) blocks (Teslovich et al., 2014), which was in fact replicated in our sample (Figure 1). These prior data were interpreted as evidence that the greater salience of food cues interferes with behavioral inhibition more than non-food cues (Teslovich et al., 2014). However, this study was limited in its interpretation given it did not specifically examine in vivo eating behaviors in relation to inhibitory control, and only used pictures of food cues. Our study builds upon these previous findings and demonstrated that, although participants committed a greater percentage of commission errors on Neutral-Go compared to Food-Go blocks, all types of commission errors related to greater in vivo energy intake at a laboratory test meal, non-specific to cue type.
Our mediation findings are consistent with the hypothesis that negative affect may lead to poorer inhibitory control, which then contributes to greater food intake, demonstrating a more complete mechanistic model of disinhibited eating and lending support to the idea that inhibitory control may represent a modifiable neurocognitive mechanism for disinhibited eating behaviors driven by negative affect. In addition to targeting negative mood, clinicians may benefit from also utilizing supplementary interventions designed to target inhibitory control skills among children and adolescents with maladaptive overeating behaviors. These interventions could include response inhibition training, which may be an effective intervention to aid in prevention of excess weight gain by reducing food consumption (Lawrence, Verbruggen, Morrison, Adams, & Chambers, 2015); or implicit priming of higher level construal thinking, which has been shown to enhance self-control and reduce food consumption without inhibitory resource demand (Fujita, Trope, Liberman, & Levin-Sagi, 2006; Price, Higgs, & Lee, 2016). Even more interestingly, when percentage of total commission errors were separated by type of Go vs. No-Go target cue, results showed that Food-Go commission errors significantly mediated the relationship between negative affect and energy intake, but Neutral-Go errors did not, contrary to hypotheses. Results of this nature seemingly contradict past research, which has interpreted blocks in which food cues are designated as “Go” and non-food toys are “No-Go” as a control condition of general inhibitory control (Teslovich et al., 2014). However, a control condition of this nature may be flawed in that it requires participants to consistently make speedy cued responses to images of food, which could contribute to “go-training effects,” a limitation that has been suggested in other published studies (Adams, Lawrence, Verbruggen, & Chambers, 2017). In theory, these go-training effects may increase food-related disinhibition as participants learn an approach response toward food cues (Adams et al., 2017). In light of the current findings, it is possible that negative affect may exacerbate these go-training effects by contributing to a stronger susceptibility to a learned approach response toward food as a maladaptive attempt to reduce negative emotions, subsequently contributing to disinhibited overeating.
It is possible the lack of significant findings for snack food intake may be due in part to use of a generally healthy sample of youth. The potentially mechanistic pathway between negative affect and inhibitory control may only drive snack intake among youth with both high weight and self-reported disinhibited eating (Shomaker et al., 2013; Shomaker et al., 2010; Tanofsky-Kraff et al., 2016) or those who meet full criteria for a mood or anxiety disorder (Khalid, Williams, & Reynolds, 2016; Weng et al., 2012). The laboratory test meal paradigm included in the current study was a multi-item buffet that varied broadly in macronutrient intake. It would be interesting for future studies to examine relationships between negative affect, inhibitory control, and snack food intake with use of alternate classical eating paradigms, such as a bogus taste test (Houben & Jansen, 2011; Lawrence, Verbruggen, Morrison, Adams, & Chambers, 2015). Bogus taste test paradigms often include primarily snack foods, and may have even more ecological validity for snack food intake than a multi-item buffet in which participants are instructed to “Let yourself go and eat as much as you want.” By comparison, bogus taste test instructions typically involve asking participants to sample different types of snack foods and rate their tastes, while being encouraged to eat as much as they want because leftovers will be discarded (Adams et al., 2017; Price, Lee, & Higgs, 2016). These instructions theoretically disguise the intention of the paradigm to measure intake, potentially capturing a more ecologically valid measure of naturalistic eating patterns.
This study had a number of strengths, including a racially diverse sample of boys and girls across the weight spectrum, as well as objective measures of body composition for covariates. Use of a well-validated and standardized laboratory buffet test meal designed to elicit disinhibited eating is considered a strength. Indeed, intake patterns during this test meal paradigm have been related to eating in the absence of hunger (Kelly et al., 2016), binge-eating (Mirch et al., 2006), loss-of-control eating (Tanofsky-Kraff et al., 2009), and emotional eating (Vannucci et al., 2013), suggesting that this paradigm may have implications for youth’s broader disinhibited eating habits and risk for adverse health outcomes. Use of self-report trait anxiety and depressive symptoms and use of food and neutral object photos in the Go/No-Go task paradigm are limitations. It could be of interest for future research to analyze momentary state negative affect, or interactions of state and trait negative affect, given it could exhibit a differential effect on inhibitory control. Cross-sectional mediation analyses limit the ability to draw any causal conclusions from the findings, and thus our findings require replication with prospective data. Given the cross-sectional nature of this analysis, it is also alternately possible that negative affect may mediate the relationship between inhibitory control and eating. Although follow-up exploratory analyses of the current cross-sectional data did not find that negative affect significantly mediated any relationships between inhibitory control and intake, future prospective data are needed to determine this relationship. Finally, it is possible that the nature of a single laboratory test meal may not reflect patterns of eating in the natural eating environment, thus potentially limiting the ecological validity of this paradigm. As such, results from the present study can only reflect relationships between negative affect, inhibitory control, and energy intake at a single meal.
In summary, the total percentage of commission errors, as well as percentage of commission errors specifically on Food-Go blocks, significantly mediated the relationship between negative affect and energy intake. These cross-sectional analyses lend support to affect theory, in that that negative affect may lead to a stronger tendency for an approach response toward food as a maladaptive attempt to seek comfort, which in turn may be associated with greater food intake. Future research should determine whether, in combination with approaches to reduce negative affect, improved inhibitory control could help prevent overeating in youths with depressive or anxiety symptoms.
Acknowledgments
Funding Sources: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number ZIA-HD00641; J. Yanovski). No funding sources had any role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: ClinicalTrials.gov ID#: NCT02390765
CONFLICT OF INTEREST: The authors have no conflicts of interest to declare. Dr. Yanovski reports grant support from Rhythm Pharmaceuticals, Inc. and Soleno Therapeutics, Inc. for work unrelated to the current submission. The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of USU, the United States Department of Defense, or the US Department of Health and Human Services.
References
- Adams RC, Lawrence NS, Verbruggen F, & Chambers CD (2017). Training response inhibition to reduce food consumption: Mechanisms, stimulus specificity and appropriate training protocols. Appetite, 109, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR (2011). From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biological Psychiatry, 69(12), e55–e68. doi: 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, & Robbins TW (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. doi: 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Barnes LL, Harp D, & Jung WS (2002). Reliability generalization of scores on the Spielberger State-Trait Anxiety Inventory. Educational and Psychological Measurement, 62(4), 603–618. [Google Scholar]
- Batterink L, Yokum S, & Stice E (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. 52(4), 1696–1703. doi: 10.1016/j.neuroimage.2010.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens JT (1997). Principles and procedures of exploratory data analysis. Psychological Methods, 2(2), 131–160. [Google Scholar]
- Bora E, Harrison BJ, Yücel M, & Pantelis C (2013). Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychological Medicine, 43(10), 2017–2026. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, & Lask BD (1996). The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders, 19(4), 391–397. [DOI] [PubMed] [Google Scholar]
- Burke NL, & Storch EA (2015). A meta-analysis of weight status and anxiety in children and adolescents. Journal of Developmental & Behavioral Pediatrics, 36(3), 133–145. [DOI] [PubMed] [Google Scholar]
- Byrne ME, Shomaker LB, Brady SM, Kozlosky M, Yanovski JA, & Tanofsky-Kraff M (2020). Associations between latent trait negative affect and patterns of food-intake among girls with loss-of-control eating. International Journal of Eating Disorders, 53, 618–624. doi: 10.1002/eat.23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME, Tanofsky-Kraff M, Kelly NR, Grammer AC, Jaramillo M, Mi SJ, … Yanovski JA (2019). Pediatric Loss-of-Control Eating and Anxiety in Relation to Components of Metabolic Syndrome. Journal of Pediatric Psychology, 44(2), 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Faulstich ME, Gresham FM, Ruggiero L, & Enyart P (1987). Children's Depression Inventory: Construct and discriminant validity across clinical and nonreferred (control) populations. Journal of Consulting and Clinical Psychology, 55(5), 755. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, … Sallis JF (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine & Science in Sports & Exercise, 35(8), 1381–1395. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Racine SE, & Klump KL (2015). Research Review: What we have learned about the causes of eating disorders–a synthesis of sociocultural, psychological, and biological research. Journal of Child Psychology and Psychiatry, 56(11), 1141–1164. [DOI] [PubMed] [Google Scholar]
- Cyders MA, & Smith GT (2008). Emotion-based dispositions to rash action: positive and negative urgency. Psychological Bulletin, 134(6), 807–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CA, Tanofsky-Kraff M, Shomaker LB, Columbo KM, Wolkoff LE, Ranzenhofer LM, & Yanovski JA (2010). An examination of the interpersonal model of loss of control eating in children and adolescents. Behaviour Research and Therapy, 48(5), 424–428. doi: 10.1016/j.brat.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, & Cooper Z (1993). The Eating Disorder Examination (12th edition). In Fairburn CG & Wilson GT (Eds.), Binge eating: Nature, assessment, and treatment. (pp. 317–360). New York, NY, US: Guilford Press. [Google Scholar]
- Fujita K, Trope Y, Liberman N, & Levin-Sagi M (2006). Construal levels and self-control. Journal of Personality and Social Psychology, 90(3), 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky-Kraff M, & Wilfley DE (2008). Disordered eating attitudes and behaviors in overweight youth. Obesity, 16(2), 257–264. doi: 10.1038/oby.2007.48 [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Smith KE, Crosby RD, Boyd HK, Dougherty E, Engel SG, & Haedt-Matt A (2018). Ecological momentary assessment of maladaptive eating in children and adolescents with overweight or obesity. International Journal of Eating Disorders, 51(6), 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedt-Matt AA, & Keel PK (2011). Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychological Bulletin, 137(4), 660–681. doi: 10.1037/a0023660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann AS, Czaja J, Rief W, & Hilbert A (2010). Personality and psychopathology in children with and without loss of control over eating. Comprehensive Psychiatry, 51(6), 572–578. [DOI] [PubMed] [Google Scholar]
- Hartmann AS, Rief W, & Hilbert A (2012). Laboratory snack food intake, negative mood, and impulsivity in youth with ADHD symptoms and episodes of loss of control eating. Where is the missing link? Appetite, 58(2), 672–678. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2017). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach: Guilford Publications. [Google Scholar]
- Heatherton TF, & Baumeister RF (1991). Binge eating as escape from self-awareness. Psychological Bulletin, 110(1), 86. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Rief W, Tuschen-Caffier B, de Zwaan M, & Czaja J (2009). Loss of control eating and psychological maintenance in children: An ecological momentary assessment study. Behaviour Research and Therapy, 47(1), 26–33. doi: 10.1016/j.brat.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Hruby A, Manson JE, Qi L, Malik VS, Rimm EB, Sun Q, … Hu FB (2016). Determinants and consequences of obesity. American Journal of Public Health, 106(9), 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. (2006). Dietary reference intakes: the essential guide to nutrient requirements (Meyers LD, Hellwig JP, & Otten JJ Eds.). Washington, DC: National Academies Press. [Google Scholar]
- Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, … Hillman CH (2012). The Relation of Adiposity to Cognitive Control and Scholastic Achievement in Preadolescent Children. Obesity, 20(12), 2406–2411. doi: 10.1038/oby.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kelly NR, Jaramillo M, Ramirez S, Altman DR, Rubin SG, Yang SB, … Yanovski JA (2020). Executive functioning and disinhibited eating in children and adolescents. Pediatric Obesity, e12614. doi: 10.1111/ijpo.12614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NR, Shomaker LB, Radin RM, Thompson KA, Cassidy OL, Brady S, … Yanovski JA (2016). Associations of sleep duration and quality with disinhibited eating behaviors in adolescent girls at-risk for type 2 diabetes. Eating Behaviors, 22, 149–155. doi: 10.1016/j.eatbeh.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenardy J, Arnow B, & Agras WS (1996). The aversiveness of specific emotional states associated with binge-eating in obese subjects. Australian and New Zealand Journal of Psychiatry, 30(6), 839–844. [DOI] [PubMed] [Google Scholar]
- Khalid S, Williams CM, & Reynolds SA (2016). Is there an association between diet and depression in children and adolescents? A systematic review. British Journal of Nutrition, 116(12), 2097–2108. doi: 10.1017/s0007114516004359 [DOI] [PubMed] [Google Scholar]
- Kovacs M (1992). The Children’s Depression Inventory (CDI) manual. Toronto, Canada. [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL (2002). 2000 CDC Growth Charts for the United States: methods and development. Vital and Health Statistics, 11(246), 1–190. [PubMed] [Google Scholar]
- Lawrence NS, Verbruggen F, Morrison S, Adams RC, & Chambers CD (2015). Stopping to food can reduce intake. Effects of stimulus-specificity and individual differences in dietary restraint. Appetite, 85, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay-Russell S, Tanofsky-Kraff M, Schvey NA, Kelly NR, Shank LM, Mi SJ, … Yanovski JA (2019). Associations of Weekday and Weekend Sleep with Children’s Reported Eating in the Absence of Hunger. Nutrients, 11(7), 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Rustemeier M, Paslakis G, Pietrowsky R, Muller A, & Herpertz S (2018). Mood and restrained eating moderate food-associated response inhibition in obese individuals with binge eating disorder. Psychiatry Research, 264, 346–353. [DOI] [PubMed] [Google Scholar]
- Mallorquí-Bagué N, Testa G, Lozano-Madrid M, Vintró-Alcaraz C, Sánchez I, Riesco N, … Megías-Robles A (2020). Emotional and non-emotional facets of impulsivity in eating disorders: From anorexia nervosa to bulimic spectrum disorders. European Eating Disorders Review. [DOI] [PubMed] [Google Scholar]
- Mirch MC, McDuffie JR, Yanovski SZ, Schollnberger M, Tanofsky-Kraff M, Theim KR, … Yanovski JA (2006). Effects of binge eating on satiation, satiety, and energy intake of overweight children. The American Journal of Clinical Nutrition, 84(4), 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Ollendick T, King N, & Bogie N (2002). Three traditional and three new childhood anxiety questionnaires: Their reliability and validity in a normal adolescent sample. Behaviour Research and Therapy, 40(7), 753–772. [DOI] [PubMed] [Google Scholar]
- Nasser JA, Gluck ME, & Geliebter A (2004). Impulsivity and test meal intake in obese binge eating women. Appetite, 43(3), 303–307. doi: 10.1016/j.appet.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, & Jansen A (2006). Why obese children cannot resist food: The role of impulsivity. Eating Behaviors, 7(4), 315–322. doi: 10.1016/j.eatbeh.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Coelho JS, Guerrieri R, Houben K, & Jansen A (2012). Specificity of the failure to inhibit responses in overweight children. Appetite, 59(2), 409–413. doi: 10.1016/j.appet.2012.05.028 [DOI] [PubMed] [Google Scholar]
- Nelson TD, Aylward BS, & Steele RG (2007). Structural equation modeling in pediatric psychology: Overview and review of applications. Journal of Pediatric Psychology, 33(7), 679–687. doi: 10.1093/jpepsy/jsm107 [DOI] [PubMed] [Google Scholar]
- Osborne JW, & Overbay A (2004). The power of outliers (and why researchers should always check for them). Practical Assessment, Research, and Evaluation, 9(1), 1–8. [Google Scholar]
- Pauli-Pott U, Albayrak Ö, Hebebrand J, & Pott W (2010). Association between Inhibitory Control Capacity and Body Weight in Overweight and Obese Children and Adolescents: Dependence on Age and Inhibitory Control Component. Child Neuropsychology, 16(6), 592–603. doi: 10.1080/09297049.2010.485980 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Kelley K (2011). Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychological Methods, 16(2), 93. [DOI] [PubMed] [Google Scholar]
- Price M, Higgs S, & Lee M (2016). Snack intake is reduced using an implicit, high-level construal cue. Health Psychology, 35(8), 923. [DOI] [PubMed] [Google Scholar]
- Price M, Lee M, & Higgs S (2016). Food-specific response inhibition, dietary restraint and snack intake in lean and overweight/obese adults: a moderated-mediation model. International Journal of Obesity, 40, 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenhofer LM, Engel SG, Crosby RD, Anderson M, Vannucci A, Cohen LA, … Tanofsky-Kraff M (2014). Using ecological momentary assessment to examine interpersonal and affective predictors of loss of control eating in adolescent girls. International Journal of Eating Disorders, 47(7), 748–757. doi: 10.1002/eat.22333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenhofer LM, Hannallah L, Field SE, Shomaker LB, Stephens M, Sbrocco T, … Tanofsky-Kraff M (2013). Pre-meal affective state and laboratory test meal intake in adolescent girls with loss of control eating. Appetite, 68, 30–37. doi: 10.1016/j.appet.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MH, Nadler EP, & Mackey ER (2018). Impulse Control in Negative Mood States, Emotional Eating, and Food Addiction are Associated with Lower Quality of Life in Adolescents with Severe Obesity. Journal of Pediatric Psychology, 43(4), 443–451. doi: 10.1093/jpepsy/jsx127 [DOI] [PubMed] [Google Scholar]
- Shank LM, Tanofsky-Kraff M, Kelly NR, Jaramillo M, Rubin SG, Altman DR, … Broadney MM (2019). The association between alexithymia and eating behavior in children and adolescents. Appetite, 142, 104381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Mooreville M, Reina SA, Courville AB, Field SE, … Yanovski JA (2013). Links of adolescent-and parent-reported eating in the absence of hunger with observed eating in the absence of hunger. Obesity, 21(6), 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Stern EA, Miller R, Zocca JM, Field SE, … Yanovski JA (2011). Longitudinal study of depressive symptoms and progression of insulin resistance in youth at risk for adult obesity. Diabetes Care, 34(11), 2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, & Yanovski JA (2011). Disinhibited eating and body weight in youth In Handbook of Behavior, Food and Nutrition (pp. 2183–2200). New York, NY: Springer. [Google Scholar]
- Shomaker LB, Tanofsky-Kraff M, Zocca JM, Courville AB, Kozlosky M, Columbo KM, … Yanovski JA (2010). Eating in the absence of hunger in adolescents: intake after a large-array meal compared with that after a standardized meal. The American Journal of Clinical Nutrition, 92(4), 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AS, Mulder C, Twisk JW, Van Mechelen W, & Chinapaw MJ (2008). Tracking of childhood overweight into adulthood: a systematic review of the literature. Obesity Reviews, 9(5), 474–488. [DOI] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Crosby RD, Engel SG, & Wonderlich SA (2019). A multimodal, naturalistic investigation of relationships between behavioral impulsivity, affect, and binge eating. Appetite, 136, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Mason TB, Johnson JS, Lavender JM, & Wonderlich SA (2018). A systematic review of reviews of neurocognitive functioning in eating disorders: The state-of-the-literature and future directions. International Journal of Eating Disorders, 51(8), 798–821. doi: 10.1002/eat.22929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, & Green BJ (1986). Normative and reliability data for the Children's Depression Inventory. Journal of Abnormal Child Psychology, 14(1), 25–39. [DOI] [PubMed] [Google Scholar]
- Sopher AB, Thornton JC, Wang J, Pierson RN, Heymsfield SB, & Horlick M (2004). Measurement of percentage of body fat in 411 children and adolescents: a comparison of dual-energy X-ray absorptiometry with a four-compartment model. Pediatrics, 113(5), 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1973). Manual for the State-trait Anxiety Inventory for Children. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Tanofsky-Kraff M, Crosby RD, Vannucci A, Kozlosky M, Shomaker LB, Brady SM, … Young JF (2016). Effect of adapted interpersonal psychotherapy versus health education on mood and eating in the laboratory among adolescent girls with loss of control eating. International Journal of Eating Disorders, 49(5), 490–498. doi: 10.1002/eat.22496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, … Yanovski JA (2009). Laboratory assessment of the food intake of children and adolescents with loss of control eating. The American Journal of Clinical Nutrition, 89(3), 738–745. doi: 10.3945/ajcn.2008.26886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Ranzenhofer LM, Yanovski SZ, Schvey NA, Faith M, Gustafson J, & Yanovski JA (2008). Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite, 51(1), 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Theim KR, Yanovski SZ, Bassett AM, Burns NP, Ranzenhofer LM, … Yanovski JA (2007). Validation of the emotional eating scale adapted for use in children and adolescents (EES-C). International Journal of Eating Disorders, 40(3), 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich T, Freidl EK, Kostro K, Weigel J, Davidow JY, Riddle MC, … Mayer L (2014). Probing behavioral responses to food: Development of a food-specific go/no-go task. Psychiatry Research, 219(1), 166–170. doi: 10.1016/j.psychres.2014.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen KR, Callesen MB, Hesse M, Kvamme TL, Pedersen MM, Pedersen MU, & Voon V (2018). Impulsivity traits and addiction-related behaviors in youth. Journal of Behavioral Addictions, 7(2), 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci A, Tanofsky-Kraff M, Crosby RD, Ranzenhofer LM, Shomaker LB, Field SE, … Yanovski SZ (2013). Latent profile analysis to determine the typology of disinhibited eating behaviors in children and adolescents. Journal of Consulting and Clinical Psychology, 81(3), 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci A, Tanofsky-Kraff M, Shomaker LB, Ranzenhofer LM, Matheson BE, Cassidy OL, … Yanovski JA (2012). Construct validity of the emotional eating scale adapted for children and adolescents. International Journal of Obesity, 36(7), 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence: PsychCorp. [Google Scholar]
- Weng T-T, Hao J-H, Qian Q-W, Cao H, Fu J-L, Sun Y, … Tao F-B (2012). Is there any relationship between dietary patterns and depression and anxiety in Chinese adolescents? Public Health Nutrition, 15(4), 673–682. [DOI] [PubMed] [Google Scholar]
- Yanovski JA (2015). Pediatric obesity. An introduction. Appetite, 93, 3–12. doi: 10.1016/j.appet.2015.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]


